Abstract
The association between variation in the fat mass and obesity-associated (FTO) gene and adulthood body mass index (BMI; weight (kg)/height (m)2) is well-replicated. More thorough analyses utilizing phenotypic data over the life course may deepen our understanding of the development of BMI and thus help in the prevention of obesity. The authors used a structural equation modeling approach to explore the network of variables associated with BMI from the prenatal period to age 31 years (1965–1997) in 4,435 subjects from the Northern Finland Birth Cohort 1966. The use of structural equation modeling permitted the easy inclusion of variables with missing values in the analyses without separate imputation steps, as well as differentiation between direct and indirect effects. There was an association between the FTO single nucleotide polymorphism rs9939609 and BMI at age 31 years that persisted after controlling for several relevant factors during the life course. The total effect of the FTO variant on adult BMI was mostly composed of the direct effect, but a notable part was also arising indirectly via its effects on earlier BMI development. In addition to well-established genetic determinants, many life-course factors such as physical activity, in spite of not showing mediation or interaction, had a strong independent effect on BMI.
Keywords: body mass index, molecular epidemiology, structural equation model
The prevalence of obesity is rapidly increasing in both developed and developing countries. Obesity predisposes people to many chronic diseases, such as the metabolic syndrome, type 2 diabetes, and cardiovascular disease (1). Recent progress in genome-wide association studies has led to the discovery of novel genetic variants associated with body mass index (BMI; weight (kg)/height (m)2) and increased risk of obesity (2–5). The strongest signals discovered to date are located in the fat mass and obesity-associated (FTO) gene, which was originally found within a study on type 2 diabetes genes, but the association was mediated by BMI (6). Since then the association between FTO and BMI has been replicated in several studies (3, 4, 7, 8). The association between FTO and BMI growth throughout the life course is still somewhat unclear, but some studies suggest that the effect starts to show at least as early as approximately age 7 years (9–11).
Genetic variants discovered so far explain only a small proportion of the variability in body weight. For instance, in the Northern Finland Birth Cohort 1966, variants in the FTO and melanocortin 4 receptor (MC4R) genes explain only 0.55% of the total variation in adult BMI (12). The heritability of BMI has been estimated to be moderate-to-high (40%–80%) (13, 14), so there are probably many common single nucleotide polymorphisms of comparable effect sizes yet to be identified and obviously stronger underlying rare variants that wait to be discovered (4). Meanwhile, it is important to study the interplay between life-course factors and the genetic variants discovered so far.
It has been suggested that the FTO gene plays a role in appetite regulation (15) and that it is associated with energy expenditure (16), energy intake (17, 18), and diminished satiety (19), whereas 2 recent studies found no evidence for an association between nutrition and FTO (20, 21). In several studies, investigators have also reported a significant effect of interaction between FTO and physical activity on BMI (22–26). However, in 1 relatively large study, Jonsson et al. (27) found no evidence for interaction between FTO and physical activity.
To identify mediators or modifiers of the genetic associations, carefully characterized cohorts are needed (28), as well as appropriate statistical methods for dealing with complex relations. Multiple regression analysis has often been used as a standard method, yet a model with several terms may produce biased and unstable estimates because of sparse data and multicollinearity (29). Standard multiple regression also ignores the presumed causal and temporal ordering of exposure variables and their interrelations (30) and thus can provide information only on direct effects conditional on all of the other variables in the model (31), whereas an appropriate path analysis can provide deeper insight into the interrelations of the variables, that is, indirect effects and mediation.
Structural equation modeling (SEM), which includes path analysis and latent variables (32), can be used to study associations between variables thought to be causally ordered along the life course. Within this modeling approach, variables are subdivided into background factors, such as parental characteristics; intermediate outcomes, such as BMI at birth and BMI during childhood; and distal outcomes, such as adult BMI. Formally, relations among these factors are specified via simultaneous equations, and then the covariance structure of the assumed model is estimated.
We used SEM to examine the effects of the FTO rs9939609 variant on adult BMI in a large, prospectively followed birth cohort on which we had data from the prenatal period to adulthood. Our aim was to obtain more information on the networks around the variables along the life course and a better understanding of the mechanisms by which the FTO polymorphism plays a role in weight gain, together with other factors. We also hypothesized that the effect of the FTO variant might be mediated or modified through behavioral factors that have been suggested to be associated with FTO, such as diet and physical activity.
MATERIALS AND METHODS
Participants
The study population consisted of persons belonging to the Northern Finland Birth Cohort 1966. Initially, all mothers with expected delivery dates in 1966 who were living in the 2 northernmost provinces of Finland, Oulu and Lapland, were invited; over 96% participated (12,055 mothers with 12,058 liveborn children) (33). Data on the prenatal and perinatal period were collected via questionnaires administered by local midwives in the antenatal clinics. In 1980, at the age of 14 years, all living cohort members with known addresses received postal questionnaires containing questions on their growth, health habits, and family situation. An abridged version of the questionnaire was sent to the parents in cases where the adolescent did not respond or to the school health nurse if neither the child nor the parent responded. The postal questionnaire was returned by 94% (n = 11,010) of the adolescents, 52% (n = 389) of the parents, and 97% (n = 354) of the nurses. In 1997, at the age of 31 years, the subjects received a postal questionnaire including questions on their health, lifestyle, and occupation, and it was returned by 75% (n = 8,767). At the same time, those subjects living in the original target area (northern Finland) or in the capital area (Helsinki) were invited to undergo a clinical examination, and 71% (n = 6,033) of those invited participated (34). At this point, blood samples were drawn and DNA was extracted for 5,753 subjects. For the present study, we included persons who had both genotype data and measured BMI data available at age 31 years. After exclusion of multiple births, 4,435 persons (2,137 men and 2,298 women) remained for analysis.
All participants gave their written, informed consent when DNA was taken at age 31 years. The University of Oulu ethics committee approved the study.
Genotyping
Genotyping of the samples was performed using the TaqMan single nucleotide polymorphism genotyping assay (Applied Biosystems, Warrington, United Kingdom) according to the manufacturer's protocol. Genotyping was carried out for 5,365 samples from the cohort. The allele frequencies of the single nucleotide polymorphism rs9939609 were not observed to deviate essentially from Hardy-Weinberg equilibrium (P = 0.33). The duplicate concordance rate was 99.9%, and the genotype success rate in the sample was 88% (n = 4,701).
Phenotypic, behavioral, and environmental variables
Data on variables related to the prenatal period or birth were obtained from the questionnaire targeted toward the mothers during pregnancy and supplemented after delivery. Maternal prepregnancy BMI and gestational age were treated as continuous variables in the analyses. The categorizations of parity, maternal smoking after the second month of pregnancy, familial socioeconomic status (based on the father's occupation, or on the mother's if single), and maternal hypertension during pregnancy are shown in Table 1.
Table 1.
Characteristics of the Northern Finland Birth Cohort 1966 Study Sample, 1965–1997
| Characteristic | Total | No. | % | Mean (SD) |
| FTO rs9939609 genotype | 4,435 | |||
| TT | 1,678 | 37.8 | ||
| AT | 2,068 | 46.6 | ||
| AA | 689 | 15.5 | ||
| Prenatal factors | ||||
| Maternal BMIa | 4,052 | 23.2 (3.2) | ||
| Maternal age, years | 4,427 | 28.2 (6.6) | ||
| Parity | 4,424 | |||
| 0 | 1,384 | 31.3 | ||
| 1–3 | 2,193 | 49.6 | ||
| ≥4 | 847 | 19.2 | ||
| Maternal smoking during second month of pregnancy | 4,325 | |||
| Nonsmoker | 3,715 | 85.9 | ||
| 1–10 cigarettes/day | 510 | 11.8 | ||
| >10 cigarettes/day | 100 | 2.3 | ||
| Blood pressure during pregnancy | 4,344 | |||
| Normotensive | 2,418 | 55.7 | ||
| Gestational hypertensionb | 779 | 17.9 | ||
| Elevated systolic blood pressure | 361 | 8.3 | ||
| Elevated diastolic blood pressure | 338 | 7.8 | ||
| Not determined/not known | 448 | 10.3 | ||
| Family SES | 4,403 | |||
| I + II (professional) | 1,027 | 23.3 | ||
| III (skilled worker) | 1,476 | 33.5 | ||
| IV (unskilled worker) | 951 | 21.6 | ||
| V (farmer) | 949 | 21.6 | ||
| Characteristics at birth | ||||
| Sex | 4,435 | |||
| Male | 2,137 | 48.2 | ||
| Female | 2,298 | 51.8 | ||
| BMI | 4,404 | 13.8 (1.3) | ||
| Gestational age, weeks | 4,278 | 40.1 (1.8) | ||
| Characteristics at age 14 years | ||||
| BMI | 3,957 | 19.4 (2.5) | ||
| Frequency of participation in sports | 4,191 | |||
| Daily | 738 | 17.6 | ||
| Every other day | 821 | 19.6 | ||
| Twice a week | 934 | 22.3 | ||
| Once a week | 688 | 16.4 | ||
| Less than once a week | 1,010 | 24.1 | ||
| Alcohol consumption | 4,238 | |||
| Nonregular intake | 4,125 | 97.3 | ||
| Regular intake | 113 | 2.7 | ||
| Smoking | 4,243 | |||
| Nonsmoker | 3,515 | 82.4 | ||
| Occasional smoker | 463 | 10.9 | ||
| Regular smoker | 265 | 6.3 | ||
| Family SES | 4,260 | |||
| I + II (professional) | 1,223 | 28.7 | ||
| III (skilled worker) | 1,463 | 34.3 | ||
| IV (unskilled worker) | 935 | 22.0 | ||
| V (farmer) | 639 | 15.0 | ||
| Characteristics at age 31 years | ||||
| BMI | 4,435 | 24.7 (4.3) | ||
| Quartile of physical activity, MET-hours per week | 4,022 | 14.8 (14.6) | ||
| 0–3.7 | 1,106 | 27.5 | ||
| 3.8–10.8 | 1,155 | 28.7 | ||
| 10.9–20.5 | 1,080 | 26.9 | ||
| ≥20.6 | 681 | 16.9 | ||
| Tertile of alcohol consumption, g/day | 4,296 | 9.2 (15.8) | ||
| Abstainer | 405 | 9.4 | ||
| 0.1–2.5 | 1,283 | 29.9 | ||
| 2.6–8.7 | 1,298 | 30.2 | ||
| ≥8.8 | 1,310 | 30.5 | ||
| Smoking | 4,162 | |||
| Nonsmoker | 2,385 | 57.3 | ||
| 1–10 cigarettes/day | 887 | 21.3 | ||
| >10 cigarettes/day | 890 | 21.4 | ||
| Unhealthy diet scorec | 4,395 | |||
| 0–1 | 1,380 | 31.4 | ||
| 2–3 | 2,495 | 56.8 | ||
| 4–5 | 520 | 11.8 | ||
| SES | 4,387 | |||
| I + II (professional) | 1,039 | 23.7 | ||
| III (skilled worker) | 1,354 | 30.9 | ||
| IV (unskilled worker) | 1,113 | 25.4 | ||
| V (farmer) | 159 | 3.6 | ||
| VI (other) | 722 | 16.5 |
Abbreviations: BMI, body mass index; FTO, fat mass and obesity-associated; MET, metabolic equivalent; SD, standard deviation; SES, socioeconomic status.
Weight (kg)/height (m)2.
Includes gestational hypertension, chronic hypertension, preeclampsia, and superimposed preeclampsia.
An unhealthy diet was defined as daily or almost daily consumption of sausage and less frequent (twice a week or less often) consumption of rye bread or crisp bread, fresh vegetables and salads, and berries or fruit. One point was given for each of these counts, and scores could range from 0 to 5.
Regarding variables assessed at age 14 years, we calculated BMI from self-reported height and weight and classified the adolescent's smoking status into 1) nonsmoker (never smoked or had tried smoking), 2) occasional smoker (smoked occasionally or about twice a week), and 3) regular smoker (smoked daily) (35). Alcohol consumption was classified into 1) nonconsumer (never drank alcohol, had merely tasted it, or had consumed alcohol occasionally) and 2) regular consumer (drank alcohol monthly or more often) (36). The subjects were asked how often they participated in sports after school hours and were classified into 5 physical activity groups: 1) daily, 2) every other day, 3) twice a week, 4) once a week, and 5) less than once a week (37). Familial socioeconomic status was coded similarly to prepregnancy socioeconomic status.
Weight and height at age 31 years were measured at the clinical examination, and BMI was calculated from those measurements. Data on other background variables were obtained from the postal questionnaire filled in at the same age. Smoking during the past year was categorized as 1) nonsmoker, 2) 1–10 cigarettes/day, or 3) >10 cigarettes/day. Alcohol consumption was measured with several questions on type, amount, and frequency of alcohol consumption, and the information was validated against 7-day food diaries. It was transformed into daily intake (g/day) (34) and was further classified into abstainers and consumers divided according to tertile of daily intake (Table 1). Frequencies of consumption of various types of food were also ascertained, and an unhealthy diet was defined as daily or almost daily consumption of sausage and less frequent (twice a week or less often) consumption of rye bread or crisp bread, fresh vegetables and salads, and berries or fruit. One point was given for each of these counts (38), and the unhealthy diet score, ranging from 0 to 5, was used in the analyses. The subjects were also asked about the frequency and duration of light and brisk physical activities. These data were transferred into metabolic equivalent (MET)-hours per week (39) and further classified into quartiles. In the calculations, an intensity value of 3 METs was used for light physical activity and 5 METs was used for brisk physical activity. The subject's own socioeconomic status was based on occupation and employment data. It was classified from I (high) to IV (low), plus farmers and others (student, pensioner, long-term unemployed, or not defined).
Statistical analyses
Associations between FTO rs9939609 and maternal prepregnancy BMI, BMI at birth, BMI at age 14 years, and BMI at age 31 years, respectively, were first analyzed with multiple linear regression models assuming an additive model for genotype. All of the outcome variables were natural logarithm-transformed to reduce skewness, and the analyses were adjusted for sex (BMI at ages 14 and 31 years) or sex and gestational age (BMI at birth). Results are presented as geometric mean values and 95% confidence intervals.
A SEM approach was used to model the assumed underlying relations between the studied variables. Four simultaneous equations were fitted with maternal prepregnancy BMI, BMI at birth, BMI at age 14 years, and BMI at age 31 years as the outcome variables and assuming additive models for the effect of FTO rs9939609. All data on the outcome variables were natural logarithm-transformed; hence, the results are presented as percentage changes with 95% confidence intervals. Estimation of the parameters was carried out by the method of maximum likelihood, assuming normal distributions for the outcome variables. The expectation-maximization algorithm (40) implemented in Mplus (41) was used in the analyses of incomplete data. The expectation-maximization algorithm relies on the assumption that data are missing at random (42). For comparison, we also estimated model parameters for complete cases only (data not shown). Model evaluations were carried out on the basis of the following goodness-of-fit indices: the comparative fit index and the root mean square error of approximation. The comparative fit index is a fit index that is independent of sample size, and the model is thought to have a good fit if the index exceeds 0.95 (43, 44). The root mean square error of approximation measures the discrepancy between the model and the observed covariance matrix and is expressed per degree of freedom, thus taking into account the complexity of the model. A value less than 0.05 indicates a good fit (45). Modification indices (46) were used in detecting model misspecifications. All of the analyses were conducted with SAS, version 9.1.3 (SAS Institute Inc., Cary, North Carolina) and Mplus, version 3.12 (41). All P values reported are 2-sided.
RESULTS
The distributions of FTO rs9939609, BMI, and background factors are presented in Table 1. Of the 4,435 study subjects, 46.6% were heterozygous for the risk A allele and 15.5% were AA homozygous. Data on maternal BMI were available for 4,052 subjects, with a mean of 23.2 (standard deviation (SD), 3.2). The subjects’ mean BMIs were 13.8 (SD, 1.3) at birth, 19.4 (SD, 2.5) at age 14 years, and 24.7 (SD, 4.3) at age 31 years.
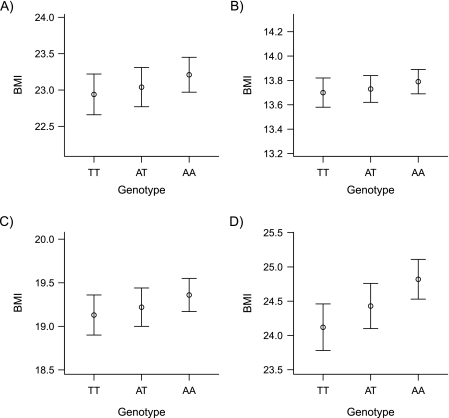
Figure 1 shows the geometric mean BMIs and back-transformed 95% confidence intervals according to FTO rs9939609 genotype. Because the Northern Finland Birth Cohort 1966 was part of the database used in the replication of the original FTO finding, these associations between BMI at ages 14 years and 31 years were reported previously by Frayling et al. (6). We additionally included maternal BMI and BMI at birth in the analyses. Carrying the risk A allele was associated with a 1.40% (95% confidence interval (CI): 0.72, 2.09) higher BMI at age 31 years (per A-allele change from an adjusted additive model corresponding to a 0.34-unit (95% CI: 0.18, 0.51) higher BMI, P = 5.1 × 10−5) and a 0.58% (95% CI: 0.00, 1.16) higher BMI at age 14 years (0.11-unit higher BMI (95% CI: 0.00, 0.22), P = 0.05). Weaker evidence of effects pointing in the same direction on maternal BMI and BMI at birth were also observed (maternal BMI: 0.55% (95% CI: −0.05, 1.14), corresponding to 0.11 units (95% CI: −0.01, 0.22), P = 0.07; BMI at birth: 0.32% (95% CI: −0.09, 0.72), corresponding to 0.04 units (95% CI: −0.01, 0.10), P = 0.12).
Figure 1.
Relation between the fat mass and obesity-associated (FTO) rs9939609 genotype and A) maternal body mass index (BMI; weight (kg)/height (m)2); B) the subject's own BMI at birth; C) BMI at age 14 years; and D) BMI at age 31 years in the Northern Finland Birth Cohort 1966, 1965–1997. Data are presented as geometric mean values (circles) with back-transformed 95% confidence intervals (bars). Results were adjusted for sex and gestational age (for birth BMI) or sex only (for BMI at ages 14 and 31 years).
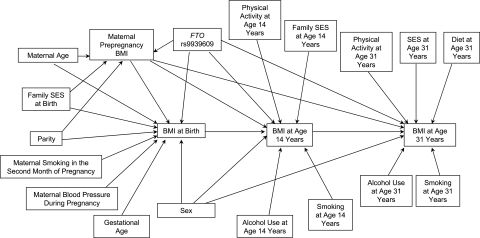
The SEM fitted to the data is depicted in Figure 2. It shows the relations we assumed to be underlying among the available variables in our study sample, based on previous knowledge of the associations (38, 47) and the correlation structure of the variables. Note that we also specified a relation between child's genotype and maternal BMI, because half of the child's genotype is inherited from the mother, and thus it partly represents the mother's genotype. The initial model was modified by removing nonsignificant associations whose inclusion would have worsened the overall model fit considerably and by adding new paths based on modification indices. Although we hypothesized that the FTO effect may be mediated through diet, unfortunately it was not possible to examine this adequately with our rather crude diet measurement, which showed no association with the FTO variant. Including a mediating path through diet would also have considerably worsened the overall model fit in terms of the comparative fit index (a drop from 0.92 to 0.74). Additionally, we tested for an interaction between the FTO variant and physical activity, but no evidence for it was observed (P > 0.20 for all interaction terms); thus, the terms were omitted from the final model. All of the mediating paths through BMI measurements were left in the model, although some of these paths showed only weak evidence of a direct association (the effect of FTO rs9939609 on BMI at birth and BMI at age 14 years).
Figure 2.
Structural equation model of relations between the fat mass and obesity-associated (FTO) rs9939609 genotype and body mass index (BMI; weight (kg)/height (m)2) fitted to data from the Northern Finland Birth Cohort 1966, 1965–1997. Four simultaneous equations were fitted using maternal BMI, BMI at birth, BMI at age 14 years, and BMI at age 31 years as the outcome variables. SES, socioeconomic status.
Because of the categorical nature of several variables in our analysis, we did not allow for correlations between variables and did not specify associations between sex, alcohol, smoking, and physical activity, for instance. When we conducted the analyses separately for men and women (data not shown), we observed sex differences in the estimated effects of these variables on BMI, but these differences did not influence the estimated effect of the FTO variant on BMI, which was our main interest in this study. Thus, we report results from the analysis conducted for men and women together.
For cross-validation of our results, we randomly assigned study subjects to a training sample and a validation sample (48); we first conducted the analyses in the training sample and then validated them in the other sample. Because the results did not differ substantially between the samples (data not shown), we merged the samples and report the results for the whole sample.
The model gave a good fit in terms of the root mean square error of approximation (0.025) and an adequate fit in terms of the comparative fit index (0.92). The regression coefficients conditional on all of the variables in the SEM analysis are shown in Table 2. Note that because of some very small effect sizes, the estimates are presented in 10−3 scale, the changes are presented in per mils (per 1,000), and the corresponding changes in mean BMI are presented in g/m2 instead of the conventional kg/m2. The standardized β coefficients are used to compare the relative importance of the independent variables, since they describe the change in the outcome variable in SD units per a 1-SD change in the continuous predictor and per the change from 0 to 1 in a binary predictor. These standardized coefficients suggest that FTO rs9939609 would have a modest effect on BMI in comparison with some of the early background exposures, which show a much stronger independent effect (e.g., the standardized regression coefficients for maternal BMI were 0.031 SD units for the FTO variant and 0.325 SD units for maternal age). However, for outcomes in adulthood, the estimated effect of the FTO variant on BMI was comparable with that of smoking and socioeconomic status (for example). The model including both genetic and life-course factors explained 20% of the total variation in maternal BMI and explained 16%, 5%, and 34% of the variation in BMI at birth, age 14 years, and age 31 years, respectively.
Table 2.
Results From Structural Equation Modeling of Relations Between the Fat Mass and Obesity-Associated (FTO) rs9939609 Genotype and Life-Course Data in the Northern Finland Birth Cohort 1966, 1965–1997
| Model | β (×10−3) | Standard Error (×10−3) | Standardized β | P Value | Change, ‰ | 95% CI | Corresponding Change in Mean BMI, g/m2 | 95% CI |
| Maternal BMIa,b | ||||||||
| FTO rs9939609 (additive model) | 6.00 | 2.73 | 0.031 | 0.03 | 6.18 | 0.64, 12.0 | 143 | 14.9, 279 |
| Maternal age, years | 6.53 | 0.39 | 0.325 | <0.001 | 6.75 | 5.94, 7.56 | 157 | 138, 176 |
| Parity | ||||||||
| 0 | Referent | |||||||
| 1–3 | 20.1 | 4.34 | 0.150 | <0.001 | 22.2 | 12.3, 33.1 | 516 | 285, 768 |
| ≥4 | 50.2 | 7.86 | 0.376 | <0.001 | 65.2 | 41.6, 92.7 | 1,512 | 965, 2,150 |
| Family SES at birth | ||||||||
| I + II (professional) | Referent | |||||||
| III (skilled worker) | 4.46 | 4.77 | 0.034 | 0.35 | 4.56 | −4.78, 14.8 | 106 | −111, 344 |
| IV (unskilled worker) | 11.8 | 5.76 | 0.088 | 0.04 | 12.5 | 0.51, 26.0 | 290 | 11.9, 602 |
| V (farmer) | 25.8 | 5.79 | 0.192 | <0.001 | 29.4 | 15.5, 44.9 | 682 | 360, 1,042 |
| BMI at birthb | ||||||||
| FTO rs9939609 (additive model) | 2.56 | 1.91 | 0.018 | 0.18 | 2.60 | −1.18, 6.52 | 35.8 | −16.3, 90.0 |
| Sex (female vs. male) | −6.00 | 2.70 | −0.062 | 0.03 | −5.83 | −10.7, −0.71 | −80.4 | −147, −9.80 |
| Gestational age, weeks | 15.2 | 0.89 | 0.288 | <0.001 | 16.4 | 14.4, 18.5 | 227 | 199, 255 |
| Log maternal BMI | 0.11 | 0.01 | 0.155 | <0.001 | 0.11 | 0.09, 0.14 | 1.60 | 1.20, 1.90 |
| Maternal age, years | −0.51 | 0.31 | −0.035 | 0.09 | −0.51 | −1.10, 0.09 | −7.00 | −15.2, 1.20 |
| Parity | ||||||||
| 0 | Referent | |||||||
| 1–3 | 30.8 | 3.42 | 0.316 | <0.001 | 36.0 | 27.2, 45.4 | 497 | 375, 627 |
| ≥4 | 43.6 | 5.53 | 0.447 | <0.001 | 54.6 | 38.7, 72.3 | 753 | 534, 997 |
| Maternal smoking | ||||||||
| Nonsmoker | Referent | |||||||
| 1–10 cigarettes/day | −11.9 | 4.45 | −0.124 | 0.01 | −11.2 | −18.7, −3.16 | −155 | −257, −43.6 |
| >10 cigarettes/day | −31.0 | 8.99 | −0.317 | 0.001 | −26.6 | −38.5, −12.5 | −367 | −531, −172 |
| Maternal blood pressure | ||||||||
| Normotensive | Referent | |||||||
| Gestational hypertensionc | −18.1 | 4.04 | −0.185 | <0.001 | −16.5 | −22.9, −9.63 | −228 | −316, −133 |
| Elevated systolic blood pressure | 8.11 | 4.98 | 0.083 | 0.10 | 8.44 | −1.64, 19.6 | 117 | −22.7, 270 |
| Elevated diastolic blood pressure | −1.68 | 5.52 | −0.019 | 0.76 | −1.66 | −11.8, 9.57 | −23.0 | −162, 132 |
| Not determined/not known | −3.57 | 4.81 | −0.036 | 0.46 | −3.51 | −12.2, 6.03 | −48.4 | −168, 83.1 |
| Family SES at birth | ||||||||
| I + II (professional) | Referent | |||||||
| III (skilled worker) | −10.3 | 3.65 | −0.106 | 0.01 | −9.82 | −16.1, −3.12 | −136 | −222, −43.1 |
| IV (unskilled worker) | −15.6 | 4.19 | −0.161 | <0.001 | −14.5 | −21.2, −7.13 | −199 | −293, −98.3 |
| V (farmer) | −15.3 | 4.29 | −0.156 | <0.001 | −14.1 | −21.1, −6.61 | −195 | −291, −91.2 |
| BMI at age 14 yearsb | ||||||||
| FTO rs9939609 (additive model) | 4.83 | 2.88 | 0.026 | 0.09 | 4.95 | −0.82, 11.1 | 96.1 | −15.8, 214 |
| Sex (female vs. male) | 8.07 | 4.11 | 0.062 | 0.05 | 8.40 | 0.02, 17.5 | 163 | 0.40, 339 |
| Log birth BMI | 0.11 | 0.02 | 0.080 | <0.001 | 0.11 | 0.06, 0.15 | 2.10 | 1.20, 2.90 |
| Log maternal BMI | 0.17 | 0.02 | 0.175 | <0.001 | 0.17 | 0.14, 0.20 | 3.30 | 2.60, 3.90 |
| Frequency of participation in sports at age 14 years | ||||||||
| Daily | Referent | |||||||
| Every other day | 1.60 | 6.47 | 0.013 | 0.80 | 1.61 | −10.5, 15.4 | 31.3 | −204, 298 |
| Twice a week | 0.99 | 6.21 | 0.007 | 0.87 | 1.00 | −10.6, 14.1 | 19.3 | −205, 273 |
| Once a week | 5.55 | 7.14 | 0.043 | 0.44 | 5.71 | −8.11, 21.6 | 111 | −157, 419 |
| Less than once a week | −1.61 | 6.75 | −0.012 | 0.81 | −1.60 | −13.8, 12.3 | −31.0 | −267, 239 |
| Alcohol consumption at age 14 years | ||||||||
| Nonregular intake | Referent | |||||||
| Regular intake | 31.6 | 12.6 | 0.248 | 0.01 | 37.2 | 7.18, 75.6 | 721 | 139, 1,467 |
| Smoking at age 14 years | ||||||||
| Nonsmoker | Referent | |||||||
| Occasional smoker | 18.0 | 6.27 | 0.138 | 0.004 | 19.7 | 5.86, 35.4 | 382 | 114, 686 |
| Regular smoker | 7.38 | 9.06 | 0.058 | 0.42 | 7.66 | −9.85, 28.6 | 149 | −191, 554 |
| Family SES at age 14 years | ||||||||
| I + II (professional) | Referent | |||||||
| III (skilled worker) | 2.08 | 4.86 | 0.017 | 0.67 | 2.10 | −7.18, 12.3 | 40.8 | −139, 239 |
| IV (unskilled worker) | −5.21 | 5.86 | −0.041 | 0.37 | −5.07 | −15.4, 6.49 | −98.4 | −298, 126 |
| V (farmer) | −1.76 | 6.13 | −0.014 | 0.77 | −1.75 | −12.9, 10.8 | −33.9 | −250, 210 |
| BMI at age 31 yearsb | ||||||||
| FTO rs9939609 (additive model) | 9.22 | 2.88 | 0.040 | 0.001 | 9.66 | 3.63, 16.0 | 239 | 89.6, 396 |
| Sex (female vs. male) | −44.8 | 4.77 | −0.278 | <0.001 | −36.1 | −41.8, −29.8 | −891 | −1,032, −737 |
| Log maternal BMI | 0.11 | 0.02 | 0.095 | <0.001 | 0.11 | 0.08, 0.15 | 2.80 | 2.00, 3.60 |
| Log BMI at age 14 years | 0.66 | 0.03 | 0.529 | <0.001 | 0.66 | 0.60, 0.72 | 16.4 | 14.9, 17.9 |
| Quartile of physical activity, MET-hours per week | ||||||||
| 0–3.7 | 26.5 | 6.83 | 0.165 | 0.001 | 30.4 | 14.0, 49.0 | 750 | 347, 1,211 |
| 3.8–10.8 | 15.1 | 5.98 | 0.093 | 0.01 | 16.3 | 3.41, 30.7 | 402 | 84.1, 759 |
| 10.9–20.5 | 2.39 | 5.59 | 0.015 | 0.67 | 2.42 | −8.21, 14.3 | 59.8 | −203, 353 |
| ≥20.6 | Referent | |||||||
| Tertile of alcohol consumption, g/day | ||||||||
| Abstainer | Referent | |||||||
| 0.1–2.5 | −3.70 | 8.39 | −0.022 | 0.66 | −3.63 | −18.2, 13.6 | −89.7 | −451, 336 |
| 2.6–8.7 | −7.56 | 8.16 | −0.048 | 0.35 | −7.29 | −21.0, 8.80 | −180 | −519, 217 |
| ≥8.8 | 8.93 | 8.43 | 0.054 | 0.29 | 9.34 | −7.32, 29.0 | 231 | −181, 716 |
| Smoking at age 31 years | ||||||||
| Nonsmoker | Referent | |||||||
| 1–10 cigarettes/day | 3.86 | 5.53 | 0.024 | 0.49 | 3.93 | −6.74, 15.8 | 97.1 | −167, 391 |
| >10 cigarettes/day | −9.52 | 5.94 | −0.059 | 0.11 | −9.08 | −19.1, 2.14 | −224 | −471, 52.8 |
| Unhealthy diet scored | 4.65 | 2.01 | 0.033 | 0.02 | 4.76 | 0.70, 8.97 | 118 | 17.3, 222 |
| SES at age 31 years | ||||||||
| I + II (professional) | Referent | |||||||
| III (skilled worker) | 15.4 | 5.67 | 0.094 | 0.01 | 16.6 | 4.34, 30.3 | 410 | 107, 749 |
| IV (unskilled worker) | 11.1 | 5.21 | 0.069 | 0.04 | 11.8 | 0.90, 23.8 | 290 | 22.3, 587 |
| V (farmer) | 18.7 | 11.3 | 0.118 | 0.10 | 20.5 | −3.48, 50.5 | 507 | −86.0, 1,248 |
| VI (other) | 19.8 | 6.88 | 0.124 | 0.004 | 21.9 | 6.52, 39.5 | 541 | 161, 976 |
Abbreviations: BMI, body mass index; CI, confidence interval; FTO, fat mass and obesity-associated; MET, metabolic equivalent; SES, socioeconomic status.
Weight (kg)/height (m)2.
The outcome variables maternal BMI and BMI at birth, age 14 years, and age 31 years were natural logarithm-transformed.
Includes gestational hypertension, chronic hypertension, preeclampsia, and superimposed preeclampsia.
An unhealthy diet was defined as daily or almost daily consumption of sausage and less frequent (twice a week or less often) consumption of rye bread or crisp bread, fresh vegetables and salads, and berries or fruit. One point was given for each of these counts, and scores could range from 0 to 5.
Table 3 shows the estimated indirect, direct, and total effects of the FTO variant on BMI, calculated assuming that the relations depicted in Figure 2 are correct. The total effects of FTO rs9939609 on maternal BMI, BMI at birth, and BMI at age 14 years were strengthened in comparison with the cross-sectional explorative analyses shown in Figure 1. For BMI at age 31 years, the evidence for the association remained strong (P = 5.0 × 10−5). The estimated direct effect of a per-A-allele change (conditional on all of the other variables in the model) on adult BMI was 0.97% (95% CI: 0.36, 1.60), which corresponds to an increase of 0.24 units (95% CI: 0.09, 0.40) in mean BMI. Indirect effects of the FTO variant were observed through maternal BMI, BMI at birth, and BMI at age 14 years (Figure 2, Table 3). The effects through BMI at birth were modest, since the association between the FTO variant and BMI at birth was of small magnitude (0.26%, 95% CI: −0.12, 0.65). Adding all of the indirect effects together, an increase of 0.49% (95% CI: 0.08, 0.92) in adult BMI was observed, which is equivalent to 0.12 units (95% CI: 0.02, 0.23). The total effect, which is the sum of the indirect and direct effects, was then 1.50% (95% CI: 0.75, 2.30), corresponding to a 0.37-unit (95% CI: 0.19, 0.57) increase in mean BMI.
Table 3.
Direct, Indirect, and Total Effects of Fat Mass and Obesity-Associated (FTO) Genotype rs9939609 on Body Mass Index During the Life Course in the Northern Finland Birth Cohort 1966, 1965–1997
| Model | β (×10−3) | Standard Error (×10−3) | Standardized β | P Value | Change, ‰ | 95% CI | Corresponding Change in Mean BMI, g/m2 | 95% CI |
| Maternal BMIa | ||||||||
| Total effect | 6.00 | 2.73 | 0.031 | 0.03 | 6.18 | 0.64, 12.0 | 143 | 14.9, 279 |
| BMI at birth | ||||||||
| Total effect | 3.24 | 1.94 | 0.023 | 0.09 | 3.30 | −0.56, 7.30 | 45.5 | −7.71, 101 |
| Total indirect effect | 0.68 | 0.32 | 0.005 | 0.03 | 0.68 | 0.06, 1.31 | 9.42 | 0.84, 18.1 |
| 1) FTO–maternal BMI–birth BMI | 0.68 | 0.32 | 0.005 | 0.03 | 0.68 | 0.06, 1.31 | 9.42 | 0.84, 18.1 |
| Direct effect | 2.56 | 1.91 | 0.018 | 0.18 | 2.60 | −1.18, 6.52 | 35.8 | −16.3, 90.0 |
| BMI at age 14 years | ||||||||
| Total effect | 6.19 | 2.95 | 0.033 | 0.04 | 6.39 | 0.41, 12.7 | 124 | 7.88, 23.5 |
| Total indirect effect | 1.36 | 0.55 | 0.007 | 0.01 | 1.37 | 0.28, 2.47 | 26.6 | 5.52, 47.8 |
| 1) FTO–maternal BMI–BMI at 14 | 1.02 | 0.48 | 0.005 | 0.03 | 1.02 | 0.08, 1.97 | 19.8 | 1.55, 38.3 |
| 2) FTO–birth BMI–BMI at 14 | 0.27 | 0.21 | 0.001 | 0.19 | 0.27 | −0.14, 0.68 | 5.28 | −2.67, 13.3 |
| 3) FTO–maternal BMI–birth BMI–BMI at 14 | 0.07 | 0.04 | 0.000 | 0.05 | 0.07 | 0.00, 0.14 | 1.40 | −0.01, 2.81 |
| Direct effect | 4.83 | 2.88 | 0.026 | 0.09 | 4.95 | −0.82, 11.1 | 92.1 | −15.8, 214 |
| BMI at age 31 years | ||||||||
| Total effect | 14.0 | 3.45 | 0.060 | 5.0 × 10−5 | 15.0 | 7.50, 23.1 | 371 | 185, 570 |
| Total indirect effect | 4.78 | 2.04 | 0.021 | 0.02 | 4.90 | 0.79, 9.17 | 121 | 19.6, 227 |
| 1) FTO–maternal BMI–BMI at 31 | 0.69 | 0.33 | 0.003 | 0.04 | 0.69 | 0.04, 1.34 | 17.0 | 1.02, 33.1 |
| 2) FTO–BMI at 14–BMI at 31 | 3.20 | 1.90 | 0.014 | 0.09 | 3.25 | −0.53, 7.17 | 80.2 | −13.0, 177 |
| 3) FTO–maternal BMI–BMI at 14–BMI at 31 | 0.67 | 0.32 | 0.003 | 0.03 | 0.67 | 0.05, 1.30 | 16.7 | 1.20, 32.2 |
| 4) FTO–birth BMI–BMI at 14–BMI at 31 | 0.18 | 0.14 | 0.001 | 0.19 | 0.18 | −0.09, 0.45 | 4.45 | −2.28, 11.2 |
| 5) FTO–maternal BMI–birth BMI–BMI at 14–BMI at 31 | 0.05 | 0.02 | 0.000 | 0.05 | 0.05 | 0.00, 0.10 | 1.19 | 0.02, 2.35 |
| Direct effect | 9.22 | 2.88 | 0.040 | 0.001 | 9.66 | 3.63, 16.0 | 239 | 89.6, 396 |
Abbreviations: BMI, body mass index; CI, confidence interval; FTO, fat mass and obesity-associated.
Weight (kg)/height (m)2.
Attrition and missing data
The subset of Northern Finland Birth Cohort 1966 subjects who participated in the clinical examination at age 31 years has been shown to be well-representative of the original study population (49). We further compared the distributions of all of the variables used in the present study between subjects who had complete data on all of the selected variables (n = 2,761) and those who had missing information on at least 1 of the variables (n = 1,674). With regard to the maternal characteristics, subjects with missing values on any of the variables used in the analyses were more likely to have a slightly older mother (mean age at delivery = 28.5 years vs. 28.0 years), a mother with more children (mean parity = 3.2 vs. 2.8), a mother belonging to a lower socioeconomic status group (proportion of unskilled workers = 25% vs. 20%), and a mother who was a heavy smoker during pregnancy (3.6% smoking >10 cigarettes/day vs. 1.6%) in comparison with subjects with complete data. The subjects with missing data on any of the variables used in the analyses were themselves more likely to be male (52% vs. 47%), to be physically inactive at age 14 years (28% vs. 22%), to be a regular smoker both at age 14 years (7.2% vs. 5.8%) and at age 31 years (28% vs. 18%), and to come from a lower socioeconomic status group both at age 14 years (proportion of unskilled workers = 25% vs. 20%) and at age 31 years (30% vs. 23%).
We fitted a SEM for complete cases only (n = 2,761; data not shown) and compared the estimates with those obtained from analysis including all cases. In general, all of the estimates pointed in the same direction and were approximately of the same magnitude as in the all-cases analysis, but the effect of the FTO variant on BMI at age 14 years was attenuated in the complete-case analyses (β = 1.22 × 10−3, 95% CI: −7.46 × 10−3, 9.89 × 10−3) as compared with the all-cases analyses (β = 4.83 × 10−3, 95% CI: −2.60 × 10−3, 12.3 × 10−3). Note that the variables identified as influencing the missingness mechanisms were included in the model used for the all-cases analysis, and therefore imbalances between completers and noncompleters were implicitly taken into account in the all-cases analyses.
DISCUSSION
We analyzed the effects of FTO rs9939609 on BMI in a large sample with good representation of the general population of northern Finland using SEM, taking advantage of data on a large selection of nongenetic exposures associated with BMI during the life course. This study provided positive evidence for an association between the FTO variant and adult BMI despite control for several factors during the life course, and weaker evidence for associations with BMI at age 14 years and maternal BMI.
In this study, we were not able to observe any mediation or modification of the genetic effect through potentially relevant health behavioral exposures. However, the study showed that nongenetic life-course factors are important determinants of BMI development in addition to genetic factors, since many of these factors had a strong independent effect on BMI. We identified mediation through earlier BMI development, which is in line with previous findings that the FTO polymorphism would start affecting BMI by the time of adolescence (6, 9–11). However, the effect of the FTO variant on adult BMI was not fully mediated via earlier BMI development, indicating that the variant continues to function actively over the later life course as well. This is an important observation, since age-varying associations may cause failure to replicate a genetic-association finding (50, 51).
Our inability to find evidence for any mediation or modification through behavioral variables may be due to the low precision of some of the variables available in the present study. For instance, our rather crude measure of unhealthy diet, which is a surrogate for total energy intake, was inadequate in the attempt to shed light on the contradictory findings about the association between nutrition and FTO. The quality of data seems to play a huge role in the detection of gene-environment interaction analyses (52). In addition, we acknowledge a need for bigger sample sizes, since complicated models with several parameters require quite substantial sample sizes, as do gene-environment interaction analyses (53, 54).
The estimation of indirect and direct effects assumes that the specified model is correct (55). We aimed to build a model that would be a good approximation of the reality by including several relevant variables in the model. An indication of successful selection and model-fitting, in spite of some inaccuracies in variable measurement, was the fact that (for instance) the assumed model explained 34% of the total variation in adult BMI. However, one drawback of these models is that they are difficult to replicate in other cohorts as such, because not many cohorts have similar data. Therefore, we conducted cross-validation within the study population itself as a sensitivity check of the estimates.
Our analyses were conducted using observations with missing values via the expectation-maximization algorithm in maximum likelihood estimation. This allowed us to use the data to their full potential, with a noteworthy increase in statistical power. This is important in studies utilizing life-course data, since attrition and missing values are common in data collected over a long period of time. The expectation-maximization algorithm relies on the assumption that data are missing at random, which cannot be tested in practice (56). However, we conducted the analysis for complete cases only, and the estimates obtained were of a similar direction and, for most of the parameters, a similar magnitude as those from the analysis using observations with missing values. Only the estimated effect of the FTO variant on BMI at age 14 years was notably attenuated in the complete-case analyses as compared with the all-cases analyses. However, the 95% confidence intervals for the parameter estimates from the analyses overlapped.
The other advantage of using SEM is that it deals with the collinearity problem efficiently. We had repeated measurements of several variables, and putting them into a single equation in the multiple regression analysis could have produced problems in estimation due to collinearity, as demonstrated in a similar kind of study including repeated BMI measurements by Gamborg et al. (57). By using SEM, we avoided this problem and also obtained interpretable estimates of indirect and direct effects.
Recently, Mendelian randomization (58) has been widely used to study the mediating effect of variables in genetic epidemiology, and SEM has been very rarely used to address causal questions. The commentary by Tu (59) also highlighted the underutilization of SEM in epidemiology. Tu concluded that SEM might be a step in the right direction in the field of epidemiology. Especially in the genetic field, the advantages of SEM have been utilized in systems biology, quantitative trait loci analysis, twin studies, animal models, and linkage analyses (e.g., see Stein et al. (60)), but its use in genetic epidemiology is still very limited. However, some promising studies that have investigated gene-environment interactions using this method have already been published (61, 62).
In conclusion, we estimated the effects of the FTO rs9939609 variant on BMI measurements taken over the life course in the largest study so far to collect extensive data from early pregnancy to adulthood, using a SEM approach, and we showed that the associations remain robust despite controlling for several relevant factors during the life course. Mediation of the FTO effect on adult BMI was observed via body mass development but not via an interaction with physical activity. Evidence for mediation or effect modification by diet could not be evaluated. More analyses of this kind should be carried out in large cohort studies with adequate statistical power that have carefully collected information over the life course. SEM proved to be an efficient analytical tool for modeling the complex networks around genetic and nongenetic variables.
Acknowledgments
Author affiliations: Institute of Health Sciences, Faculty of Medicine, University of Oulu, Oulu, Finland (Marika Kaakinen, Marjo-Riitta Järvelin); Biocenter Oulu, University of Oulu, Oulu, Finland (Marika Kaakinen, Karl-Heinz Herzig, Marjo-Riitta Järvelin); Department of Mathematical Sciences, Faculty of Science, University of Oulu, Oulu, Finland (Esa Läärä); Department of Lifecourse and Services, National Institute of Health and Welfare, Oulu, Finland (Anneli Pouta, Marjo-Riitta Järvelin); Institute of Clinical Medicine/Obstetrics and Gynaecology, Faculty of Medicine, University of Oulu, Oulu, Finland (Anna-Liisa Hartikainen); Finnish Institute of Occupational Health, Oulu, Finland (Jaana Laitinen, Tuija H. Tammelin); LIKES Research Center for Sport and Health Sciences, Jyväskylä, Finland (Tuija H. Tammelin); Institute of Biomedicine/Physiology, Faculty of Medicine, University of Oulu, Oulu (Karl-Heinz Herzig); Department of Psychiatry, Kuopio University Hospital, Kuopio, Finland (Karl-Heinz Herzig); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, United Kingdom (Ulla Sovio, Paul Elliott, Marjo-Riitta Järvelin); Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford, Oxford, United Kingdom (Amanda J. Bennett, Mark I. McCarthy); Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, United Kingdom (Mark I. McCarthy); Institute for Molecular Medicine, University of Helsinki, Helsinki, Finland (Leena Peltonen); Broad Institute, Harvard University and Massachusetts Institute of Technology, Cambridge, Massachusetts (Leena Peltonen); Wellcome Trust Sanger Institute, Cambridge, United Kingdom (Leena Peltonen); and Medical Statistics Unit, Department of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom (Bianca De Stavola).
This work was supported by the Academy of Finland (project grants 104781 and 120315 and the Center of Excellence in Complex Disease Genetics); University Hospital Oulu (Oulu, Finland); Biocenter Oulu, University of Oulu (Oulu, Finland); the European Commission (European Birth-Life Course Studies (EURO-BLCS), Framework 5 award QLG1-CT-2000-01643); the US National Heart, Lung, and Blood Institute (grant 5R01HL087679-02), through the STAMPEED Program (grant 1RL1MH083268-01); the US National Institute of Mental Health (grant 5R01MH63706:02); the ENGAGE Project (grant HEALTH-F4-2007-201413); and the United Kingdom Medical Research Council (studentship grant G0500539). DNA extraction, sample quality control, biobank upkeep, and aliquoting were performed at the National Public Health Institute, Biomedicum Helsinki (Helsinki, Finland); these activities were supported financially by the Academy of Finland and Biocentrum Helsinki.
The authors thank Professor Paula Rantakallio (launch of the Northern Finland Birth Cohort 1966 and initial data collection), Sarianna Vaara (data collection), Tuula Ylitalo (administration), Markku Koiranen (data management), Outi Tornwall, (DNA biobanking) and Minttu Jussila (DNA biobanking).
The authors recently lost their distinguished colleague and coauthor Prof. Leena Peltonen. They acknowledge her major contributions in human genetics.
Parts of this work were presented in poster form at the 2nd Nordic-Baltic Biometric Conference, Tartu, Estonia, June 10–12, 2009 and at the 59th Annual Meeting of the American Society for Human Genetics, Honolulu, Hawaii, October 20–24, 2009.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- FTO
fat mass and obesity-associated
- MET
metabolic equivalent
- SD
standard deviation
- SEM
structural equation modeling
References
- 1.Smith SC., Jr Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3 suppl 1):S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Loos RJ, Lindgren CM, Li S, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40(6):768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 4.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyre D, Delplanque J, Chèvre JC, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41(2):157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 6.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39(6):724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 8.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7) doi: 10.1371/journal.pgen.0030115. e115. (doi: 10.1371/journal.pgen.0030115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakanen M, Raitakari OT, Lehtimäki T, et al. FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity. J Clin Endocrinol Metab. 2009;94(4):1281–1287. doi: 10.1210/jc.2008-1199. [DOI] [PubMed] [Google Scholar]
- 10.Haworth CM, Carnell S, Meaburn EL, et al. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity (Silver Spring) 2008;16(12):2663–2668. doi: 10.1038/oby.2008.434. [DOI] [PubMed] [Google Scholar]
- 11.Jess T, Zimmermann E, Kring SI, et al. Impact on weight dynamics and general growth of the common FTO rs9939609: a longitudinal Danish cohort study. Int J Obes (Lond) 2008;32(9):1388–1394. doi: 10.1038/ijo.2008.110. [DOI] [PubMed] [Google Scholar]
- 12.Sabatti C, Service SK, Hartikainen AL, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41(1):35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjelmborg JB, Fagnani C, Silventoinen K, et al. Genetic influences on growth traits of BMI: a longitudinal study of adult twins. Obesity (Silver Spring) 2008;16(4):847–852. doi: 10.1038/oby.2007.135. [DOI] [PubMed] [Google Scholar]
- 14.Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29(1):49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- 15.Fredriksson R, Hägglund M, Olszewski PK, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149(5):2062–2071. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- 16.Do R, Bailey SD, Desbiens K, et al. Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes. 2008;57(4):1147–1150. doi: 10.2337/db07-1267. [DOI] [PubMed] [Google Scholar]
- 17.Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 2008;16(8):1961–1965. doi: 10.1038/oby.2008.318. [DOI] [PubMed] [Google Scholar]
- 18.Timpson NJ, Emmett PM, Frayling TM, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88(4):971–978. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardle J, Carnell S, Haworth CM, et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93(9):3640–3643. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 20.Stutzmann F, Cauchi S, Durand E, et al. Common genetic variation near MC4R is associated with eating behaviour patterns in European populations. Int J Obes (Lond) 2009;33(3):373–378. doi: 10.1038/ijo.2008.279. [DOI] [PubMed] [Google Scholar]
- 21.Johnson L, van Jaarsveld CH, Emmett PM, et al. Dietary energy density affects fat mass in early adolescence and is not modified by FTO variants. PLoS One. 2009;4(3) doi: 10.1371/journal.pone.0004594. e4594. (doi: 10.1371/journal.pone.0004594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57(1):95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 23.Cauchi S, Stutzmann F, Cavalcanti-Proença C, et al. Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. J Mol Med. 2009;87(5):537–546. doi: 10.1007/s00109-009-0451-6. [DOI] [PubMed] [Google Scholar]
- 24.Rampersaud E, Mitchell BD, Pollin TI, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med. 2008;168(16):1791–1797. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vimaleswaran KS, Li S, Zhao JH, et al. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am J Clin Nutr. 2009;90(2):425–428. doi: 10.3945/ajcn.2009.27652. [DOI] [PubMed] [Google Scholar]
- 26.Franks PW, Jablonski KA, Delahanty LM, et al. Assessing gene-treatment interactions at the FTO and INSIG2 loci on obesity-related traits in the Diabetes Prevention Program. Diabetologia. 2008;51(12):2214–2223. doi: 10.1007/s00125-008-1158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson A, Renström F, Lyssenko V, et al. Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults. Diabetologia. 2009;52(7):1334–1338. doi: 10.1007/s00125-009-1355-2. [DOI] [PubMed] [Google Scholar]
- 28.Manolio TA. Cohort studies and the genetics of complex disease. Nat Genet. 2009;41(1):5–6. doi: 10.1038/ng0109-5. [DOI] [PubMed] [Google Scholar]
- 29.Robins JM, Greenland S. The role of model selection in causal inference from nonexperimental data. Am J Epidemiol. 1986;123(3):392–402. doi: 10.1093/oxfordjournals.aje.a114254. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives [editorial] Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 31.De Stavola BL, Nitsch D, dos Santos Silva I, et al. Statistical issues in life course epidemiology. Am J Epidemiol. 2006;163(1):84–96. doi: 10.1093/aje/kwj003. [DOI] [PubMed] [Google Scholar]
- 32.Bollen KA. Structural Equations With Latent Variables. New York, NY: John Wiley & Sons, Inc; 1989. [Google Scholar]
- 33.Rantakallio P. Groups at risk in low birth weight infants and perinatal mortality. Acta Paediatr Scand. 1969;193(suppl):1–71. [PubMed] [Google Scholar]
- 34.Järvelin MR, Sovio U, King V, et al. Early life factors and blood pressure at age 31 years in the 1966 Northern Finland Birth Cohort. Hypertension. 2004;44(6):838–846. doi: 10.1161/01.HYP.0000148304.33869.ee. [DOI] [PubMed] [Google Scholar]
- 35.Isohanni I, Järvelin MR, Rantakallio P, et al. Juvenile and early adulthood smoking and adult educational achievements—a 31-year follow-up of the Northern Finland 1966 Birth Cohort. Scand J Public Health. 2001;29(2):87–95. doi: 10.1177/14034948010290020501. [DOI] [PubMed] [Google Scholar]
- 36.Rantakallio P. Family background to and personal characteristics underlying teenage smoking. Background to teenage smoking. Scand J Soc Med. 1983;11(1):17–22. doi: 10.1177/140349488301100104. [DOI] [PubMed] [Google Scholar]
- 37.Tammelin T, Laitinen J, Näyhä S. Change in the level of physical activity from adolescence into adulthood and obesity at the age of 31 years. Int J Obes Relat Metab Disord. 2004;28(6):775–782. doi: 10.1038/sj.ijo.0802622. [DOI] [PubMed] [Google Scholar]
- 38.Laitinen J, Pietiläinen K, Wadsworth M, et al. Predictors of abdominal obesity among 31-y-old men and women born in Northern Finland in 1966. Eur J Clin Nutr. 2004;58(1):180–190. doi: 10.1038/sj.ejcn.1601765. [DOI] [PubMed] [Google Scholar]
- 39.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington, DC: US Department of Health and Human Services; 2008. ( http://www.health.gov/paguidelines/pdf/paguide.pdf). (Accessed November 23, 2009) [Google Scholar]
- 40.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Series B Stat Methodol. 1977;39(1):1–38. [Google Scholar]
- 41.Muthén L, Muthén B. Mplus: Statistical Analysis With Latent Variables. Los Angeles, CA: Muthén and Muthén; 1998. [Google Scholar]
- 42.Little R, Rubin D. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 43.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 44.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 45.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage Publications; 1993. pp. 445–455. [Google Scholar]
- 46.Jöreskog KG, Sörbom D. LISREL 7: A Guide to the Program and Applications. Chicago, IL: SPSS, Inc; 1988. [Google Scholar]
- 47.Järvelin MR, Elliott P, Kleinschmidt I, et al. Ecological and individual predictors of birthweight in a Northern Finland Birth Cohort 1986. Paediatr Perinat Epidemiol. 1997;11(3):298–312. doi: 10.1111/j.1365-3016.1997.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 48.Cudeck R, Browne MW. Cross-validation of covariance structures. Multivariate Behav Res. 1983;18:147–167. doi: 10.1207/s15327906mbr1802_2. [DOI] [PubMed] [Google Scholar]
- 49.Sovio U, King V, Miettunen J, et al. Cloninger's temperament dimensions, socio-economic and lifestyle factors and metabolic syndrome markers at age 31 years in the Northern Finland Birth Cohort 1966. J Health Psychol. 2007;12(2):371–382. doi: 10.1177/1359105307074301. [DOI] [PubMed] [Google Scholar]
- 50.Lasky-Su J, Lyon HN, Emilsson V, et al. On the replication of genetic associations: timing can be everything! Am J Hum Genet. 2008;82(4):849–858. doi: 10.1016/j.ajhg.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyon HN, Emilsson V, Hinney A, et al. The association of a SNP upstream of INSIG2 with body mass index is reproduced in several but not all cohorts. PLoS Genet. 2007;3(4) doi: 10.1371/journal.pgen.0030061. e61. (doi: 10.1371/journal.pgen.0030061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong MY, Day NE, Luan JA, et al. The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? Int J Epidemiol. 2003;32(1):51–57. doi: 10.1093/ije/dyg002. [DOI] [PubMed] [Google Scholar]
- 53.Dempfle A, Scherag A, Hein R, et al. Gene-environment interactions for complex traits: definitions, methodological requirements and challenges. Eur J Hum Genet. 2008;16(10):1164–1172. doi: 10.1038/ejhg.2008.106. [DOI] [PubMed] [Google Scholar]
- 54.Freathy RM, Timpson NJ, Lawlor DA, et al. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes. 2008;57(5):1419–1426. doi: 10.2337/db07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole SR, Hernán MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 56.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 57.Gamborg M, Andersen PK, Baker JL, et al. Life course path analysis of birth weight, childhood growth, and adult systolic blood pressure. Am J Epidemiol. 2009;169(10):1167–1178. doi: 10.1093/aje/kwp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 59.Tu YK. Commentary: is structural equation modelling a step forward for epidemiologists? Int J Epidemiol. 2009;38(2):549–551. doi: 10.1093/ije/dyn346. [DOI] [PubMed] [Google Scholar]
- 60.Stein CM, Song Y, Elston RC, et al. Structural equation model-based genome scan for the metabolic syndrome. BMC Genet. 2003;31(4 suppl 1):S99. doi: 10.1186/1471-2156-4-S1-S99. (doi: 10.1186/1471-2156-4-S1-S99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song XY, Lee SY, Ma RC, et al. Phenotype-genotype interactions on renal function in type 2 diabetes: an analysis using structural equation modelling. Diabetologia. 2009;52(8):1543–1553. doi: 10.1007/s00125-009-1400-1. [DOI] [PubMed] [Google Scholar]
- 62.Nock NL, Larkin EK, Morris NJ, et al. Modeling the complex gene × environment interplay in the simulated rheumatoid arthritis GAW15 data using latent variable structural equation modeling. BMC Proc. 2007;1(suppl 1) doi: 10.1186/1753-6561-1-s1-s118. S118. (doi: 10.1186/1753-6561-1-s1-s118) [DOI] [PMC free article] [PubMed] [Google Scholar]