Abstract
Parasites have been argued to influence clutch size evolution, but past work and theory has largely focused on within-species optimization solutions rather than clearly addressing among-species variation. The effects of parasites on clutch size variation among species can be complex, however, because different parasites can induce age-specific differences in mortality that can cause clutch size to evolve in different directions. We provide a conceptual argument that differences in immunocompetence among species should integrate differences in overall levels of parasite-induced mortality to which a species is exposed. We test this assumption and show that mortality caused by parasites is positively correlated with immunocompetence measured by cell-mediated measures. Under life history theory, clutch size should increase with increased adult mortality and decrease with increased juvenile mortality. Using immunocompetence as a general assay of parasite-induced mortality, we tested these predictions by using data for 25 species. We found that clutch size increased strongly with adult immunocompetence. In contrast, clutch size decreased weakly with increased juvenile immunocompetence. But, immunocompetence of juveniles may be constrained by selection on adults, and, when we controlled for adult immunocompetence, clutch size decreased with juvenile immunocompetence. Thus, immunocompetence seems to reflect evolutionary differences in parasite virulence experienced by species, and differences in age-specific parasite virulence appears to exert opposite selection on clutch size evolution.
Keywords: life history, evolution, age-specific mortality
Understanding why evolved clutch size differs among species is a central issue in life history theory (1–6). Most attention has focused on the roles of food limitation (refs. 1–3; reviewed in ref. 4) and nest predation (5, 7, 8). Recently, however, parasitism has been suggested to play an important role in clutch size evolution (9–16). Investigators have argued that species that live in conditions with increased abundance of ectoparasites should evolve reduced clutch size (10, 17). However, if ectoparasites have long generation times, they are thought to favor increased clutch size (10, 11, 15–18). These previous arguments have neglected the potential age-specific differences in mortality of different parasites and pathogens and the well-established body of life history theory related to age-specific mortality (e.g., refs. 6 and 19). For example, species exposed to a greater abundance of parasites that attack juveniles probably incur greater juvenile mortality even if parasites have long generation times compared with species exposed to fewer parasites. The resulting increase in juvenile mortality can favor reduced clutch size among species (6, 19) rather than an increase in clutch size as predicted within species (11, 15, 16, 18). In short, the effects of parasites on clutch size can depend on their mortality effects and how exposure to this mortality differs across species.
Parasite-induced mortality is defined as virulence (20–22). The same parasite species can differ in virulence among host species, and different parasite species can vary in their virulence and abundance within and among host species in an age-specific way (20, 23–28) that yields different mortality effects on adults (e.g., refs. 13, 14, and 28) vs. juveniles (e.g., refs. 26 and 29). Consequently, no single parasite type will reflect the general and age-specific mortality effects of the full range of parasites to which species may be exposed. Given such complexities, then how do we assess potential effects of parasites? We believe the answer lies in assessing the general effects of parasites on mortality (i.e., virulence). Life history theory predicts that increases in adult mortality should favor an increase in clutch size and fecundity, whereas an increase in juvenile mortality should favor a decrease (6, 19, 30). These predictions have been verified by field studies and experiments (31, 32). Given that different parasites can exert different mortality effects on adults vs. juveniles (see above), then parasite effects should be examined in an age-specific context. However, this requires some means of assessing age-specific mortality effects induced by parasites. We suggest that one approach is to use immunocompetence as a general measure of parasite-induced mortality under the following logic.
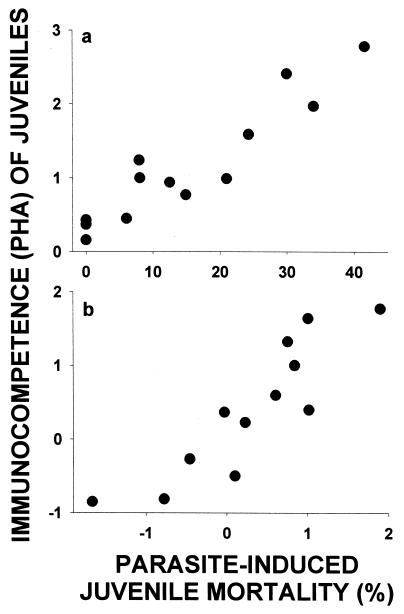
Intraspecific field studies have shown that mortality is reduced for individuals with increased immunocompetence (33–40), yielding a negative proximate relationship within species (Fig. 1a). Given that immunocompetence has a genetic component (41, 42), then selection should favor evolution of increased levels of immunocompetence in species that are exposed to higher parasite-induced mortality (Fig. 1a). Indeed, species that are subject to higher exposure to parasites because of their nest sites, sociality, migration habits, or geographic location show increased investment in immunocompetence (43–46). Thus, differences in immunocompetence among species should reflect the levels of mortality experienced (Fig. 1a) such that clutch size should decrease with juvenile immunocompetence, but increase with adult immunocompetence (Fig. 1b) under life history theory (i.e., refs. 6, 19, and 30).
Figure 1.
(a) Proximate (within-species) and evolutionary (among-species) relationships between immunocompetence and parasite virulence, which is defined as parasite-induced mortality. Studies show that individuals with higher immunocompetence have reduced mortality within species (33–40). Given this benefit, then species that are exposed to higher parasite virulence and, hence, that experience higher parasite-induced mortality should evolve greater immunocompetence. (b) Assuming that immunocompetence measured for juveniles vs. adults reflects levels of parasite-induced mortality experienced, then species with higher adult immunocompetence should evolve larger clutches, whereas species with higher juvenile immunocompetence should evolve smaller clutches under life history theory.
Here, we conduct an initial exploratory examination of these ideas and predictions. We begin by testing the assumption that immunocompetence increases with parasite-induced mortality. Then, we use measured levels of immunocompetence for juveniles vs. adults to examine whether they predict clutch size differences among species as predicted by life history theory (e.g., refs. 6, 9, and 30).
Methods
We first test the predicted relationship between immunocompetence and mortality (Fig. 1a). We needed a standardized measure of immunocompetence for comparisons. We used T lymphocyte cell-mediated responses to phytohaemagluttinin (PHA) as an initial assessment of immunocompetence. PHA tests of T cell responses seem a reasonable first approach to assessing immunocompetence because: (i) they have been measured across a diversity of species (Table 1), (ii) they have high repeatability (e.g., refs. 38, 47), (iii) they show genetic heritability (33), (iv) they are not confounded by parasite infection levels, like other measures such as immunoglobulins and white blood cells (e.g., see ref. 36), because studies that experimentally inoculated nests with parasites did not find a change in PHA compared with control nests (48, 49), (v) whereas PHA only measures one aspect (T cell) of immunocompetence, it has been show to be strongly correlated with other components of the immune system, such as B-cells, across species (50), and (vi) PHA measures of immunocompetence are positively correlated with survival of both nestlings (33, 35, 37, 39) and adults (34, 36, 38). The latter studies were tests within species and, thus, represent proximate responses, and we ask here whether the opposite evolutionary relationship exists among species (i.e., Fig. 1a). We obtained data on PHA responses of both adults and juveniles from published sources as well as supplementary unpublished data provided by the authors of published sources (Table 1). All data were collected by authors to test other hypotheses than the ones we test here. Data on mortality effects of parasites on adults are rare and, so, we focused on mortality effects of parasites on juveniles as a first approximation of whether immunocompetence is positively related to parasite virulence across species (i.e., Fig. 1a). We estimated parasite-induced mortality as the difference in number of young fledged in low or no parasite nests compared with nests with high parasite density. In particular, we subtracted the difference in number of young fledged in low vs. high parasite nests and divided by the mean number of young fledged in low parasite nests to obtain the percentage reduction in reproductive success from parasites. To test predicted relationships between clutch size vs. adult and juvenile immunocompetence (Fig. 1b), we obtained data on population estimates of clutch sizes of each species by using estimates from the same population where immunocompetence measures were taken whenever possible. Immune responses can increase with body mass (50), so we test for possible allometric (i.e., body mass) effects and control them when significant.
Table 1.
Clutch size (CS), mortality from parasites (Mort), phytohaemaglutinin measures of immunocompetence of young (Yg) and adults (Ad), body mass (Mass), and characterization of southern (South) hemisphere and hole-nesting (Hole) of study species, and references (Ref) for each species
| Species | CS | Mort | Yg | Ad | Mass | South | Hole | Ref |
|---|---|---|---|---|---|---|---|---|
| Delichon urbica | 4.03 | 24.30 | 1.59 | 0.40 | 18.3 | No | No | 51 |
| Ficedula hypoleuca | 5.73 | 6.03 | 0.45 | 0.20 | 12.5 | No | Yes | 52* |
| H. albigularis | 2.80 | 0.45 | 0.22 | 21.3 | Yes | No | 50 | |
| H. cucullata | 2.97 | 1.41 | 0.40 | 27.1 | Yes | No | 50 | |
| H. daurica | 4.50 | 12.50 | 0.94 | 19.1 | No | No | 50, 53 | |
| H. dimidiata | 2.40 | 0.75 | 0.25 | 11.0 | Yes | No | 50 | |
| H. fuligula | 2.80 | 1.32 | 0.30 | 22.4 | Yes | No | 50 | |
| H. rustica | 4.67 | 7.94 | 1.24 | 0.50 | 18.9 | No | No | 50, 54 |
| Luscinia svecica | 5.80 | 0.43 | 18.3 | No | No | 55† | ||
| Merops apiaster | 6.00 | 1.60 | 55.1 | No | No | 56‡ | ||
| Oenanthe leucura | 4.28 | 0 | 0.16 | 0.35 | 35.0 | No | No | 38 |
| Panurus biarmicus | 5.79 | 0.68 | 14.7 | No | No | C‡ | ||
| Parus major | 8.85 | 14.90 | 0.77 | 14.4 | No | Yes | 37, 49 | |
| Passer domesticus | 4.60 | 0.48 | 30.4 | No | No | 36 | ||
| Passer montanus | 4.95 | 30.00 | 2.41 | 23.5 | No | Yes | 57§ | |
| Petrochelidon pyrrhonota | 3.41 | 41.60 | 2.78 | 0.34 | 21.6 | No | No | 50, 58, 59 |
| Petrochelidon spilodera | 2.40 | 34.00 | 1.97 | 0.28 | 20.6 | Yes | No | 50, 60 |
| Pica pica | 6.10 | 0.79 | 224.0 | No | No | 39 | ||
| Riparia cincta | 3.20 | 0.90 | 0.31 | 21.5 | Yes | No | 50 | |
| R. paludicola | 2.90 | 0.93 | 0.40 | 13.1 | Yes | No | 50 | |
| R. riparia | 5.16 | 21.00 | 0.99 | 0.51 | 13.5 | No | No | 50, 61 |
| Serinus serinus | 4.00 | 0.80 | 11.5 | No | No | 62 | ||
| Sturnus vulgaris | 5.10 | 8.04 | 1.00 | 79.7 | No | Yes | 63 | |
| Tachycineta bicolor | 5.40 | 0 | 0.37 | 0.18 | 20.1 | No | Yes | 50 |
| Turdus merula | 4.04 | 1.28 | 95.9 | No | No | 64 |
J. Moreno, personal communication.
J. Lifjeld, personal communication.
H. Hoi, personal communication.
F. de Lope, personal communication.
Because we are testing potential evolutionary relationships across species, we corrected for possible phylogenetic effects using independent contrasts (i.e., ref. 65) based on regressions through the origin (i.e., refs. 5, 66, and 67). Our phylogenetic hypotheses for North American, European, and South African species were taken from other references (5, 50, 67).
Results
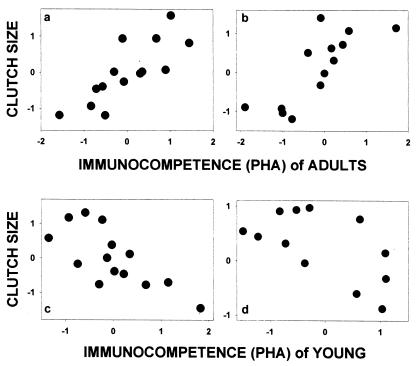
We found that parasite-induced mortality of young was strongly positively correlated to juvenile immunocompetence corrected for body mass across species (Fig. 2a). This relationship remained after phylogenetic effects were corrected by using independent contrasts (Fig. 2b). Given that differences in immunocompetence among species seem to reflect differences in parasite-induced mortality, we then tested the predicted relationships between immunocompetence and clutch size (i.e., Fig. 1b). We found that the two hole-nesting species were outliers when clutch size was examined relative to immunocompetence of adults across all species (Fig. 3a). Thus, we included hole-nesting as a dummy variable in the analysis, along with body mass, and found that clutch size was strongly positively related to immunocompetence of adults (Fig. 3b), and also, when possible phylogenetic effects were controlled by using independent contrasts (Fig. 3c).
Figure 2.
Immunocompetence of young (i.e., nestlings) is positively related to parasite virulence, as measured by parasite-induced mortality incurred by nestlings for (a) raw data (r = 0.94, P < 0.0001, n = 13) because mass is nonsignificant (rP = 0.33, P = 0.3, n = 13) and (b) corrected for possible phylogenetic effects using independent contrasts and body mass (rP = 0.89, P < 0.0001, n = 12).
Figure 3.
Clutch size is positively related to immunocompetence of adults as measured by phytohaemagluttinin (PHA) tests for (a) raw data, (b) when corrected for body mass and hole-nesting (rP = 0.73, P = 0.002, n = 17), and (c) when corrected for body mass, hole-nesting and possible phylogenetic effects using independent contrasts (rP = 0.73, P = 0.002, n = 16). Clutch size shows a nonsignificant tendency to be negatively related to immunocompetence of young (d) for raw data (r = −0.23, P = 0.31, n = 22) because body mass was nonsignificant (rP = 0.26, P = 0.25), and (e) when corrected for latitude (rP = −0.33, P = 0.15, n = 22), and (f) when corrected for body mass, latitude, and possible phylogenetic effects using independent contrasts (rP = −0.16, P = 0.49, n = 21). Solid symbols represent open-nesting north temperate species. Gray symbols represent open-nesting south temperate species, and open symbols represent hole-nesting north temperate species.
Clutch size was not correlated with immunocompetence of young when corrected for body mass (Fig. 3d). However, south temperate (South African) species showed an independent relationship (Fig. 3d). Yet, when latitude (Fig. 3e) as well as phylogenetic effects (Fig. 3f) were controlled, the correlation remained nonsignificant. The evolution of immunocompetence of young may be genetically correlated with the evolution of adult immunocompetence. If this is the case, then the independent variation in juvenile immunocompetence is best evaluated in a multiple regression in which the effect of adult immunocompetence is removed in addition to body mass, hole-nesting, and latitude. When possible effects of juvenile immunocompetence were removed, the correlation between clutch size and adult immunocompetence remained strongly positive (Fig. 4a) even when controlled for phylogeny (Fig. 4b). When the possible effects of adult immunocompetence were removed, we found a negative relationship between clutch size and immunocompetence of young (Fig. 4c), even when controlled for phylogeny (Fig. 4d).
Figure 4.
Clutch size was examined in a multiple regression with immunocompetence of adults and young, body mass, hole-nesting, and latitude. Clutch size (a) increased with adult immunocompetence (rP = 0.82, P = 0.002, n = 14) and (b) even when controlled for phylogeny using independent contrasts (rP = 0.74, P = 0.009, n = 13), whereas clutch size (c) decreased with juvenile immunocompetence (rP = −0.74, P = 0.010, n = 14) and (d) even when controlled for phylogeny using independent contrasts (rP = −0.55, P = 0.094, n = 13).
Discussion
Parasites differ in their age-specific virulence and, hence, in the direction and intensity of selection on clutch size, making a relationship between clutch size and any single parasite type unlikely. We argued that immunocompetence should integrate the overall level of age-specific parasite-induced mortality experienced by different species and, thus, yield a positive correlation between parasite-induced mortality and immunocompetence across species. This evolutionary prediction across species is opposite that predicted at the proximate level within species (i.e., Fig. 1a), but such opposing proximate and ultimate relationships are common (68). The negative proximate response has been clearly documented (33–40). The positive evolutionary relationship has been indirectly indicated by work showing increased investment in immunocompetence organs among species that are exposed to more parasites (43–46). Yet, given that parasites can differ in their virulence, exposure to more parasites does not directly address whether parasite-induced mortality is related to investment in immunocompetence. We directly tested this relationship and found that immunocompetence of juveniles was, in fact, strongly related to parasite-induced mortality of juveniles. The strength of this result is actually a bit surprising. First, parasite-induced mortality has been measured by comparing low abundance or absence of ectoparasites vs. high abundance. Yet, different studies increased or decreased ectoparasites to different extents, yielding an expectation of noise in mortality estimates. Moreover, studies often manipulated or studied effects of a single ectoparasite and, thus, may not have measured the full effects of differing parasites on juvenile mortality. To be sure, some studies were based on the cumulative effect of all ectoparasites, and remaining studies were based on the predominant parasite that may yield the strongest indication of overall parasite effects on mortality. We suspect that broader tests of this relationship may find a noisier one. Nonetheless, our results show that a clear signal exists and supports the assumption that immunocompetence reflects the relative levels of mortality selection from parasite and disease exposure.
Previous theory (e.g., refs. 10–11 and 17) has largely focused on the potential mortality effects of parasites on offspring. Yet, parasites can also affect adult survival (e.g., refs. 13, 14, 69), and life history theory predicts opposite directional selection from juvenile vs. adult mortality (5, 6, 19, 30) (see Fig. 1b). We found opposite responses of clutch size to mortality as indexed by immunocompetence of juveniles vs. adults (Fig. 4). The positive correlation between adult immunocompetence and clutch size was very strong once hole-nesting was taken into account. Hole-nesters were clear outliers, and it is likely that hole-nesting imposes independent selection (e.g., see refs. 1, 2, 5, and 67) yielding parallel relationships with different intercepts, but data from more species are needed to test such possibilities.
The raw relationship of clutch size with juvenile immunocompetence was very weak and differed between northern and southern regions (Fig. 3d). Yet, when adult immunocompetence was taken into account, the relationship between clutch size and juvenile immunocompetence became clear (Fig. 4 c and d), whereas controlling for juvenile immunocompetence did not really influence the relationship between clutch size and adult immunocompetence (Fig. 4). Such results may indicate that selection on adult immunocompetence may constrain expression of immunocompetence in juveniles; e.g., species that benefit from strong expression of immunocompetence by adults may begin development of this expression during early growth and thereby constrain juvenile expression of immunocompetence to relatively greater levels. Yet, the very weak or nonexistent raw relationships of juvenile immunocompetence with clutch size (Fig. 3) may also indicate the complexity of differing selection exerted by differing parasites (e.g., refs. 11, 15, 16, 18) and emphasize the need for much more research on the effects of parasites on clutch size evolution. Nonetheless, the very fact that the relationships of clutch size with adult vs. juvenile immunocompetence were strongly opposite (Fig. 4) provides compelling support for the notion that age-specific differences in selection caused by parasites and diseases can favor opposing selection on clutch size as predicted by life history theory.
Ultimately, our results suggest that parasites and diseases exert selection on differential expression of immunocompetence and clutch sizes among species. Other factors, such as nest predation and food limitation (1, 3–5, 8), also influence evolution of clutch size, and exploration into the relative roles and influences of the simultaneous action of these selective forces in shaping interspecific variation in clutch size evolution should prove a fertile area of future investigation. Indeed, some evidence shows that these factors may interact directly. For example, variation in food and feeding rates of young can influence the development of their immune system and ability to resist parasites (e.g., refs. 12, 33, 51, and 62). Moreover, development of the immune system may compete with growth and development of young (50, 70), but increases in length of developmental periods can increase exposure to predation risk. Conversely, high predation risk may favor increased developmental rates (1–5), which may constrain development of immunocompetence (70). On the other hand, other potential selective forces may not interact and instead yield separate selection on life histories; the fact that hole-nesting species were clear outliers (Fig. 3a) may reflect one environmental condition with differing selection pressures. In short, life history strategies are the result of multiple selection pressures, and immunocompetence and parasites represent one set of selection agents that can influence evolution of life histories in complex ways and, thus, deserve greater attention.
Acknowledgments
We thank two anonymous reviewers for helpful comments. This work was supported by the National Science Foundation (DEB-9981527 to T.E.M.), Centre National de la Recherche Scientifique (to T.E.M., A.P.M., and J.C.), and the Spanish Ministry of Education (project PB97-1233-coz-01 to S.M.).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lack D. Ibis. 1947;90:25–45. [Google Scholar]
- 2.Lack D. The Natural Regulation of Animal Numbers. Oxford: Clarendon; 1954. [Google Scholar]
- 3.Lack D. Ecological Adaptations for Breeding in Birds. London: Methuen; 1968. [Google Scholar]
- 4.Martin T E. Annu Rev Ecol Syst. 1987;18:453–487. [Google Scholar]
- 5.Martin T E. Ecol Monogr. 1995;65:101–127. [Google Scholar]
- 6.Roff D A. Evolution of Life Histories. Englewood Cliffs, NJ: Prentice–Hall; 1992. [Google Scholar]
- 7.Slagsvold T. Oecologia. 1982;54:159–169. doi: 10.1007/BF00378388. [DOI] [PubMed] [Google Scholar]
- 8.Martin T E, Martin P R, Olson C R, Heidinger B J, Fontaine J J. Science. 2000;287:1482–1485. doi: 10.1126/science.287.5457.1482. [DOI] [PubMed] [Google Scholar]
- 9.Møller A P. Funct Ecol. 1991;5:351–359. [Google Scholar]
- 10.Poiani A. Oikos. 1993;68:455–462. [Google Scholar]
- 11.Richner H, Heeb P. Oikos. 1995;73:435–441. [Google Scholar]
- 12.Christe P, Richner H, Oppliger A. Behav Ecol. 1996;7:127–131. [Google Scholar]
- 13.Oppliger A, Christe P, Richner H. Nature (London) 1996;381:565. doi: 10.1038/381565a0. [DOI] [PubMed] [Google Scholar]
- 14.Oppliger A, Christe P, Richner H. Behav Ecol. 1997;8:148–152. [Google Scholar]
- 15.Perrin N, Christe P, Richner H. Oikos. 1996;75:317–320. [Google Scholar]
- 16.Richner H, Tripet F. Oikos. 1999;86:535–538. [Google Scholar]
- 17.Moss W W, Camin J H. Science. 1970;168:1000–1003. doi: 10.1126/science.168.3934.1000. [DOI] [PubMed] [Google Scholar]
- 18.Richner H. Zoology. 1998;101:333–344. [Google Scholar]
- 19.Law R. Am Nat. 1979;114:399–417. [Google Scholar]
- 20.Anderson R M, May R M. Infectious Diseases of Humans: Dynamics and Control. Oxford, U.K.: Oxford Univ. Press; 1991. [Google Scholar]
- 21.Read A F. Trends Microbiol. 1994;2:73–76. doi: 10.1016/0966-842x(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 22.Frank S. Q Rev Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- 23.Møller A P, Christe P, Lux E. Q Rev Biol. 1999;74:3–20. doi: 10.1086/392949. [DOI] [PubMed] [Google Scholar]
- 24.Møller A P, Allander K, Dufva R. In: Population Biology of Passerine Birds: An Integrated Approach. Blondel J, Gosler A, Lebreton J-D, McCleery R H, editors. Berlin: Springer; 1990. pp. 269–280. [Google Scholar]
- 25.Weatherhead P J, Metz K J, Bennett G F, Irwin R E. Behav Ecol Sociobiol. 1993;33:13–23. [Google Scholar]
- 26.Merino S, Potti J. Oikos. 1995;73:95–103. [Google Scholar]
- 27.Van Riper C, Van Riper S G, Goff M L, Laird M. Ecol Monogr. 1986;56:327–344. [Google Scholar]
- 28.Atkinson C, Dusek R J, Woods K L, Iko W M. J Wildl Dis. 2000;36:197–204. doi: 10.7589/0090-3558-36.2.197. [DOI] [PubMed] [Google Scholar]
- 29.Richner H, Oppliger A, Christe P. J Anim Ecol. 1993;62:703–710. [Google Scholar]
- 30.Michod R E. Am Nat. 1979;113:531–550. [Google Scholar]
- 31.Crowl T A, Covich A P. Science. 1990;247:949–951. doi: 10.1126/science.247.4945.949. [DOI] [PubMed] [Google Scholar]
- 32.Reznick D A, Bryga H, Endler J A. Nature (London) 1990;346:357–359. [Google Scholar]
- 33.Saino N, Calza S, Møller A P. J Anim Ecol. 1997;66:827–836. [Google Scholar]
- 34.Saino N, Calza S, Møller A P. Proc Natl Acad Sci USA. 1997;94:549–552. doi: 10.1073/pnas.94.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christe P, Møller A P, de Lope F. Oikos. 1998;83:175–179. [Google Scholar]
- 36.Gonzalez G, Sorci G, Møller A P, Ninni P, Haussy C, de Lope F. J Anim Ecol. 1999;68:1225–1234. [Google Scholar]
- 37.Hõrak P, Tegelmann L, Ots I, Møller A P. Oecologia. 1999;121:316–322. doi: 10.1007/s004420050934. [DOI] [PubMed] [Google Scholar]
- 38.Soler M, Martin-Vivaldi M, Marin J-M, Møller A P. Behav Ecol. 1999;10:281–286. [Google Scholar]
- 39.Soler J J, Møller A P, Soler M, Martinez J G. Evol Ecol Res. 1999;1:189–210. [Google Scholar]
- 40.Merino S, Martinez J, Møller A P, Sanabria L, de Lope F, Perez J, Rodriguez-Caabeiro F. Anim Behav. 1999;58:219–222. doi: 10.1006/anbe.1999.1127. [DOI] [PubMed] [Google Scholar]
- 41.Wakelin D, Blackwell J M, editors. Genetics of Resistance to Bacterial and Parasitic Infection. London: Taylor and Francis; 1988. [Google Scholar]
- 42.Wakelin D, Apanius V. In: Host-Parasite Evolution. General Principles and Avian Models. Clayton D H, Moore J, editors. Oxford: Oxford Univ. Press; 1997. pp. 30–58. [Google Scholar]
- 43.Møller A P. Proc R Soc London Ser B. 1997;264:561–566. [Google Scholar]
- 44.Møller A P. Oikos. 1998;82:265–270. [Google Scholar]
- 45.Møller A P, Erritzøe J. Evolution. KS: Lawrence; 1996. 50, 2066–2072. [Google Scholar]
- 46.Møller A P, Erritzøe J. Evol Ecol. 1998;12:945–953. [Google Scholar]
- 47.Saino N, Calza S, Ninni P, Møller A P. J Anim Ecol. 1999;68:999–1009. [Google Scholar]
- 48.Saino N, Calza S, Møller A P. Oikos. 1998;81:217–228. [Google Scholar]
- 49.Brinkhof M W G, Heeb P, Kölliker M, Richner H. Proc R Soc London Ser B. 1999;266:2315–2322. [Google Scholar]
- 50.Møller, A. P., Merino, S., Brown, C. R. & Robertson, R. J. (2001) Am. Nat., in press. [DOI] [PubMed]
- 51.de Lope F, Gonzalez G, Perez J J, Møller A P. Oecologia. 1993;95:234–240. doi: 10.1007/BF00323495. [DOI] [PubMed] [Google Scholar]
- 52.Moreno J, Sanz J J, Arriero E. Proc R Soc London Ser B. 1999;266:1105–1109. [Google Scholar]
- 53.Merino, S., Martinez, J., Møller, A. P., Barbosa, A., de Lope, F. & Rodriguez-Caabeiro, F. (2001) Behav. Ecol., in press.
- 54.Møller A P. Ecology. 1990;71:2345–2357. [Google Scholar]
- 55.Johnsen A, Andersen V, Sunding C, Lifjeld J T. Nature (London) 2000;406:296–299. doi: 10.1038/35018556. [DOI] [PubMed] [Google Scholar]
- 56.Cramp S, editor. The Birds of the Western Palearctic. Oxford, U.K.: Oxford Univ. Press; 1988. [Google Scholar]
- 57.Pinowski B, Pinowski J. Int Stud Sparrows. 1977;15:1–30. [Google Scholar]
- 58.Brown C R, Brown M B. Ecology. 1986;67:1206–1218. [Google Scholar]
- 59.Brown C R, Brown M B. Coloniality in the Cliff Swallow. Chicago: Univ. Chicago Press; 1996. [Google Scholar]
- 60.Burgerjon J J. Ostrich. 1964;35:77–85. [Google Scholar]
- 61.Szep T, Møller A P. Oecologia. 1999;119:9–15. doi: 10.1007/s004420050755. [DOI] [PubMed] [Google Scholar]
- 62.Hoi-Leitner, M., Romero-Pujante, M., Hoi, H. & Pavlova, A. (2001) Behav. Ecol. Sociobiol., in press.
- 63.Gwinner H, Oltrogge M, Trost L, Nienaber U. Anim Behav. 2000;59:301–309. doi: 10.1006/anbe.1999.1306. [DOI] [PubMed] [Google Scholar]
- 64.Preault M. Strategie d'Appariement et Selection Sexualle Chez le Merle Noir, Turdus merula, en Milieu Urbain. DEA thesis. Paris: Université Pierre et Marie Curie; 1999. [Google Scholar]
- 65.Felsenstein J. Am Nat. 1985;125:1–15. [Google Scholar]
- 66.Harvey P, Pagel M D. The Comparative Method in Evolutionary Biology. Oxford, U.K.: Oxford Univ. Press; 1991. [Google Scholar]
- 67.Martin T E, Clobert J. Am Nat. 1996;147:1028–1046. [Google Scholar]
- 68.Martin T E, Scott J, Menge C. Proc R Soc London Ser B. 2000;267:2287–2294. doi: 10.1098/rspb.2000.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richner H, Christe P, Oppliger A. Proc Natl Acad Sci USA. 1995;92:1192–1194. doi: 10.1073/pnas.92.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ricklefs R E. Proc Natl Acad Sci USA. 1992;89:4722–4725. doi: 10.1073/pnas.89.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]