This study by the groups of Drs. Barry Byrne and Cathryn Mah at the University of Florida examines the safety and efficacy of AAV-mediated gene delivery in a canine model of glycogen storage disease type Ia (GSDIa). The authors find that intraportal delivery of AAV8 encoding glucose-6-phosphatase-α (G6Pase) followed 20 weeks later by intraportal administration of AAV1 encoding G6Pase led to significant correction of the GSDIa phenotype.
Abstract
Glycogen storage disease type Ia (GSDIa; von Gierke disease; MIM 232200) is caused by a deficiency in glucose-6-phosphatase-α. Patients with GSDIa are unable to maintain glucose homeostasis and suffer from severe hypoglycemia, hepatomegaly, hyperlipidemia, hyperuricemia, and lactic acidosis. The canine model of GSDIa is naturally occurring and recapitulates almost all aspects of the human form of disease. We investigated the potential of recombinant adeno-associated virus (rAAV) vector-based therapy to treat the canine model of GSDIa. After delivery of a therapeutic rAAV2/8 vector to a 1-day-old GSDIa dog, improvement was noted as early as 2 weeks posttreatment. Correction was transient, however, and by 2 months posttreatment the rAAV2/8-treated dog could no longer sustain normal blood glucose levels after 1 hr of fasting. The same animal was then dosed with a therapeutic rAAV2/1 vector delivered via the portal vein. Two months after rAAV2/1 dosing, both blood glucose and lactate levels were normal at 4 hr postfasting. With more prolonged fasting, the dog still maintained near-normal glucose concentrations, but lactate levels were elevated by 9 hr, indicating that partial correction was achieved. Dietary glucose supplementation was discontinued starting 1 month after rAAV2/1 delivery and the dog continues to thrive with minimal laboratory abnormalities at 23 months of age (18 months after rAAV2/1 treatment). These results demonstrate that delivery of rAAV vectors can mediate significant correction of the GSDIa phenotype and that gene transfer may be a promising alternative therapy for this disease and other genetic diseases of the liver.
Introduction
Glycogen storage disease type Ia (GSDIa) is an autosomal recessive disorder that results from a deficiency of functional microsomal enzyme glucose-6-phosphatase-α (G6Pase, EC 3.1.3.9). As a result of G6Pase deficiency, glucose from stored glycogen or gluconeogenesis is unavailable during periods of fasting. Affected individuals are unable to maintain glucose homeostasis, ultimately resulting in severe hypoglycemia. Clinical pathology includes profound enlargement of the liver and kidneys because of excess glycogen accumulation, and shunting into alternative pathways results in hyperlipidemia, hyperuricemia, and lactic acidosis (Chen and Burchell, 1995; Chou et al., 2002; Wolfsdorf et al., 2003). Current therapy is palliative, with the aim of controlling hypoglycemia by providing continuous sources of glucose via frequent feedings, continuous overnight feeding by nasogastric tube, and/or oral administration of uncooked cornstarch or other starches (Chen et al., 1984; Smit et al., 1984, 1988; Fernandes et al., 1988; Bhattacharya et al., 2007). Affected patients can now survive to adulthood, but long-term complications remain common, including hepatic adenomas, hepatocellular carcinoma, renal disease, gout, osteoporosis, and pulmonary hypertension (Smit et al., 1990; Mundy et al., 2003, 2005; Ozen, 2007).
Both mouse and canine models of GSDIa exist. The mice recapitulate many, but not all, of the features of human GSDIa (Lei et al., 1996; Kim et al., 2007). The Maltese–Beagle canine model of GSDIa originated from a naturally occurring Maltese colony carrying the p.M121I mutation (Kishnani et al., 1997, 2001). The canine model manifests the same pathophysiology seen in the mouse model of GSDIa. Furthermore, unlike in the murine model, the canine model presents with profound lactic acidosis, which is an important feature in the GSDIa human population.
Although nutritional therapy is effective in moderating disease, alternative therapies such as novel longer-acting starches and/or gene therapy strategies are attractive as they may provide significant improvements in quality of life. There exist several efforts toward developing gene therapy strategies for the treatment of GSDIa. rAAV2/1 and rAAV2/8 vectors, that is, recombinant adeno-associated virus serotype 2 pseudotyped with capsids from AAV1 and AAV8, have mediated prolonged survival in addition to sustained correction of glucose homeostasis and normalization of triglyceride, cholesterol, and uric acid levels in the mouse model of GSDIa (Chen and Burchell, 1995; Sun et al., 2002; Ghosh et al., 2006; Koeberl et al., 2006). Similar studies using a helper-dependent adenoviral (HDAd) vector also gave rise to correction of a mouse model of GSDIa with increased median survival rates (Sun et al., 2002; Koeberl et al., 2007b). More recently, Koeberl and colleagues demonstrated prolonged survival and improved glucose homeostasis in the canine model, using an rAAV2/8 vector (Koeberl et al., 2007a, 2008). In the current study, we further evaluate the correction resulting from delivery of rAAV-G6Pase in the canine model of disease.
Materials and Methods
Packaging, purification, and delivery of recombinant AAV vectors
The recombinant AAV plasmid pUF11-G6Pase has been described previously (Ghosh et al., 2006). Recombinant AAV particles based on serotypes 8 and 1 were produced with pUF11-G6Pase and were generated, purified, and titered at the University of Florida Powell Gene Therapy Center Vector Core Laboratory (Gainesville, FL) as previously described (Zolotukhin et al., 2002).
Animal studies
All animal studies were performed in accordance with guidelines of the University of Florida Institutional Animal Care and Use Committee. The vector-treated GSDIa dog was administered rAAV2/8-UF11-G6Pase via jugular vein injection at 1 day old and the second dose of vector, rAAV2/1-UF11-G6Pase, was administered via portal vein injection.
Histological and biochemical evaluation
Specific G6Pase enzyme activity was measured from microsomal preparations from liver biopsies in 100-μl reactions containing 50 mM sodium cacodylate buffer, 10 mM glucose 6-phosphate, and 2 mM EDTA as previously described (Lei et al., 1996; Ghosh et al., 2006). Samples were incubated at 30°C for 10 min, absorbance was read at 820 nm, and nonspecific activity was subtracted.
Histochemical analysis of G6Pase activity in situ was performed by the method of lead trapping of phosphate generated by G6P hydrolysis as previously described (Teutsch, 1981; Ghosh et al., 2006).
Blood glucose and lactate measurements were taken using handheld Freestyle Flash (Abbot Diabetes Care, Alameda, CA) and Lactate Pro LT-1710 (Arkray KDK, Tokyo, Japan) meters, respectively, or alternatively with a clinical Hitachi 911 chemistry analyzer.
Histology (periodic acid–Schiff [PAS] and hematoxylin–eosin [H&E] staining) was performed by the University of Florida Molecular Pathology Core. Images were taken with an Axio Skope (Carl Zeiss Microimaging, Oberkochen, Germany).
Glycogen quantitation
Quantitation of glycogen content was assessed on perchloric acid-extracted liver biopsy samples from non-gene therapy-treated dogs, dogs receiving gene therapy treatment 5 months after rAAV8 injection and 6 months after rAAV1 administration, wild-type dogs, and heterozygous dogs, using proton magnetic resonance spectroscopy (1H-MRS) on a 500-MHz DRX spectrometer (Bruker AXS, Madison, WI) at the University of Florida Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility, as previously described (Mah et al., 2007).
Vector analysis
Vector genome quantitation was performed by the University of Florida National Gene Vector Laboratory (NGVL) Toxicology Core, using real-time PCR with primers and probes directed against the ubiquitously expressing hybrid cytomegalovirus immediate-early enhancer chicken β-actin (CBA) promoter as previously described (Conlon et al., 2005).
Anti-AAV8 antibody titers were determined as follows. Plates (Dynex Immulon, 4BX; Dynatech Laboratories, Chantilly, VA) were coated with 1.29 × 109 AAV2 particles (standard) or AAV8 (unknowns) per well overnight at 4°C in coating buffer (0.1 M NaHCO3, pH 8.4) and blocked at 37°C for 2 hr with 10% fetal bovine serum (FBS; Mediatech, Herndon, VA). Human sera known to be positive against AAV2 were used as a standard and unknowns (the experimental dog sera) were serially diluted and incubated overnight at 4°C. Detection antibody, horseradish peroxidase (HRP)-conjugated rabbit anti-canine immunoglobulin (Sigma-Aldrich, St. Louis, MO) or anti-human immunoglobulin, was added to the wells at a 1:10,000 dilution and incubated at 37°C for 2 hr. Detection was accomplished with TMB peroxidase substrate (KPL, Gaithersburg, MD) and the reaction was stopped with 1 M H3PO4. The plates were read at 450 nm and data were tabulated relative to a standard curve, using the seropositive reference.
Results and Discussion
Nutritional supplementation can prolong the life span of a GSDIa dog
Without treatment, GSDIa dogs die at birth and the historical average survival of GSDIa dogs, even with medical treatment, is approximately 5–8 weeks (Brix et al., 1995; Koeberl et al., 2007a). Unlike in the patient population, raw cornstarch did not act as an efficient source of glucose in our GSDIa dog, and we determined that oral supplementation with glucose at an average rate of 1.18 g/kg body weight per hour was most effective at maintaining normal blood glucose levels. The GSDIa dog receiving solely glucose supplementation survived up to 5.5 months of age and succumbed to complications resulting from acute pancreatitis. Whether the pancreatitis was associated with GSDIa-related pathology is unknown. The untreated dog did not manifest elevated blood triglyceride or cholesterol levels when compared with normal canine laboratory standards; however, postmortem pathology did reveal significant fat and glycogen accumulation in the liver as well as the presence of multifocal tubular degeneration in the kidneys (data not shown), both hallmarks of GSDIa. The degree of liver and kidney pathology that resulted from the pancreatitis is not known. Although this case indicated that frequent oral glucose supplementation may prolong the life span of GSDIa dogs, the ideal nutritional regimen for such dogs remains unknown. However, a nutritional regimen incorporating a complex carbohydrate source with a lower glycemic index or a higher percentage of calories derived from protein sources would likely provide additional benefits.
Delivery of rAAV2/8 mediates short-term improved glucose homeostasis
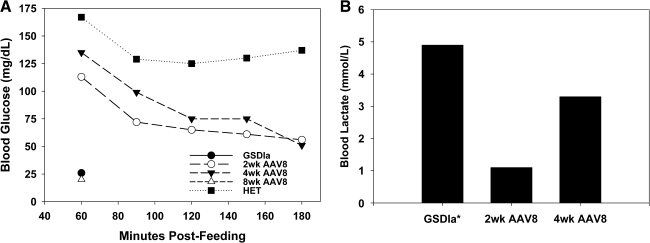
Prior work has shown that rAAV2/8 vectors could mediate the highest levels of hepatic G6Pase expression, as compared with rAAV2- and rAAV2/1-based vectors, in the mouse model (Ghosh et al., 2006; Koeberl et al., 2006). As such, we decided to use an rAAV2/8 vector expressing G6Pase under the control of the CBA promoter (rAAV2/8-CBA-G6Pase) in the canine model. Although it is possible that constitutive G6Pase overexpression could lead to other complications such as potential persistent hyperglycemia, we wanted to assess in the canine model the vectors that had given the highest levels of correction in the mouse model, to better understand what levels of correction can be achieved with the current strongest vectors as well as to provide insight into possible future optimization of vectors. rAAV2/8-CBA-G6Pase (5 × 1013 vector genomes [VG]/kg) was administered intravenously to a 1-day-old affected GSDIa puppy. As shown in Fig. 1A, at 4 weeks postinjection the treated dog had normal blood glucose levels after 2 hr of fasting; however, levels dropped below the lower limit of normal (:62 mg/dl) by 3 hr to 56 mg/dl with a concurrent elevation in blood lactate levels above the upper normal limit (92.1 mmol/liter) to 3.3 mmol/liter (Fig. 1B). Between 4 and 8 weeks after vector delivery, the dog began to experience periodic episodes of lethargy and vomiting, which was associated with elevated blood lactate levels greater than 4 mmol/liter. By 8 weeks postinjection, the rAAV2/8-treated animal was no longer able to sustain normal fasting blood glucose levels and had blood glucose levels similar to that of the untreated GSDIa dog by 1 hr of fasting (Fig. 1A). Dietary glucose supplementation was resumed to the levels required for an untreated dog to prevent episodes of hypoglycemia.
FIG. 1.
Neonatal delivery of rAAV2/8 results in short-term improvement of fasting glucose homeostasis and blood lactate levels. (A) Fasting blood glucose levels of untreated (no gene therapy; GSDIa) and heterozygous littermate control (HET) and the rAAV2/8-treated GSDIa dog at 2, 4, and 8 weeks postinjection. (B) Blood lactate levels of untreated (GSDIa) and rAAV2/8-treated GSDIa dogs at 2 and 4 weeks postinjection after 3 hr of fasting. *GSDIa, dog blood lactate level taken at 1 hr of fasting, after which fasting was discontinued to avoid complications.
Similar results in which intravenous rAAV2/8 delivery to neonates does not result in sustained correction have been shown by Wang and colleagues and Cunningham and colleagues, who demonstrated a dramatic decline in hepatic transgene expression after neonatal vector administration over time. This phenomenon was attributed to the dilution and degradation of vector genomes during the rapid growth and development of the liver rather than as a result of immune response to the vector and/or transgene product (Wang et al., 2005; Cunningham et al., 2008, 2009). We also noted similar kinetics in the mouse model of GSDIa, using rAAV2/8-CBA-G6Pase (J. Chou, data not shown). These results are in contrast to work by Koeberl and colleagues, in which neonatal administration of therapeutic rAAV2/8 vectors to GSDIa mice and dogs did not result over time in a diminishing ability to maintain glucose homeostasis after a 2-hr fast (Koeberl et al., 2008). The reasons for these differences are unknown. It is possible that the difference in precise timing of vector delivery and degree of liver development (delivery between 1 and 7 days in mice as compared with 12 days by Koeberl and colleagues; delivery at 1 vs. 3 days of age in the GSDIa dogs) may have contributed to the differences in transgene expression kinetics. It is also possible that the choice of promoter may have influenced the differences in expression persistence. The current study used the CBA promoter, as did the studies by Wang and colleagues and Chou and colleagues, whereas the studies by Cunningham and colleagues used the liver-specific promoter (LSP1). On the other hand, the study by Koeberl and colleagues used a G6Pase promoter and it is possible that use of the transgene's native promoter may have contributed to the persistence; however, the mechanism of persistence is not known.
Redosing with an rAAV2/1 vector results in correction of the GSDIa phenotype
Because the rAAV2/8-treated dog was unable to maintain fasting normoglycemia, the animal was redosed with rAAV2/1-CBA-G6Pase (1 × 1012 VG/kg) delivered via portal vein injection at 20 weeks of age. Although the presence of circulating anti-rAAV2/8 antibodies at the time of redosing was actually 1.9-fold below background (levels detected in dogs not exposed to vector [n = 4; 3 wild-type, 1 GSDIa]), an rAAV2/1 vector was used to avoid any other potential inhibition of transduction that may have been elicited from the first dose of vector. At 5 weeks after rAAV2/1 delivery, all glucose supplementation was completely discontinued. As shown in Fig. 2A, at 8 weeks after rAAV2/1 delivery, the dog was able to maintain normal blood glucose levels (66 mg/dl) after 5 hr of fasting and levels remained nearly normal at 9 hr of fasting (56 mg/dl). These results represented a dramatic improvement of the metabolic status of the dog as compared with the dog receiving rAAV2/8-only treatment. Corresponding blood lactate levels (Fig. 2B) did show an increase to above-normal levels after 9 hr of fasting, indicating that correction was partial.
FIG. 2.
Redosing with rAAV2/1 results in improved fasting glucose homeostasis and blood lactate levels. (A) Fasting blood glucose levels of the rAAV2/8 plus rAAV2/1-treated GSDIa dog, 8 weeks after rAAV2/1 delivery (open circles), wild-type (WT) control (solid circles), and an untreated (no gene therapy) GSDIa dog (solid triangle). (B) Fasting blood lactate levels of an untreated GSDIa dog (GSDIa); the gene therapy-treated GSDIa dog (rAAV2/1 plus rAAV2/8) at 2, 4, and 9 hr of fasting; and control heterozygous (HET; n = 5) and wild-type (n = 4) dogs after 12 hr of fasting.
At 6 months after rAAV2/1 delivery (11 months of age), the rAAV-treated GSDIa dog was still robust and able to be maintained without any supplemental dietary glucose/carbohydrates. At this time point, absolute hepatic G6Pase activity was 6.3% of wild-type levels (Fig. 3A). As shown in Table 1, quantitative real-time PCR determined an overall vector copy number of 1.32 × 104 vector genome copies per microgram of DNA in the liver (it was not possible to differentiate between rAAV2/8- and rAAV2/1-derived vectors, as both contained the same vector genome). It is interesting that a tiny increase in hepatic G6Pase activity from 3.8% wild-type activity (measured from tissue isolated just before the rAAV2/1 delivery at 20 weeks of age and Fig. 3B showing in situ histochemical staining for G6Pase activity) resulted in such a significant difference in metabolic control in the animal, suggesting that perhaps ∼6% of wild-type activity provides a critical therapeutic threshold. In addition to improved glucose homeostasis, we also noted a moderate improvement in liver histology. In Fig. 3C, both periodic acid–Schiff (PAS) and hematoxylin and eosin (H&E) staining revealed markedly abnormal hepatic ultrastructure in the dog 5 months after treatment with rAAV2/8, with substantial fat infiltrates in cells. At 3 months postdelivery of the rAAV2/1 vector, although PAS staining did reveal excess glycogen storage, overall hepatocyte structure was more normal.
FIG. 3.
Hepatic G6Pase enzyme activity and liver histology are improved in the gene therapy-treated dog. (A) Liver G6Pase activity in wild-type (WT; n = 3), heterozygous (HET; n = 3), and gene therapy-treated dogs 20 weeks after rAAV2/8 delivery (AAV8), a gene therapy-treated dog 6 months after redosing with rAAV1 (AAV8 + 1), and an untreated dog, which did not receive gene therapy (GSDIa). (B) Histochemical analysis of G6Pase activity in the liver of an untreated dog (22 weeks old, no gene therapy; GSDIa), a wild-type dog (WT, 20 weeks old), and an rAAV2/8-treated dog (AAV8) 20 weeks postinjection. Brown staining is indicative of lead trapping of phosphate generated by G6P hydrolysis by active G6Pase. (C) Periodic acid–Schiff (PAS) and hematoxylin and eosin (H&E) staining of liver tissue from a gene therapy-treated dog 20 weeks after rAAV2/8 delivery (AAV8) and 6 months after rAAV2/1 redosing (AAV8 + 1). (D) 1H-MRS spectra of hepatic glycogen in a heterozygous (Het) dog, a wild-type (WT) dog, an untreated (G6Pase–/–) dog, and a gene therapy-treated dog 20 weeks after rAAV2/8 delivery (rAAV2/8) and 6 months after rAAV2/1 redosing (rAAV2/8 + 2/1).
Table 1.
Determination of Vector Genome Copy Numbera
| Subject | DNA loaded (μg) | Vector genome copy no. (per μg DNA) |
|---|---|---|
| AAV-tx GSDIa | 1.00 | 13,249 |
| WT | 1.00 | 0 |
| GSDIa | 1.00 | 0 |
Abbreviations: AAV-tx GSDIa, AAV-treated GSDIa animal; GSDIa, untreated GSDIa animal; WT, wild type.
AAV vector-derived genomes were detectable by real-time PCR in liver 6 months after secondary dosing. One microgram of DNA isolated from the liver was analyzed by quantitative real-time PCR and copy numbers are reported.
In addition to histological assessment of glycogen content, hepatic glycogen in liver biopsy samples was also quantified by MRS. As shown in Fig. 3D, 5 months after rAAV2/8 delivery, the glycogen peak was 56% less than glycogen levels seen in the animal that did not receive gene therapy, and at 6 months after rAAV2/1 treatment the levels of hepatic glycogen were reduced even more, to only 26% of levels in the non-gene therapy dog.
At 15 months after rAAV2/1 delivery, the dog continued to be maintained in the absence of supplemental dietary glucose; however, fasting studies did show some diminishment in metabolic correction as normal blood glucose and lactate levels could be maintained after only 4 hr of fasting (blood glucose, 61 mg/dl and blood lactate, 2.2 mmol/liter at 4 hr) and by 6 hr blood glucose levels were below normal (26 mg/dl) and lactate levels were elevated (2.9 mmol/liter).
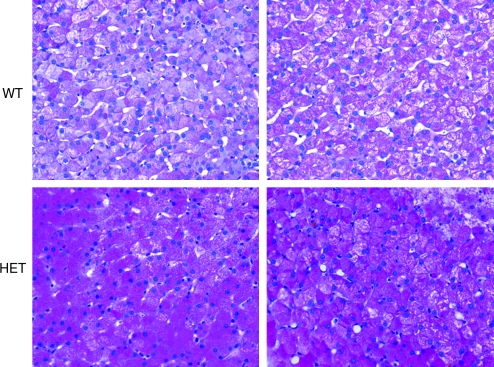
A curious finding in our studies was that heterozygous (carrier) dogs exhibited some subtle abnormal characteristics. Although the human carrier population does not exhibit any overt pathology or complications of GSDIa, parents of affected children do describe anecdotal accounts of symptoms that could be attributed to the disease, such as shakiness and hypoglycemia with fasting. As shown in Fig. 3A, carrier dogs have approximately 50% wild-type levels of G6Pase enzyme activity. In Fig. 2B, after a 12-hr fast, blood lactate levels are elevated as compared with those of wild-type dogs and are slightly above normal laboratory standards for dogs (carrier, n = 4; wild type, n = 5). As shown in Figs. 3D and 4, MRS data showed greater levels of hepatic glycogen (∼2.9 times) in the carrier dog as compared with the wild-type animal, which was also observed by PAS staining. Whether these findings also occur in the human population and whether there is any influence on metabolic function remain to be seen. However, these results do suggest that although 50% of WT G6Pase levels will result in an overall normal phenotype, these levels may not confer complete correction at the basic biochemical, histological, and physiologic levels in canines.
FIG. 4.
Heterozygous dogs have increased hepatic glycogen as compared with wild-type dogs. PAS staining of liver from two wild-type animals (top row; 1.4 and 3.9 years old, respectively) and two heterozygous animals (bottom row; 1.4 [littermate to WT animal directly above] and 4.7 years old).
In contrast to the previously reported gene therapy in GSDIa dogs, the dog reported in this paper achieved normal lactate concentrations after gene therapy. Koeberl and colleagues noted that although their dogs did show normal blood glucose levels after a 2-hr fast (longer time points were not presented), they exhibited high (significantly greater than 2.1 mmol/liter) blood lactate levels after a 2-hr fast, even at 1 month posttreatment (Koeberl et al., 2008). It is also of interest to note that the 2-hr fasting blood lactate levels in their control carrier animals were also high (>4 mmol/liter), above what is considered normal in dogs. Direct comparison of the absolute G6Pase activity between the studies is difficult as the activity data for the carrier animals between our study and those reported by Koeberl and colleagues differ by greater than 100-fold (64.9 μmol/mg/min vs. ∼8 μmol/g/min, respectively). However, these results also corroborate our findings that neonatal delivery of rAAV2/8 alone is not enough to confer complete correction of GSDIa and that carriers of GSDIa may not have completely normal pathophysiology. That notwithstanding, collectively these works do demonstrate that AAV-mediated gene therapy does result in significant improvements in the pathophysiological effects of GSDIa and that with modifications in the gene therapy strategy such as vector serotype, promoter used, and timing of vector delivery, these vectors hold great promise as a potential treatment for GSDIa.
Acknowledgments
The authors gratefully acknowledge the following groups: the University of Florida Powell Gene Therapy Center Vector Core Laboratory, which produced the rAAV vectors used in this study; the UF GSD Puppy Care Team and the University of Florida Animal Care Services veterinary staff for assistance in animal care; the University of Florida Molecular Pathology Core; and the University of Florida Powell Gene Therapy Center Toxicology Core. This work was supported by grants from the Children's Fund for Glycogen Storage Disease Research and the National Institutes of Health (NHLBI P01 HL59412-06, NIDDK P01 DK58327-03). Additional philanthropic support was provided by the Scott Miller Glycogen Storage Disease Program Fund and the Matthew Ehrman GSD Research Fund. B.J.B., Johns Hopkins University, and the University of Florida could be entitled to patent royalties for inventions described in this paper.
Author Disclosure Statement
No competing financial interests exist.
References
- Bhattacharya K. Orton R.C. Qi X. Mundy H. Morley D.W. Champion M.P. Eaton S. Tester R.F. Lee P.J. A novel starch for the treatment of glycogen storage diseases. J. Inherit. Metab. Dis. 2007;30:350–357. doi: 10.1007/s10545-007-0479-0. [DOI] [PubMed] [Google Scholar]
- Brix A.E. Howerth E.W. Conkie-Rosell A. Peterson D. Egnor D. Wells M.R. Chen Y.T. Glycogen storage disease type Ia in two littermate Maltese puppies. Vet. Pathol. 1995;32:460–465. doi: 10.1177/030098589503200502. [DOI] [PubMed] [Google Scholar]
- Chen Y.T. Burchell A. Glycogen storage diseases. In: Scriver C.R., editor; Sly W.S., editor; Valle D., editor. The Metabolic and Molecular Bases of Inherited Disease. 7th. McGraw-Hill; New York: 1995. pp. 905–934. [Google Scholar]
- Chen Y.T. Cornblath M. Sidbury J.B. Cornstarch therapy in type I glycogen-storage disease. N. Engl. J. Med. 1984;310:171–175. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- Chou J.Y. Matern D. Mansfield B.C. Chen Y.T. Type I glycogen storage diseases: Disorders of the glucose-6-phosphatase complex CHOU2002. Curr. Mol. Med. 2002;2:121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- Conlon T.J. Cossette T. Erger K. Choi Y.K. Clarke T. Scott-Jorgensen M. Song S. Campbell-Thompson M. Crawford J. Flotte T.R. Efficient hepatic delivery and expression from a recombinant adeno-associated virus 8 pseudotyped α1-antitrypsin vector. Mol. Ther. 2005;12:867–875. doi: 10.1016/j.ymthe.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Cunningham S.C. Dane A.P. Spinoulas A. Logan G.J. Alexander I.E. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol. Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- Cunningham S.C. Spinoulas A. Carpenter K.H. Wilcken B. Kuchel P.W. Alexander I.E. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spfash mice. Mol. Ther. 2009;17:1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J. Leonard J.V. Moses S.W. Odièvre M. Di Rocco M. Schaub J. Smit G.P. Ullrich K. Durand P. Glycogen storage disease: Recommendations for treatment. Eur. J. Pediatr. 1988;147:226–228. doi: 10.1007/BF00442683. [DOI] [PubMed] [Google Scholar]
- Ghosh A. Allamarvdasht M. Pan C.J. Sun M.S. Mansfield B.C. Byrne B.J. Chou J.Y. Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer. Gene Ther. 2006;13:321–329. doi: 10.1038/sj.gt.3302650. [DOI] [PubMed] [Google Scholar]
- Kim S.Y. Chen L.Y. Yiu W.H. Weinstein D.A. Chou J.Y. Neutrophilia and elevated serum cytokines are implicated in glycogen storage disease type Ia. FEBS Lett. 2007;581:3833–3838. doi: 10.1016/j.febslet.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani P.S. Bao Y. Wu J.Y. Brix A.E. Lin J.L. Chen Y.T. Isolation and nucleotide sequence of canine glucose-6-phosphatase mRNA: Identification of mutation in puppies with glycogen storage disease type Ia. Biochem. Mol. Med. 1997;61:168–177. doi: 10.1006/bmme.1997.2600. [DOI] [PubMed] [Google Scholar]
- Kishnani P.S. Faulkner E. Vancamp S. Jackson M. Brown T. Boney A. Koeberl D. Chen Y.T. Canine model and genomic structural organization of glycogen storage disease type Ia (GSD Ia) Vet. Pathol. 2001;38:83–91. doi: 10.1354/vp.38-1-83. [DOI] [PubMed] [Google Scholar]
- Koeberl D.D. Sun B.D. Damodaran T.V. Brown T. Millington D.S. Benjamin D.K., Jr. Bird A. Schneider A. Hillman S. Jackson M. Beaty R.M. Chen Y.T. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. doi: 10.1038/sj.gt.3302774. [DOI] [PubMed] [Google Scholar]
- Koeberl D.D. Kishnani P.S. Chen Y.T. Glycogen storage disease types I and II: Treatment updates. J. Inherit. Metab. Dis. 2007a;30:159–164. doi: 10.1007/s10545-007-0519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberl D.D. Sun B. Bird A. Chen Y. Oka K. Chan L. Efficacy of helper-dependent Adenovirus vector-mediated gene therapy in murine glycogen storage disease type Ia. Mol. Ther. 2007b;15:1253–1258. doi: 10.1038/sj.mt.6300188. [DOI] [PubMed] [Google Scholar]
- Koeberl D.D. Pinto C. Sun B. Li S. Kozink D.M. Benjamin D.K., Jr. Demaster A.K. Kruse M.A. Vaughn V. Hillman S. Bird A. Jackson M. Brown T. Kishnani P.S. Chen Y.T. AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol. Ther. 2008;16:665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- Lei K.J. Chen H. Pan C.J. Ward J.M. Mosinger B., Jr. Lee E.J. Westphal H. Mansfield B.C. Chou J.Y. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat. Genet. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- Mah C. Pacak C.A. Cresawn K.O. Deruisseau L.R. Germain S. Lewis M.A. Cloutier D.A. Fuller D.D. Byrne B.J. Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors. Mol. Ther. 2007;15:501–507. doi: 10.1038/sj.mt.6300100. [DOI] [PubMed] [Google Scholar]
- Mundy H.R. Hindmarsh P.C. Matthews D.R. Leonard J.V. Lee P.J. The regulation of growth in glycogen storage disease type 1. Clin. Endocrinol. 2003;58:332–339. doi: 10.1046/j.1365-2265.2003.01717.x. [DOI] [PubMed] [Google Scholar]
- Mundy H.R. Georgiadou P. Davies L.C. Cousins A. Leonard J.V. Lee P.J. Exercise capacity and biochemical profile during exercise in patients with glycogen storage disease type I. J. Clin. Endocrinol. Metab. 2005;90:2675–2680. doi: 10.1210/jc.2004-0890. [DOI] [PubMed] [Google Scholar]
- Ozen H. Glycogen storage diseases: New perspectives. World J. Gastroenterol. 2007;13:2541–2553. doi: 10.3748/wjg.v13.i18.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G.P. Berger R. Potasnick R. Moses S.W. Fernandes J. The dietary treatment of children with type I glycogen storage disease with slow release carbohydrate. Pediatr. Res. 1984;18:879–881. doi: 10.1203/00006450-198409000-00015. [DOI] [PubMed] [Google Scholar]
- Smit G.P. Ververs M.T. Belderok B. Van Rijn M. Berger R. Fernandes J. Complex carbohydrates in the dietary management of patients with glycogenosis caused by glucose-6-phosphatase deficiency. Am. J. Clin. Nutr. 1988;48:95–97. doi: 10.1093/ajcn/48.1.95. [DOI] [PubMed] [Google Scholar]
- Smit G.P. Fernandes J. Leonard J.V. Matthews E.E. Moses S.W. Odièvre M. Ullrich K. The long-term outcome of patients with glycogen storage diseases. J. Inherit. Metab. Dis. 1990;13:411–418. doi: 10.1007/BF01799498. [DOI] [PubMed] [Google Scholar]
- Sun M.S. Pan C.J. Shieh J.J. Ghosh A. Chen L.Y. Mansfield B.C. Ward J.M. Byrne B.J. Chou J.Y. Sustained hepatic and renal glucose-6-phosphatase expression corrects glycogen storage disease type Ia in mice. Hum. Mol. Genet. 2002;11:2155–2164. doi: 10.1093/hmg/11.18.2155. [DOI] [PubMed] [Google Scholar]
- Teutsch H.F. Chemomorphology of liver paraenchyma: Qualitative histochemical distribution patterns and quantitative sinusoidal profiles of G6Pase, G6PDH and malic enzyme activity and of glycogen content. Prog. Histochem. Cytochem. 1981;14:1–92. [PubMed] [Google Scholar]
- Wang Z. Zhu T. Qiao C. Zhou L. Wang B. Zhang J. Chen C. Li J. Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Wolfsdorf J.I. Weinstein D.A. Glycogen storage diseases. Rev. Endocr. Metab. Disord. 2003;4:95–102. doi: 10.1023/a:1021831621210. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S. Potter M. Zolotukhin I. Sakai Y. Loiler S. Fraites T.J., Jr. Chiodo V.A. Phillipsberg T. Muzyczka N. Hauswirth W.W. Flotte T.R. Byrne B.J. Snyder R.O. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]