Abstract
Context:
Regaining full, active range of motion (AROM) after trauma to the wrist is difficult.
Objective:
To report the cases of 6 patients who lacked full range of motion (ROM) in the wrist due to trauma. The treatment regimen was thermal 3-MHz ultrasound and joint mobilizations.
Design:
Case series.
Setting:
University therapeutic modalities laboratory.
Patients or Other Participants:
Six patients (2 women, 4 men) from the university population lacked a mean AROM of 21.7° of flexion and 26.8° of extension approximately 2.1 years after trauma or surgery.
Main Outcome Measure(s):
I assessed changes in flexion and extension AROM before and after each treatment. Treatment consisted of 6 minutes of 3-MHz continuous ultrasound at an average intensity of 1.4 W/cm2 on the dorsal and volar aspects of the wrist, immediately followed by approximately 10 minutes of joint mobilizations. After posttreatment ROM was recorded, ice was applied to the area for about 20 minutes. Once the patient achieved full AROM or did not improve on 2 consecutive visits, he or she was discharged from the study.
Results:
By the sixth treatment, 5 participants achieved normal flexion AROM, and 3 exceeded the norm. All 6 achieved normal extension AROM, and 4 exceeded the norm. All returned to normal activities and normal use of their hands. One month later, they had, on average, maintained 93% of their final measurements.
Conclusions:
A combination of thermal ultrasound and joint mobilizations was effective in restoring AROM to wrists lacking ROM after injury or surgery.
Keywords: rehabilitation, deep heat, therapeutic modalities
Patients who experience reduced active range of motion (AROM) for long periods of time, especially when those impairments affect the fine motor movements of the wrists and hands, have their lives altered. Envision what your life would be like if you could flex and extend one of your wrists only halfway. For a functioning wrist, AROM should be near 80° of flexion and 75° of extension as measured by a goniometer.1–4
Clinicians know that combining heat and joint mobilizations can increase physiologic and accessory movements.5–7 These results, however, have only been shown on large muscles and joints. For example, pulsed short-wave diathermy, a form of deep heat, has been used clinically to apply heat to larger joints with decreased range of motion (ROM) after a period of immobilization.5,8,9 Pulsed short-wave diathermy is appropriate for patients with ankle, knee, and shoulder injuries due to the size and depth of the area that must be heated.10,11 This application, in conjunction with joint mobilizations, has been an effective treatment regimen for those patients. Based on the results, I have used ultrasound on small joints such as the wrist and hand.
With today's modern ultrasound units, tissues at the wrist can be heated to 5°C to 6°C (41.0°F to 42.8°F) using 6 minutes of 1.4-W/cm2, 3-MHz ultrasound.12,13 This treatment reaches the sufficient depth and level of vigorous heating to increase collagen extensibility and produce other positive physiologic responses that are important in regaining and maintaining AROM with joint mobilizations.14,15 Therefore, my goal was to investigate the efficacy of a regime consisting of 3-MHz ultrasound and joint mobilizations on patients whose wrists could not reach 80° to 85° of flexion and extension.16
Ultrasound at an intensity of 1.4 W/cm2 and frequency of 3 MHz raises tissue temperature approximately 5°C to 6°C (41.0°F to 42.8°F) in 6 minutes.12,13,17 These settings increase collagen extensibility, inhibit sympathetic activity, and improve the viscoelastic properties of the tissues.14,15 In a stiff joint, heat combined with joint mobilizations can enhance collagen extensibility and improve joint physiologic and accessory movements.
Quantifying AROM is a difficult task because it varies from person to person and author to author. For this study, I have used AROM, which is appropriate to entry-level knowledge of goniometric measurement,3,4 with normal flexion as 80° and normal extension as 75°.
In this case series, I will describe a unique treatment protocol, combining ultrasound and joint mobilization, used in 6 patients who sustained severe wrist injuries.
CASE DESCRIPTIONS
History
A summary of each patient's history is provided in Table 1. The patients included in this case series were referred to the laboratory or clinic due to a long-term, significant decrease in wrist ROM, active and passive, that was present after rehabilitation and limited normal daily and recreational activities. Two students were referred by friends, 2 were self-referred students of mine, 1 student was referred by a certified athletic trainer, and a university electrician was referred by a certified athletic trainer. Patients were excluded if they had a compromised peripheral vascular system, decreased sensation in the affected area, or implanted pacemaker or neurostimulator. The Brigham Young University Human Subjects Review Board approved the methods used in this case series, and all patients signed informed consent forms.
Table 1.
Patient Histories
On the first visit, participants were asked a series of questions:
How long has it been since your injury?
Describe how the injury happened (mechanism of injury).
If you are in pain, point to where it hurts.
Describe the pain (dull, ache, burning, tingling, numb, etc).
If you were immobilized in a brace or a cast, how many weeks were you immobilized?
Describe any physical therapy or home exercises that you participated in.
What was the duration of this treatment?
What do you want to get out of the therapy?
Each patient's main concern was regaining flexion and extension. (Ulnar and radial deviation was severely limited in 1 volunteer; these motions were successfully restored, but I do not report those data here.) If more information was needed, I requested the physician's notes regarding the patient's treatment or radiographs. These were obtained for patients 4 and 6. The next section presents a short history of each patient. All listed ROMs are AROMs.
Patient 1, a 23-year-old man, sustained a fracture of the distal radius while he was snowboarding. Treatment involved cast immobilization for 2 months. He did not have any traditional physical therapy but was given a home exercise program and still lacked 5° of flexion and 25° of extension.
Patient 2 was a 22-year-old man. At age 19, he underwent surgery for a ganglion cyst, at which time the surgeon accidently severed 3 wrist extensor tendons. The patient went to another surgeon for repair of the severed tendons and was placed in a cock-up splint for 3 months. After the initial repair, he had undergone traditional physical therapy consisting of ultrasound and stretching (but not joint mobilizations). He had been out of the country for the previous 3 years and had not performed any therapy on his wrist during that time. When he reported to the laboratory, he lacked 38° of flexion and 12° of extension.
Patient 3, a 21-year-old woman, had also undergone ganglion cyst excision. Her surgery was successful, but she reported to the laboratory about 5 weeks after surgery, having had no physical therapy. She lacked 42° of flexion and 25° of extension.
Patient 4 was a 21-year-old soccer goalie. Two and a half years earlier, he experienced an undiagnosed scaphoid fracture and developed avascular necrosis. The physician performed surgery using a bone graft from the thumb and immobilized the wrist for 12 weeks. Once the cast was removed, the athletic trainer began a course of whirlpool and stretching. Patient 4 was told by his orthopaedic surgeon that “no further gains would occur in his wrist ROM.” When the patient was referred to me, he had full flexion but lacked 25° of extension.
Patient 5 was a 42-year-old university electrician. While racing his motorcycle, he fractured his scaphoid. Later, he underwent surgery and a bone graft to assist in repair of the scaphoid. He was in a cast for 4 months and pursued a typical physical therapy regimen (whirlpool and stretching). When he reported to the laboratory, he lacked 3° of flexion and 29° of extension.
Patient 6 was a 23-year-old woman. She was referred 6 weeks after wrist surgery to repair 2 injuries: a triangular fibrocartilage tear and a lunate that the surgeon stated had “disintegrated over time.” Also, scar tissue had developed during the past 9 years of playing high school and recreational sports. She had no physical therapy and lacked 42° of flexion and 45° of extension.
Half of these patients pursued physical therapy after surgery. During the physical therapy sessions, each received moist heat in the form of silicate gel hot packs or whirlpool. Some received heat treatment via ultrasound. After the heat treatment, most pursued exercise in the form of active or passive stretching, joint mobilization, or weight training. (The therapeutic exercises differed slightly for each patient.) An ice pack was applied at the end of the session.
Examination
The movements measured were extension and flexion in the early patients. (I measured pronation and supination in patient 6 but did not use these data in the study.) Because their surgeries or injuries had occurred some time earlier, most of their radial and ulnar deviation had returned. Also, as flexion and extension improved, so did the compensatory movements of radial and ulnar deviation.
All AROM measurements were obtained using a 10.16-cm plastic 360° universal goniometer (scale marked in 1° increments) for increased validity of measurement.18–20 However validity may range from approximately 4° to 6° when using a goniometer. In this study, I measured ROM 3 times and then averaged the 3 measures. The ROM measurement was performed as follows:
Flexion: The patient sat next to the table with the forearm supported on the table in pronation. The wrist was in the neutral position, and the fingers were extended. The axis was the distal styloid process of the ulna. The stationary arm was placed parallel to and over the lateral midline of the ulna, in line with the olecranon process. The moving arm was placed along the lateral midline of the fifth metacarpal. The forearm was stabilized as wrist flexion was performed over the edge of the table.
Extension. The same landmarks and positioning were used for extension. Extension was performed with the fingers held loosely in flexion, so that full extension of the joint could be reached.
These landmarks were standardized for each patient, ensuring reliability and validity of measurement, and were based on entry-level goniometric measurements.18–20 The same goniometer was used for all measurements to limit error from differences between goniometers. The goniometer was not masked during measurements.
Intervention
After the initial examination, the treatment protocol of 3-MHz ultrasound followed by joint mobilizations and concluding with a crushed-ice pack was initiated. During each treatment session, AROM was measured before the ultrasound treatment and after the joint mobilizations but before ice-pack application. Participants were instructed not to start any new or additional home exercise programs other than those they were already pursuing at the start of the intervention.
The 3-MHz ultrasound (Omnisound 3000C; Accelerated Care Plus, Reno, NV) was applied to the dorsal and ventral aspects of the wrist for 6 minutes (each side) at 1.4 W/cm2. Ultrasound at these settings increases the tissue temperature approximately 5°C to 6°C (41.0°F to 42.8°F) and maintains half of this increase for about 10 to 15 minutes.5,13 Joint mobilizations were then applied to the wrist for 10 minutes.
Joint mobilizations were applied depending upon each patient's tolerance. This was determined subjectively (asking the patient to identify his or her pain level and if the pressure should be lessened) and objectively (noting tissue-response guarding, muscle spasm, and muscle contraction). The joint mobilizations included Maitland grade III (large-amplitude movements as long as the resistance was encountered somewhere in that range) and Maitland grade IV (small-amplitude movement as long as the resistance was encountered somewhere in that range). Static glide techniques (Ola Grimsby) were also used.5,7
More specifically, to improve flexion, the following joint mobilizations were performed: traction, dorsal glides of the radial-carpal joint, scaphoid, and lunate. To improve extension, the following joint mobilizations were performed: traction, ventral glides of the radial-carpal joint, scaphoid, and lunate. Individual mobilizations of the carpal bones were used as needed to improve both flexion and extension. Each sustained or oscillatory mobilization was performed for a minimum of 30 seconds with 3 repetitions.
After the posttreatment measurements, a crushed-ice pack was applied for 20 to 30 minutes with the wrist in neutral position. The purpose of the ice-pack application was 3-fold. First, it helped to limit any secondary ischemic injury if inflammation occurred from the stress of the joint mobilizations.21,22 Second, it reduced pain from any microtrauma caused by the treatment.23 Third, it enhanced plastic elongation of the tissues (ie, the ability of the tissues to stay at the ROM reached during the joint mobilization and stretching).15 However, because the wrist was in a neutral position during the cooling, these effects were probably mild. The intervention was applied 2 to 3 times per week for 3 to 6 visits (Table 2). Patients were discharged if ROM did not improve after 2 consecutive visits or when the target ROM was reached.
Table 2.
Patients' Progress
RESULTS
At the end of 6 or fewer sessions, all patients except 1 had achieved or surpassed treatment goals for flexion AROM, and all patients reached or surpassed the treatment goals for extension AROM (Table 2; Figures 1 and 2). Overall, patients 1 through 6 improved their ROM in flexion and extension by 10° and 25°, 38° and 12°, 35° and 33°, 7° and 26°, 3° and 34°, and 52° and 50°, respectively. At 1 month after the final treatment session, 5 patients had maintained 100% of their flexion AROM and all 6 patients had maintained 100% of their extension AROM. In all, patients maintained 93% of their discharge ROM.
Figure 1.
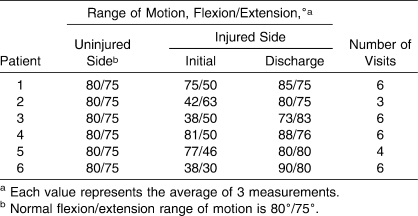
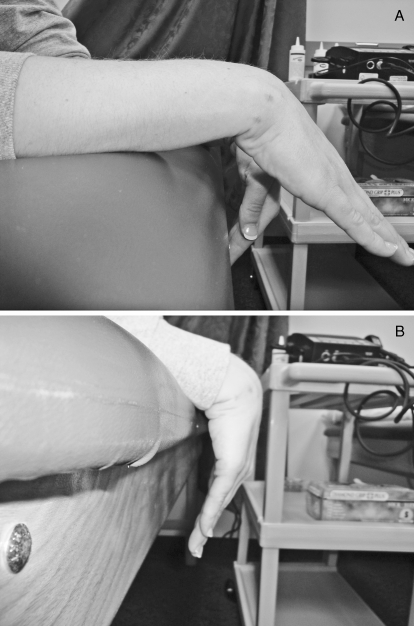
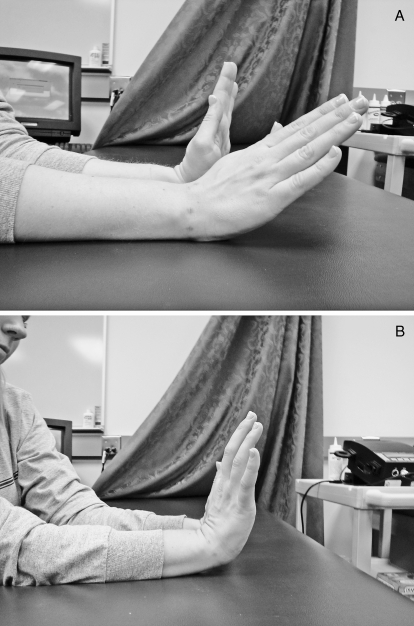
Patient 6 demonstrating flexion. A, Before any treatments. B, After the sixth treatment.
Figure 2.
Patient 6 demonstrating extension. A, Before any treatments. B, After the sixth treatment.
DISCUSSION
I performed this study to investigate the efficacy of a 3-MHz ultrasound and joint mobilization regimen on wrists with reduced flexion and extension elastic barriers. This protocol was effective, as demonstrated by all patients except 1 achieving or surpassing treatment AROM goals for flexion (80°) and all patients achieving or surpassing treatment goals for extension (75°). I will now discuss how the results of this study compare with those of previous studies, why ultrasound was the appropriate modality for this study, and why joint mobilizations were the appropriate intervention for these patients.
For the ankle joint capsule, which contains tissues similar to those of the wrist, Seiger and Draper9 studied 4 patients with ankle fractures that were repaired via open reduction, internal fixation using metal implants. The treatment regimen consisted of pulsed short-wave diathermy to the medial and lateral ankle (2 induction drums) for 20 minutes at 27.12 MHz, 800 pulses per second, 400 microseconds, and 48 W. These settings increase tissue temperature approximately 4°C to 5°C (39.2°F to 41.0°F).8,16 Immediately after diathermy treatment, mobilizations were administered to the ankle, and ice was applied for 20 minutes. Dorsiflexion ROM improved 15°, 15°, 10°, and 14° in the 4 patients. No discomfort, pain, or burning was reported during or after treatment.
Ultrasound was used in the present study for 2 reasons. First, it heats small areas about twice the size of the soundhead. The crystal was 5 cm2, with an effective radiating area of 4.5 cm2, making it ideal for heating 1 side of the wrist at a time. Second, ultrasound at 3 MHz heats to 2.5 centimeters deep, which again is ideal for targeting the superficial tissues of the wrist.24 The average treatment time of 6 minutes at 1.4 W/cm2 (with the Omnisound 3000C) heats tissues by 5°C to 6°C (41.0°F to 42.8°F).13 This is well within the range for changing the viscoelastic properties of collagen.16
I selected wrist joint mobilizations due to their effectiveness in improving AROM in both contractile and noncontractile tissues, such as hypomobile joint contractures and scar tissue.5,7 Joint mobilizations were performed immediately after the ultrasound treatment because the heat dissipates rapidly via thermal conduction away from the site via the vascular system.11,13,24 The temperature rise in skeletal muscle decreases rapidly within the first 10 to 15 minutes after the ultrasound application is completed (depending on the intensity and frequency used and depth of the target tissues).11,13,24 However, the current study was performed on noncontractile tissue, in which the rate of cooling might be slower, allowing more time to perform joint mobilization. Regardless, the sooner the clinician can start mobilizing the joints after heating the tissues, the better the outcome because the tissues will start to cool down as soon as the ultrasound treatment has ended.13,25
Passive stretching was not my main focus because it does not aim at the gliding or sliding component that is often missing when a joint has contracted.5,7 Kaltenborn5 believed that more ROM is gained in a hypomobile joint when joint mobilizations are performed than when basic stretching is performed. Stretching only works the angular components (ie, flexion, extension), whereas joint mobilization works the accessory components, the most important being gliding and sliding.
Indications for joint glide mobilization include pain, muscle spasm, guarding, reversible joint hypomobility, and functional immobility.7 The indications I focused on in this case series were hypomobility and immobility. The barrier that prevents movement in the direction of motion loss is called the restrictive barrier.7 Thus, my goal in using wrist joint mobilizations was to move the restrictive barrier as far into the direction of motion loss as possible. In order to determine hypomobility, normal and pathologic end feels must be understood. At the elbow joint, normal extension is a “hard” end feel, as the olecranon of the ulna comes into contact with the olecranon process. A pathologic end feel requiring joint glide mobilizations occurs when extension is halted before full extension is reached. The halting would not feel hard or like bone to bone but firm, as if the capsule were tight.26 At the elbow joint, normal flexion is a “soft” end feel, as the biceps brachii muscle comes into contact with the forearm muscles. A pathologic end feel requiring joint glide mobilizations occurs when flexion is halted before full flexion is reached. The halting would not feel soft but firm, as if the capsule was tight.26
In his text, Kaltenborn5 stated that mobilization preceded by heat application often produces greater mobility gains, and ice application after mobilization can better preserve mobility gains.5 He even listed ultrasound and diathermy as effective deep-heat applications.5
Fractures of the scaphoid have high complication rates including posttraumatic arthritis, avascular necrosis, loss of function, and difficulty regaining the last few degrees of extension. My 2 patients with this injury both achieved full ROM in flexion and extension, and both regained full use of their hands.
Probably the most interesting and challenging case was patient 2, who had 3 wrist extensor tendons severed accidentally. He could flex the wrist only to 43°. After just 3 treatments (every other day), his ROM increased to 80°. I suggested that a few more treatments might restore his wrist flexion equal to his opposite wrist (85°). It was the end of the semester, however; he was content with the progress he had made and chose to discontinue treatment.
The university electrician (patient 5) was 42 years old, about 20 years older than the other patients. His age alone might have led to slower healing, but he did reach full ROM in just 4 treatments (lacked only 3° of flexion), and he lacked 29° for extension. He was only able to receive 4 treatments, so I might have encouraged him a little more at the start. Also, he was accustomed to using his wrist more than the other patients, due to the nature of his job, which could have led to a quicker recovery.
LIMITATIONS
Although these results were quite good, some limitations are evident. A lack of blinding of the goniometric measurements could have decreased validity of study outcomes.27 The patients were not blinded to their treatment conditions, and I did not include a control group. In retrospect, it might have been a good idea during the ultrasound treatment to rest the opposite side of the wrist on a hot pack to prolong the heating of the tissues.
To determine the full implications of this case series, additional research is needed to identify the effectiveness of this protocol using a greater number of research participants involved in a randomized experiment, with a control group and outcome measures including ROM, pain, edema, and function. Future investigators need to include blinding of the clinician to the experimental group and to the goniometric measurements.
CONCLUSIONS
Thermal ultrasound used in concert with joint mobilizations was an effective regimen aimed at restoring ROM in hypomobile wrists postinjury or when immobilized after surgery. Ultrasound also increased patient comfort during the treatment and minimized posttreatment soreness.1,27,28
REFERENCES
- 1.Palmer L. M., Epler M. E. Fundamentals of Musculoskeletal Assessment Techniques. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 2.Magee D. J. Orthopedic Physical Assessment. 2nd ed. Philadelphia, PA: WB Saunders; 1992. pp. 182–183. [Google Scholar]
- 3.Starkey C., Ryan J. Evaluation of Orthopedic and Athletic Injuries. 2nd ed. Vol. 540 Philadelphia, PA: FA Davis; 2002. [Google Scholar]
- 4.Hoppenfeld S. Physical Examination of the Spine and Extremities. Vol. 91 Norwalk, CT: Appleton-Century-Crofts; 1976. [Google Scholar]
- 5.Kaltenborn F. M. Manual Mobilization of the Joints: The Kaltenborn Method of Joint Examination and Treatment. Vol 1. 6th ed. Vol. 78 Oslo, Norway: Olaf Norlis Bokhandel; 2002. [Google Scholar]
- 6.Guler-Uysal F., Kozanoglu E. Comparison of the early response to two methods of rehabilitation in adhesive capsulitis. Swiss Med Wkly. 2004;134:353–358. doi: 10.4414/smw.2004.10630. [DOI] [PubMed] [Google Scholar]
- 7.Greenman P. E. Principles of Manual Medicine. 2nd ed. Vol. 542 Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- 8.Draper D. O., Castel J. C., Castel D. Low-watt pulsed shortwave diathermy and metal-plate fixation of the elbow. Athl Ther Today. 2004;9(5):27–31. [Google Scholar]
- 9.Seiger C., Draper D. O. Use of pulsed shortwave diathermy and joint mobilizations to increase ankle range of motion in the presence of surgical implanted metal: a case series. J Orthop Sports Phys Ther. 2006;36(9):669–677. doi: 10.2519/jospt.2006.2198. [DOI] [PubMed] [Google Scholar]
- 10.Draper D. O., Knight K., Fukiwara T., Castel J. C. Temperature change in human muscle during and after pulsed short wave diathermy. J Orthop Sports Phys Ther. 1999;29(1):13–22. doi: 10.2519/jospt.1999.29.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Garrett C. L., Draper D. O., Knight K. L. Heat distribution in the lower leg from pulsed short-wave diathermy and ultrasound treatments. J Athl Train. 2000;35(1):50–55. [PMC free article] [PubMed] [Google Scholar]
- 12.Draper D. O., Castel J. C., Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995;22(4):142–150. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]
- 13.Draper D. O., Ricard M. D. Rate of temperature decay following 3 MHz ultrasound: the stretching window revealed. J Athl Train. 1995;30(4):304–307. [PMC free article] [PubMed] [Google Scholar]
- 14.Dyson M. The use of ultrasound in sports physiotherapy. In: Grisogono V., editor. Sports Injuries. Edinburgh, United Kingdom: Churchill Livingstone; 1989. [Google Scholar]
- 15.Lehmann J. F., Masock A. J., Warren C. G., Koblanski J. N. Effect of therapeutic temperatures on tendon extensibility. Arch Phys Med Rehabil. 1970;51(8):481–487. [PubMed] [Google Scholar]
- 16.Hengered E., Banks K., editors. Maitland's Peripheral Manipulation. 4th ed. Vol. 401 Oxford, United Kingdom: Butterworth-Heinemann; 2005. [Google Scholar]
- 17.Knight K. L., Draper D. O. Therapeutic Modalities: The Art and Science. Vol. 285 Baltimore, MD: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 18.Boone D. C., Azen S. P., Lin C-M., Spence C., Baron C., Lee L. Reliability of goniometric measurements. Phys Ther. 1978;58(11):1355–1360. doi: 10.1093/ptj/58.11.1355. [DOI] [PubMed] [Google Scholar]
- 19.Gajdosik R. L., Bohannon R. W. Clinical measurement of range of motion: review of goniometry emphasizing reliability and validity. Phys Ther. 1987;67(12):1867–1872. doi: 10.1093/ptj/67.12.1867. [DOI] [PubMed] [Google Scholar]
- 20.Low J. L. The reliability of joint measurement. Physiotherapy. 1976;62(7):227–229. [PubMed] [Google Scholar]
- 21.Leadbetter W. B., Buckwalter J. A., Gordon S. L., editors. Sports-Induced Inflammation: Clinical and Basic Science Concepts. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1990. pp. 55–101. [Google Scholar]
- 22.Knight K. L. The effects of hypothermia on inflammation and swelling. Athl Train J Natl Athl Train Assoc. 1976;11(1):7–10. [Google Scholar]
- 23.Merrick M. A. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train. 2002;37(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes B. T., Merrick M. A., Sandrey M. A., Cordova M. L. 3-MHz ultrasound heats deeper into the tissues than we originally theorized. J Athl Train. 2004;39(2):230–234. [PMC free article] [PubMed] [Google Scholar]
- 25.Rose S., Draper D. O., Schulthies S. S., Durrant E. The stretching window, part two: rate of thermal decay in deep muscle following 1-MHz ultrasound. J Athl Train. 1996;31(3):139–143. [PMC free article] [PubMed] [Google Scholar]
- 26.Cyriax J. Textbook of Orthopaedic Medicine: Diagnosis of Soft Tissue Lesions. 8th ed. London, United Kingdom: Bailliere Tindall; 1982. [Google Scholar]
- 27.Kaltenborn F. M. Manual Mobilization of the Joints: The Kaltenborn Method of Joint Examination and Treatment. Vol 2. 4th ed. Oslo, Norway: Olaf Norlis Bokhandel; 2003. [Google Scholar]
- 28.Lehmann J. F. Therapeutic Heat and Cold. 4th ed. Baltimore, MD: Williams & Wilkins; 1990. pp. 528–530. [Google Scholar]