Abstract
Background
The fruit fly, Drosophila melanogaster, is a well-established model organism for probing the molecular and cellular basis of physiological and immune system responses of adults or late stage larvae to bacterial challenge. However, very little is known about the consequences of bacterial infections that occur in earlier stages of development. We have infected mid-second instar larvae with strains of Pseudomonas fluorescens to determine how infection alters the ability of larvae to survive and complete development.
Methodology/Principal Findings
We mimicked natural routes of infection using a non-invasive feeding procedure to study the toxicity of the three sequenced P. fluorescens strains (Pf0-1, SBW25, and Pf-5) to Drosophila melanogaster. Larvae fed with the three strains of P. fluorescens showed distinct differences in developmental trajectory and survival. Treatment with SBW25 caused a subset of insects to die concomitant with a systemic melanization reaction at larval, pupal or adult stages. Larvae fed with Pf-5 died in a dose-dependent manner with adult survivors showing eye and wing morphological defects. In addition, larvae in the Pf-5 treatment groups showed a dose-dependent delay in the onset of metamorphosis relative to control-, Pf0-1-, and SBW25-treated larvae. A functional gacA gene is required for the toxic properties of wild-type Pf-5 bacteria.
Conclusions/Significance
These experiments are the first to demonstrate that ingestion of P. fluorescens bacteria by D. melanogaster larvae causes both lethal and non-lethal phenotypes, including delay in the onset of metamorphosis and morphological defects in surviving adult flies, which can be decoupled.
Introduction
The fruit fly, Drosophila melanogaster, is a well-established model organism for the study of host response to microbial infection and animal development. To protect against infection, D. melanogaster employs a suite of cellular and humoral defense mechanisms, making it a good reference organism in which to dissect host responses (reviewed in [1], [2], [3], [4], [5], [6]). For example, D. melanogaster employ mechanical barriers (such as the cuticle and tightly connected epithelial cells) to reduce the entry of environmental pathogens. In addition, a rapid defense is mounted through the secretion of a battery of inducible effector molecules (i.e. antimicrobial peptides such as Diptericin and reactive oxygen species), activation of phenoloxidase through a complement-like protease cascade (which leads to the production of reactive compounds and melanin), clotting of the hemolymph, and phagocytosis and encapsulation of foreign objects by blood cells (hemocytes), all of which are tightly regulated mechanisms for neutralizing intruders while maintaining a balanced microbiota.
D. melanogaster larvae and adults are exposed to a variety of environmental bacteria in their natural habitats. Among these are species of Pseudomonas, a diverse genus of gamma proteobacteria commonly found in soil, water, or in association with plants or animals. Certain strains of Pseudomonas entomophila [7] and Pseudomonas aeruginosa [8], [9] are known pathogens of D. melanogaster, but this pathogenicity is thought to be uncommon for the genus. Of 28 strains representing a spectrum of Pseudomonas spp. that were tested by Vodovar et al. [7], only P. entomophila exhibited pathogenicity against D. melanogaster. Nevertheless, genes with predicted functions in insect toxicity are present in genomic sequences of other Pseudomonas spp., including fitD in Pseudomonas fluorescens strains Pf-5 and CHA0 [10], and tc-like toxins in Pseudomonas syringae pv. syringae strain B728a and P. fluorescens Pf0-1 [11]. A recent study demonstrated that fitD confers strong insecticidal activity, which was exhibited when Pf-5 or the closely-related strain CHA0 was injected into larvae of two insect species, the tobacco hornfworm Manduca sexta and the greater wax moth Galleria mellonella [10]. These results highlighted the exciting possibility that strains of P. fluorescens have additional previously-unappreciated insecticidal activities.
This study was initiated to determine the oral toxicity of P. fluorescens against the model organism D. melanogaster, and to characterize the effect of the microbial infection throughout host development. The immune response to high doses of pathogenic and non-pathogenic bacteria has been studied extensively in adult D. melanogaster and late stage larvae. Very few experiments have examined the impact of exposure to bacteria at earlier developmental stages when the animals are undergoing rapid growth of both body tissues and rapid divisions of the imaginal disc cells, which will generate the adult body. We developed a new protocol to feed timed cultures of D. melanogaster larvae with bacterial strains without handling the larvae, hence reducing the risk of injuries and stress prior to infection. Using this non-invasive protocol, we observed three distinct and strain-specific responses of D. melanogaster to infection by three strains of P. fluorescens (Pf0-1, SBW25 and Pf-5) with fully sequenced genomes [12], [13]. Oral ingestion of strain Pf0-1 had little effect on larvae. In contrast, strains SBW25 and Pf-5 were toxic to D. melanogaster, with SBW25 causing a systemic melanization response and Pf-5 causing a dose-dependent lethality and delayed metamorphosis coupled with morphological defects in adults. Induction of an immune response after oral infection with Pf-5, SBW25 and Pf0-1 was also observed by using a diptericin-lacZ reporter. Using the novel larval feeding assay developed herein, we could assess the effect of bacterial infection on larval growth rate, development of the imaginal discs and timing of metamorphosis, thereby establishing the toxicity of P. fluorescens SBW25 and Pf-5 to D. melanogaster and revealing intriguing new insights into the coupled effects of microbial infection on host defense response and development.
Results
Pf-5 and SBW25 reduced D. melanogaster survival
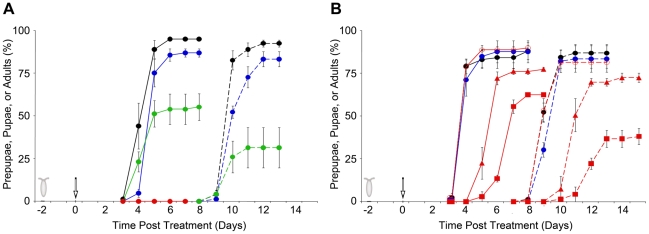
We determined the effect of three strains of P. fluorescens on larval and pupal survival in a newly-developed, non-invasive feeding assay (Fig. 1). Second instar CantonS-A (CS-A) wild-type larvae were fed with a yeast suspension containing high cell densities of bacteria or, as a control treatment, yeast suspension containing no added bacterial cells. In the control treatments, 86±4% of the larvae survived to become adults (Fig. 2, Fig. S1). Similarly, 87±1% of larvae fed with P. fluorescens strain Pf0-1 at doses ranging from 103 to 109 cfu/plate survived to adulthood (Fig. 2, Fig. S1), which is not different from the survival of larvae in the control treatment group (Χ2 = 0.95, Df = 6, P = 0.99). In contrast, only 30±10% of larvae fed with a yeast suspension containing 107 or 109 cells of SBW25 survived to adulthood (Fig. 2A, Fig. S1), which is significantly different from the control treatment group (Χ2 = 61.1, Df = 1, P<0.00001). Insects in the SBW25 treatment died at two different stages: approximately half (58±8%) of the larvae pupariated and, of those pupae, only about half (49±13%) emerged as adults. Thus, ingestion of SBW25 by larvae caused individuals at both feeding and non-feeding stages to die. The consequence of ingesting Pf-5 bacteria was more severe; no larvae fed Pf-5 at the highest dose tested, 109 cfu/plate, pupariated (Fig. 2, Fig. S1).
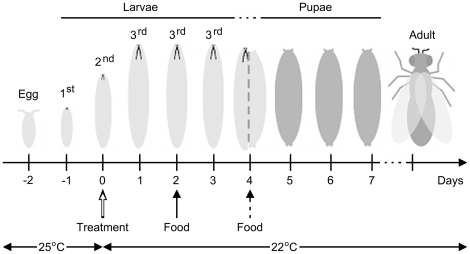
Figure 1. Timeline of the experimental protocol.
Above the daily timeline are cartoons of the developmental stage that most closely replicates the control-treatment group trajectory (see Material and Methods for details). On Day −2, eggs were transferred from the egglaying plates to non-nutritive agar plates. The split image on Day 4 indicates the transition period between wandering larvae and prepupal stages around the onset of metamorphosis. Treatments distributed on the agar surface on Day 0 (open arrow) were composed of a yeast suspension, which served as a food source for the larvae, alone (control) or amended with bacterial inoculum. An additional yeast supplement (food) was added on Day 2 to all plates (solid arrow) and every 2 days as long as feeding larvae were visible (dashed arrow). Using this method, larvae were never directly handled and the only disturbance was the application of treatments or food to the surface of the plate. The plates were housed in an incubator on a 12L:12D cycle initially at 25°C; on Day 2, the temperature was shifted to 22°C to slow down larval development.
Figure 2. Developmental time course after bacterial inoculation.
The percentage of larvae that pupariated, counted as prepupae and/or pupae (solid lines), or emerged as adults (dashed lines) were determined for two different experiments (A and B) in which second instar larvae were fed with a yeast suspension having no bacteria (black) or amended with bacterial strains Pf0-1 (blue), SBW25 (green, Fig. 2A), Pf-5 (red), or a Pf-5 gacA mutant (open red circle, Fig. 2B). On the timeline in this and Figure 3, the egg-laying period is denoted by the egg cartoon and the treatment by an open arrow at Day 0. Bacterial treatment as cfu/plate: (A) Pf0-1, 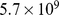 ; SBW25,
; SBW25, 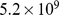 ; Pf-5,
; Pf-5, 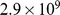 , or (B) Pf0-1,
, or (B) Pf0-1, 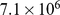 ; Pf-5 gacA mutant,
; Pf-5 gacA mutant, 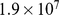 ; Pf-5 (squares),
; Pf-5 (squares), 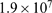 ; Pf-5 (triangles),
; Pf-5 (triangles), 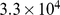 .
.
Other wild-type D. melanogaster strains also showed high mortality after feeding with Pf-5 and SBW25 bacteria (Fig. S1; data not shown). When larvae of the Oregon-R (OR) or other two CS wild-type strains were fed Pf-5 at 107 cfu/plate, none pupariated. At a lower dose of 104 cfu/plate, few OR or CS larvae survived to adulthood (3±3% and 7±4%). OR larvae fed SBW25 at either 104 or107 cfu/plate had reduced survival to adulthood (Fig. S1). Thus, ingestion of strains Pf-5 and SBW25 significantly reduced larval and pupal survival rates of D. melanogaster.
Living cells and GacA are required for toxicity of strain Pf-5
After determining that ingestion of Pf-5 killed larvae, we investigated factors contributing to this toxicity. We first tested whether living Pf-5 cells were required for lethality by feeding larvae heat-killed Pf-5 cells at a dose of 109 cfu/plate. Because 91±1% of larvae survived to adulthood (Fig. 3, Fig. S1), similar to the rate of survival in control and Pf0-1-treatment groups, we conclude that the toxicity of Pf-5 to D. melanogaster larvae requires intact live bacterial cells or a heat-labile molecule.
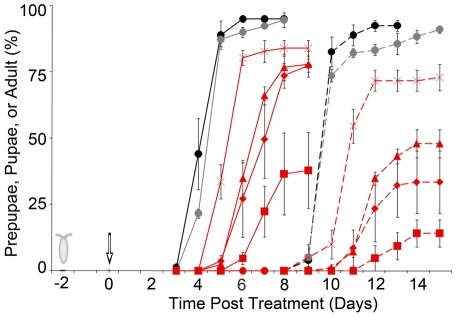
Figure 3. Developmental time course after inoculation with different doses of Pf-5.
The percentage of larvae that pupariated, counted as prepupae and/or pupae (solid lines), or emerged as adults (dashed lines) after inoculation with Pf-5 (filled red symbols or star), killed Pf-5 (killed 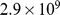 cfu/plate, gray circles) or control (black circles ) treatment were determined for each time point. The Pf-5 cell densities as cfu/plate were
cfu/plate, gray circles) or control (black circles ) treatment were determined for each time point. The Pf-5 cell densities as cfu/plate were 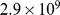 (circles),
(circles), 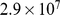 (squares),
(squares), 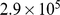 (diamonds),
(diamonds), 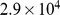 (triangles),
(triangles), 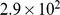 (stars) cfu/plate.
(stars) cfu/plate.
Because the GacS/GacA two component regulatory system controls the production of virulence factors and secondary metabolites in Pseudomonas spp. [14] including strain Pf-5 [15], [16], [17], and is required for virulence of P. entomophila against D. melanogaster
[7], we tested the toxicity of a Pf-5 gacA mutant. Whereas 90±1% of larvae pupariated, fewer survived to adulthood (81±3%) when fed a yeast suspension containing Pf-5 gacA mutant cells at a dose of 107 cfu/plate; only 38±5% of larvae fed the same dose of wild-type Pf-5 survived (Fig. 2B). Thus, GacA had a major effect on the insect toxicity exhibited by Pf-5. The frequency of adult survival of Pf-5 gacA-treated larvae (averaging 71±6% for three different experiments at doses of 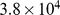 ,
, 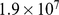 , and
, and 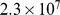 cfu/plate) was found to be significantly lower compared to that of control larvae (Χ2 = 7.91, Df = 2, P = 0.02). Thus, we conclude that the lethality associated with ingestion of Pf-5 bacteria by D. melanogaster larvae is largely dependent on GacA-regulated functions, but there must also be one or more Pf-5 components unrelated to GacA that also contribute to this lethality.
cfu/plate) was found to be significantly lower compared to that of control larvae (Χ2 = 7.91, Df = 2, P = 0.02). Thus, we conclude that the lethality associated with ingestion of Pf-5 bacteria by D. melanogaster larvae is largely dependent on GacA-regulated functions, but there must also be one or more Pf-5 components unrelated to GacA that also contribute to this lethality.
Larval and pupal survival after Pf-5-treatment was dose-dependent
Larval survival was strongly dependent on the initial cell density of Pf-5 added to the diet. No larvae survived to later stages when fed with a yeast suspension containing Pf-5 at 109cfu/plate, and both the frequency of pupariation and survival to adulthood increased as the dose of Pf-5 was reduced (Figs 2B, 3, Fig. S1). Chi-squared analysis revealed that the adult survival of larvae fed low doses of Pf-5 (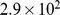 to
to 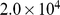 cfu/plate) was not significantly different to that of larvae in the control group (84±3%, Χ2 = 2.08, Df = 4, P = 0.71). At intermediate doses of Pf-5 (
cfu/plate) was not significantly different to that of larvae in the control group (84±3%, Χ2 = 2.08, Df = 4, P = 0.71). At intermediate doses of Pf-5 (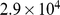 to
to 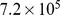 cfu/plate), there was a significant difference in adult survival compared to the control treatment group (62±6%, Χ2 = 66.95, Df = 8, P<0.00001). This difference was the result of a significant reduction in the number of pupae surviving to become adults (Figs 2B, 3; Χ2 = 37.89, Df = 8, P<0.00001). For the larvae exposed to high treatment doses of Pf-5 (1.7×107 to 2.9×109 cfu/plate), adult survival was significantly reduced (17±4%, Χ2 = 229.73, Df = 4, P<0.00001) due to a reduction in both the number of larvae that pupariated and pupae that survived to adult emergence (Χ2 = 118.2, Df = 4, P<0.0000; Χ2 = 21.1, Df = 4, P<0.00003, respectively). For larvae fed Pf-5 at 107cfu/plate, the frequency of adult survival was highly variable, ranging from 0–38%, suggesting that slight differences in the timing of the bacterial treatment have a larger effect at this high dose than at lower doses. To examine the effect of developmental age, we compared the survival of first and second instar larvae fed the same doses of Pf-5. No first instar larvae fed Pf-5 at 104 or 106 survived to the pupal stage, whereas second instar larvae fed the same doses were able to pupariate (data not shown). Therefore, Pf-5 ingestion caused both larval and pupal mortality in a dose-dependent fashion.
cfu/plate), there was a significant difference in adult survival compared to the control treatment group (62±6%, Χ2 = 66.95, Df = 8, P<0.00001). This difference was the result of a significant reduction in the number of pupae surviving to become adults (Figs 2B, 3; Χ2 = 37.89, Df = 8, P<0.00001). For the larvae exposed to high treatment doses of Pf-5 (1.7×107 to 2.9×109 cfu/plate), adult survival was significantly reduced (17±4%, Χ2 = 229.73, Df = 4, P<0.00001) due to a reduction in both the number of larvae that pupariated and pupae that survived to adult emergence (Χ2 = 118.2, Df = 4, P<0.0000; Χ2 = 21.1, Df = 4, P<0.00003, respectively). For larvae fed Pf-5 at 107cfu/plate, the frequency of adult survival was highly variable, ranging from 0–38%, suggesting that slight differences in the timing of the bacterial treatment have a larger effect at this high dose than at lower doses. To examine the effect of developmental age, we compared the survival of first and second instar larvae fed the same doses of Pf-5. No first instar larvae fed Pf-5 at 104 or 106 survived to the pupal stage, whereas second instar larvae fed the same doses were able to pupariate (data not shown). Therefore, Pf-5 ingestion caused both larval and pupal mortality in a dose-dependent fashion.
Larvae fed with a yeast suspension containing high doses of Pf-5 tended to die at an earlier stage of development than larvae fed with lower doses. For example, when Pf-5 was added at 109cfu/plate, many larvae died as second instars, some molted into third instar larvae but none were able to pupariate. At doses of 104 to 107 cfu/plate, most larvae survived to become third instars but many were unable to pupariate or died as pre-pupae. Pre-pupae that did not continue development characteristically failed to evert their anterior spiracles or progress beyond the mid-bubble stage and so did not complete pupal ecdysis [18]. At lower doses of Pf-5 (102–103), most larvae pupariated and a few pupae died at later stages of metamorphosis. We found similar survival rates when the total treatment dose was divided into two equal applications on Day 0 and 1 (data not shown) or was delivered as a single dose on Day 0, suggesting that it is the population of bacterial cells that is important, not the feeding regimen, for determining survival. Thus, we have found a strong dose-dependent correlation of survival after ingestion of Pf-5.
Pf-5, but not Pf0-1 or SBW25, causes developmental delay
We noticed that larvae exposed to Pf-5 treatment frequently failed to pupariate even when viable for many days, suggesting that they were arrested or delayed in their development. In addition, this delay in pupariation also appeared to be dose-dependent. Therefore, we calculated the relative duration of the larval stage for larvae fed different concentrations of Pf-5 (Fig. 4A). Larvae fed Pf-5 at lower doses delayed metamorphosis for up to a day, whereas larvae fed higher doses pupariated with up to a three-day delay. In experiments with high doses of Pf-5 (≥107 cfu/plate), some larvae survived for days but were frequently unable to pupariate. In contrast, the duration of the pupal stage for most Pf-5 treatment groups was similar to that of controls (i.e within a half day), indicating that there is no systematic developmental delay during this stage (Fig. 4B). Thus, as shown in Figure 4C, the overall delay to adult emergence for Pf-5 treatments reflects the delay in pupariation and is accompanied by a reduction in the number of pupae able to eclose as adults. Larvae of other D. melanogaster wild-type strains exposed to Pf-5 bacterial treatments also had delayed pupariation. When OR larvae were fed with 4.5×104 cfu/plate Pf-5, pupariation was delayed for three days (Fig. 4A). Larvae from two other CS strains also showed a delay in pupariation (data not shown). The extended, dose-dependent developmental delay is specific to the Pf-5 treatment; larvae fed high doses of SBW25, killed Pf-5, or the Pf-5 gacA mutant cells pupariated and emerged as adults at a similar time as control larvae (Fig. 4). Only those CS-A larvae fed the highest dose of Pf0-1 (109 cfu/plate) exhibited any delay in pupariation, and that delay was very slight, whereas larvae fed with lower doses of Pf0-1 pupariated at the same time as control treated larvae (Fig. 4A).
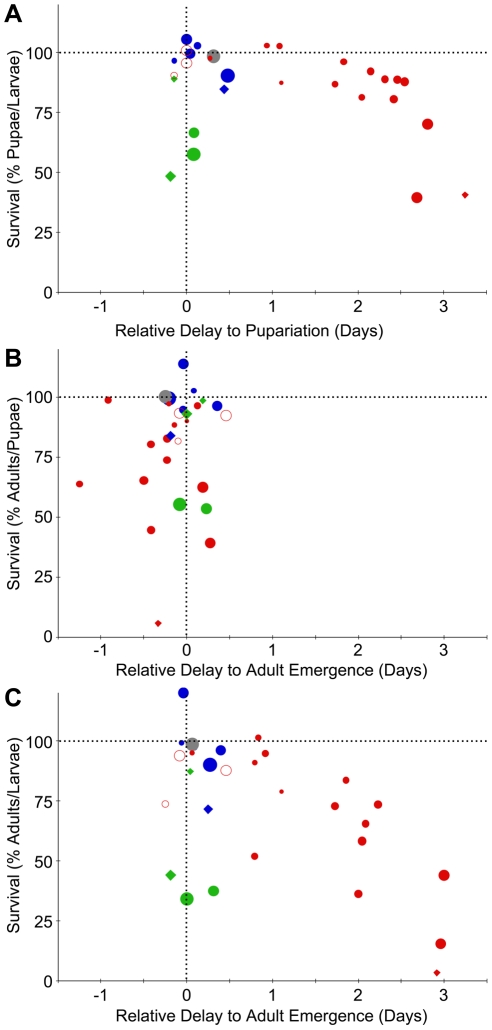
Figure 4. Dose-dependent effects of P. fluorescens strains on survival and developmental delay.
For each experimental treatment, the percent pupal or adult survival is normalized to that of the appropriate control, which has been set to 100%, and the relative delay to pupariation is normalized to that of the appropriate control, which has been set at 0. In this figure CS-A larval treatment groups are represented using circles and OR larval treatment groups by diamonds. The size of each data point is scaled to the inoculation dose (highest dose/largest circle is 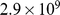 cfu/plate; smallest dose/smallest circle is
cfu/plate; smallest dose/smallest circle is 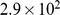 cfu/plate; highest dose/largest diamond is
cfu/plate; highest dose/largest diamond is 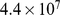 ; lowest dose/smallest diamond is
; lowest dose/smallest diamond is 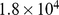 ); Pf0-1 (blue), SBW25 (green), Pf-5 (red), killed Pf-5 (gray) and a Pf-5 gacA mutant (open red circle). (A) The relative developmental delay (see Materials and Methods) during the larval stage (X-axis) was plotted against the percentage of larvae that survived to pupariate (Y-axis) for six different experiments. (B) The relative developmental delay during the pupal stage (X-axis) was plotted against the percentage of pupae that survived to adult survivors (Y-axis). (C) The relative developmental delay during larval and pupal stages (X-axis) was plotted against the percentage of larvae that survived to adult (Y-axis).
); Pf0-1 (blue), SBW25 (green), Pf-5 (red), killed Pf-5 (gray) and a Pf-5 gacA mutant (open red circle). (A) The relative developmental delay (see Materials and Methods) during the larval stage (X-axis) was plotted against the percentage of larvae that survived to pupariate (Y-axis) for six different experiments. (B) The relative developmental delay during the pupal stage (X-axis) was plotted against the percentage of pupae that survived to adult survivors (Y-axis). (C) The relative developmental delay during larval and pupal stages (X-axis) was plotted against the percentage of larvae that survived to adult (Y-axis).
In D. melanogaster, the onset of metamorphosis is gated by the attainment of a critical weight in the third instar larval stage in response to hormonal signaling associated with the growth of the imaginal discs, which generate the adult external structures [19], [20], [21], [22]. We measured the surface area of the wing blade as a convenient indicator of body size to determine whether larval developmental delay was correlated with changes in body size. Wing surface area was inversely related to the dose of Pf-5 in the diet (Fig. S2). At 107 cfu/plate, the treatment with the highest cell density of Pf-5 compatible with survival to adulthood, the wing surface area of surviving adults differed significantly from those of control, Pf0-1, and SBW25 treatments, suggesting that the delay in pupariation may be a compensatory mechanism: after exposure to lower populations of Pf-5 cells, the delay allows larvae the time needed to reach their critical weight and normal body size.
Pf-5 and SBW25 cause structural and behavioral defects in adult flies
Larval ingestion of Pf-5 and SBW25 produced several larval and adult phenotypes in addition to developmental delay and/or lethality. Immediately after the control-,Pf0-1- or SBW25-infested yeast suspensions were added to the assay plates, the larvae remained in place or moved to the region of the plate where the yeast was applied. In contrast, larvae responded to the Pf-5 inoculum by moving away from the site of application towards the edges of the plate. For example, about half of the larvae (61±18%) moved to the side of the dish or wall within two hours of applying a yeast suspension having 109 cfu of Pf-5 to the middle of the plate (data not shown). Although we do not know the nature of the repulsive agent in Pf-5, it was associated with high concentrations of live cells of Pf-5. Larvae did not move away from yeast suspensions having a high dose (109 cfu/plate) of killed Pf-5 cells or a lower dose (≤107 cfu/plate) of viable Pf-5 cells.
Because D. melanogaster larvae were found to cease feeding after exposure to P. entomophila [7], [23], we tested if they responded the same way to strains Pf-5, SBW25, or Pf0-1. However, when larvae were fed a bacterial suspension containing food dye, the dye was found in the guts of some or most live larvae at one hour and for up to one day, suggesting that larvae continued to feed after exposure to these three P. fluorescens strains at the concentrations that we tested (data not shown).
Larvae fed moderate to high concentrations of Pf-5 (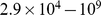 cfu/plate) had a very characteristic appearance that differed from larvae fed control, Pf-5 gacA mutant or killed Pf-5 treatments (Fig. 5A–F). After moderate to high Pf-5 treatments, second and third instar larvae were lethargic and the lateral sheets of fat body organ lost their opaque appearance. At death, the larvae were nearly clear and lacked any visible fat body (Fig. 5F). These third instar larvae remained very small and the main tracheal trunks, which stretch dorsally along the full length of the body, had a convoluted trajectory due to the failure of the larvae to extend to full size (Fig. 5D–F). Control third instar larvae typically dig into the agar of the plate in their search for food, whereas the larvae ingesting high doses of Pf-5 (107–109) remained on the surface of the plate.
cfu/plate) had a very characteristic appearance that differed from larvae fed control, Pf-5 gacA mutant or killed Pf-5 treatments (Fig. 5A–F). After moderate to high Pf-5 treatments, second and third instar larvae were lethargic and the lateral sheets of fat body organ lost their opaque appearance. At death, the larvae were nearly clear and lacked any visible fat body (Fig. 5F). These third instar larvae remained very small and the main tracheal trunks, which stretch dorsally along the full length of the body, had a convoluted trajectory due to the failure of the larvae to extend to full size (Fig. 5D–F). Control third instar larvae typically dig into the agar of the plate in their search for food, whereas the larvae ingesting high doses of Pf-5 (107–109) remained on the surface of the plate.
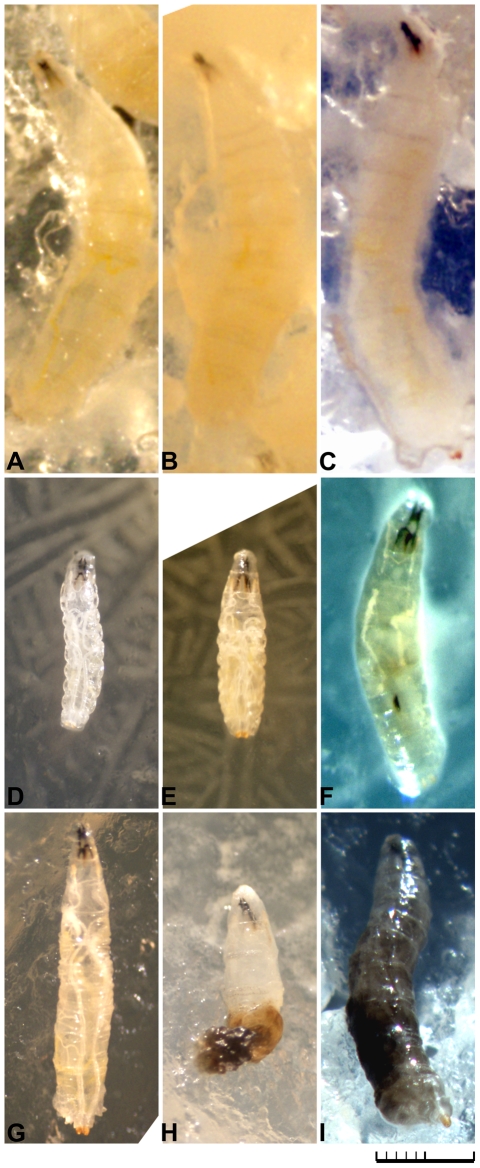
Figure 5. Effects of P. fluorescens strains on the morphology of third instar larvae.
Third instar larvae on the surface of the treatment plates were photographed using a digital camera mounted on a dissecting scope. Bar is 1 mm with 0.1 mm divisions. (A) 96 hour post control treatment. (B) 72 hour post Pf0-1 treatment (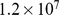 cfu/plate). (C) 72 hour post Pf-5 gacA mutant treatment (
cfu/plate). (C) 72 hour post Pf-5 gacA mutant treatment (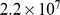 cfu/plate). (D) 48 hour post Pf-5 treatment (
cfu/plate). (D) 48 hour post Pf-5 treatment ( cfu/plate), showing small body size and convoluted longitudinal tracheal trunks. (E) 72 hour post Pf-5 treatment (
cfu/plate), showing small body size and convoluted longitudinal tracheal trunks. (E) 72 hour post Pf-5 treatment (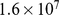 cfu/plate), showing small body size and convoluted tracheal trunks. (F) 72 hour post Pf-5 treatment (
cfu/plate), showing small body size and convoluted tracheal trunks. (F) 72 hour post Pf-5 treatment (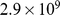 cfu/plate), showing loss of fat body opacity and a melanotic nodule. (G) 72 hour post SBW25 treatment (2.9×107 cfu/plate), with no melanization within the body cavity. (H) 72 hour post SBW25 treatment (
cfu/plate), showing loss of fat body opacity and a melanotic nodule. (G) 72 hour post SBW25 treatment (2.9×107 cfu/plate), with no melanization within the body cavity. (H) 72 hour post SBW25 treatment (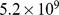 cfu/plate) showing melanization within the posterior third of the body. (I) 72 hour post SBW25 treatment (
cfu/plate) showing melanization within the posterior third of the body. (I) 72 hour post SBW25 treatment ( cfu/plate) showing complete melanization reaction.
cfu/plate) showing complete melanization reaction.
We observed a striking physical difference in immune response between larvae ingesting SBW25 compared to Pf-5. In the SBW25-treated group, larvae had two fates. About half of the larvae appeared similar to control larvae, whereas the subset of larvae that died developed a systemic melanization reaction followed by death (Fig. 5A, G–I). The first evidence of melanization occurred in the middle of the third instar stage with the appearance of black nodules and epidermal lesions. Subsequently, larvae developed widespread blackening within the body cavity in the posterior abdomen near the malphigian tubules (Fig. 5H). Over the course of the next 6–12 hours, melanization spread throughout the entire body cavity and the animals died swollen and fully extended (Fig. 5I). Larvae that did not develop a melanization reaction successfully pupariated, but in the middle of the pupal stage, about half of these pupae developed a systemic melanization reaction and died. We also noted that many adult flies died post-eclosion after a systemic melanization reaction occurred. By contrast, the Pf-5-treated larvae had the occasional small black nodules associated with tissues or black lesions in the epidermis, but did not show the whole body melanization response exhibited by a subset of larvae that ingested SBW25 (Fig. 5F). In order to determine whether the ingestion of P. fluorescens strains induced an immune response, we used a line of D. melanogaster expressing β-galactosidase under the control of the promoter of an immune challenge inducible gene encoding an antimicrobial peptide, diptericin-lacZ. Second instar larvae were fed Pf0-1, Pf-5 and SBW25 and then dissected as feeding or wandering third instar larvae for β-galactosidase activity. We found that third instar larvae exposed to all three bacterial strains or the Pf-5 gacA mutant expressed β-galactosidase in their gut and fat body suggesting that their immune system had been activated (Fig. S3).
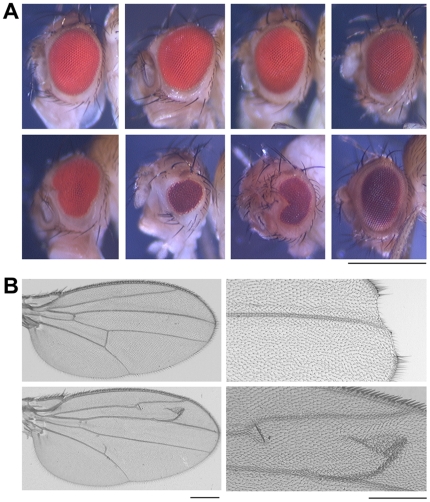
The adult survivors of Pf-5 and SBW25 treatments showed morphological defects in specific external structures (Fig. 6, Table S1). In pilot experiments, flattened cuticle preparations of adult survivors of bacterially treated larvae were examined and eyes, head capsule, legs and wings were the only structures in which defects were detected (data not shown). The most common defects in the eye were an abnormally small size and/or the presence of anterior and posterior nicks at the equator, sometimes accompanied by small duplications of bristles and head capsule epidermis (Fig. 6A). A few flies from the Pf0-1-treatment groups had small eye phenotypes but, by Chi-squared analysis, the frequency was not significant compared to the frequency found in the control-treatment group (4%, Χ2 = 1.09, Df = 3, P = 0.78). Treatment with any dose of live Pf-5 bacteria caused the majority of the surviving flies to have an eye phenotype that was significantly different from that of the control (91%, Χ2 = 245.78, Df = 7, P<0.00001). Similar eye defects were found in survivors of Pf-5 treatment of larvae from the OR or the two other CS strains (Fig. S4). At low to moderate doses of Pf-5, we observed wing defects, such as the presence of extra wing veins and loss of small portions of the wing margin (Fig. 6B), however the average frequency of wing defects spanning all tested doses was not significant (12%, Χ2 = 6.50, Df = 7, P = 0.37). On rare occasions, we saw defects in the leg joints but there was no consistent pattern (1%, Χ2 = 0.09, Df = 7, P = 0.99). Somewhat surprisingly, unlike the frequency of survival or developmental delay, the frequency of eye and wing defects in Pf-5 treated animals did not show an obvious dose-response relationship. Flies that developed after treatment with Pf-5 gacA mutant or heat-killed cells had fewer defects than those treated with live Pf-5 cells (6%, Χ2 = 0.77, Df = 2, P = 0.95; 23%, Χ2 = 3.40 [n = 1 inoculation dose]; respectively), suggesting that these phenotypes may be a consequence of GacA function. Only the highest dose of SBW25 had a small eye phenotype (87%, Χ2 = 17.30, [n = 1 inoculation dose]), suggesting that a differential affect on adult survival and morphology by the three P. fluorescens strains accompanies the previously demonstrated distinct survival and developmental delay phenotypes.
Figure 6. Morphological defects in adult survivors after infection with Pf-5.
(A) Eye images from control and bacterial treatment groups. Top panel from left to right: control; 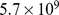 cfu/plate, Pf0-1;
cfu/plate, Pf0-1; 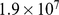 cfu/plate Pf-5 gacA mutant;
cfu/plate Pf-5 gacA mutant; 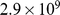 cfu/plate killed Pf-5. Bottom panel from left to right:
cfu/plate killed Pf-5. Bottom panel from left to right: 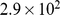 cfu/plate Pf-5;
cfu/plate Pf-5; 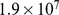 cfu/plate Pf-5;
cfu/plate Pf-5; 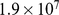 cfu/plate Pf-5;
cfu/plate Pf-5; 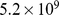 cfu/plate SBW25. Magnification bar = 500 µm. (B) Images of wing blades from adult flies after control (upper left) or Pf-5 at
cfu/plate SBW25. Magnification bar = 500 µm. (B) Images of wing blades from adult flies after control (upper left) or Pf-5 at 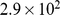 cfu/plate (bottom left, right panel) treatments. Developmental defects visible in these wings include loss of cells at the distal margin (upper right) and/or the presence of ectopic wing veins (bottom left and right). Magnification bar = 100 µm.
cfu/plate (bottom left, right panel) treatments. Developmental defects visible in these wings include loss of cells at the distal margin (upper right) and/or the presence of ectopic wing veins (bottom left and right). Magnification bar = 100 µm.
Colonization of D. melanogaster larvae by P. fluorescens
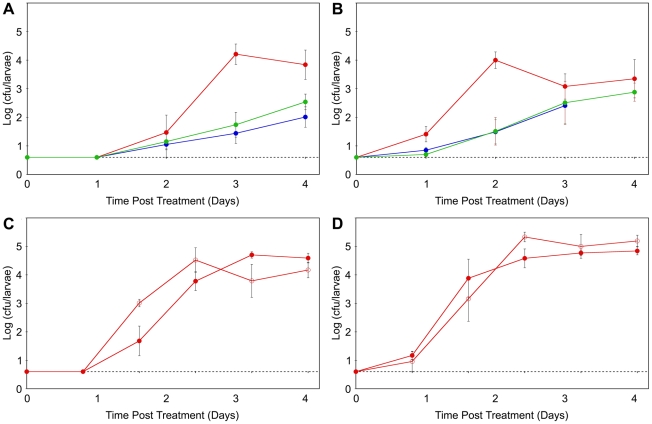
Because of the dose- and strain-dependent effects of P. fluorescens on D. melanogaster survival and development, the population sizes of the three strains were assessed from surface-sterilized larvae (Fig. 7). All three strains increased to an internal population size of 102 to 105cfu/larva over the course of the experiment. The population size of Pf-5 bacteria generally exceeded those of SBW25 or Pf0-1. In a second experiment, population sizes of the gacA mutant were compared to those of Pf-5 and found to be similar. Therefore, the three strains of P. fluorescens colonized internal tissues (including the gut cavity) of the larvae and, at least for strain Pf-5, the capacity to establish internal populations was independent of gacA.
Figure 7. Population sizes of P. fluorescens in larvae of D. melanogaster.
Second instar larvae were inoculated with strains Pf0-1 (blue), SBW25 (green), or Pf-5 (red) (A and B), or the Pf-5 gacA mutant (open circle) or wildtype Pf-5 (filled circle) (C and D). Each day following inoculation, larvae were individually surface sterilized, homogenized and bacterial numbers were estimated by culturing. Each point represents the average of five larvae. Bacterial population sizes in the inocula were 104 cfu/plate (A and C), or 107 cfu/plate (B and D). The dotted line denotes the limit of detection. On Day 4, larvae inoculated with Pf0-1 at 107 cfu/plate had pupariated and were not sampled. Error bars denote standard errors.
Discussion
In nature, D. melanogaster live in an environment full of microorganisms, yet only a few microbes are known to be lethal to wild-type D. melanogaster larvae and/or adults, including Pseudomonas aeruginosa, Pseudomonas entomophila, Serratia marcescens and Beauveria bassiana ([7], [8], [24], [25]. Here, we demonstrate that the insecticidal activity of P. fluorescens strain Pf-5, which was observed previously after injection of the tobacco hornworm Manduca sexta and the greater wax moth Galleria mellonella [10], is also exhibited when Pf-5 is introduced into D. melanogaster through a more natural route including direct contact and feeding of larvae. Strain SBW25 also caused lethality at both feeding and non-feeding stages, but to a lesser extent than Pf-5. As reported previously [26], insect lethality is not universal to P. fluorescens strains, and strain Pf0-1 exhibited no detectable toxicity to larvae after ingestion. Nevertheless, ingestion of Pf0-1, like Pf-5 and SBW25, induced an immune response in larvae, assessed using a transgenic line of D. melanogaster containing a diptericin-lacZ reporter gene fusion. Diptericin plays a critical role in defense of Drosophila against Gram-negative bacteria, including P. entomophila [23], being one of the anti-microbial peptides regulated by the Imd immune response pathway. Therefore, as all three strains of P. fluorescens were able to induce Diptericin gene expression, the difference in their toxicities may reflect their differential capacities to overcome or thwart different aspects of the immune response of Drosophila. The results of this study place P. fluorescens strains Pf-5 and SBW25 in a small group of bacteria that cause mortality of D. melanogaster larvae through feeding or physical contact.
Ingestion of either P. fluorescens Pf-5 or SBW25 was lethal to larval D. melanogaster from two different wild-type strains, but the bacterial strains caused markedly different, dose-dependent host responses. When high doses of Pf-5 were ingested, nearly all larvae died and most failed to molt to the third instar stage. This response is similar to that reported previously for P. entomophila: 70% of wild-type third instar larvae died after ingestion of high doses of P. entomophila [7]. In the present study, dose-response relationships were also explored, and developmental delay was the most striking consequence of ingestion of lower, sub-lethal doses of Pf-5. Although larvae exposed to sub-lethal doses of Pf-5 pupariated, many of these pupae later died, indicating that the consequences of bacterial ingestion extended into non-feeding stages. In addition, the marked delay in metamorphosis was accompanied by phenotypic changes, such as reduced larval body size and loss of fat body integrity. One of these phenotypes, small larval body size, is also a consequence of ingestion of P. entomophila [7]. Occasionally, Pf-5-treated larvae also exhibited a few small melanized nodules, a reaction that is unlikely to be sufficient to cause death. Very rarely, Pf-5-treated larvae turned light brown, but the color change appeared to be post-mortem. In contrast, a striking systemic melanization throughout the hemolymph typically preceded death for the subset of larvae that died following ingestion of strain SBW25. This melanization response is considered to be symptomatic of an overactive immune system, which can be lethal [2]. Correspondingly, in the present study, all larvae or pupae that exhibited systemic melanization later died. Those larvae that did not develop the systemic melanization appeared to develop into pupae at normal rates, indicating that SBW25 did not cause the developmental delay associated with sub-lethal doses of Pf-5.
Many of the developmental and morphological phenotypes associated with Pf-5 ingestion by larvae, including small body size, loss of fat body integrity and prolonged larval development, are reminiscent of larval responses to starvation. During the feeding stages, larvae grow by increasing the size of terminally differentiated polyploid cells in larval tissues, and the adult precursor cells in the imaginal disc undergo numerous divisions. Attainment of normal larval body size has been shown to require continuous acquisition of dietary protein, whereas the continued division of imaginal disc cells is largely independent of nutrition; instead, these cells require circulating growth factors, which are produced by fat bodies [27]. Early in the third instar, larvae assess their critical weight, a checkpoint used to determine the timing at which to initiate metamorphosis [21]. If starved before the critical weight assessment, larvae delay the onset of metamorphosis, whereas larvae starved after this checkpoint metamorphose early and at a smaller size [19], [20]. Wild-type larvae starved from hatching, by feeding only with sucrose, survive for up to a week [28]. Therefore, the three-day delay in metamorphosis and reduced larval body size observed in this study are consistent with a starvation response of larvae at early stages of development prior to the assessment of larval critical weight. Vodovar et al. [7] and Liehl et al. [23] observed that oral infection with P. entomophila caused a cessation in food uptake by third instar larvae of D. melanogaster. In our experiments, larvae were provided with sufficient nutrition to complete development and appeared to continue to feed. Therefore, we propose that ingestion of Pf-5 may block larvae from feeding or impair their ability to use or obtain the necessary nutrition to maintain their growth and development. An interesting, although as yet untested, possibility is that Pf-5 ingestion may alter the activity of molecular regulators of larval cell growth and nutrition, such as the insulin receptor/phosphinositol kinase signaling and the TOR (target of rapamycin) pathway, which are known to phenocopy starvation in fed larvae [28], [29], [30]. Alternatively, a delay in morphogenesis can also result from damage to the developing imaginal discs, which regulate critical size in Drosophila [20], [22], and larvae fed the highest doses of Pf-5 compatible with survival produced smaller adult flies as evidenced by atypically small wings. These results suggest that at least some of the delay in metamorphosis may be due to damage or slower cell divisions of the imaginal disc cells in response to Pf-5 infection.
Ingestion of Pf-5 or SBW25 caused specific morphological defects in two different strains of wild-type adult survivors. The defects in the eye, leg and wing discs are similar to those found in adults with mutations in certain genes, such as cut, or mutations that disrupt hormonal signaling necessary for initiating pattern formation of the imaginal discs (e.g. [31]). The similarity between the eye and wing phenotypes suggest that ectopic apoptosis of cells within the imaginal discs result in the absence of ommatidia in the eye and loss of wing margin cells in larvae ingesting Pf-5 or SBW25. It is possible that modulation of or interference with the host's immune system by microbial factors could lead to the morphological defects found in Pf-5- and SBW25-treated survivors. For example, peptidoglycan-recognition proteins have been shown to activate the D. melanogaster immune system through NF-κB-like factors that can substitute for one another in the developing animal [4], [32]. Furthermore, RNAi experiments of PGRP-SC1/2 in infected D. melanogaster larvae led to increased developmental defects, including wing margin defects and lethality [33]. Alternatively, it is possible that bacterial factors interact with other non-immune-related developmental pathways to create the morphological defects we observe.
The three strains of P. fluorescens evaluated in this study caused remarkably different host responses when fed to second instar D. melanogaster larvae. This observation is not surprising given the enormous genomic diversity of the strains, which share only ca. 60% of their proteomes [13], [34]. Furthermore, strain variation in virulence to D. melanogaster is well recognized in P. aeruginosa [35], a genus that is far more homogenous than P. fluorescens as presently defined [13], [36]. An obvious reason for the differential effects of the P. fluorescens strains on the insect is the distinctive genetic compositions of these bacteria. The genomes of Pf-5 and SBW25 each contain ca. 1500–1600 genes that are lineage-specific, having no defined orthologs in the genomes of either the other strain or Pf0-1 [13], [34]. Certain of these lineage-specific genes may have direct roles in the lethality and developmental delay observed in Drosophila following bacteria ingestion. For example, genes for the biosynthesis of at least eight secondary metabolites toxic to certain eukaryotes are among the products of lineage-specific genes in the Pf-5 genome [12], [34]. Among the lineage-specific genes in the SBW25 genome are those encoding a type III secretion system [37], an export pathway found in many Gram-negative bacteria that injects bacterial effectors into the cytosol of eukaryotic cells [38]. The type III secretion apparatus and effectors are key factors in the pathogenicity of P. aeruginosa to D. melanogaster [39], [40], but this machinery is lacking in P. fluorescens strains Pf-5 and Pf0-1 and P. entomophila [12], [41]. A key role of Type III secretion is in overcoming host defense response [42], [43], although these pathways represent only one mechanism that bacteria can employ to silence the host immune system, thereby allowing them to invade and colonize the animal. In this study, we found that all three strains of P. fluorescens, including the relatively benign strain Pf0-1, became established in the gut cavity or internal tissues of Drosophila larvae and induced host expression of the gene encoding the antimicrobial peptide, Diptericin. Internal population sizes of the lethal strain Pf-5 were higher than those of the other two strains, which could reflect an enhanced capacity to suppress host immune responses, the expanded availability of nutritional substrates as a consequence of host cellular breakdown, or other factors. In P. aeruginosa, pathogenicity is mutifactorial, with many bacterial genes contributing to mortality of Drosophila [44]; and combinatorial, with distinct sets of genes required for pathogenicity of different strains [45]. Similar levels of complexity are likely to operate in P. fluorescens, and differences in gene regulation as well as composition among the three strains could contribute to their varied effects on host response.
A gacA mutant of P. fluorescens strain Pf-5 exhibited attenuated toxicity against D. melanogaster, but GacA was not required for colonization of internal tissues of the larvae. gacA mutants of P. aeruginosa [44] and P. entomophila [23] also display reduced toxicity against D. melanogaster. The GacS/GacA signal transduction system is highly conserved among Pseudomonas spp., acting via the positive control of regulatory RNAs to serve as a master regulator of genes encoding for diverse cellular functions, including chronic and acute infection. In P. fluorescens Pf-5, the GacS/GacA system controls multiple phenotypes, including secondary metabolite and exoenzyme production, stress response, and motility [15], [16], [17], [46]. Furthermore, a recent transcriptomic analysis identified more than 600 genes expressed under the control of GacA, further highlighting the central role of the GacS/GacA pair in cellular function of the bacterium [16]. Among the genes positively controlled by GacA are those with demonstrated roles in Drosophila lethality of other Pseudomonas spp. Examples include aprA, which encodes an extracellular metalloprotease that contributes to oral toxicity of P. entomophila [23] and hcnA, involved in the biosynthesis of hydrogen cyanide, one of many factors contributing to the injectable lethality of P. aeruginosa [44], [47]. The GacA regulon of Pf-5 includes many other secondary metabolites, toxins, secretory systems, and exoenzymes that could contribute to the gacA-regulated lethality and developmental delay observed herein. Identification of the specific genes and phenotypes functioning in the newly-recognized ingestible toxicity of Pf-5 represents a promising avenue for inquiry that is likely to reveal novel factors functioning in host-microbe interactions.
The most striking finding of this study is the observed link between the physiological response to microbial infection and alterations in the growth, development and differentiation of D. melanogaster larvae and adults. A second significant finding is the identification of two strains of P. fluorescens that cause mortality of wild-type D. melanogaster when ingested. While other groups have focused on the immune response of larvae or adult flies to bacterial infection, we characterized the effects of larval infection on the animals throughout development, thereby identifying novel host responses to bacterial infection. A new non-invasive feeding protocol that mimicked natural routes of microbial infection, which was developed in this study, was key in establishing a link between microbial infection and developmental delay in the P. fluorescens-Drosophila interaction. Our study demonstrates strain-specific effects of P. fluorescens on D. melanogaster morphogenesis and mortality, and opens the door to further investigation into strain-specific and GacA-mediated mechanisms of pathogenicity in a new model system. The combination of complete genomic sequence data, genetic tractability, and sophisticated analytical tools for D. melanogaster and P. fluorescens will facilitate future characterization of the cellular, molecular and developmental components of the host-microbe interactions observed in this study.
Materials and Methods
Bacterial strains and culture conditions
Three strains of Pseudomonas fluorescens having fully sequenced genomes were evaluated: strain Pf-5 was isolated from soil in Texas, USA [48]; strain SBW25 was isolated from the phyllosphere of sugar beet in Oxfordshire, England [49]; and strain Pf0-1 was isolated from an agricultural soil in the USA [50]. Cultures of SBW25 and Pf0-1 were obtained from Joseph Raaijmakers, Wageningen University, and Mark Silby, Tufts University. A Pf-5 gacA mutant (JL4577) was constructed previously by replacing 626 bp (nt 1 to 626) internal to gacA with aphI, which confers kanamycin resistance [16]. Inoculum for larval feeding experiments was obtained by culturing strains of P. fluorescens on King's Medium B (KMB) [51] and incubating plates overnight at 27°C. Strains were then inoculated into culture tubes containing 5 ml of KMB broth and incubated with shaking (200 rpm) overnight prior to harvest by centrifugation. Cells were washed once, resuspended in sterile deionized water and diluted to an appropriate OD600 for each experiment. The initial number of colony forming units (cfu) for each experiment was determined by spreading samples from serial dilutions on KMB.
Drosophila melanogaster cultures
Four isogenic wild-type lines of D. melanogaster were used: A CantonS-A (CS-A) line of D. melanogaster, obtained from Jeffrey C. Hall, Brandeis University; a Canton-S (CS) line obtained from William W. Mattox, MD Anderson Cancer Center; a Canton-S (CS) line from Jadwiga Giebultowicz, Oregon State University; and an Oregon-R (OR) line from Chris Q. Doe, University of Oregon. All Drosophila lines were maintained at 25°C, in a 12 hour light and 12 hour dark cycle (12L∶12D), on standard cornmeal, dextrose, yeast and agar media with Nipagin (p-hydroxybenzoic acid methyl ester, Sigma Aldrich, Inc., St. Louis, MO, USA). A fly line containing a diptericin-lacZ fusion with isogenic CS-A second and third chromosomes, y, w, DDI;+;+ [52] was employed in experiments evaluating the capacity of P. fluorescens to trigger an immune response involving activation of the antimicrobial peptide Diptericin by detection of β-galactosidase activity using standard methods.
Non-invasive insect toxicity assay
To test the toxicity of P. fluorescens strains to D. melanogaster, we developed a non-invasive assay (Fig. 1), which required transferring insects only at the egg stage, thereby minimizing the risk of stress or wounding prior to infection. To reduce fungal and bacterial contamination, all experiments were performed using autoclaved medium, sterile plates, pipettes, microcentrifuge tubes and ethanol-rinsed utensils. Yeast supplements for larvae and adults were prepared from yeast (Fleischmann's Active Dry yeast, Fenton, MO, USA) microwaved 2–3 times for 30 seconds to kill the yeast cells. To obtain eggs, females were collected as virgins, mated with males for 4–6 days in standard food vials, and then transferred to egg-laying chambers with apple juice agar plates prepared without Nipagin (Apple agar).
The experimental design is outlined in Figure 1. On the first day of the experiment (Day −2), flies were transferred to Petri plates containing Apple agar supplemented by yeast grains (20 mg), and plates were incubated for four hours at 25°C to allow egg lay. From these plates, thirty eggs were transferred aseptically to the surface of non-nutritive agar (2% wt/vol agar in water) having 2–3 mg yeast grains distributed on the agar surface in a 35 mm Petri plate. Plates were incubated at 25°C. The number of first instar larvae per plate was determined on Days −1 and 0 by counting the number of empty egg cases. On Day 0, 200 µl of a yeast suspension was added to the middle of the plate to serve as a food source for second instar larvae. The yeast suspension was prepared by dissolving 0.2 g yeast in 1.2 ml of sterile water or a bacterial suspension, prepared as described above. The plates were transferred to 22°C and, starting at Day 2, larvae were fed with 100 µl of a yeast suspension (0.2 mg yeast/1.2 ml sterile water) at 48 hour intervals as long as live larvae were observed in the dish. Three replica plates were established for every treatment group in the development and survival experiments. The developmental stage was determined at 24-hour intervals and the number of pupae counted until adult emergence. Adult flies were anesthetized with CO2 and then fixed in alcohol. Larval development was documented through images captured by a digital camera mounted on a dissecting scope with images adjusted only for contrast and brightness in Photoshop (Adobe Systems Inc., San Jose, CA, USA) as needed.
We established that the assay procedures were sufficient for D. melanogaster larval survival and development by assessing the proportion of CS-A eggs that survived in control treatments. In these control plates, 92±0.5% of the eggs transferred to the assay plates (n = 6,928 eggs on 231 plates in seven experiments) hatched into first instar larvae and 86±4% of the larvae survived to adulthood. This level of adult survival is consistent with a null hypothesis expectation of 100% survival to adulthood for larvae in our control treatment regimen by Chi-squared analysis (Χ2 = 3.57, Df = 6, P = 0.73359). Based upon this statistical analysis, the procedures for rearing flies were shown to provide an experimental system for assessing the consequence of natural infection of larvae of D. melanogaster without introducing artifacts or stress associated with transfer or manipulation of the insect beyond the egg stage of development.
Calculation of the duration of developmental stages
Because some bacterial treatments lengthened the duration of insect development, we quantified the developmental delay as follows. First, for each experiment, the duration of larval development post-inoculation was empirically determined as the interval (hours) between inoculation and the time when pupariation had reached 50% of the total number of pupae for the control group (T1/2 pupae control), based on repeated counts of pupae and prepupae over the course of the experiment (as in Figs 2 and 3). Second, for each treatment group in a given experiment, the duration of larval development was determined as the interval between inoculation and the time when pupariation had reached 50% of the total number of pupae for each treatment (T1/2 pupae treatment). For within and between experiment comparisons, the difference between the duration of the larval stage for a bacterial treatment and the duration of the larval stage for control larvae was calculated as 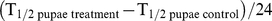 hours/day. Similarly, for each experiment we determined the duration of pupal development as the interval between T1/2 pupae control, and the time when 50% of flies had emerged from the pupal case (T1/2 adult control). The difference between the duration of pupal development for each treatment group and the duration of the pupal stage for control pupae was calculated as
hours/day. Similarly, for each experiment we determined the duration of pupal development as the interval between T1/2 pupae control, and the time when 50% of flies had emerged from the pupal case (T1/2 adult control). The difference between the duration of pupal development for each treatment group and the duration of the pupal stage for control pupae was calculated as 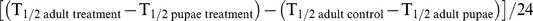 hours/day. The difference in the duration of both larval and pupal stages for each treatment group was calculated as
hours/day. The difference in the duration of both larval and pupal stages for each treatment group was calculated as 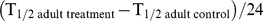 hours/day.
hours/day.
Bacterial colonization of larvae
Population sizes of the P. fluorescens strains internal to larvae of D. melanogaster were estimated from surface-sterilized larvae. Six larvae were placed in a drop of the sterilizing solution (2.5 ml bleach and 45 µl 0.01% Triton-X in 10 ml water) for 60 seconds and then transferred serially through four drops of sterile water before being gently blotted and placed individually into a 1.5 ml microcentrifuge tube. Each larva was then homogenized in 50 µl of sterile distilled water, serially diluted, and the dilutions were spread on KMB. Plates were incubated overnight at 27°C prior to counting colonies. The experiment was done twice with similar results, and results from one experiment are presented.
Morphological defect analysis
All adults that emerged were fixed in ethanol and then examined through a dissecting scope for defects in their external structures. In pilot experiments, flattened adult cuticle preparations were made by macerating the flies in hot 10% KOH solution and placing in a mounting medium (Permount™, Fisher Scientific Research, Pittsburgh PA). These adults were examined for external morphological defects. We discovered morphological defects in eyes, wings and legs. For subsequent experiments, flies that had been fixed in ethanol were examined under a dissecting scope and scored for defects in these structures. Eye defects included smaller eye size and nicks at the edge of the equator. Wing defects included extra or abnormal veins and loss of wing margin cells. Abnormal leg joints were observed in some adults. Digital images of eyes were captured directly into a camera mounted onto a dissecting scope. Final images were only adjusted slightly for contrast and brightness.
Wing size was used as a criterion to estimate adult body size. From each adult fly, one wing was removed and mounted on a slide in glycerol. Images of each wing were captured at 4× and 10× magnification using an Olympus Vanox A2 research grade microscope with a digital camera. Photoshop images were imported into Image Pro 4000 (Media Cybernetics, Bethesda, MD, USA) for surface area measurements. The average wing blade surface area was determined for 10 adult flies from each treatment (except for treatments having low survival rates resulting in less than 10 surviving adults). To evaluate the statistical significance of treatment effects a one-way ANOVA was calculated with the Tukey test for contrast (α = 0.05; P<0.001; F = 7.64) using SigmaStat (Systat Software, Inc.).
Supporting Information
(0.04 MB DOC)
Adult survival after bacterial treatment. Adult survival for each treatment group (circles represent CS-A and diamonds represent OR) is plotted against the treatment dose. For each treatment group, the adult survival is normalized to the survival of the appropriate control, which has been set at 100%. Each point is scaled according to the dose (cfu/plate) for each bacterial treatment. The size of each data point is scaled to the inoculation dose (highest dose/largest circle = 2.9×109 cfu/plate; smallest dose/smallest circle = 2.9×102 cfu/plate; highest dose/largest diamond = 4.4×107; lowest dose/smallest diamond = 1.8×104); Pf0-1 (blue), SBW25 (green), Pf-5 (red), killed Pf-5 (gray) and a gacA mutant of Pf-5 (open red circle).
(0.10 MB TIF)
Average wing blade surface area for adult survivors. Lanes (1) control, (2) 5.7×109 cfu/plate Pf0-1, (3) 5.2×109 cfu/plate SBW25, (4) 2.9×109 cfu/plate killed Pf-5, (5) 2.9×102 cfu/plate Pf-5, (6) 2.9×104 cfu/plate Pf-5, 7) 2.9×105 cfu/plate Pf-5, (8) 2.9×107 cfu/plate Pf-5. Asterisk indicates significant difference in wing-surface area from the control. See Materials and Methods for details and calculations.
(0.14 MB TIF)
Diptericin-lacZ expression in third instar larvae from control and bacterial treatments. Fat body cells from third instar y,w DDI larvae, which were dissected open and then stained for β-galactosidase activity.(A) Segment of larval fat body (delimited by arrows), positioned over body wall musculature, from a control larva in which only a few cells express faint β-galactosidase activity. (B) Labeled fat body cells, positioned over body wall musculature, from a larva fed Pf0-1 at 107 cfu/plate.(C) Isolated segment of larval fat body from a larva fed SBW25 at 107 cfu/plate. (D) Isolated segment of larval fat body from a larva fed Pf-5 at 107 cfu/plate. (E) Isolated segment of larval fat body from a larva fed gacA mutant Pf-5 at 107 cfu/plate.
(3.65 MB TIF)
Morphological defects in adult survivors of Pf-5 treated OR and CS adults. Whole mount eye images from OR adult (left panel) inoculated with 4.5×104 cfu/plate Pf-5 and CS adults (middle and right panels) inoculated with 1.7×104 cfu/plate Pf-5. The center of the eye in the right panel was damaged during handling.
(0.80 MB TIF)
Acknowledgments
We are grateful to Brenda Shaffer and Ed Davis for assistance in colonization studies, and Joseph Raaijmakers and Mark Silby for providing bacterial cultures. We also thank Dr. Dennis Hruby for support for Dr. Olcott.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This project was supported by National Research Initiative Competitive Grants 2006-35319-17427 and 2008-35600-18770 from the United States Department of Agriculture Cooperative State Research, Education, and Extension Service to JEL and by National Institutes of Health Grant 9RO1 GM085818-14A2 to BJT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dionne MS, Ghori N, Schneider DS. Drosophila melanogaster is a genetically tractable model host for Mycobacterium marinum. Infect Immun. 2003;71:3540–3550. doi: 10.1128/IAI.71.6.3540-3550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dionne MS, Schneider DS. Models of infectious diseases in the fruit fly Drosophila melanogaster. Dis Model Mech. 2008;1:43–49. doi: 10.1242/dmm.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govind S. Innate immunity in Drosophila: Pathogens and pathways. Insect Science. 2008;15:29–43. doi: 10.1111/j.1744-7917.2008.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual Review Immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 5.Leulier F, Royet J. Maintaining immune homeostasis in fly gut. Nat Immunol. 2009;10:936–938. doi: 10.1038/ni0909-936. [DOI] [PubMed] [Google Scholar]
- 6.Vallet-Gely I, Lemaitre B, Boccard F. Bacterial strategies to overcome insect defences. Nat Rev Microbiol. 2008;6:302–313. doi: 10.1038/nrmicro1870. [DOI] [PubMed] [Google Scholar]
- 7.Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci U S A. 2005;102:11414–11419. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibley CD, Duan K, Fischer C, Parkins MD, Storey DG, et al. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 2008;4:e1000184. doi: 10.1371/journal.ppat.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye YH, Chenoweth SF, McGraw EA. Effective but costly, evolved mechanisms of defense against a virulent opportunistic pathogen in Drosophila melanogaster. PLoS Pathog. 2009;5:e1000385. doi: 10.1371/journal.ppat.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pechy-Tarr M, Bruck DJ, Maurhofer M, Fischer E, Vogne C, et al. Molecular analysis of a novel gene cluster encoding an insect toxin in plant-associated strains of Pseudomonas fluorescens. Environ Microbiol. 2008;10:2368–2386. doi: 10.1111/j.1462-2920.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- 11.ffrench-Constant RH, Dowling A, Waterfield NR. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon. 2007;49:436–451. doi: 10.1016/j.toxicon.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Paulsen IT, Press CM, Ravel J, Kobayashi DY, Myers GS, et al. Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat Biotechnol. 2005;23:873–878. doi: 10.1038/nbt1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silby MW, Cerdeno-Tarraga AM, Vernikos GS, Giddens SR, Jackson RW, et al. Genomic and genetic analyses of diversity and plant interactions of Pseudomonas fluorescens. Genome Biol. 2009;10:R51. doi: 10.1186/gb-2009-10-5-r51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapouge K, Schubert M, Allain FH, Haas D. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol Microbiol. 2008;67:241–253. doi: 10.1111/j.1365-2958.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 15.Corbell N, Loper JE. A global regulator of secondary metabolite production in Pseudomonas fluorescens Pf-5. J Bacteriol. 1995;177:6230–6236. doi: 10.1128/jb.177.21.6230-6236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan KA, Johnson A, Shaffer BT, Ren Q, Kidarsa TA, et al. Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ Microbiol. 2010;12:899–915. doi: 10.1111/j.1462-2920.2009.02134.x. [DOI] [PubMed] [Google Scholar]
- 17.Whistler CA, Corbell NA, Sarniguet A, Ream W, Loper JE. The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor sigmaS and the stress response in Pseudomonas fluorescens Pf-5. J Bacteriol. 1998;180:6635–6641. doi: 10.1128/jb.180.24.6635-6641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- 19.Mirth CK, Riddiford LM. Size assessment and growth control: how adult size is determined in insects. Bioessays. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- 20.Mirth CK, Truman JW, Riddiford LM. The ecdysone receptor controls the post-critical weight switch to nutrition-independent differentiation in Drosophila wing imaginal discs. Development. 2009;136:2345–2353. doi: 10.1242/dev.032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nijhout HF. The control of growth. Development. 2003;130:5863–5867. doi: 10.1242/dev.00902. [DOI] [PubMed] [Google Scholar]
- 22.Stieper BC, Kupershtok M, Driscoll MV, Shingleton AW. Imaginal discs regulate developmental timing in Drosophila melanogaster. Dev Biol. 2008;321:18–26. doi: 10.1016/j.ydbio.2008.05.556. [DOI] [PubMed] [Google Scholar]
- 23.Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2006;2:e56. doi: 10.1371/journal.ppat.0020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci U S A. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, et al. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007;3:e173. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daborn PJ, Waterfield N, Silva CP, Au CP, Sharma S, et al. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc Natl Acad Sci U S A. 2002;99:10742–10747. doi: 10.1073/pnas.102068099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Britton JS, Edgar BA. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125:2149–2158. doi: 10.1242/dev.125.11.2149. [DOI] [PubMed] [Google Scholar]
- 28.Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 29.Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 2000;14:2689–2694. doi: 10.1101/gad.845700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennan CA, Ashburner M, Moses K. Ecdysone pathway is required for furrow progression in the developing Drosophila eye. Development. 1998;125:2653–2664. doi: 10.1242/dev.125.14.2653. [DOI] [PubMed] [Google Scholar]
- 32.Stein D, Goltz JS, Jurcsak J, Stevens L. The Dorsal-related immunity factor (Dif) can define the dorsal-ventral axis of polarity in the Drosophila embryo. Development. 1998;125:2159–2169. doi: 10.1242/dev.125.11.2159. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, et al. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2006;2:e14. doi: 10.1371/journal.ppat.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross H, Loper JE. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep. 2009;26:1408–1446. doi: 10.1039/b817075b. [DOI] [PubMed] [Google Scholar]
- 35.Lutter EI, Faria MM, Rabin HR, Storey DG. Pseudomonas aeruginosa cystic fibrosis isolates from individual patients demonstrate a range of levels of lethality in two Drosophila melanogaster infection models. Infect Immun. 2008;76:1877–1888. doi: 10.1128/IAI.01165-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathee K, Narasimhan G, Valdes C, Qiu X, Matewish JM, et al. Dynamics of Pseudomonas aeruginosa genome evolution. Proc Natl Acad Sci U S A. 2008;105:3100–3105. doi: 10.1073/pnas.0711982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston GM, Bertrand N, Rainey PB. Type III secretion in plant growth-promoting Pseudomonas fluorescens SBW25. Mol Microbiol. 2001;41:999–1014. doi: 10.1046/j.1365-2958.2001.02560.x. [DOI] [PubMed] [Google Scholar]
- 38.Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 39.Fauvarque MO, Bergeret E, Chabert J, Dacheux D, Satre M, et al. Role and activation of type III secretion system genes in Pseudomonas aeruginosa-induced Drosophila killing. Microb Pathog. 2002;32:287–295. doi: 10.1006/mpat.2002.0504. [DOI] [PubMed] [Google Scholar]
- 40.Pielage JF, Powell KR, Kalman D, Engel JN. RNAi screen reveals an Abl kinase-dependent host cell pathway involved in Pseudomonas aeruginosa internalization. PLoS Pathog. 2008;4:e1000031. doi: 10.1371/journal.ppat.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vodovar N, Vallenet D, Cruveiller S, Rouy Z, Barbe V, et al. Complete genome sequence of the entomopathogenic and metabolically versatile soil bacterium Pseudomonas entomophila. Nat Biotechnol. 2006;24:673–679. doi: 10.1038/nbt1212. [DOI] [PubMed] [Google Scholar]
- 42.Coburn B, Sekirov I, Finlay BB. Type III secretion systems and disease. Clin Microbiol Rev 2007. 2007;20:535–549. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant SR, Fisher EJ, Chang JH, Mole BM, Dangl JL. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu Rev Microbiol. 2006;60:425–449. doi: 10.1146/annurev.micro.60.080805.142251. [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, Park SY, Heo YJ, Cho YH. Drosophila melanogaster-based screening for multihost virulence factors of Pseudomonas aeruginosa PA14 and identification of a virulence-attenuating factor, HudA. Infect Immun. 2008;76:4152–4162. doi: 10.1128/IAI.01637-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DG, Urbach JM, Wu G, Liberati NT, Feinbaum RL, et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006;7:R90. doi: 10.1186/gb-2006-7-10-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stockwell VO, Loper JE. The sigma factor RpoS is required for stress tolerance and environmental fitness of Pseudomonas fluorescens Pf-5. Microbiology. 2005;151:3001–3009. doi: 10.1099/mic.0.28077-0. [DOI] [PubMed] [Google Scholar]
- 47.Broderick KE, Chan A, Balasubramanian M, Feala J, Reed SL, et al. Cyanide produced by human isolates of Pseudomonas aeruginosa contributes to lethality in Drosophila melanogaster. J Infect Dis. 2008;197:457–464. doi: 10.1086/525282. [DOI] [PubMed] [Google Scholar]
- 48.Howell CR, Stipanovic RD. Control of Rhizoctonia solani in cotton seedlings with Pseudomonas fluorescens and with an antibiotic produced by the bacterium. Phytopathology. 1979;69:480–482. [Google Scholar]
- 49.Bailey MJ, Lilley AK, Thompson IP, Rainey PB, Ellis RJ. Site directed chromosomal marking of a fluorescent pseudomonad isolated from the phytosphere of sugar beet; stability and potential for marker gene transfer. Mol Ecol. 1995;4:755–763. doi: 10.1111/j.1365-294x.1995.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 50.Compeau G, Al-Achi BJ, Platsouka E, Levy SB. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl Environ Microbiol. 1988;54:2432–2438. doi: 10.1128/aem.54.10.2432-2438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King EO, Ward MK, Raney DE. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 52.Rutschmann S, Jung AC, Hetru C, Reichhart JM, Hoffmann JA, et al. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity. 2000;12:569–580. doi: 10.1016/s1074-7613(00)80208-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(0.04 MB DOC)
Adult survival after bacterial treatment. Adult survival for each treatment group (circles represent CS-A and diamonds represent OR) is plotted against the treatment dose. For each treatment group, the adult survival is normalized to the survival of the appropriate control, which has been set at 100%. Each point is scaled according to the dose (cfu/plate) for each bacterial treatment. The size of each data point is scaled to the inoculation dose (highest dose/largest circle = 2.9×109 cfu/plate; smallest dose/smallest circle = 2.9×102 cfu/plate; highest dose/largest diamond = 4.4×107; lowest dose/smallest diamond = 1.8×104); Pf0-1 (blue), SBW25 (green), Pf-5 (red), killed Pf-5 (gray) and a gacA mutant of Pf-5 (open red circle).
(0.10 MB TIF)
Average wing blade surface area for adult survivors. Lanes (1) control, (2) 5.7×109 cfu/plate Pf0-1, (3) 5.2×109 cfu/plate SBW25, (4) 2.9×109 cfu/plate killed Pf-5, (5) 2.9×102 cfu/plate Pf-5, (6) 2.9×104 cfu/plate Pf-5, 7) 2.9×105 cfu/plate Pf-5, (8) 2.9×107 cfu/plate Pf-5. Asterisk indicates significant difference in wing-surface area from the control. See Materials and Methods for details and calculations.
(0.14 MB TIF)
Diptericin-lacZ expression in third instar larvae from control and bacterial treatments. Fat body cells from third instar y,w DDI larvae, which were dissected open and then stained for β-galactosidase activity.(A) Segment of larval fat body (delimited by arrows), positioned over body wall musculature, from a control larva in which only a few cells express faint β-galactosidase activity. (B) Labeled fat body cells, positioned over body wall musculature, from a larva fed Pf0-1 at 107 cfu/plate.(C) Isolated segment of larval fat body from a larva fed SBW25 at 107 cfu/plate. (D) Isolated segment of larval fat body from a larva fed Pf-5 at 107 cfu/plate. (E) Isolated segment of larval fat body from a larva fed gacA mutant Pf-5 at 107 cfu/plate.
(3.65 MB TIF)
Morphological defects in adult survivors of Pf-5 treated OR and CS adults. Whole mount eye images from OR adult (left panel) inoculated with 4.5×104 cfu/plate Pf-5 and CS adults (middle and right panels) inoculated with 1.7×104 cfu/plate Pf-5. The center of the eye in the right panel was damaged during handling.
(0.80 MB TIF)