Abstract
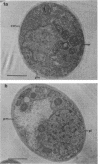
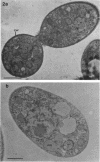
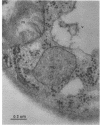
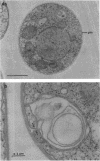
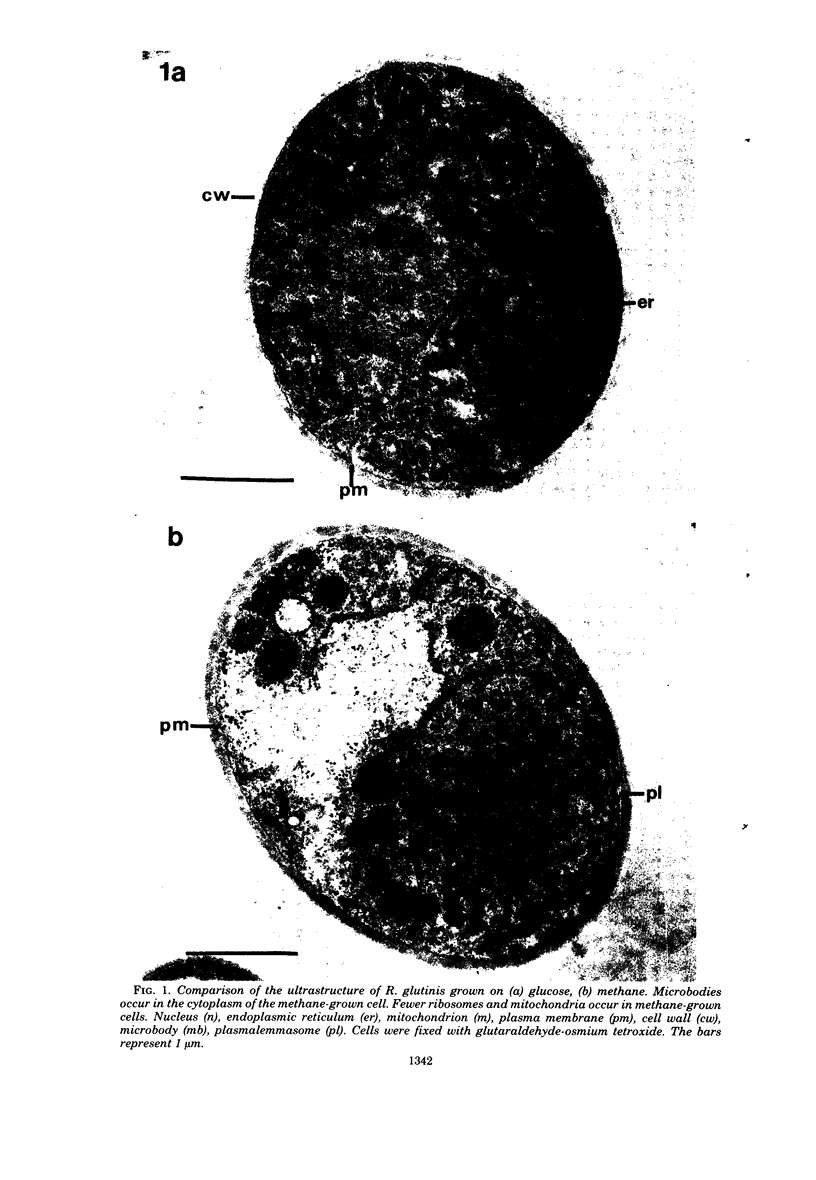
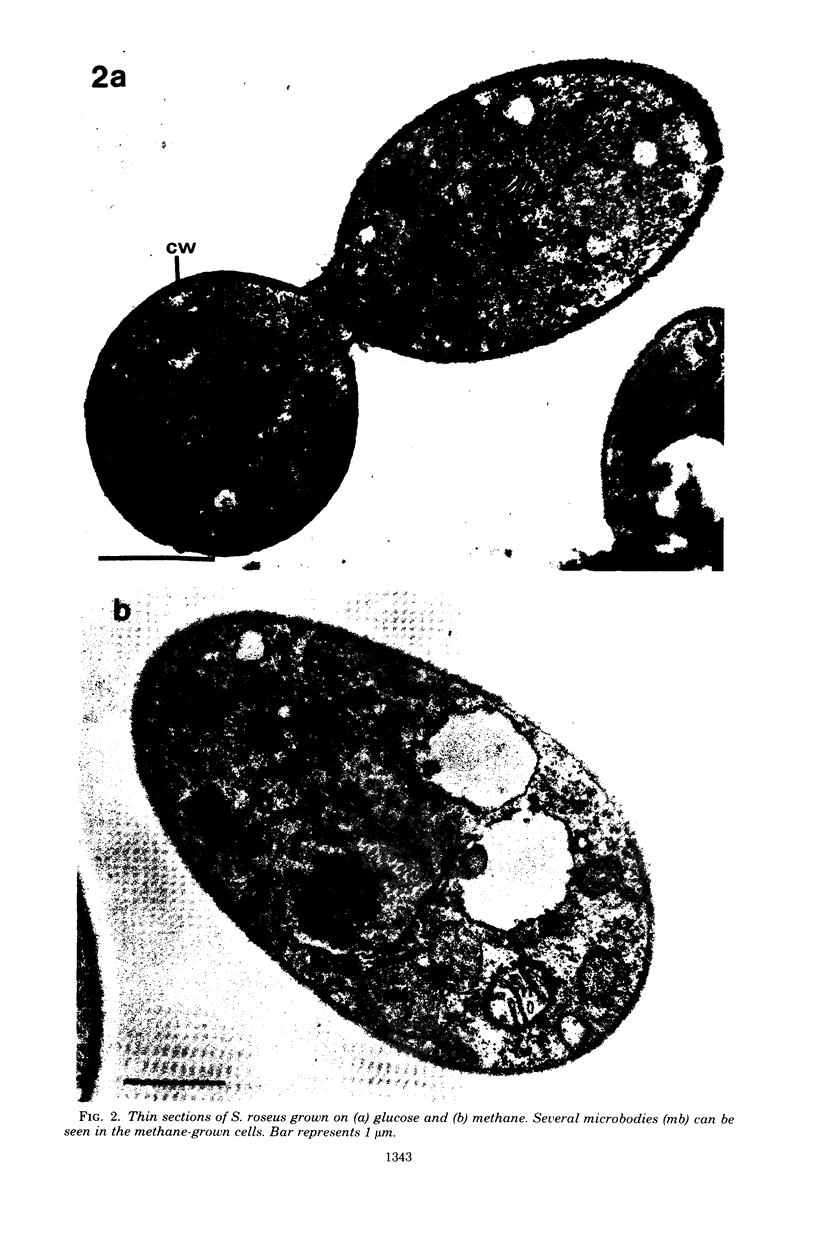
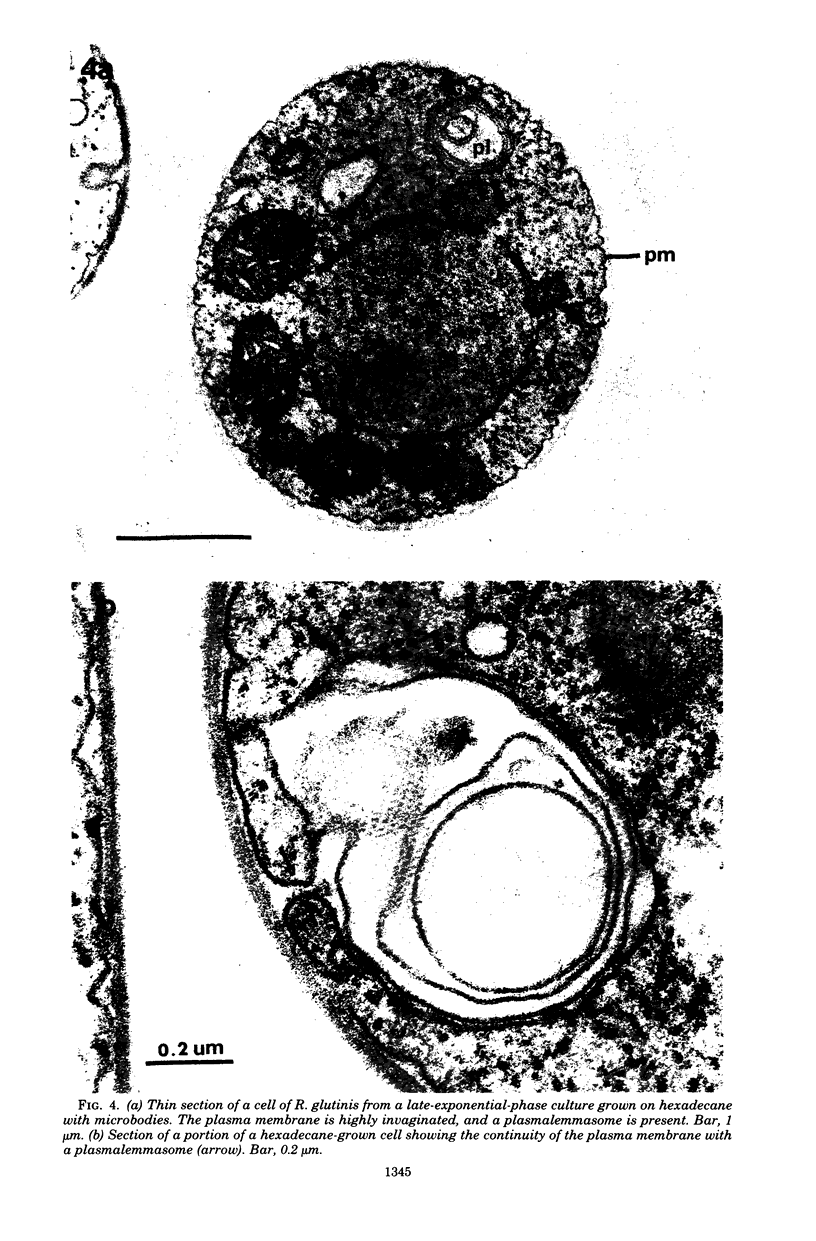
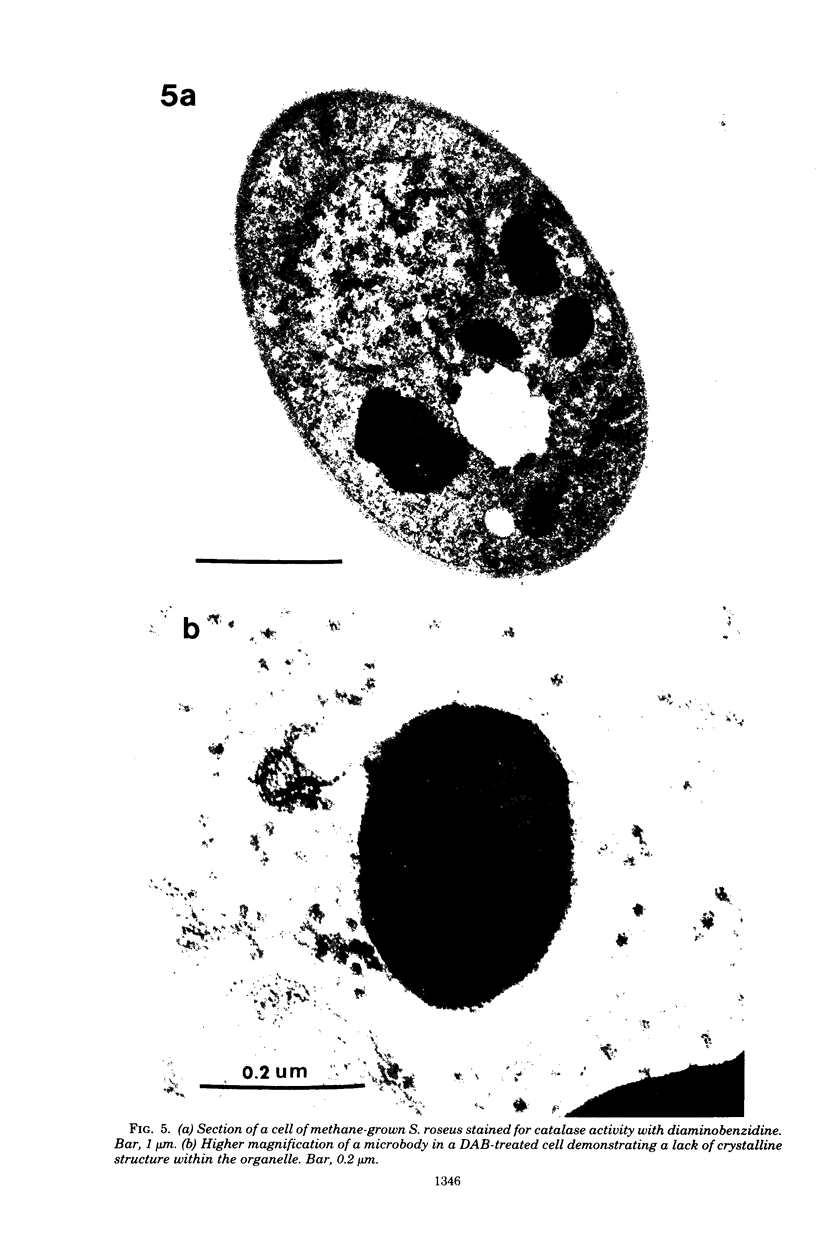
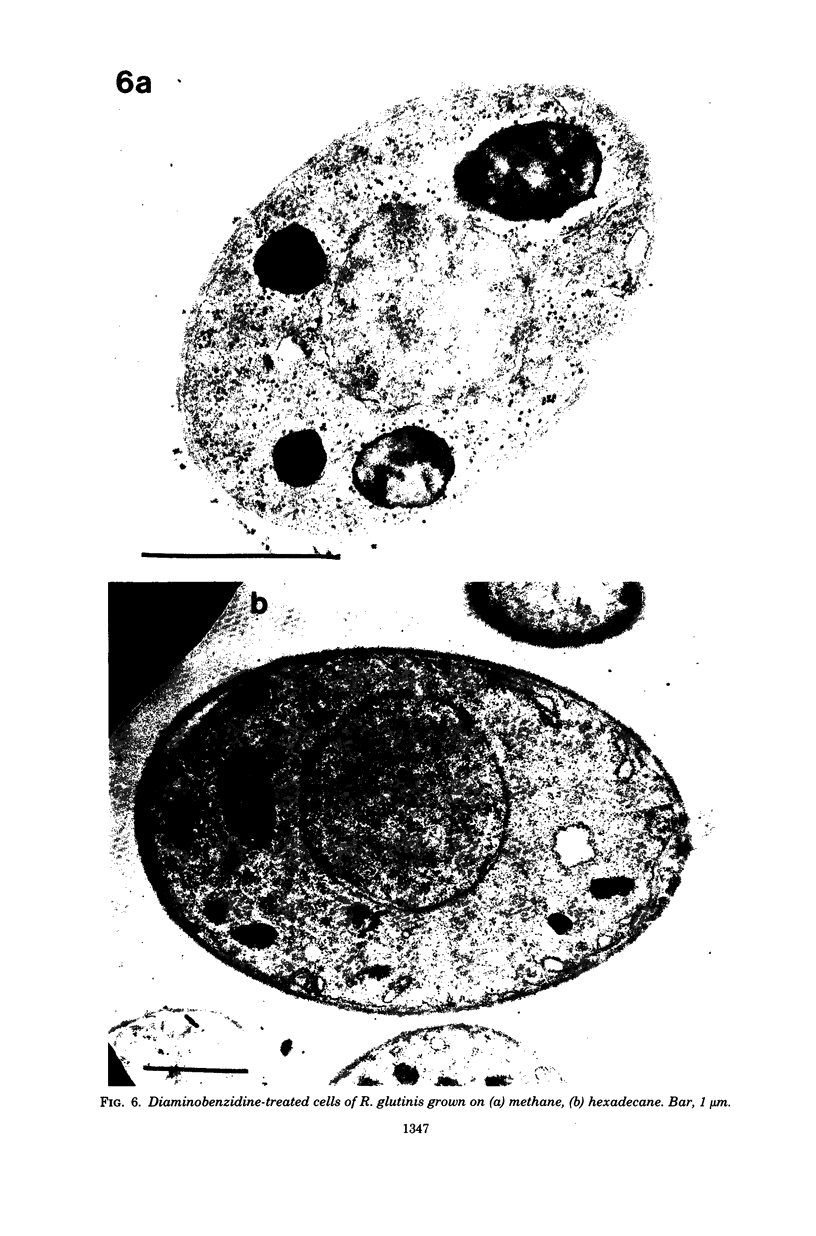
The cellular structure of two yeast strains capable of growth on methane was investigated by electron microscopy. Microbodies were observed in cells of Sporobolomyces roseus strain Y and Rhodotorula glutinis strain CY when grown on methane but rarely when grown on glucose. The size of the microbodies and the number observed per cell in a thin section did not increase with culture age. No crystalline organization was observed within these organelles. Similar microbodies were also observed in cells of R. glutinis CY grown on hexadecane. The plasma membranes of both methane and hexadecane-grown cells exhibited increased invagination compared to that of glucose-grown cells. Catalase activity was detected in the microbodies of alkane-grown cells by using 3,3'-diaminobenzidine as a cytochemical stain. The data presented suggest that microbodies, and the catalase contained within them, play a role in eucaryotic methane metabolism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. The biochemistry of methylotrophic micro-organisms. Sci Prog. 1975 Summer;62(246):167–206. [PubMed] [Google Scholar]
- CONTI S. F., HIRSCH P. BIOLOGY OF BUDDING BACTERIA. 3. FINE STRUCTURE OF RHODOMICROBIUM AND HYPHOMICROBIUM SPP. J Bacteriol. 1965 Feb;89:503–512. doi: 10.1128/jb.89.2.503-512.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- Fukui S., Kawamoto S., Yasuhara S., Tanaka A., Osumi M. Microbody of methanol-grown yeasts. Localization of catalase and flavin-dependent alcohol oxidase in the isolated microbody. Eur J Biochem. 1975 Nov 15;59(2):561–566. doi: 10.1111/j.1432-1033.1975.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Fukui S., Tanaka A., Kawamoto S., Yasuhara S., Teranishi Y., Osumi M. Ultrastructure of methanol-utilizing yeast cells: appearance of microbodies in relation to high catalase activity. J Bacteriol. 1975 Jul;123(1):317–328. doi: 10.1128/jb.123.1.317-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H. P., Szabo A., Avers C. J. Cytochemical localization of catalase activity in yeast peroxisomes. J Bacteriol. 1970 Oct;104(1):581–584. doi: 10.1128/jb.104.1.581-584.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvík J., Munk V., Dostálek M. Ultrastructural changes in the yeast Candida lipolytica caused by penetration of hydrocarbons into the cell. Experientia. 1968 Oct 15;24(10):1066–1068. doi: 10.1007/BF02138754. [DOI] [PubMed] [Google Scholar]
- Munk V., Dostálek M., Volfová O. Cultivation of yeast on gas oil. Biotechnol Bioeng. 1969 May;11(3):383–391. doi: 10.1002/bit.260110310. [DOI] [PubMed] [Google Scholar]
- Osumi M., Miwa N., Teranishi Y., Tanaka A., Fukui S. Ultrastructure of Candida yeasts grown on n-alkanes. Appearance of microbodies and its relationship to high catalase activity. Arch Microbiol. 1974;99(3):181–201. doi: 10.1007/BF00696234. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenkamp R., Sahm H., Hinkelmann W., Wagner F. Alcohol oxidase and catalase in peroxisomes of methanol-grown Candida boidinii. Eur J Biochem. 1975 Nov 1;59(1):231–236. doi: 10.1111/j.1432-1033.1975.tb02446.x. [DOI] [PubMed] [Google Scholar]
- Sahm H., Roggenkamp R., Wagner F., Hinkelmann W. Microbiodies in methanol-grown Candida boidinii. J Gen Microbiol. 1975 Jun;88(2):218–222. doi: 10.1099/00221287-88-2-218. [DOI] [PubMed] [Google Scholar]
- Shennan J. L., Levi J. D. The growth of yeasts on hydrocarbons. Prog Ind Microbiol. 1974;13:1–57. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Yasuhara S., Kawamoto S., Fukui S., Osumi M. Development of Microbodies in the yeast Kloeckera growing on methanol. J Bacteriol. 1976 May;126(2):919–927. doi: 10.1128/jb.126.2.919-927.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani Y., Kato N., Yamada H. Utilization of methanol by yeasts. Adv Appl Microbiol. 1978;24:165–186. doi: 10.1016/s0065-2164(08)70639-7. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Harder W. Cytochemical studies on the localization of methanol oxidase and other oxidases in peroxisomes of methanol-grown Hansenula polymorpha. Arch Microbiol. 1976 Dec 1;111(1-2):123–135. doi: 10.1007/BF00446559. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Veenhuis M., Kreger-van Rij N. J., Harder W. Microbodies in methanol-assimilating yeasts. Arch Microbiol. 1975;102(1):41–44. doi: 10.1007/BF00428343. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Veenhuis M., Vermeulen C. A., Harder W. Cytochemical localization of catalase activity in methanol-grown Hansenula polymorpha. Arch Microbiol. 1975 Nov 7;105(3):261–267. doi: 10.1007/BF00447145. [DOI] [PubMed] [Google Scholar]