Abstract
Context:
Cold-water immersion is recommended for the immediate field treatment of exertional heat stroke. However, concerns exist over potential overcooling of hyperthermic individuals during cold-water immersion.
Objective:
To evaluate the recommendation that removing previously hyperthermic individuals from a cold-water bath at a rectal temperature (Tre) of 38.6°C would attenuate overcooling.
Design:
Controlled laboratory study.
Setting:
University research laboratory.
Patients or Other Participants:
Participants included 6 men and 4 women (age = 22 ± 3 years, height = 172 ± 10 cm, mass = 67.8 ± 10.7 kg, body fat percentage = 17.1% ± 4.5%, maximum oxygen consumption = 59.3 ± 8.7 mL·kg−1·min−1).
Intervention(s):
After exercising at an ambient temperature of 40.0°C for 38.5 ± 9.4 minutes, until Tre reached 39.5°C, participants were immersed in a 2.0°C circulated water bath until Tre decreased to either 37.5°C or 38.6°C. Subsequently, participants were removed from the water bath and recovered for 20 minutes at an ambient temperature of 25°C.
Main Outcome Measure(s):
Rectal and esophageal temperatures were measured continuously during the immersion and recovery periods.
Results:
Because of the experimental design, the overall time of immersion was greater during the 37.5°C trial (16.6 ± 5.7 minutes) than the 38.6°C trial (8.8 ± 2.6 minutes) (t9 = −4.740, P = .001). During the recovery period after cold-water immersion, both rectal (F1,9 = 50.540, P < .001) and esophageal (F1,6 = 20.365, P = .007) temperatures remained greater in the 38.6°C trial than in the 37.5°C trial. This was evidenced by low points of 36.47°C ± 0.70°C and 37.19°C ± 0.71°C for rectal temperature (t9 = 2.975, P = .016) and of 35.67°C ± 1.27°C and 36.72°C ± 0.95°C for esophageal temperature (t6 = 3.963, P = .007) during the recovery period of the 37.5°C and 38.6°C trials, respectively.
Conclusions:
Immersion for approximately 9 minutes to a rectal temperature cooling limit of 38.6°C negated any risk associated with overcooling hyperthermic individuals when they were immersed in 2°C water.
Keywords: core temperature, heat stress, recovery
Key Points.
Cold-water immersion provides the greatest cooling rates possible for the treatment of hyperthermic individuals. However, the use of cold-water immersion might pose a risk of overcooling hyperthermic individuals as the result of a lack of rectal temperature monitoring, differences in the temporal response of various indices of core temperature, or a core temperature afterdrop after the cold-water immersion intervention.
After cold-water immersion, rectal temperature continued to decrease and reached a low point of approximately 36.5°C and 37.2°C after rectal temperature cooling limits of 37.5°C and 38.6°C, respectively. Similarly, esophageal temperature reached a low point of approximately 35.7°C and 36.7°C after rectal temperature cooling limits of 37.5°C and 38.6°C, respectively.
Immersion of hyperthermic individuals in 2°C water for approximately 9 minutes to a rectal temperature cooling limit of 38.6°C negated the risk of overcooling in a laboratory setting.
From a clinical standpoint, the dangers associated with hyperthermia far outweigh those associated with overcooling of hyperthermic individuals. As such, cooling of hyperthermic individuals should be undertaken as quickly as possible.
Many athletic, occupational, and military activities potentially can expose individuals to a risk of exertional heat stroke.1–3 The length of time that core temperature remains above critical values is the main criterion determining the survival of individuals with exertional heat stroke.1 Therefore, any treatment strategy should be aimed at reducing core temperature immediately and as quickly as possible.1 Authors4–7 of many systematic reviews have concluded that cold-water immersion (between 2°C and 10°C) is the most effective strategy for the rapid treatment of exertional heat stroke. In fact, the American College of Sports Medicine1 and the National Athletic Trainers' Association8 currently recommend the use of cold-water immersion for the treatment of exertional heat stroke.
Cold-water immersion is recognized as the most effective cooling strategy for hyperthermic individuals because of the heat-transfer properties of water. Although cold-water immersion itself is a safe treatment intervention, extreme prudence is required when planning to remove individuals from the water bath, given the powerful cooling properties of cold water. In fact, the American College of Sports Medicine9 recognized that overcooling of hyperthermic individuals potentially places them at risk for hypothermia (ie, core temperature <35°C). Many investigators10–14 have emphasized the potential overcooling of hyperthermic individuals when using cold-water immersion as a treatment intervention. For example, Moran et al15 reported 2 cases in which soldiers with heat stroke became hypothermic as a result of excessive cooling. Furthermore, Proulx et al16 showed that core body temperatures can drop to levels considered hypothermic subsequent to a cold-water immersion treatment (core temperature afterdrop); however, individuals in their study16 were removed from the water bath at a normal rectal temperature of 37.5°C.
To counter the potential risk of overcooling hyperthermic individuals, researchers5,11–13 have proposed that individuals should be removed from cold water at rectal temperatures ranging from 38°C to 39°C. However, these recommendations are based on anecdotal evidence and, thus, offer somewhat arbitrary cut-off rectal temperature values. To our knowledge, only Proulx et al17 have attempted to experimentally, albeit indirectly, determine safe cooling limits from exercise-induced hyperthermia. Based on thermometric calculations, Proulx et al17 determined that for water temperatures of less than 10°C, 100% of the heat gained during exercise had been removed when rectal temperature reached 38.6°C. Yet calculating changes in body heat content using thermometry recently has been shown to be unreliable.18,19 Therefore, a safe rectal temperature cooling limit to prevent overcooling of hyperthermic individuals has not been validated experimentally.
Thus, the purpose of our study was to evaluate the recommendation of Proulx et al17 that removing hyperthermic individuals from cold-water immersion at a rectal temperature of 38.6°C effectively avoids potential overcooling and subsequent hypothermia. We evaluated the hypothesis that removing hyperthermic individuals at a rectal temperature of 38.6°C rather than removing them at 37.5°C would prevent core temperatures from decreasing to levels considered hypothermic and would attenuate the core temperature afterdrop reported to occur subsequent to removing individuals from a cold-water bath at 2°C.
METHODS
Ten healthy, physically active people (6 men, 4 women; age = 22 ± 3 years, height = 172 ± 10 cm, mass = 67.8 ± 10.7 kg, body fat percentage = 17.1% ± 4.5%, maximum oxygen consumption = 59.3 ± 8.7 mL·kg−1·min−1) volunteered for this study. Physically active was defined as exercising at least 3 times each week at a medium intensity (≥12 on the Borg20 scale) for at least 20 minutes. Maximum oxygen consumption was measured during a progressive treadmill running protocol conducted 5 to 7 days before the experimental trial, and the data were used to select the submaximal workload for the experimental exercise phase of the study. Body density also was measured using the hydrostatic weighing technique, and body fat percentage was calculated using the Siri21 equation. Women were tested in the follicular phase of the menstrual cycle, which was defined as 1 to 5 days after the onset of their self-reported menstruation. All participants gave written consent, and the experimental protocol was approved by the University of Ottawa Research Ethics Committee.
Instrumentation
Rectal temperature was measured using a pediatric thermocouple probe (Mon-a-therm model 503-0021; Covidien-Nellcor, Boulder, CO) inserted to a minimum of 12 cm past the anal sphincter. Esophageal temperature was measured by placing a pediatric thermocouple probe (Mon-a-therm model 503-0021; Covidien-Nellcor) of approximately 2 mm in diameter through the participant's nostril. The location of the probe tip in the esophagus was estimated to be at the level of the eighth and ninth thoracic vertebrae.22 Skin temperature was monitored at 12 sites by Type T thermocouples (Concept Engineering, Old Saybrook, CT). Because the head and the chest were not entirely immersed in water, the area-weighted mean skin temperature was calculated by assigning the following regional percentages: upper back, 12%; lower back, 12.5%; abdomen, 12.5%; biceps, 9.5%; forearm, 9.5%; hand, 2%; quadriceps, 12%; hamstrings, 12%; front calf, 9%; and back calf, 9%.23,24 All temperature data were collected and digitized with LabVIEW software (version 7.0; National Instruments, Austin, TX) at 5-second intervals displayed graphically on a computer screen and recorded in spreadsheet format on a hard disk.
Experimental Protocol
Each participant took part in 2 experimental trials that were separated by a minimum of 48 hours. All trials were performed at the same time of day to avoid circadian variations in skin and core temperatures. Participants were instructed to fast for at least 3 hours before experimentation and were instructed to consume 2 slices of whole wheat toast with butter and orange juice during the last meal. The participants wore shorts, athletic shoes, and a sports bra (women) and were fitted with the appropriate instruments upon arrival at the laboratory.
After instrumentation, the participants remained resting in the upright seated posture for 15 minutes at an ambient air temperature of approximately 25°C and a relative humidity of approximately 35%. Next, they entered a temperature-controlled chamber regulated at an ambient temperature of 40.0°C and a relative humidity of approximately 18% and ran on a treadmill at 65% of their predetermined maximum oxygen consumption until rectal temperature reached 39.5°C. Participants then were transferred (approximately 1.5 minutes) and immersed in a recumbent position in a circulated water bath (model J-315; Jacuzzi Spas International, Chino, CA) maintained at 2.0°C. Before entering the circulated water bath, participants were fitted with neoprene mitts and socks to reduce discomfort. Participants remained in the water until (1) rectal temperature reached 37.5°C or (2) rectal temperature reached 38.6°C. Participants subsequently were removed from the water bath, were seated on a chair, and were wrapped with towels for 20 minutes at an ambient air temperature of approximately 25°C and a relative humidity of approximately 35%.
Statistical Analyses
For each trial, baseline resting and end-exercise temperatures, exercise duration, cooling times, overall rectal cooling rates (start to end of immersion), and nadir rectal and esophageal temperatures during the recovery period were analyzed using paired-samples t tests. During the recovery period after cold-water immersion, rectal (n = 10) and esophageal (n = 7) temperatures also were analyzed using 2-way analyses of variance with repeated measures on time (1, 3, 5, 10, 15, and 20 minutes) and on rectal temperature cooling limit (37.5°C, 38.6°C). Paired-samples t tests were used to perform pairwise post hoc comparisons. The α level was set at .05 and was adjusted during multiple comparisons to maintain the rate of type I error at 5% during the Holm-Bonferroni post hoc analysis. The data are presented as mean ± SD unless otherwise indicated. All analyses were performed using SPSS (version 17.0 for Windows; SPSS Inc, Chicago, IL).
RESULTS
Exercise Period
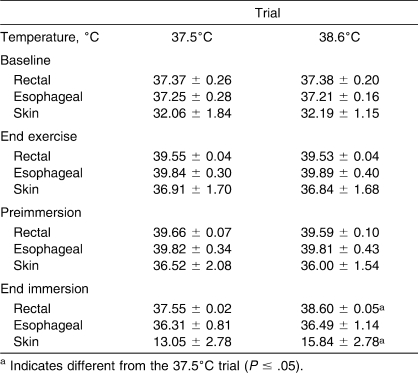
Baseline rectal (t9 = 0.101, P = .92), esophageal (t9 = 0.384, P = .71), and mean skin (t9 = 0.264, P = .8) temperatures (Table 1) did not differ between the 37.5°C and 38.6°C trials. Exercise time taken to reach a rectal temperature of 39.5°C was not different between trials: 35.6 ± 7.4 minutes for the 37.5°C trial and 38.8 ± 9.9 minutes for the 38.6°C trial (t9 = 0.812, P = .44). All participants met this criterion. Exercise resulted in an increase in rectal temperature (from baseline) of 2.20°C ± 0.29°C and 2.18°C ± 0.26° for the 37.5°C and the 38.6°C trials, respectively (t9 = 0.233, P = .82). Rectal (t9 = −0.751, P = .47), esophageal (t9 = 0.303, P = .77), and mean skin (t9 = −0.123, P = .90) temperatures were similar between trials at the end of the exercise (Table 1).
Table 1.
Rectal, Esophageal, and Skin Temperatures at Baseline Rest, End of Exercise, Before Cold-Water Immersion, and at the End of Cold-Water Immersion (Mean ± SD)
Water Immersion
Rectal (t9 = −2.021, P = .07), esophageal (t9 = −0.136, P = .90), and mean skin (t9 = −0.801, P = .44) temperatures at the start of immersion in the water bath did not differ between trials (Table 1). The overall time of immersion was greater during the 37.5°C trial (16.6 ± 5.7 minutes) than the 38.6°C trial (8.8 ± 2.6 minutes) (t9 = −4.740, P = .001). As expected from the experimental design, rectal (t9 = 76.437, P < .001) and mean skin (t9 = 2.752, P = .02) temperatures at the end of immersion were lower during the 37.5°C trial. However, we found no differences between trials for esophageal temperature at the end of immersion (t6 = 1.138, P = .30) (Table 1). We also found no differences in the overall rectal temperature cooling rates between the 2 trials: 0.14°C/min ± 0.05°C/min for the 37.5°C trial and 0.12°C/min ± 0.04°C/min for the 38.6°C trial (t9 = −1.543, P = .16).
Recovery Period After Water Immersion
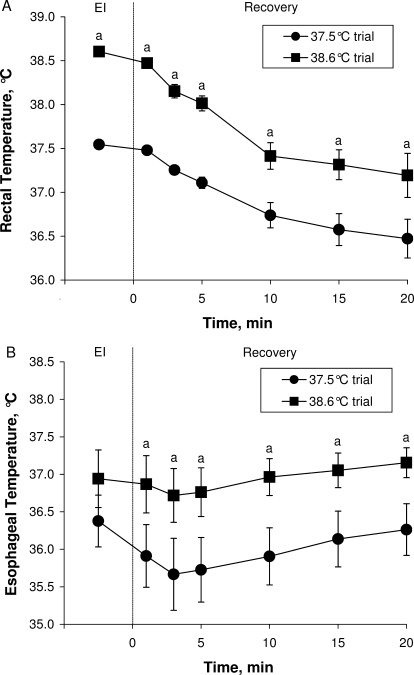
After exiting the water bath, rectal temperature decreased as a function of recovery time (F5,45 = 40.955, P < .001) and was different between trials (F1,9 = 50.540, P < .001). This was evidenced by a greater rectal temperature throughout the recovery period after the 38.6°C trial (Figure). Similarly, esophageal temperature changed as a function of recovery time (F5,30 = 0.555, P = .03), with an initial decrease followed by a slow but gradual rise toward end-immersion values (Figure). Esophageal temperature remained greater after the 38.6°C trial than after the 37.5°C trial (F1,6 = 20.365, P = .007). Rectal temperatures reached nadirs of 36.47°C ± 0.70°C and 37.19°C ± 0.71°C (t9 = 2.975, P = .02), and esophageal temperatures reached nadirs of 35.67°C ± 1.27°C and 36.72°C ± 0.95°C (t6 = 3.963, P = .007) after participants were removed from the water bath at rectal temperature cooling limits of 37.5°C and 38.6°C, respectively. After the 38.6°C trial, rectal (37.19°C ± 0.71°C; t9 = 0.741, P = .48) and esophageal (37.15°C ± 0.53°C; t6 = 0.246, P = .81) temperatures returned to levels comparable to baseline values at the end of the 20-minute postimmersion recovery. However, after the 37.5°C trial, rectal (36.47°C ± 0.70°C; t9 = 3.866, P = .004) and esophageal (36.26°C ± 0.91°C; t6 = 2.674, P = .04) temperatures were still lower than baseline values after the 20-minute postimmersion recovery.
Figure.
A, Rectal and B, esophageal temperatures during a 20-minute recovery period after a cold-water (2°C) immersion with a rectal temperature cooling limit of 37.5°C or 38.6°C. Before the cold-water immersion period, participants were rendered hyperthermic (ie, rectal temperature of 39.5°C) by exercising in the heat. Values are mean ± SE for a group of 10 participants (6 men, 4 women). Abbreviation: EI, end immersion. a Indicates different from the 37.5°C trial (P ≤ .05).
DISCUSSION
Cold-water immersion is the criterion standard intervention for the field treatment of exertional heat stroke. However, cold-water immersion might pose a risk of overcooling hyperthermic individuals as a result of (1) a lack of rectal temperature monitoring, (2) differences in the temporal response of various indices of core temperature, or (3) a core temperature afterdrop after the cold-water immersion intervention. Although safe cooling limits have been established,17 they were based on indirect calculations of changes in body heat content, which recently have been shown to be inaccurate.18,19 Therefore, our study provides empirical evidence demonstrating that a rectal temperature cooling limit of 38.6°C (mean immersion time of approximately 9 minutes) negated each of the aforementioned risks when hyperthermic individuals were immersed in 2°C water. On the other hand, removing these individuals at a rectal temperature of 37.5°C posed a greater risk of overcooling, as evidenced by a longer immersion time (mean of approximately 17 minutes) and by lower postimmersion rectal and esophageal temperatures.
Lack of Core Temperature Monitoring
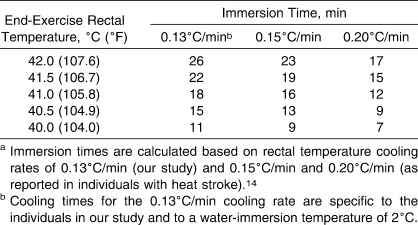
From a clinical standpoint, the dangers associated with hyperthermia far outweigh those associated with overcooling hyperthermic individuals. Therefore, cooling hyperthermic individuals should be undertaken as quickly as possible, even if a measurement of core temperature is not readily available. Although rectal temperature should be monitored when treating hyperthermic individuals, this measurement might not be available in a field setting or emergency situation. In this case, Casa et al5 recommended that cooling times should not exceed 15 to 20 minutes. In our study, it took only 8.8 ± 2.6 minutes (range, 5.8 to 11.8 minutes) of cooling to attain a safe rectal temperature cooling limit of 38.6°C during immersion in 2°C water. However, our study was limited ethically in the level of hyperthermia achieved during exercise (rectal temperature = 39.5°C). In reality, exertional heat stroke is exemplified by core body temperatures greater than 40°C. Based on the average rectal temperature cooling rate from our study (0.13°C/min), it is possible to calculate the cooling times required to decrease rectal temperature to 38.6°C for individuals with rectal temperatures typically associated with exertional heat stroke. These cooling times for end-exercise rectal temperatures of 40°C (104°F), 40.5°C (104.9°F), 41°C (105.8°F), 41.5°C (106.7°F), and 42°C (107.6°F) are presented in Table 2. Furthermore, Costrini14 reported that rectal temperature cooling rates in individuals with heat stroke were greater than those of hyperthermic individuals, ranging from 0.15°C/min to 0.20°C/min. Therefore, we also have included immersion times based on these cooling rates in Table 2. Considering that the main objective of any treatment strategy for hyperthermic individuals is to reduce their core temperatures below the threshold level for tissue injury (ie, approximately 40°C),1 our data further support the suggestion that cooling time should not exceed 15 to 20 minutes5 when a measurement of rectal temperature is not available in a field setting or emergency situation.
Table 2.
Calculated Immersion Times Required to Decrease Rectal Temperatures Associated With Exertional Heat stroke to a Safe Level of 38.6°C (101.2°F)a
Differences in Temporal Response Between Indices of Core Temperature
Even when the core temperature of hyperthermic individuals is monitored during a cold-water immersion intervention, a potential risk of overcooling is still present because of differences in the temporal response of various core temperature indices. Rectal temperature is the criterion standard measurement of core temperature available in a field setting25,26 and should be used whenever possible to monitor a hyperthermic individual's core temperature during a cold-water immersion treatment.1,5 However, rectal temperature has a relatively slow response time compared with esophageal temperature, both during exercise and subsequent cold-water immersion.17,27,28 Differences between rectal and esophageal temperatures are mostly due to differences in tissue mass and blood flow distribution at the sites of measurement.29,30 However, both measurements of core temperature provide equally valuable information. Esophageal temperature best represents the temperature of the heart and of the blood perfusing the brain, whereas rectal temperature best represents the temperature of vital organs located in the visceral area. In our study, esophageal temperature was recorded simultaneously with rectal temperature to highlight the fact that although hyperthermic individuals are cooled to a “normal” resting rectal temperature during a cold-water intervention, temperatures elsewhere in the body might be at levels that are considered hypothermic. For example, in our study when individuals were removed from the water bath at a normothermic rectal temperature of 37.5°C, average esophageal temperature was approximately 36.38°C. On the other hand, when individuals were removed from the water bath at a safe rectal temperature cooling limit of 38.6°C, esophageal temperature was approximately 36.94°C. The approximately 0.5°C greater esophageal temperature at the end of the 38.6°C immersion attenuated the subsequent afterdrop and reduced the likelihood that values would reach the hypothermic threshold of 35°C.
Core Temperature Afterdrop
A core temperature afterdrop is characterized by a sustained decrease in core temperature after removal from cold-water immersion and occurs because of conductive and convective (via the blood) heat transfer from the periphery to the core.31,32 This phenomenon has been observed16,33 with cold-water immersion after exercise-induced hyperthermia. In our study, when participants were removed from the water bath at a rectal temperature of 37.5°C, their rectal temperatures subsequently attained a low point of approximately 36.5°C at the end of the recovery period. However, if participants were removed at a rectal temperature of 38.6°C, rectal temperature only reached a low point of approximately 37.2°C. Furthermore, esophageal temperatures were prevented from dropping below 36.0°C when using a rectal temperature cooling limit of 38.6°C, with a nadir point of only approximately 36.7°C (range, 35.3°C–38.0°C), as compared with approximately 35.7°C (range, 34.4°C–37.5°C), which was achieved when using a rectal temperature cooling limit of 37.5°C. Nonetheless, given that core temperature continued to decrease regardless of rectal temperature cooling limit, it needs to be monitored after the removal of previously hyperthermic individuals from a cold water bath. One of the authors (D.J.C.) anecdotally reported that some individuals treated for hyperthermia demonstrate a hypothermic freefall, despite being removed from the water bath, at rectal temperatures as high as 38.8°C. He observed this rare circumstance in individuals who had exertional heat stroke and who had initial precooling temperatures that were greater than 42.5°C. In these instances, rectal temperature might drop to as low as 33.5°C, which may be due to impaired thermoregulatory function because the drop in rectal temperature (eg, >5°C) is much greater than the rectal temperature afterdrop (≤1°C).
Limitations
Our study provides empirical data to support the safe rectal temperature cooling limit of 38.6°C proposed by Proulx et al.17 However, the cooling times in our study are specific to a 2°C cold-water immersion treatment and ultimately will vary according to immersion temperature. Future authors should consider examining safe cooling limits at various water temperatures (eg, 10°C–20°C). Cooling times and the core temperature response to cold-water immersion will differ depending on the physical characteristics of the individuals being immersed.34,35 Lemire et al35 recently demonstrated that rectal temperature cooling rates during 2°C water immersion after exercise-induced hyperthermia were strongly associated with an individual's lean body mass-to-body surface area ratio. Therefore, bigger, leaner hyperthermic individuals might require longer cooling times to achieve a safe rectal temperature cooling limit of 38.6°C. Finally, because of the unknown external validity of experimental trials performed in a laboratory, we must be cautious in extending these findings to the field environment. The participants in our study were exposed to controlled hyperthermia; therefore, it is uncertain how comparable these data are for cooling individuals who have had thermoregulatory failure.
CONCLUSIONS
Cold-water immersion provides the greatest cooling rates possible for the treatment of hyperthermic individuals. However, the use of cold-water immersion may pose a risk of overcooling hyperthermic individuals as a result of a lack of rectal temperature monitoring, differences in the temporal response of various indices of core temperature, or a core temperature afterdrop after the cold-water immersion intervention. Our results provide strong empirical evidence that immersion for approximately 9 minutes to a rectal temperature cooling limit of 38.6°C will negate each of these risks when hyperthermic individuals are immersed in 2°C water.
Acknowledgments
This research was supported by grant No. RGPIN-298159-2004 from the Natural Sciences and Engineering Research Council (Dr Kenny). Dr Kenny was supported by a University of Ottawa Research Chair Award. We thank all the participants who volunteered for this study.
REFERENCES
- 1.American College of Sports Medicine. Armstrong L. E., Casa D. J., et al. American College of Sports Medicine position stand: exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 2.Bonauto D., Anderson R., Rauser E., Burke B. Occupational heat illness in Washington State, 1995–2005. Am J Ind Med. 2007;50(12):940–950. doi: 10.1002/ajim.20517. [DOI] [PubMed] [Google Scholar]
- 3.Carter R., III, Cheuvront S. N., Williams J. O., et al. Epidemiology of hospitalizations and deaths from heat illness in soldiers. Med Sci Sports Exerc. 2005;37(8):1338–1344. doi: 10.1249/01.mss.0000174895.19639.ed. [DOI] [PubMed] [Google Scholar]
- 4.McDermott B. P., Casa D. J., Ganio M. S., et al. Acute whole-body cooling for exercise-induced hyperthermia: a systematic review. J Athl Train. 2009;44(1):84–93. doi: 10.4085/1062-6050-44.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casa D. J., McDermott B. P., Lee E. C., Yeargin S. W., Armstrong L. E., Maresh C. M. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141–149. doi: 10.1097/jes.0b013e3180a02bec. [DOI] [PubMed] [Google Scholar]
- 6.Smith J. E. Cooling methods used in the treatment of exertional heat illness. Br J Sports Med. 2005;39(8):503–507. doi: 10.1136/bjsm.2004.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadad E., Rav-Acha M., Heled Y., Epstein Y., Moran D. S. Heat stroke: a review of cooling methods. Sports Med. 2004;34(8):501–511. doi: 10.2165/00007256-200434080-00002. [DOI] [PubMed] [Google Scholar]
- 8.Binkley H. M., Beckett J., Casa D. J., Kleiner D. M., Plummer P. E. National Athletic Trainers' Association position statement: exertional heat illnesses. J Athl Train. 2002;37(3):329–343. [PMC free article] [PubMed] [Google Scholar]
- 9.Castellani J. W., Young A. J., Ducharme M. B., Giesbrecht G. G., Glickman E., Sallis R. E. American College of Sports Medicine position stand: prevention of cold injuries during exercise. Med Sci Sports Exerc. 2006;38(11):2012–2029. doi: 10.1249/01.mss.0000241641.75101.64. [DOI] [PubMed] [Google Scholar]
- 10.Knochel J. P. Environmental heat illness: an eclectic review. Arch Intern Med. 1974;133(5):841–864. [PubMed] [Google Scholar]
- 11.Sandor R. P. Heat illness: on-site diagnosis and cooling. Physician Sportsmed. 1997;25(6):35–40. doi: 10.3810/psm.1997.06.1400. [DOI] [PubMed] [Google Scholar]
- 12.Harker J., Gibson P. Heat-stroke: a review of rapid cooling techniques. Intensive Crit Care Nurs. 1995;11(4):198–202. doi: 10.1016/s0964-3397(95)80073-5. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro Y., Seidman D. S. Field and clinical observations of exertional heat stroke patients. Med Sci Sports Exerc. 1990;22(1):6–14. [PubMed] [Google Scholar]
- 14.Costrini A. Emergency treatment of exertional heatstroke and comparison of whole body cooling techniques. Med Sci Sports Exerc. 1990;22(1):15–18. [PubMed] [Google Scholar]
- 15.Moran D. S., Heled Y., Shani Y., Epstein Y. Hypothermia and local cold injuries in combat and non-combat situations: the Israeli experience. Aviat Space Environ Med. 2003;74(3):281–284. [PubMed] [Google Scholar]
- 16.Proulx C. I., Ducharme M. B., Kenny G. P. Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol. 2003;94(4):1317–1323. doi: 10.1152/japplphysiol.00541.2002. [DOI] [PubMed] [Google Scholar]
- 17.Proulx C. I., Ducharme M. B., Kenny G. P. Safe cooling limits from exercise-induced hyperthermia. Eur J Appl Physiol. 2006;96(4):434–445. doi: 10.1007/s00421-005-0063-y. [DOI] [PubMed] [Google Scholar]
- 18.Jay O., Reardon F. D., Webb P., et al. Estimating changes in mean body temperature for humans during exercise using core and skin temperatures is inaccurate even with a correction factor. J Appl Physiol. 2007;103(2):443–451. doi: 10.1152/japplphysiol.00117.2007. [DOI] [PubMed] [Google Scholar]
- 19.Jay O., Kenny G. P. The determination of changes in body heat content during exercise using calorimetry and thermometry. J Hum Environ Syst. 2007;10(1):19–29. [Google Scholar]
- 20.Borg G. A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 21.Siri W. E. Gross composition of the body. In: Lawrence J. H., Tobias C. A., editors. Advances in Biological and Medical Physics. New York, NY: Academic; 1956. pp. 239–280. [DOI] [PubMed] [Google Scholar]
- 22.Mekjavic I. B., Rempel M. E. Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol. 1990;69(1):376–379. doi: 10.1152/jappl.1990.69.1.376. [DOI] [PubMed] [Google Scholar]
- 23.Hardy J. D., DuBois E. F. The technique of measuring radiation and convection. J Nutr. 1938;15(5):461–475. [Google Scholar]
- 24.Layton R. P., Mints W. H., Jr, Annis J. F., Rack M. J., Webb P. Calorimetry with heat flux transducers: comparison with a suit calorimeter. J Appl Physiol. 1983;54(5):1361–1367. doi: 10.1152/jappl.1983.54.5.1361. [DOI] [PubMed] [Google Scholar]
- 25.Casa D. J., Becker S. M., Ganio M. S., et al. Validity of devices that assess body temperature during outdoor exercise in the heat. J Athl Train. 2007;42(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- 26.Ganio M. S., Brown C. M., Casa D. J., et al. Validity and reliability of devices that assess body temperature during indoor exercise in the heat. J Athl Train. 2009;44(2):124–135. doi: 10.4085/1062-6050-44.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagnon D., Lemire B. B., Jay O., Kenny G. P. Aural canal, esophageal, and rectal temperatures during exertional heat stress and subsequent recovery period. J Athl Train. 2010;45(2):157–163. doi: 10.4085/1062-6050-45.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor N. A., Caldwell J. N., Van den Heuvel A. M., Patterson M. J. To cool, but not too cool: that is the question. Immersion cooling for hyperthermia. Med Sci Sports Exerc. 2008;40(11):1962–1969. doi: 10.1249/MSS.0b013e31817eee9d. [DOI] [PubMed] [Google Scholar]
- 29.Moran D. S., Mendal L. Core temperature measurement: methods and current insights. Sports Med. 2002;32(14):879–885. doi: 10.2165/00007256-200232140-00001. [DOI] [PubMed] [Google Scholar]
- 30.Sawka M. N., Pandolf K. B. Physical exercise in hot climates: physiology, performance, and biomedical issues. In: Pandolf K. B., Burr R. E., editors. Medical Aspects of Harsh Environments. Vol 1. Washington, DC: Office of the Surgeon General at TMM Publications, Borden Institute, Walter Reed Army Medical Center; 2001. pp. 87–133. http://www.bordeninstitute.army.mil/published_volumes/harshEnv1/Ch3-PhysicalExerciseinHotClimates.pdf. Accessed May 25, 2010. [Google Scholar]
- 31.Mittleman K. D., Mekjavić I. B. Effect of occluded venous return on core temperature during cold water immersion. J Appl Physiol. 1988;65(6):2709–2713. doi: 10.1152/jappl.1988.65.6.2709. [DOI] [PubMed] [Google Scholar]
- 32.Romet T. T. Mechanism of afterdrop after cold water immersion. J Appl Physiol. 1988;65(4):1535–1538. doi: 10.1152/jappl.1988.65.4.1535. [DOI] [PubMed] [Google Scholar]
- 33.Clements J. M., Casa D. J., Knight J. C., et al. Ice-water immersion and cold-water immersion provide similar cooling rates in runners with exercise-induced hyperthermia. J Athl Train. 2002;37(2):146–150. [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson G. S. Human morphology and temperature regulation. Int J Biometeorol. 1999;43(3):99–109. doi: 10.1007/s004840050123. [DOI] [PubMed] [Google Scholar]
- 35.Lemire B. B., Gagnon D., Jay O., Kenny G. P. Differences between sexes in rectal cooling rates after exercise-induced hyperthermia. Med Sci Sports Exerc. 2009;41(8):1633–1639. doi: 10.1249/MSS.0b013e31819e010c. [DOI] [PubMed] [Google Scholar]