Boyer and colleagues in the laboratory of Dr. Ronald Crystal at Cornell Medical College constructed adenovirus vaccine vectors containing pathogen-specific antigens fused to the pIX capsid protein. Using a mouse model of Yersinia pestis infection, they demonstrate that this vaccine platform has strong adjuvant properties that can stimulate more protective immune responses than equivalent recombinant protein-based subunit vaccines administered with conventional adjuvant.
Abstract
The aerosol form of the bacterium Yersinia pestis causes pneumonic plague, a rapidly fatal disease that is a biothreat if deliberately released. At present, no plague vaccines are available for use in the United States, but subunit vaccines based on the Y. pestis V antigen and F1 capsular protein show promise when administered with adjuvants. In the context that adenovirus (Ad) gene transfer vectors have a strong adjuvant potential related to the ability to directly infect dendritic cells, we hypothesized that modification of the Ad5 capsid to display either the Y. pestis V antigen or the F1 capsular antigen on the virion surface would elicit high V antigen- or F1-specific antibody titers, permit boosting with the same Ad serotype, and provide better protection against a lethal Y. pestis challenge than immunization with equivalent amounts of V or F1 recombinant protein plus conventional adjuvant. We constructed AdYFP-pIX/V and AdLacZ-pIX/F1, E1–, E3– serotype 5 Ad gene transfer vectors containing a fusion of the sequence for either the Y. pestis V antigen or the F1 capsular antigen to the carboxy-terminal sequence of pIX, a capsid protein that can accommodate the entire V antigen (37 kDa) or F1 protein (15 kDa) without disturbing Ad function. Immunization with AdYFP-pIX/V followed by a single repeat administration of the same vector at the same dose resulted in significantly better protection of immunized animals compared with immunization with a molar equivalent amount of purified recombinant V antigen plus Alhydrogel adjuvant. Similarly, immunization with AdLacZ-pIX/F1 in a prime–boost regimen resulted in significantly enhanced protection of immunized animals compared with immunization with a molar-equivalent amount of purified recombinant F1 protein plus adjuvant. These observations demonstrate that Ad vaccine vectors containing pathogen-specific antigens fused to the pIX capsid protein have strong adjuvant properties and stimulate more robust protective immune responses than equivalent recombinant protein-based subunit vaccines administered with conventional adjuvant, suggesting that F1-and/or V-modified capsid Ad-based recombinant vaccines should be considered for development as anti-plague vaccines.
Introduction
Aerosol transmission of virulent Yersinia pestis is a threat as a biological weapon because it results in pneumonic plague, a rapidly fatal disease (Perry and Fetherston, 1997; Inglesby et al., 2000; Titball et al., 2004; Prentice and Rahalison, 2007). Yersinia pestis is sensitive to antibiotics, but mortality associated with plague is high and multidrug-resistant Y. pestis isolates have been identified (Galimand et al., 1997; Inglesby et al., 2000; Guiyoule et al., 2001). At the present time, there are no plague vaccines licensed for use in the United States. Both live attenuated and killed whole cell vaccines against plague have been developed, but the killed whole cell vaccine provides poor protection against pneumonic plague and the live vaccine is associated with significant adverse effects (Meyer, 1970; Russell et al., 1995; Jefferson et al., 2000; Zilinskas, 2006).
Current vaccine development efforts are largely focused on the Y. pestis V antigen and the capsular F1 antigen as the primary targets (Titball and Williamson, 2001, 2004; Williamson et al., 2005; Cornelius et al., 2007; Morris, 2007; Smiley, 2008). V antigen, required for translocation of bacterial effector proteins into host cells via a type III secretion system, induces expression of host interleukin (IL)-10 and suppresses tumor necrosis factor (TNF)-α through unknown mechanisms (Perry and Fetherston, 1997; Sarker et al., 1998; Pettersson et al., 1999; Auerbuch and Isberg, 2007; Pouliot et al., 2007; Reithmeier-Rost et al., 2007). The F1 capsular antigen is the major protein component of the gel-like capsule that surrounds the bacterium and likely contributes to avoidance of phagocytosis (Cavanaugh and Randall, 1959; Perry and Fetherston, 1997; Titball et al., 2004).
Vaccination of mice with Alhydrogel adjuvant plus V antigen or F1 protein elicits protection against a respiratory Y. pestis challenge (Leary et al., 1995; Anderson et al., 1996, 1998; Andrews et al., 1996; Heath et al., 1998; Titball and Williamson, 2001, 2004; Jones et al., 2006; Williamson et al., 2007). To improve the efficacy of F1 and V antigen-based vaccines, we have explored the use of E1–,E3– replication-deficient adenoviruses (Ads) as adjuvants that may more effectively enhance immune responses against F1 and V antigen. By adding the sequences of V antigen or F1 to the 3′ end of the capsid pIX coding sequence of an E1–,E3– serotype 5 Ad, we created two Ad-based plague vaccine vectors, AdYFP-pIX/V and AdLacZ-pIX/F1, that display the V antigen or F1 protein, respectively, on the surface of the virion for immune recognition. We hypothesized that these vectors would function similarly to V antigen or F1 protein immunogens administered with Alhydrogel in eliciting immunity, but that the adjuvant effects of Ad would be superior. After immunization of mice with either AdYFP-pIX/V or AdLacZ-pIX/F1, the antigen-specific serum antibody titers elicited and the protective efficacy against a lethal intranasal Y. pestis challenge were more robust compared with immunization with equimolar amounts of the protein subunits combined with conventional Alhydrogel adjuvant.
Materials and Methods
Adenoviral vectors
The recombinant Ad vectors used in this study were replication-defective E1–,E3– human adenoviral vectors based on the Ad5 genome. The expression cassettes were inserted into the E1 region and contain (5′–3′) the human cytomegalovirus intermediate-early promoter/enhancer, the transgene, and the simian virus 40 poly(A) stop signal. The vectors express a marker gene encoding yellow fluorescent protein (YFP) or β-galactosidase (LacZ). For the V antigen capsid-modified vector, AdYFP-pIX/V, the V antigen human codon-optimized coding sequence was fused to the C terminus of protein IX. For the F1 capsid-modified vector, AdLacZ-pIX/F1, the N-terminal 14 amino acids were deleted from the human codon-optimized F1 coding sequence and the resulting coding sequence was fused to the C terminus of protein IX. AdYFP-pIX/V, AdLacZ-pIX/F1, and the control vectors AdYFP and AdLacZ (identical to the pIX-modified vaccines but without the capsid modifications) were produced in 293 cells and purified by centrifugation twice by passage through a CsCl gradient as previously described (Rosenfeld et al., 1991, 1992). The titer of each recombinant Ad preparation was determined spectrophotometrically and expressed as particle units (pu) (Mittereder et al., 1996).
Purification of recombinant V antigen and F1 protein
Recombinant V antigen from Yersinia pestis was produced by inserting the V antigen coding sequence into the T7 promoter-driven prokaryotic expression plasmid pRSET (Invitrogen, Carlsbad, CA) to generate the pRSET-V plasmid, expressing V antigen as a histidine-tag fusion protein. pRSET-V was transformed into the BL21(DE3) pLysS strain of Escherichia coli and expression of V antigen was induced with isopropyl-β-d-thiogalactopyranoside (IPTG). V antigen was affinity purified by passage through a nickel–nitrilotriacetic acid (Ni–NTA) Superflow column (Qiagen, Valencia, CA) under native conditions. The purity of the protein was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) (NuPAGE system; Invitrogen) and its identity was confirmed by Western analysis with a rabbit anti-V antigen antibody (kindly provided by S. Bavari, U.S. Army Medical Research Institute for infectious diseases [USAMRIID], Fort Detrick, MD).
Recombinant F1 protein from Yersinia pestis was produced by inserting the F1 protein coding sequence into the T7 promoter-driven prokaryotic expression plasmid pRSET (Invitrogen) to generate the pRSET-F1 plasmid, expressing F1 as a histidine-tag fusion protein. After transforming the plasmid into the BL21(DE3) pLysS strain of E. coli, protein expression was induced with IPTG and the protein was affinity purified by passage through a Ni–NTA Probond column (Invitrogen) under hybrid conditions. The purity of the protein was confirmed by SDS–PAGE (NuPAGE system; Invitrogen) and its identity was confirmed by Western analysis with a polyclonal rabbit anti-F1 antibody (kindly provided by S. Bavari, USAMRIID).
Western analysis
To assess the presence of V antigen and F1 protein in the viral capsid, Ad vectors (1010 PU) were denatured by heating at 95°C for 5 min in NuPAGE sample buffer (Invitrogen) and separated by SDS–polyacrylamide (4–12%) gel electrophoresis (NuPAGE system; Invitrogen). The gel was transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) and exposed to blocking solution (5% fat-free milk [blot grade; Bio-Rad] in phosphate-buffered saline [PBS], pH 7.4) for 1 hr. For detection of V antigen, the membrane was then incubated with a 1:5000 dilution of anti-V antigen polyclonal antiserum for 1 hr. A peroxidase-conjugated goat anti-rabbit IgG secondary antibody (AbCam, Cambridge, MA) was then added at a dilution of 1:10,000 for 1 hr of incubation, followed by detection with chemiluminescent peroxidase substrate (ECL Plus reagent; GE Healthcare Life Sciences, Piscataway, NJ). For detection of F1 protein, the membrane was incubated with a 1:5,000 dilution of a polyclonal rabbit anti-F1 antibody for 1 hr. A peroxidase-conjugated goat anti-rabbit IgG secondary antibody (AbCam) was then added at a dilution of 1:10,000 for 1 hr of incubation, followed by detection with ECL Plus reagent.
Assessment of anti-V antigen and anti-F1 humoral responses
Female BALB/c mice were obtained from Jackson Laboratory (Bar Harbor, ME). The animals were housed under specific pathogen-free conditions and used at 6 to 8 weeks of age. The mice were immunized intramuscularly in a prime–boost regimen with AdYFP-pIX/V or AdLacZ-pIX/F1 vector diluted in 100 μl of PBS to the specified dose (109 or 1011 pu), or with the corresponding theoretically calculated molar equivalent amount of purified recombinant antigen plus Alhydrogel (HCI Biosector, Frederikssund, Denmark) adjuvant (diluted to a final concentration of 1%). Four weeks after prime vaccination, the groups were boosted with the same immunogen at the same dose received in the primary immunization. Naive mice and mice immunized with AdYFP or AdLacZ were used as negative controls. The dose of vector selected for immunization was based on our previous experience in murine immunity studies and our experience in administering recombinant Ad vectors to normal human volunteers. A dose of 1011 PU in a mouse scales (by weight) to 1013 PU in humans, which has been successfully administered by our group with no adverse effects (Crystal et al., 2002; Harvey et al., 2002).
To assess the ability of AdYFP-pIX/V or purified recombinant V antigen to generate anti-V antigen-specific antibodies in vivo, at 6 weeks after prime immunization, serum was collected via the tail vein, centrifuged at 8000 × g for 20 min, and stored at −20°C. Anti-V antigen antibody levels in mouse serum were assessed by ELISA, using flat-bottomed 96-well EIA/RIA plates (Corning, New York, NY) coated with 0.5 μg of recombinant V antigen per well in a total volume of 100 μl of 0.05 M carbonate buffer, pH 7.4, overnight at 4°C. The plates were washed with PBS and blocked with 5% dry milk in PBS for 1 hr at 23°C. Serial serum dilutions were added to each well and incubated for 1 hr at 23°C. The plates were washed three times with PBS containing 0.05% Tween (PBS–Tween) and 100 μl/well of 1:10,000 diluted peroxidase-conjugated sheep anti-mouse IgG (Sigma-Aldrich, St. Louis, MO) in PBS containing 1% dry milk was added and incubated for 1 hr at 23°C. Absorbance at 415 nm was read with a microplate reader (Bio-Rad). Antibody titers were calculated according to a log(OD) – log(dilution) interpolation model and a cutoff value equal to 2-fold the absorbance of background.
A similar method was used to calculate anti-F1 antibody levels from serum collected from AdLacZ-pIX/F1- or purified recombinant F1-immunized mice. In this case, the ELISA 96-well plates were coated with 0.3 μg of recombinant F1 protein per well.
Yersinia pestis CO92 lethal respiratory tract challenge
To assess the ability of AdYFP-pIX/V, AdLacZ-pIX/F1, purified recombinant V antigen, and purified recombinant F1 protein to protect mice against a lethal challenge with Y. pestis, 6 weeks after prime immunization each mouse was challenged intranasally with Y. pestis CO92 (Sofer-Podesta et al., 2009). The Y. pestis challenge studies were carried out at the Public Health Research Institute (PHRI) at the International Center for Public Health (Newark, NJ) under BSL3 conditions. Yersinia pestis CO92 was grown aerobically in heart infusion broth (Difco; Becton Dickinson, Franklin Lakes, NJ) at 30°C, and diluted in saline solution at doses from 103 to 106 colony-forming units (CFU). The challenge dose used was 5 × 103 CFU. Twenty-five microliters of bacterial suspension was used for intranasal infection of mice; bacterial dose was controlled by plating on Yersinia selective agar (YSA; Oxoid, Hampshire, UK) and counting colonies for CFU determination. Survival was monitored daily for 14 days.
Statistical analyses
Data are presented as means ± standard error of the mean. Statistical analyses were performed using the nonpaired two-tailed Student t test, assuming equal variance. Survival evaluation was carried out by Kaplan–Meier analysis. Statistical significance was determined at p < 0.05.
Results
Construction of genetically pIX-modified vectors and detection of V antigen and F1 protein on the capsid of modified virions
The recombinant Ad vectors AdYFP-pIX/V and AdLacZ-pIX/F1, encoding either the V antigen coding sequence or the F1 capsular antigen coding sequence fused to the C terminus of pIX, were constructed with the AdEasy vector system (Fig. 1). In vitro studies demonstrated that these vectors expressed yellow fluorescent protein (YFP) or β-galactosidase (LacZ) as marker transgenes.
FIG. 1.
Capsid-modified Ad vectors. The constructs used in this study are replication-defective E1–,E3– serotype 5 Ad gene transfer vectors containing (5′ to 3′) the cytomegalovirus immediate early promoter/enhancer (CMV), a marker gene, and the simian virus 40 poly(A) stop signal. Top: Ad5 genome. Middle: AdYFP-pIX/V expresses YFP as a transgene and has the V antigen coding sequence fused in frame to the C terminus of protein IX. Bottom: AdLacZ-pIX/F1 expresses LacZ as a transgene and has the coding sequence for the F1 capsular antigen fused in frame to the C terminus of protein IX. Expression of the pIX/V or pIX/F fusion protein is under the control of the pIX promoter.
After growth and purification of the vectors, incorporation of the modified pIX into viral capsids was analyzed by Western blotting (Fig. 2). The probing of electrophoretically resolved AdYFP-pIX/V and AdLacZ-pIX/F1 purified virions with either an anti-V antigen antibody or an anti-F1 antibody detected the presence of bands of the expected molecular sizes for a pIX/V fusion protein (55 kDa) or a pIX/F1 fusion protein (32 kDa) as compared with purified recombinant V antigen (37 kDa) or F1 protein (15 kDa).
FIG. 2.
Detection of V antigen or F1 antigen on the surface of capsid-modified Ad vectors by Western analysis. (A) Purified AdYFP-pIX/V virions, purified AdYFP virions as a negative control, or a molar equivalent amount of purified recombinant V antigen that corresponds to the amount of V antigen displayed on the capsid of AdYFP-pIX/V were assessed by Western analysis with an anti-V antigen polyclonal antiserum. Lane 1, AdYFP, 1010 pu; lane 2, AdYFP-pIX/V, 1010 pu; lane 3, purified recombinant V antigen, 0.15 μg. (B) Purified AdLacZ-pIX/F1 virions, purified AdLacZ virions as a negative control, or a molar equivalent amount of purified recombinant V antigen that corresponds to the amount of V antigen displayed on the capsid of AdYFP-pIX/V were assessed by Western analysis with a polyclonal rabbit F1 antibody. Lane 4, AdLacZ, 1010 pu; lane 5, AdLacZ-pIX/F1, 1010 pu; lane 6, purified recombinant F1 antigen, 0.05 μg. The molecular weights of pIX/V, pIX/F1, V antigen, and F1 are indicated.
In addition to determining the molecular weight of the pIX/V and pIX/F1 fusion proteins, this experiment was also designed to detect an amount of purified recombinant V antigen or F1 protein theoretically calculated to be the molar equivalent amount of V antigen or F1 protein present on the virion surface. Although this is not a quantitative measurement of the absolute amount of protein present in the viral capsid, the intensity of the signal corresponding to the amount of V antigen detected on the AdYFP-pIX/V capsid and the intensity of the calculated molar equivalent amount of purified recombinant V antigen were comparable (Fig. 2, compare lanes 2 and 3). Similar results were obtained by comparing AdLacZ-pIX/F1 with the calculated molar equivalent amount of purified recombinant F1 antigen (Fig. 2, compare lanes 5 and 6), confirming that the expected amount of V antigen or F1 protein was displayed on the virion surface.
Humoral immune responses and protection against challenge with Yersinia pestis conferred by immunization with AdYFP-pIX/V versus purified recombinant V antigen plus adjuvant
To assess the humoral response against V antigen after immunization with AdYFP-pIX/V or recombinant V antigen plus adjuvant, BALB/c mice were intramuscularly vaccinated after a prime–boost regimen, and the serum anti–V antigen IgG response was determined 6 weeks after prime immunization. Mice receiving 109 pu of AdYFP-pIX/V or 15 ng of recombinant V antigen (the molar equivalent amount of protein present in 109 pu of capsid-modified virions) and boosted 4 weeks later with the same immunogen at the same dose had higher antibody titers than mice receiving only the prime immunization (Fig. 3A and B). In addition, mice receiving 1011 pu of AdYFP-pIX/V and boosted 4 weeks later with the same dose and vector show no substantial differences in anti-V antigen antibody titers than those receiving only the prime immunization (Fig. 4A). Mice vaccinated with 1.5 μg of recombinant V antigen (the molar equivalent amount of protein present in 1011 pu of capsid-modified virions) and boosted 4 weeks afterward with the same dose of V antigen, exhibited higher antibody titers than mice that received only the initial vaccination with V antigen/adjuvant, but still lower than that achieved with the Ad vaccine (Fig. 4B). No serum antibody titers were detected in naive or AdYFP-immunized mice.
FIG. 3.
Anti-V antigen antibodies and survival of mice immunized in a prime–boost regimen with AdYFP-pIX/V or V antigen/Alhydrogel. BALB/c mice (n = 8 to 16 per group) were immunized with AdYFP-pIX/V or purified V antigen/Alhydrogel. A subset of animals received a single repeat administration of the same immunogens at the same dose 4 weeks after primary immunization. (A) Serum anti-V antigen titers, AdYFP-pIX/V (109 particle units, pu). Six weeks after the prime immunization serum antibody levels were measured in an anti-V antigen-specific ELISA (p < 0.0001, AdYFP-pIX/V unboosted or boosted vs. AdYFP). (B) Serum anti-V antigen titers, purified recombinant V antigen (15 ng, the molar equivalent amount of protein present in 109 pu of AdYFP-pIX/V) with Alhydrogel (p < 0.009, V antigen unboosted or boosted vs. naive). (C) Survival, AdYFP-pIX/V. Six weeks after prime immunization with 109 pu of AdYFP-pIX/V, mice were challenged with a lethal dose of Yersinia pestis CO92, and survival of the animals was monitored for 14 days. Naive mice and mice that received AdYFP (1011 pu) intramuscularly were included as negative controls (p = 0.1437, AdYFP-pIX/V vs. AdYFP; p < 0.0001, AdYFP-pIX/V plus boost versus AdYFP). (D) Survival, V antigen/Alhydrogel. Naive mice were used as negative controls (p = 0.0093, V antigen vs. naive; p = 0.0248, V antigen plus boost vs. naive).
FIG. 4.
Anti-V antigen antibodies and survival of mice immunized in a prime–boost regimen with AdYFP-pIX/V or V antigen/Alhydrogel. BALB/c mice (n = 16 to 24 per group) were immunized with AdYFP-pIX/V or purified V antigen/Alhydrogel. A subset of animals received a single repeat administration of the same immunogens at the same dose 4 weeks after primary immunization. (A) Serum anti-V titers, AdYFP-pIX/V (1011 particle units, pu). Six weeks after the prime immunization serum antibody levels were measured in an anti-V antigen-specific ELISA (p < 0.00007, AdYFP-pIX/V unboosted or boosted vs. AdYFP). (B) Serum anti-V titers purified recombinant V antigen (1.5 μg, the molar equivalent amount of protein present in 1011 pu of AdYFP-pIX/V) with Alhydrogel (p < 0.0005, V antigen unboosted or boosted vs. naive). (C) Survival, AdYFP-pIX/V. Six weeks after prime immunization with 1011 pu of AdYFP-pIX/V, mice were challenged with a lethal dose of Yersinia pestis CO92, and survival of the animals was monitored for 14 days. Naive mice and mice that received AdYFP (1011 pu) intramuscularly were included as negative controls (p < 0.0001, AdYFP-pIX/V vs. AdYFP; p < 0.0001, AdYFP-pIX/V plus boost vs. AdYFP). (D) Survival, V antigen/Alhydrogel. Naive mice were used as negative controls (p = 0.3014, V antigen vs. naive; p < 0.0001, V antigen plus boost vs. naive).
The ability of AdYFP-pIX/V or recombinant V antigen plus adjuvant to confer protective immunity was evaluated by challenging the immunized mice with the fully virulent Y. pestis CO92 strain. Six weeks after prime immunization, the mice were infected intranasally with 5 × 103 CFU of Y. pestis. Overall, immunization with AdYFP-pIX/V followed by a single repeat administration of the same vector at the same dose showed enhanced protection compared with mice immunized with a molar equivalent of V antigen plus adjuvant (Fig. 3). Of the mice primed and boosted with 109 pu of AdYFP-pIX/V, 75% were protected (Fig. 3C), whereas only 12.5% of the mice receiving a prime–boost immunization with 15 ng of V antigen survived (Fig. 3D). A low percentage of animals receiving just the prime immunization with either 109 pu of AdYFP-pIX/V or 15 ng of V antigen survived, whereas mice vaccinated with AdYFP or naive mice were not protected against the challenge.
When mice were vaccinated with 1011 pu of AdYFP-pIX/V, a high survival rate was observed in the presence or absence of a secondary immunization (100 and 90% respectively; Fig. 4C). In contrast, only 50% of the mice receiving 1.5 μg of V antigen in a prime–boost regimen survived and animals that received only a single administration of V antigen showed almost no protection, comparable to the naive control (Fig. 4D). Control mice immunized with AdYFP were not protected.
It is not likely that the pIX/V antigen fusion protein exists in a conformation that resembles native V antigen, and consequently, pIX/V would not form functional dimers as native V antigen does. This may result in display of a different array of protective epitopes on pIX/V relative to native V antigen. Although this is relevant to consider for future studies in nonhuman primates and humans, there are V antigen epitopes commonly recognized by immune sera from V antigen-immunized mice and nonhuman primates that are protected against Y. pestis challenge (Cornelius et al., 2008), suggesting that the robust protective immunity elicited in AdYFP-pIX/V-immunized mice may translate to other species.
Humoral immune responses and protection against challenge with Yersinia pestis conferred by immunization with AdLacZ-pIX/F1 versus purified recombinant F1 antigen plus adjuvant
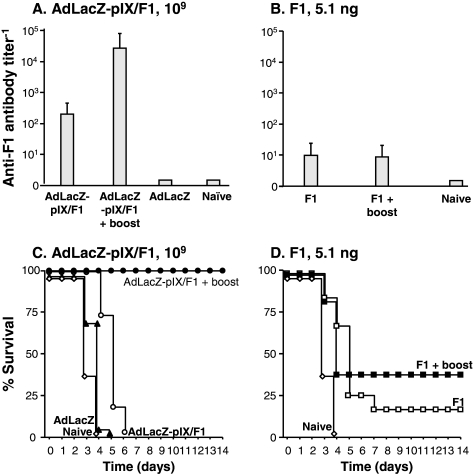
To evaluate humoral responses against the F1 capsular antigen, mice were intramuscularly immunized with AdLacZ-pIX/F1 or recombinant F1 protein plus adjuvant in a prime–boost regimen, and the anti-F1 antigen IgG titers were assessed in serum 6 weeks after prime immunization. Animals immunized with 109 or 1011 pu of AdLacZ-pIX/F1 and boosted 4 weeks later with the same vector at the same dose showed higher antibody titers than mice just receiving the prime immunization (Figs. 5A and 6A, respectively). In contrast, mice vaccinated with 5.1 ng or 0.5 μg of recombinant F1 (molar equivalent amount of protein as present in 109 or 1011 pu of capsid-modified virions, respectively) followed by the same dose of F1 at 4 weeks after prime immunization show no significant boosting effect (Figs. 5B and 6B, respectively). No F1-specific antibody titers were detected in naive or AdLacZ-immunized mice.
FIG. 5.
Anti-F1 antibodies and survival of mice immunized in a prime–boost regimen with AdLacZ-pIX/F1 or F1/Alhydrogel. BALB/c mice (n = 11 to 22 per group) were immunized with AdLacZ-pIX/F1 or purified F1/Alhydrogel. A subset of animals received a single repeat administration of the same immunogens at the same dose 4 weeks after primary immunization. (A) Serum anti-F1 titers, AdLacZ-pIX/F1 (109 particle units, pu). Six weeks after the prime immunization serum antibody levels were measured in an anti-F1-specific ELISA (p < 0.01, AdLacZ-pIX/F1 unboosted or boosted vs. AdLacZ). (B) Serum anti-F1 titers, purified recombinant F1 (5.1 ng, the molar equivalent amount of protein present in 109 pu of AdLacZ-pIX/F1) with Alhydrogel (p < 0.01, F1 unboosted or boosted vs. naive). (C) Survival, AdLacZ-pIX/F1. Six weeks after prime immunization with 109 pu of AdLacZ-pIX/F1, mice were challenged with a lethal dose of Yersinia pestis CO92, and survival of the animals was monitored for 14 days. Naive mice and mice that received AdLacZ (1011 pu) intramuscularly were included as negative controls (p < 0.0001, AdLacZ-pIX/F1 vs. AdLacZ; p < 0.0001, AdLacZ-pIX/F1 plus boost vs. AdLacZ). (D) Survival, F1/Alhydrogel. Naive mice were used as negative controls (p < 0.0007, F1 vs. naive; p = 0.0044, F1 plus boost vs. naive).
FIG. 6.
Anti-F1 antibodies and survival of mice immunized in a prime–boost regimen with AdLacZ-pIX/F1 or F1/Alhydrogel. BALB/c mice (n = 15 to 31 per group) were immunized with AdLacZ-pIX/F1 or purified F1/Alhydrogel. A subset of animals received a single repeat administration of the same immunogens at the same dose 4 weeks after primary immunization. (A) Serum anti-F1 titers, AdLacZ-pIX/F1 (1011 particle units, pu). Six weeks after the prime immunization serum antibody levels were measured by anti-F1-specific ELISA (p < 0.0003, AdLacZ-pIX/F1 unboosted or boosted vs. AdLacZ). (B) Serum anti-F1 titers, purified recombinant F1 (0.5 μg, the molar equivalent amount of protein present in 1011 pu of AdLacZ-pIX/F1) with Alhydrogel (p < 0.0005, F1 unboosted vs. naive). (C) Survival, AdLacZ-pIX/F1. Six weeks after prime immunization with 1011 pu of AdLacZ-pIX/F1, mice were challenged with a lethal dose of Yersinia pestis CO92, and survival of the animals was monitored for 14 days. Naive mice and mice that received AdLacZ (1011 pu) intramuscularly were included as negative controls (p < 0.0007, AdLacZ-pIX/F1 vs. AdLacZ; p < 0.0001, AdLacZ-pIX/F1 plus boost vs. AdLacZ. (D) Survival, F1/Alhydrogel. Naive mice were used as negative controls (p = 0.4187, F1 vs. naive; p < 0.0001, F1 plus boost vs. naive).
To asses the ability of AdLacZ-pIX/F1 or recombinant F1 protein plus adjuvant to protect mice against a lethal challenge with Y. pestis, 6 weeks after prime immunization vaccinated mice were infected intranasally with 5 × 103 CFU of Y. pestis. As seen with AdYFP-pIX/V, immunization with AdLacZ-pIX/F1 followed by a single repeat administration of the same vector at the same dose showed enhanced protection relative to mice immunized with an equimolar dose of purified recombinant F1 protein plus adjuvant. Animals primed and boosted with 109 or 1011 pu of AdLacZ-pIX/F1 were significantly protected against challenge (100%, Fig. 5C and 90%, Fig. 6C, respectively), whereas mice receiving a prime–boost immunization with 5.1 ng or 0.5 μg of F1 protein were minimally protected against challenge (30%, Fig. 5D, and 20%, Fig. 6D, respectively).
Animals receiving just a prime immunization with either dose of AdLacZ-pIX/F1 or F1 protein showed no, or a low percentage of, survival (0% for mice receiving 109 or 1011 PU of AdLacZ-pIX/F1, Figs. 5C and 6C, respectively; 20% for mice receiving 5.1 ng of F1, Fig. 5D; and 0% for mice receiving 0.5 μg of F1, Fig. 6D). Naive or AdLacZ-immunized mice were not protected against the challenge.
Although the conformation of pIX/V or pIX/F1 fusion proteins likely differs from that of native V antigen or F1 protein and the exact same array of protective epitopes may not be displayed, immunization with AdYFP-pIX/V or AdLacZ-pIX/F1 provides a more robust and enhanced protective immune response compared with immunization with purified recombinant V antigen or F1 protein.
Discussion
In this study, replication-defective adenoviral vectors containing capsid modifications that incorporate relevant Yersinia pestis immunogens as fusions to the pIX capsid protein were evaluated as vaccine candidates against pneumonic plague. Two vaccine vectors were constructed, AdYFP-pIX/V and AdLacZ-pIX/F1, to display the Y. pestis V antigen or F1 capsular antigen, respectively, on the virion surface for presentation to the immune system. Expression of these antigens on the surface of the resulting recombinant viruses was confirmed by Western analysis. A single immunization with AdYFP-pIX/V at a dose of 1011 pu conferred 90% protection against Y. pestis challenge. In addition, immunization with AdYFP-pIX/V at a dose of either 109 or 1011 pu followed by a single repeat administration of the same vector at the same dose resulted in substantial protection of immunized animals compared with immunization with a comparable molar amount of purified recombinant V antigen plus Alhydrogel adjuvant. Similarly, immunization with AdLacZ-pIX/F1 in a prime–boost regimen resulted in substantial protection of immunized animals compared with immunization with a comparable molar amount of purified recombinant F1 protein plus adjuvant. By comparison with AdYFP-pIX/V or AdLacZ-pIX/F1, the comparable molar amounts of purified V antigen or F1 protein were substantially lower (V antigen, 7- to 700-fold lower; F1, 20- to 2000-fold lower) than the amounts of V antigen or F1 protein demonstrated to be optimally effective in protecting immunized mice against Y. pestis challenge in previous studies (10 μg, each protein; Williamson et al., 2007), demonstrating the robust immune enhancement when these proteins are presented in the context of Ad.
Yersinia pestis vaccines
Plague is a potentially fatal infection in humans caused by the bacterium Yersinia pestis (Perry and Fetherston, 1997; Titball et al., 2004; Prentice and Rahalison, 2007). The currently recommended therapy for plague is antibiotics, but these drugs are only partially effective once symptoms of pneumonic plague develop and some antibiotic-resistant isolates of Y. pestis have been identified (Galimand et al., 1997; Inglesby et al., 2000; Guiyoule et al., 2001). There is no licensed Y. pestis vaccine for use in the United States (Titball and Williamson, 2004; Smiley, 2008). Killed whole cell vaccines consisting of heat-inactivated Y. pestis have been available since the late 1890s, but although these vaccines protect against the bubonic form of the disease, they do not protect against pneumonic plague (Russell et al., 1995; Titball and Williamson, 2001, 2004; Titball et al., 2004). In the mid-1900s, a formalin-killed whole cell vaccine was licensed and sold as Plague Vaccine, USP (Meyer, 1970). Although controlled clinical trials were not conducted, there is indirect evidence from vaccinated military personnel that it protects against bubonic plague (Cavanaugh et al., 1974). However, there are a variety of side effects associated with this vaccine and it does not provide protection against pneumonic plague (Titball and Williamson, 2001, 2004; Cornelius et al., 2007). A live-attenuated vaccine based on the pigmentation-negative mutant strain of Y. pestis EV76 was developed in the former Soviet Union and has been available since 1908 (Russell et al., 1995; Zilinskas, 2006). This vaccine induces a high degree of immune variability in humans and presents the risk that the bacteria could revert to virulence in vivo.
The development of a safe and effective plague vaccine has been focused on using recombinant protein subunits of Y. pestis, specifically the V antigen and the F1 capsular antigen (Titball and Williamson, 2001, 2004; Williamson et al., 2005; Cornelius et al., 2007; Morris, 2007; Smiley, 2008). Antibodies against V antigen and F1 confer protection against both bubonic and pneumonic plague in mice, guinea pigs, and nonhuman primates (Leary et al., 1995; Anderson et al., 1996, 1998; Andrews et al., 1996; Heath et al., 1998; Jones et al., 2003, 2006; Santi et al., 2006; Bashaw et al., 2007; Mett et al., 2007; Williamson et al., 2007; Cornelius et al., 2008; Chichester et al., 2009; Mizel et al., 2009). Vaccine formulations consisting of combined V antigen and F1 protein subunits or a fusion protein between F1 and V antigen have entered clinical trials and appear to be safe, well tolerated, and immunogenic (Williamson et al., 2005; Morris, 2007). However, these F1 and V antigen-based vaccines have demonstrated variability in protection of different nonhuman primate species against aerosolized Y. pestis challenge, suggesting that further refinement of these vaccines would be useful.
Adenovirus-based vaccine vectors
Recombinant Ads are highly immunogenic and, consequently, attractive vaccine candidates, particularly for biodefense (Boyer et al., 2005). Ads are stable, easy to manipulate, can be produced inexpensively at high titer, and can be purified by commonly available methods (Hackett and Crystal, 2004). Importantly, Ad vectors infect a wide variety of cell types in vivo, including dendritic cells and other antigen-presenting cells. The Ad vector may act as an adjuvant by inducing a strong immune/inflammatory response and by promoting differentiation of immature dendritic cells into antigen-presenting cells (Song et al., 1997; Zhong et al., 1999; Morelli et al., 2000; Zhang et al., 2001; Korst et al., 2002; Hackett and Crystal, 2004).
Standard Ad-based vaccine vectors are based on the concept of delivering the relevant antigen as a transgene for expression and stimulation of immunity in the host after administration (Tatsis and Ertl, 2004; Chiuchiolo et al., 2006). It has also been possible to incorporate immunogenic epitopes into permissive sites of hexon, a major capsid protein, and the resulting vaccines exhibit robust protective efficacy (Worgall et al., 2005, 2007). However, the addition of foreign immunogens into hexon is limited by size; insertions of only up to 53 amino acids in the hypervariable region 5 of hexon are tolerated for viable virus recovery (McConnell et al., 2006; Matthews et al., 2008). In contrast, studies on the minor capsid protein, pIX, have indicated that up to 1000 kDa can be fused to the C terminus of the protein, allowing surface exposure of the foreign protein without substantial impact on the formation of virions (Dmitriev et al., 2002; Campos et al., 2004; Meulenbroek et al., 2004; Le et al., 2005; Li et al., 2005; Matthews et al., 2006; Vellinga et al., 2007). We exploited this technology for vaccine development purposes by generating pIX fusions that display the full-length V antigen or F1 proteins on the virion surface to maximize the number of relevant protective epitopes available for recognition. This study demonstrates that Ad-based vaccines containing relevant immunogens on the capsid as fusions to pIX function similarly to recombinant protein-based vaccines, but with the added benefit of the strong adjuvant effect of Ad. Future studies evaluating the contribution of T cell responses may provide additional insight into the enhanced protective immune response conferred by AdYFP-pIX/V and AdLacZ-pIX/F1. Collectively, capsid-modified Ad gene transfer vectors displaying relevant immunogens on their surface via the viral pIX protein are effective vaccine platforms that elicit robust protective immunity relative to recombinant protein-based vaccines.
Acknowledgments
The authors thank N. Mohamed for help in preparing this manuscript; and Hong-Ching Yeung for technical assistance. These studies were supported, in part, by U54 AI057158 and by a gift from Robert A. Belfer to support the development of an antibioterrorism vaccine.
Author Disclosure Statement
The authors have no conflict of interest.
References
- Anderson G.W., Jr Leary S.E. Williamson E.D. Titball R.W. Welkos S.L. Worsham P.L. Friedlander A.M. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 1996;64:4580–4585. doi: 10.1128/iai.64.11.4580-4585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G.W., Jr Heath D.G. Bolt C.R. Welkos S.L. Friedlander A.M. Short- and long-term efficacy of single-dose subunit vaccines against Yersinia pestis in mice. Am. J. Trop. Med. Hyg. 1998;58:793–799. doi: 10.4269/ajtmh.1998.58.793. [DOI] [PubMed] [Google Scholar]
- Andrews G.P. Heath D.G. Anderson G.W., Jr Welkos S.L. Friedlander A.M. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 1996;64:2180–2187. doi: 10.1128/iai.64.6.2180-2187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbuch V. Isberg R.R. Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect. Immun. 2007;75:3561–3570. doi: 10.1128/IAI.01497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashaw J. Norris S. Weeks S. Trevino S. Adamovicz J.J. Welkos S. Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin. Vaccine Immunol. 2007;14:605–616. doi: 10.1128/CVI.00398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J.L. Kobinger G. Wilson J.M. Crystal R.G. Adenovirus-based genetic vaccines for biodefense. Hum. Gene Ther. 2005;16:157–168. doi: 10.1089/hum.2005.16.157. [DOI] [PubMed] [Google Scholar]
- Campos S.K. Parrott M.B. Barry M.A. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol. Ther. 2004;9:942–954. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh D.C. Randall R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J. Immunol. 1959;83:348–363. [PubMed] [Google Scholar]
- Cavanaugh D.C. Elisberg B.L. Llewellyn C.H. Marshall J.D., Jr Rust J.H., Jr Williams J.E. Meyer K.F. Plague immunization. V. Indirect evidence for the efficacy of plague vaccine. J. Infect. Dis. 1974;129(Suppl. 40):S37–S40. doi: 10.1093/infdis/129.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- Chichester J.A. Musiychuk K. Farrance C.E. Mett V. Lyons J. Mett V. Yusibov V. A single component two-valent LcrV–F1 vaccine protects non-human primates against pneumonic plague. Vaccine. 2009;27:3471–3474. doi: 10.1016/j.vaccine.2009.01.050. [DOI] [PubMed] [Google Scholar]
- Chiuchiolo M.J. Boyer J.L. Krause A. Senina S. Hackett N.R. Crystal R.G. Protective immunity against respiratory tract challenge with Yersinia pestis in mice immunized with an adenovirus-based vaccine vector expressing V antigen. J. Infect. Dis. 2006;194:1249–1257. doi: 10.1086/507644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C. Quenee L. Anderson D. Schneewind O. Protective immunity against plague. Adv. Exp. Med. Biol. 2007;603:415–424. doi: 10.1007/978-0-387-72124-8_38. [DOI] [PubMed] [Google Scholar]
- Cornelius C.A. Quenee L.E. Overheim K.A. Koster F. Brasel T.L. Elli D. Ciletti N.A. Schneewind O. Immunization with recombinant V10 protects cynomolgus macaques from lethal pneumonic plague. Infect. Immun. 2008;76:5588–5597. doi: 10.1128/IAI.00699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R.G. Harvey B.-G. Wisnivesky J.P. O'Donoghue K.A. Chu K.W. Maroni J. Muscat J.C. Pippo A.L. Wright C.E. Kaner R.J. Leopold P.L. Kessler P.D. Rasmussen H.S. Rosengart T.K. Hollmann C. Analysis of risk factors for local delivery of low and intermediate dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum. Gene Ther. 2002;13:65–100. doi: 10.1089/10430340152712647. [DOI] [PubMed] [Google Scholar]
- Dmitriev I.P. Kashentseva E.A. Curiel D.T. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J. Virol. 2002;76:6893–6899. doi: 10.1128/JVI.76.14.6893-6899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimand M. Guiyoule A. Gerbaud G. Rasoamanana B. Chanteau S. Carniel E. Courvalin P. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 1997;337:677–680. doi: 10.1056/NEJM199709043371004. [DOI] [PubMed] [Google Scholar]
- Guiyoule A. Gerbaud G. Buchrieser C. Galimand M. Rahalison L. Chanteau S. Courvalin P. Carniel E. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis. 2001;7:43–48. doi: 10.3201/eid0701.010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett N.R. Crystal R.G. Adenovirus vectors for gene therapy. In: Gene Therapy: Therapeutic Mechanisms and Strategies. In: Lasic D, editor; Templeton N.S., editor. Marcel Dekker; New York: 2004. pp. 17–40. [Google Scholar]
- Harvey B.-G. Maroni J. O'Donoghue K.A. Chu K.W. Muscat J.C. Pippo A.L. Wright C.E. Hollmann C. Wisnivesky J.P. Kessler P.D. Rasmussen H.S. Rosengart T.K. Crystal R.G. Safety of local delivery of low and intermediate dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum. Gene Ther. 2002;13:15–63. doi: 10.1089/10430340152712638. [DOI] [PubMed] [Google Scholar]
- Heath D.G. Anderson G.W., Jr Mauro J.M. Welkos S.L. Andrews G.P. Adamovicz J. Friedlander A.M. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1–V antigen fusion protein vaccine. Vaccine. 1998;16:1131–1137. doi: 10.1016/s0264-410x(98)80110-2. [DOI] [PubMed] [Google Scholar]
- Inglesby T.V. Dennis D.T. Henderson D.A. Bartlett J.G. Ascher M.S. Eitzen E. Fine A.D. Friedlander A.M. Hauer J. Koerner J.F. Layton M. McDade J. Osterholm M.T. O'Toole T. Parker G. Perl T.M. Russell P.K. Schoch-Spana M. Tonat K. Working Group on Civilian Biodefense. Plague as a biological weapon: Medical and public health management. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- Jefferson T. Demicheli V. Pratt M. Vaccines for preventing plague. Cochrane Database Syst. Rev. 2000;2:CD000976. doi: 10.1002/14651858.CD000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.M. Griffin K.F. Hodgson I. Williamson E.D. Protective efficacy of a fully recombinant plague vaccine in the guinea pig. Vaccine. 2003;21:3912–3918. doi: 10.1016/s0264-410x(03)00379-7. [DOI] [PubMed] [Google Scholar]
- Jones T. Adamovicz J.J. Cyr S.L. Bolt C.R. Bellerose N. Pitt L.M. Lowell G.H. Burt D.S. Intranasal Protollin/F1-V vaccine elicits respiratory and serum antibody responses and protects mice against lethal aerosolized plague infection. Vaccine. 2006;24:1625–1632. doi: 10.1016/j.vaccine.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Korst R.J. Mahtabifard A. Yamada R. Crystal R.G. Effect of adenovirus gene transfer vectors on the immunologic functions of mouse dendritic cells. Mol. Ther. 2002;5:307–315. doi: 10.1006/mthe.2002.0538. [DOI] [PubMed] [Google Scholar]
- Le L.P. Li J. Ternovoi V.V. Siegal G.P. Curiel D.T. Fluorescently tagged canine adenovirus via modification with protein IX-enhanced green fluorescent protein. J. Gen. Virol. 2005;86:3201–3208. doi: 10.1099/vir.0.80968-0. [DOI] [PubMed] [Google Scholar]
- Leary S.E. Williamson E.D. Griffin K.F. Russell P. Eley S.M. Titball R.W. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 1995;63:2854–2858. doi: 10.1128/iai.63.8.2854-2858.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Le L. Sibley D.A. Mathis J.M. Curiel D.T. Genetic incorporation of HSV-1 thymidine kinase into the adenovirus protein IX for functional display on the virion. Virology. 2005;338:247–258. doi: 10.1016/j.virol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Matthews Q.L. Sibley D.A. Wu H. Li J. Stoff-Khalili M.A. Waehler R. Mathis J.M. Curiel D.T. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol. Imaging. 2006;5:510–519. [PMC free article] [PubMed] [Google Scholar]
- Matthews Q.L. Yang P. Wu Q. Belousova N. Rivera A.A. Stoff-Khalili M.A. Waehler R. Hsu H.C. Li Z. Li J. Mountz J.D. Wu H. Curiel D.T. Optimization of capsid-incorporated antigens for a novel adenovirus vaccine approach. Virol. J. 2008;5:98. doi: 10.1186/1743-422X-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M.J. Danthinne X. Imperiale M.J. Characterization of a permissive epitope insertion site in adenovirus hexon. J. Virol. 2006;80:5361–5370. doi: 10.1128/JVI.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett V. Lyons J. Musiychuk K. Chichester J.A. Brasil T. Couch R. Sherwood R. Palmer G.A. Streatfield S.J. Yusibov V. A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine. 2007;25:3014–3017. doi: 10.1016/j.vaccine.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Meulenbroek R.A. Sargent K.L. Lunde J. Jasmin B.J. Parks R.J. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion: Generation of fluorescent virus through the incorporation of pIX-GFP. Mol. Ther. 2004;9:617–624. doi: 10.1016/j.ymthe.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Meyer K.F. Effectiveness of live or killed plague vaccines in man. Bull. World Health Org. 1970;42:653–666. [PMC free article] [PubMed] [Google Scholar]
- Mittereder N. March K.L. Trapnell B.C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S.B. Graff A.H. Sriranganathan N. Ervin S. Lees C.J. Lively M.O. Hantgan R.R. Thomas M.J. Wood J. Bell B. Flagellin–F1–V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin. Vaccine Immunol. 2009;16:21–28. doi: 10.1128/CVI.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli A.E. Larregina A.T. Ganster R.W. Zahorchak A.F. Plowey J.M. Takayama T. Logar A.J. Robbins P.D. Falo L.D. Thomson A.W. Recombinant adenovirus induces maturation of dendritic cells via an NF-κB-dependent pathway. J. Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.R. Development of a recombinant vaccine against aerosolized plague. Vaccine. 2007;25:3115–3117. doi: 10.1016/j.vaccine.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Perry R.D. Fetherston J.D. Yersinia pestis: Etiologic agent of plague. Clin. Microbiol. Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson J. Holmstrom A. Hill J. Leary S. Frithz-Lindsten E. von Euler-Matell A. Carlsson E. Titball R. Forsberg A. Wolf-Watz H. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- Pouliot K. Pan N. Wang S. Lu S. Lien E. Goguen J.D. Evaluation of the role of LcrV-Toll-like receptor 2-mediated immunomodulation in the virulence of Yersinia pestis. Infect. Immun. 2007;75:3571–3580. doi: 10.1128/IAI.01644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice M.B. Rahalison L. Plague. Lancet. 2007;369:1196–1207. doi: 10.1016/S0140-6736(07)60566-2. [DOI] [PubMed] [Google Scholar]
- Reithmeier-Rost D. Hill J. Elvin S.J. Williamson D. Dittmann S. Schmid A. Wilharm G. Sing A. The weak interaction of LcrV and TLR2 does not contribute to the virulence of Yersinia pestis. Microbes Infect. 2007;9:997–1002. doi: 10.1016/j.micinf.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.A. Siegfried W. Yoshimura K. Yoneyama K. Fukayama M. Stier L.E. Paakko P.K. Gilardi P. Stratford-Perricaudet L.D. Perricaudet M. Jallat S. Pavirani A. Lecocq J.-P. Crystal R.G. Adenovirus-mediated transfer of a recombinant α1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.A. Yoshimura K. Trapnell B.C. Yoneyama K. Rosenthal E.R. Dalemans W. Fukayama M. Bargon J. Stier L.E. Stratford-Perricaudet L. Perricaudet M. Guggino W.B. Pavirani A. Lecocq J.-P. Crystal R.G. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Russell P. Eley S.M. Hibbs S.E. Manchee R.J. Stagg A.J. Titball R.W. A comparison of plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine. 1995;13:1551–1556. doi: 10.1016/0264-410x(95)00090-n. [DOI] [PubMed] [Google Scholar]
- Santi L. Giritch A. Roy C.J. Marillonnet S. Klimyuk V. Gleba Y. Webb R. Arntzen C.J. Mason H.S. Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc. Natl. Acad. Sci. U.S.A. 2006;103:861–866. doi: 10.1073/pnas.0510014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker M.R. Neyt C. Stainier I. Cornelis G.R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley S.T. Current challenges in the development of vaccines for pneumonic plague. Expert Rev. Vaccines. 2008;7:209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer-Podesta C. Ang J. Hackett N.R. Senina S. Perlin D. Crystal R.G. Boyer J.L. Adenovirus-mediated delivery of an anti-V antigen monoclonal antibody protects mice against a lethal Yersinia pestis challenge. Infect. Immun. 2009;77:1561–1568. doi: 10.1128/IAI.00856-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. Kong H.L. Carpenter H. Torii H. Granstein R. Rafii S. Moore M.A. Crystal R.G. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N. Ertl H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titball R.W. Williamson E.D. Vaccination against bubonic and pneumonic plague. Vaccine. 2001;19:4175–4184. doi: 10.1016/s0264-410x(01)00163-3. [DOI] [PubMed] [Google Scholar]
- Titball R.W. Williamson E.D. Yersinia pestis (plague) vaccines. Expert Opin. Biol. Ther. 2004;4:965–973. doi: 10.1517/14712598.4.6.965. [DOI] [PubMed] [Google Scholar]
- Titball R.W. Williamson E.D. Dennis D.T. Plague. In: Plotkin S.A., editor; Orenstein W.A, editor. Vaccines. W.B. Saunders; Philadelphia: 2004. pp. 999–1010. [Google Scholar]
- Vellinga J. de Vrij J. Myhre S. Uil T. Martineau P. Lindholm L. Hoeben R.C. Efficient incorporation of a functional hyper-stable single-chain antibody fragment protein-IX fusion in the adenovirus capsid. Gene Ther. 2007;14:664–670. doi: 10.1038/sj.gt.3302908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.D. Flick-Smith H.C. Lebutt C. Rowland C.A. Jones S.M. Waters E.L. Gwyther R.J. Miller J. Packer P.J. Irving M. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 2005;73:3598–3608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.D. Stagg A.J. Eley S.M. Taylor R. Green M. Jones S.M. Titball R.W. Kinetics of the immune response to the (F1 + V) vaccine in models of bubonic and pneumonic plague. Vaccine. 2007;25:1142–1148. doi: 10.1016/j.vaccine.2006.09.052. [DOI] [PubMed] [Google Scholar]
- Worgall S. Krause A. Rivara M. Hee K.K. Vintayen E.V. Hackett N.R. Roelvink P.W. Bruder J.T. Wickham T.J. Kovesdi I. Crystal R.G. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J. Clin. Invest. 2005;115:1281–1289. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgall S. Krause A. Qiu J. Joh J. Hackett N.R. Crystal R.G. Protective immunity to Pseudomonas aeruginosa induced with a capsid-modified adenovirus expressing P aeruginosa. OprF. J. Virol. 2007;81:13801–13808. doi: 10.1128/JVI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Chirmule N. Gao G.P. Qian R. Croyle M. Joshi B. Tazelaar J. Wilson J.M. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- Zhong L. Granelli-Piperno A. Choi Y. Steinman R.M. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur. J. Immunol. 1999;29:964–972. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Zilinskas R.A. The anti-plague system and the Soviet biological warfare program. Crit. Rev. Microbiol. 2006;32:47–64. doi: 10.1080/10408410500496896. [DOI] [PubMed] [Google Scholar]