In this report, transposition with the piggyBac system is used to introduce a chimeric antigen receptor (CAR) into T lymphocytes. The CD19-specific CAR is expressed long term in expanding T cell populations from its integrated locus and leads to CD19-dependent killing of tumor targets. Chromosomal analysis indicated no signs of genotoxicity.
Abstract
Nonviral integrating vectors can be used for expression of therapeutic genes. piggyBac (PB), a transposon/transposase system, has been used to efficiently generate induced pluripotent stems cells from somatic cells, without genetic alteration. In this paper, we apply PB transposition to express a chimeric antigen receptor (CAR) in primary human T cells. We demonstrate that T cells electroporated to introduce the PB transposon and transposase stably express CD19-specific CAR and when cultured on CD19+ artificial antigen-presenting cells, numerically expand in a CAR-dependent manner, display a phenotype associated with both memory and effector T cell populations, and exhibit CD19-dependent killing of tumor targets. Integration of the PB transposon expressing CAR was not associated with genotoxicity, based on chromosome analysis. PB transposition for generating human T cells with redirected specificity to a desired target such as CD19 is a new genetic approach with therapeutic implications.
Introduction
Tcells can be genetically modified to redirect specificity through the introduction of full-length αβ T cell receptors, which recognize antigen in the context of major histocompatibility complex (MHC) or through the introduction of chimeric antigen receptors (CARs) to recognize cell surface antigen independent of MHC (Rossig and Brenner, 2003; Biagi et al., 2007). Approaches to introduce CARs are viral (transduction with retrovirus/lentivirus) (Zanzonico et al., 2006; Lu et al., 2007) or nonviral using DNA plasmids (Fewell et al., 2005; Schmieder et al., 2007; Schertzer and Lynch, 2008) or mRNA (Smits et al., 2004; Van Tendeloo et al., 2007; Wiehe et al., 2007). Electrotransfer of DNA plasmids has been adapted for clinical trials to introduce CAR transgenes into primary T cells (Cooper et al., 2003; Gonzalez et al., 2004; Jensen, 2007; Park et al., 2007). However, the integration efficiency of introduced naked DNA plasmids is low, resulting in lengthy periods of ex vivo culturing under selection pressure to recover T cells expressing stable CAR integrants. We and others have reported that the Sleeping Beauty (SB) transposon/transposase could be used to improve the efficiency of gene transfer to express CAR and α/β T cell receptor in T-cells (Huang et al., 2008; Singh et al., 2008; Jones et al., 2009) and that this system may be adapted for clinical trials (Williams, 2008; Xue et al., 2009).
We now extend these observations to demonstrate that an alternative transposon/transposase system, namely piggyBac (PB), can also be used to introduce a CAR to redirect T-cell specificity for CD19 expressed on malignant (and normal) B cells. The PB transposon, derived from the cabbage looper moth Trichoplusia ni, was originally identified in the genome of baculovirus-infected insect cells, giving rise to the name piggyBac (Cary et al., 1989; Fraser et al., 1995, 1996). The original PB element was approximately 2.4 kb with identical 13-base pair (bp) terminal inverted repeats and additional asymmetric 19-bp internal repeats (Elick et al., 1997; Li et al., 2001, 2005). PB is typically thought to mediate precise excision of transposon segments in mouse (Ding et al., 2005) and human cells through a cut-and-paste mechanism, resulting in complementary TTAA overhangs on the ends of the donor DNA and ligation of these ends to restore the donor site to its pretransposon sequence (Cary et al., 1989; Ding et al., 2005; Fraser et al., 1995; Wu et al., 2006; Wilson et al., 2007; Mitra et al., 2008). PB has been used as a vector for reprogramming murine and human embryonic fibroblasts (Woltjen et al., 2009), and for introduction of the reprogramming factor Klf4 into murine epistem cells (Guo et al., 2009).
To evaluate the capability of PB as a vector for application in gene therapy we generated primary human T cells with redirected specificity for CD19, using the PB transposon/transposase system. We constructed a PB transposon expressing a second-generation CD19-specific CAR designated CD19RCD28. We demonstrate that electroporation of primary human T cells with this PB transposon plasmid in the presence of codon-optimized PB transposase resulted in efficient integration of the CAR transgene, and numeric expansion of the CD19 CAR+ T cells to clinically significant numbers could be readily achieved by recursive propagation on γ-irradiated K562-derived designer artificial antigen-presenting cells (aAPCs).
Materials and Methods
Plasmids
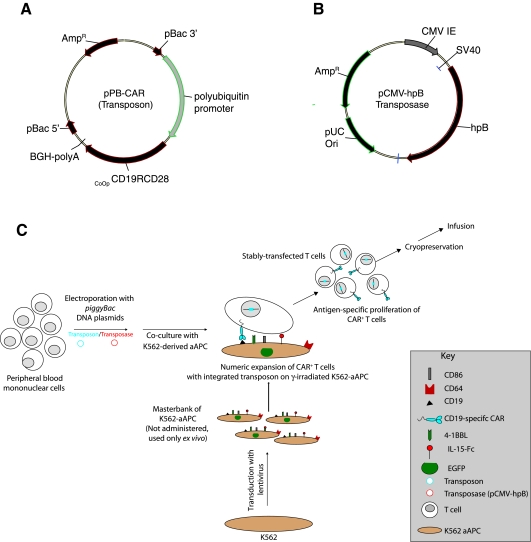
The donor plasmid pXLBacIIPUbnlsEGFP (Wu et al., 2006), derived from pBSII-ITR1 (Li et al., 2005), was a kind gift from J. Kaminski (Medical College of Georgia, Augusta, GA); it is a minimal PB vector with terminal repeats of 308 and 238 bp at the 5′ and 3′ ends, respectively. The codon-optimized second-generation CD19RCD28 (CoOpCD19RCD28) CAR (Singh et al., 2008) was subcloned into the pXLBacIIPUbnlsEGFP vector by replacing the enhanced green fluorescent protein (EGFP) sequence with the CAR sequence to create CoOpCD19RCD28/pXLBacIIUbnls (pPB-CAR) (Fig. 1A). The PB transposase was also codon optimized for expression in human cells (GenScript, Piscataway, NJ) and modified to include a 5′ SacII restriction site immediately upstream of a strong Kozak initiation signal and a 3′ PsiI restriction site after the stop codon. SacII/PsiI-digested CoOp piggyBac transposase (hpB) was then subcloned into SacII/PsiI-digested pCMV-piggyBac as described elsewhere (Wilson et al., 2007) to create pCMV-hpB (Fig. 1B).
FIG. 1.
Schematic of the two PB DNA plasmids electrotransferred. (A) CoOpCD19RCD28/pXLBacIIUbnls (pPB-CAR, Transposon): polyubiquitin promoter; CoOpCD19RCD28, codon-optimized CD19RCD28 CAR; pBac3′ and pBac5′, PB-inverted/direct repeats; BGH-polyA, polyadenylation signal from bovine growth hormone; AmpR, ampicillin resistance gene. (B) pCMV-hpB (Transposase): hpB, codon-optimized PB-transposase; CMV IE, CMV enhancer/promoter; pUC ori, minimal E. coli origin of replication. (C) Scheme for electroporation with PB plasmids and propagation on CD19+ K562-derived artificial antigen-presenting cells (aAPCs). Electroporation with transposon (blue) provides only transient expression unless incorporated into a transposon vector that can be cleaved from the plasmid and integrated into a host genome by a source of transposase (red). On the day after electroporation, T cells are cocultured with γ-irradiated K562 genetically modified to coexpress CD19, CD64, CD86, CD137L (4-1BBL), and cell surface membrane-bound IL-15 (fusion of IL-15 cytokine peptide and human Fc region), with the addition of IL-2, resulting in expansion of stably transfected CAR+ T cells to clinically significant numbers.
Cell lines and primary human T cells
Daudi cells (human Burkitt's lymphoma cell line; cat. no. CCL-213) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The GFP+ U251T glioblastoma cell line (a kind gift from W. Debinski, Wake Forest University, Winston-Salem, NC) was transfected with the ΔCD19/pSBSO vector and stable transfectants expressing truncated CD19 (Serrano et al., 2006) were established. Both GFP+ U251T cells and CD19+GFP+ U251T cells (transfected to express truncated CD19) cells, were cultured in Dulbecco's modified Eagle's medium (Hyclone, Logan, UT) supplemented with 2 mM GlutaMAX-1 (GIBCO; Invitrogen, Carlsbad, CA) and 10% heat-inactivated fetal calf serum (FCS). Human T cells were isolated by density gradient centrifugation over Ficoll-Paque PLUS (GE Healthcare Biosciences, Uppsala, Sweden), from peripheral blood obtained from the Gulf Coast Regional Blood Center (Houston, TX) after consent had been obtained.
Artificial antigen-presenting cells
K562 cells transduced with lentivirus to coexpress CD19, CD64, CD86, CD137 ligand (CD137L), and membrane-bound interleukin (IL)-15 (coexpressed with GFP), referred to as clone 4 (Fig. 1C), were kindly provided by C. June (University of Pennsylvania, Philadelphia, PA) and used as artificial antigen-presenting cells (aAPCs) for in vitro expansion of genetically modified T cells in culture medium.
Electroporation of T cells and selective outgrowth of CAR+ T cells
On day 0 of a culture cycle, 107 mononuclear cells from peripheral blood were resuspended in 100 μL of Amaxa Nucleofector solution (human CD34+ cell Nucleofector kit, cat. no. VPA-1003; Lonza, Basel, Switzerland), mixed with 15 μg of supercoiled plasmids pPB-CAR and pCMV-hpB (7.5 μg each), transferred to a cuvette, electroporated (Program U-14), and cultured overnight as described earlier (Singh et al., 2008). The next day (day 1) the cells were stimulated with γ-irradiated (100 Gy) K562-aAPCs (clone 4) at a 1:1 ratio of T cells to aAPCs. The γ-irradiated aAPCs were re-added every 7 days at a 1:1 ratio of T cells to aAPCs. Recombinant human IL-2 (rhIL-2; Chiron, Emeryville, CA) was added to the cultures at 50 U/mL on a Monday–Wednesday–Friday schedule beginning on day 1 of each 7-day T cell expansion cycle. T cells were enumerated every 7 days and viable cells were counted on the basis of trypan blue exclusion.
Flow cytometry
Fluorochrome-conjugated reagents were obtained from BD Biosciences (San Jose, CA) unless otherwise indicated: peridinin chlorophyll protein–cyanine 5.5 (PerCP–Cy5.5)-conjugated anti-human CD4 (cat no. 341654), allophycocyanin (APC)-conjugated anti-human CD8 (cat. no. 555369), phycoerythrin (PE)-conjugated anti-human CD27 (cat. no. 555441), PerCP–Cy5.5-conjugated anti-human CD28 (cat. no. 337181), APC-conjugated anti-human CD62L (cat. no. 559772), and PE-conjugated anti-human CCR7 (cat. no. FAB197P; R&D Systems, Minneapolis, MN). R-phycoerythrin-conjugated goat F(ab′)2 anti-human IgG(γ) (cat. no. H10104; Caltag Laboratories/Invitrogen, Burlingame, CA) or fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-human IgG(γ) (cat. no. 109-096-170; Jackson ImmunoResearch Laboratories, West Grove, PA) was used at 1:20 dilution to detect cell surface expression of the CD19-specific CAR, CD19RCD28. Blocking of nonspecific antibody binding was achieved with FACS wash buffer (2% fetal bovine serum [FBS] in phosphate-buffered saline [PBS]). Data acquisition was done with a FACSCalibur (BD Biosciences) using CellQuest version 3.3 (BD Biosciences). Analyses and calculation of median fluorescence intensity (MFI) was undertaken with FlowJo version 7.2.2 (TreeStar, Ashland, OR).
Chromium release assay
The cytolytic activity of T cells was determined in a 4-hr chromium release assay (CRA) (Cooper et al., 2003). CD19-specific T cells were incubated with 5 × 103 51Cr-labeled target cells in a V-bottomed 96-well plate (Costar; Corning Life Sciences, Lowell, MA). The percentage of specific cytolysis was calculated from the release of 51Cr, as described earlier, using a TopCount NXT (PerkinElmer Life and Analytical Sciences, Waltham, MA). Data are reported as means ± standard deviation (SD).
Video time-lapse microscopy
To visualize killing of tumor targets by PB-modified CD19-specific T cells, we undertook imaging by video time-lapse microscopy (VTLM), using a BioStation IM Cell-S1/Cell-S1-P system (Nikon, Melville, NY). U251T cells were chosen as targets on the basis of an ability to identify living and dying/dead cells by phase-contrast dynamic morphology (Serrano et al., 2006). Parental GFP+ U251T cells (green) were used as CD19– targets whereas CD19+GFP+ U251T cells (transfected to express truncated CD19), stained according to the manufacturer's protocol with PKH-26 red fluorescent dye (cat. no. MINI26; Sigma-Aldrich, St. Louis, MO), which fluoresced orange (green plus red), were used as CD19+ targets. CD19– and CD19+ U251T targets were mixed at a 1:1 ratio (0.25 × 106 cells per target) and plated overnight on a T-35 mm glass bottom plate (Fisher Scientific, Hampton, NH) in culture medium. PB-modified CD19RCD28+ T cells (0.2 × 106 in 200 μL of culture medium) were added to the adherent U251T targets and were immediately imaged every 200 sec at 37°C for up to 4 hr. Each image was recorded at 1600 × 1200 pixels with a × 20 objective, using a phase-contrast along with fluorescence channel 1 to observe orange CD19+GFP+ U251T cells and fluorescence channel 2 to observe green CD19–GFP+ U251T cells with an exposure time of 1/125 and 1/5 sec, respectively. Adherent live U251T cells appear flat and spread out whereas dying cells round up and implode. Movies (available at www.liebertonline.com/hum) showing the killing events were made with Microsoft Windows Movie Maker software, version 5.1 (Microsoft, Redmond, WA).
Automated cell counting
Automated cell counting was accomplished with a Cellometer (Nexcelom Bioscience, Lawrence, MA). A T-cell suspension (20 μL) and 0.2% trypan blue were mixed at a 1:1 ratio and 20 μL was loaded onto a disposable counting chamber and inserted into the Cellometer to automatically obtain concentration and live and dead cell counts. Data and images were saved and analyzed.
DNA polymerase chain reaction for PB transposase
Polymerase chain reaction (PCR) over 30 cycles with DNA isolated from PB-modified and expanded T cell cultures, using PB transposase-specific primers 5′-ACGAGCACATCCTGTCTGCTCTGCTGCAG-3′ and 5′- ACATATCGATGTTGTGCTCCCGGCAGAT-3′, was carried out in a thermal cycler (PTC-200 DNA engine cycler; Bio-Rad, Hercules, CA). The housekeeping gene GAPDH, encoding glyceraldehyde-3-phosphate dehydrogenase, was also amplified in the same samples, using forward primer 5′-TCTCCAGAACATCATCCCTGCCAC-3′ (80 ng/μL) and reverse primer 5′-TGGGCCATGAGGTCCACCACCCTG-3′ (80 ng/μL). The PCR products were separated on a 0.8% agarose gel, using 4 μL of each sample per lane. The gel was stained with ethidium bromide (0.1 mg/mL), destained with distilled water, and visualized with a VersaDoc 4000 gel documentation system (Bio-Rad).
Fluorescence in situ hybridization
Exponentially growing genetically modified T cells (5 × 106) were harvested after 21 days of coculture on aAPCs and incubated with demecolcine (0.04 μg/mL; GIBCO-BRL/Invitrogen, Grand Island, NY) for 45 min at 37°C. The treated cells were centrifuged and exposed to 75 mM KCl for 20 min, after which they were fixed in a methanol–acetic acid mixture (3:1), washed three times with the fixative, and dropped on glass slides for air drying. CD19RCD28-specific DNA probe was labeled by nick translation with Spectrum green (Vysis/Abbott Molecular, Des Plaines, IL). Hybridization with the fixed T cells was performed according to the manufacturer's protocol. The slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and the images were captured with a Quips Pathvysion System; (Applied Imaging, Santa Clara, CA). To determine the number of integrants, 40 to 50 individual metaphase spreads were analyzed.
Chromosome banding analysis
Exponentially growing PB-modified T cells cultures were incubated for 2 hr at 37°C with colcemid (20 μL, 0.04 μg/mL) per 10 mL of culture medium followed by KCl (0.075 mol/liter) at room temperature for 15 min, fixed with acetic acid–methanol (1:3), and washed three times on a glass slide. For Giemsa banding, slides treated with trypsin were stained with Giemsa stain according to standard techniques described previously (Singh et al., 2008). Ten Giemsa-banded metaphases were photographed and 5 complete karyotypes were prepared with a karyotyping system from Applied Imaging.
Results
PB-mediated gene transfer and selected propagation of CAR+ T cells
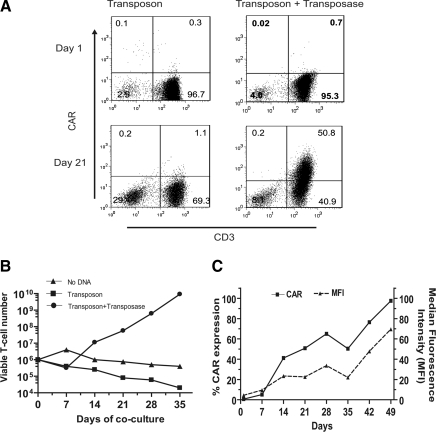
To evaluate whether the PB system can render primary human T cells specific for CD19, peripheral blood mononuclear cells (PBMCs, containing quiescent T cells) were electroporated with pPB-CAR (to express CAR transposon; Fig. 1A) in the absence and presence (in trans) of pCMV-hpB (to express transposase) (Fig. 1B). After electrotransfer the T cells expressing CAR were propagated on γ-irradiated K562-aAPCs expressing CD19 antigen and the desired costimulatory molecules CD86, CD137L, and membrane-bound IL-15 (Fig. 1C). After 21 days of coculture on aAPCs, CD3+ T cells expressing CAR increased to 50% (∼70-fold improvement in CAR expression) in cultures electroporated with both PB transposon and transposase, whereas the CAR expression remained undetectable on T cells electroporated with transposon alone (∼1%) (Fig. 2A). These data are consistent with the PB transposase improving gene transfer efficiency such that the CAR+ T cells could be selectively propagated on recursive additions of aAPCs. At the end of 3 weeks, 106 T cells modified with transposon and transposase had increased by 56-fold and the CAR+ T cells continued to numerically expand thereafter when cultured on aAPCs (Fig. 2B). The outgrowth of CAR+ T cells resulted in 97% of cells expressing CD19RCD28, with a density (MFI) peaking at 70 arbitrary units by day 49 (Fig. 2C).
FIG. 2.
CAR expression on T cells after electrotransfer of PB vector(s) and selected outgrowth of CAR+ T cells upon coculture with aAPCs. (A) Expression of CD19RCD28 CAR on CD3+ T cells by flow cytometry with anti-Fc antibody after electrotransfer of PB transposon with or without PB transposase at 24 hr and 3 weeks of coculture on γ-irradiated K562-derived aAPCs (clone 4). (B) Kinetics of T cell growth on coculture with aAPCs. (C) CAR expression over time. Percentage expression of CAR and MFI (surrogate for density) on T cells cotransfected with PB transposon and transposase upon coculture with K562-aAPCs.
Redirected function of CAR+ T cells after electrotransfer of PB plasmids
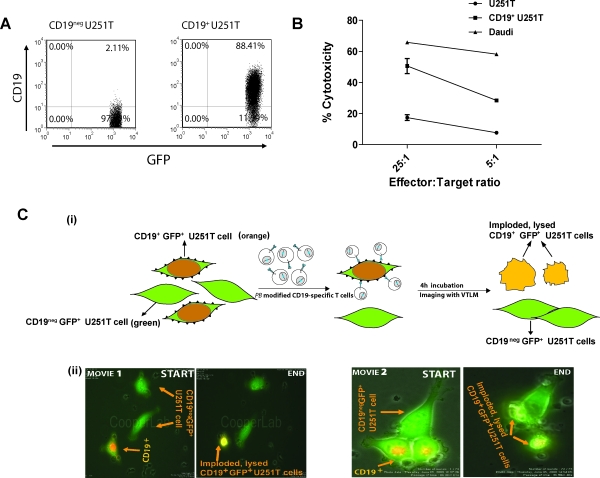
The genetically modified and numerically expanded T cells were evaluated for redirected killing of CD19– (parental) and CD19+ U251T (transfected) (Fig. 3A) tumor targets. Their specificity of killing was revealed by a 3-fold increase in lysis of CD19+ U251T cells (51% specific lysis) over background lysis of CD19– U251T cells at an effector-to-target ratio of 25:1, as shown in Fig. 3B. Further, the genetically modified T cells also demonstrated redirected cytotoxicity against CD19+ human Burkitt's lymphoma Daudi cells (65% at a 25:1 effector-to-target ratio; Fig. 3B), confirming their redirected ability to target B cell lymphomas.
FIG. 3.
Redirected specificity of PBMCs genetically modified with the PB system. (A) GFP+ U251T targets were transfected with truncated CD19-expressing plasmid and stable transfectants were analyzed for CD19 expression by flow cytometry. (B) Killing of CD19+ target cells (CD19-expressing human Burkitt's lymphoma or Daudi cells, U251T CD19– glioblastoma cells, and U251T cells transfected to express truncated CD19) in a standard 4-hr CRA. Points represent mean specific lysis of triplicate wells at two effector-to-target (E:T) cell ratios; error bars represent the SD. (C) VTLM to evaluate tumor killing by PB-modified CAR+ T cells. (i) To distinguish GFP+CD19+ from GFP+CD19– U251T cells, the red fluorescent dye PHK-26 was preloaded onto CD19+ target cells, which resulted in cells appearing orange (a merging of GFP [green] with PHK [red]). The CD19-negative and -positive targets mixed at a 1:1 ratio were plated overnight. PB-modified CAR+ T cells were added to these targets after overnight plating at an E:T ratio of 10:1. Cells were cocultured for 4 hr and imaged by VTLM. CD19+ tumor targets, which were engaged, disengaged, and killed by the T cells, imploded and lysed and are shown as greenish-yellow irregular cells, whereas live CD19– tumor targets remained flat and spread out (green). (ii) Two movies, one at low power (movie 1) and one at high power (movie 2), show tumor cell killing by PB-modified CAR+ T cells. In each case the killing events measured over 2 hr were condensed to 12–14 sec for visualization.
Visualization of CD19+ tumor cell killing by genetically modified T cells
We employed VTLM to directly visualize killing of CD19+ U251T tumor cells by genetically modified CAR+ T cells. The CD19– parental and CD19+ transfected U251T targets were admixed at a ratio of 1:1 before adding PB-modified CD19-specific T cells and killing was directly visualized over 4 hr to reveal the engagement/disengagement of T cells (small, irregular bodies shown moving across image frames) to adherent spindle-shaped green U251T tumor cells. After contact with the genetically modified T cells, the CD19+ U251T orange tumor cells were observed to round up and implode whereas the CD19– green U251T tumor cells did not (Fig. 3C, panel i). Two movies (supplementary file video 1 and supplementary file video 2), representing killing events, each over 4 hr of imaging, are available at www.liebertonline.com/hum for viewing (Fig. 3C, panel ii). These microscopy data validate the CRA experiments and show that the PB-modified CD19+ T cells are redirected to specifically lyse CD19+ tumor cells.
Memory and effector phenotype of PB-modified CAR+ T cells
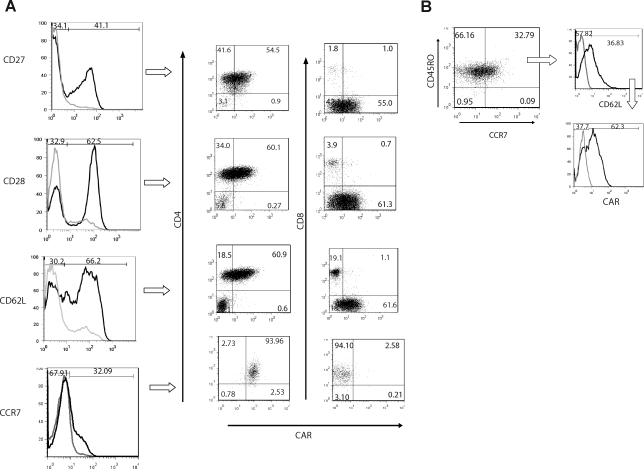
It is recognized from human trials and experiments with nonhuman primates and mice that adoptive transfer of central memory (CM) T cells can lead to long-lived immune response (Sallusto et al., 1999; Berger et al., 2008; Rolle et al., 2008). Therefore, flow cytometry was used to investigate the detection of cell surface markers on T cells associated with CM after PB transposition and propagation. We demonstrated that numerically expanded PB-modified CAR+ T cells expressed both CM markers (Sallusto et al., 1999; Ochsenbein et al., 2004; Bachmann et al., 2005) and determinants of the effector memory (EM) phenotype (Fig. 4A). Analysis of CD45RO+CCR7+ T cells (mostly CD4+CAR+ T cells) revealed that 36% expressed CD62L, defining them as TCM phenotype (Fig. 4B), and that a further 62% of the TCM cells were CAR+. CD4+ and CD8+ T cells expressing CAR also expressed CD27, CD28, and CD62L, which is also consistent with preservation of the memory cell phenotype. These data demonstrate that the combination of electrotransfer of the PB system and aAPCs can be used to propagate populations of CAR+ T cells with a phenotype predictive of long-term human engraftment.
FIG. 4.
Characterization of CAR+ T cells on PBMCs after electrotransfer of PB vectors. (A) Immunophenotype of memory cell markers (CD27, CD28, CD62L, and CCR7) on PB-modified T cells generated after 4 weeks of coculture on aAPCs. Histograms presented as solid black lines reveal the percentage of T cells expressing CD27, CD28, CD62L, and CCR7 in the lymphocyte-gated population. T cells expressing the memory cell markers were analyzed for coexpression of CAR and CD4 or CD8. (B) The central memory phenotype (TCM) of T cells generated after coculture. CD45RO and CCR7 double-positive T cells were analyzed for the expression of CD62L. In addition, TCM cells, defined as CD45RO+CCR7+CD62L+, were analyzed for coexpression of CAR.
Lack of autonomous proliferation of T cells
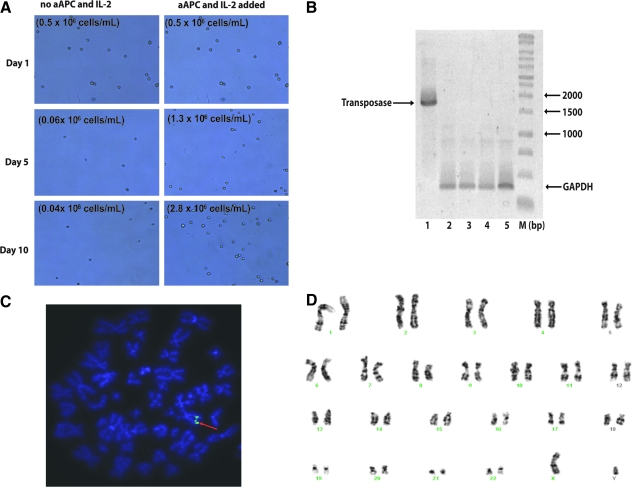
Gene transfer with the PB system may cause genotoxicity and the potential for aberrant T-cell growth. Therefore we cultured T cells in the absence/presence of K562-aAPCs and cytokine (IL-2, 50 U/mL) and demonstrated that the PB-modified CD19-specific T cells survive and sustain proliferation only in the presence of K562-aAPCs and IL-2 (Fig. 5A).
FIG. 5.
Lack of autonomous proliferation after electrotransfer with PB vectors and safety issues. (A) T-cell proliferation analyses directly imaged with a Cellometer in the absence/presence of K562-aAPCs and IL-2. Data show primarily dead T cells (shriveled) when K562-aAPCs and IL-2 are removed compared with healthy (refractile, rounded) T cells when K562-aAPCs and IL-2 are present. (B) Lack of integration of PB transposase by genomic PCR from genetically modified and propagated peripheral blood-derived T cells. DNA was isolated from T cells after mock electroporation (lanes 2 and 4, 50 and 100 ng of genomic DNA, respectively), from T cells 28 days after electroporation with the two-plasmid PB system (lanes 3 and 5, 50 and 100 ng of genomic DNA, respectively). Lane 1, pCMV-hpB plasmid DNA (1 ng) loaded as a positive control. PCR was carried out with transposase-specific primers and GAPDH-specific primers in the same reaction. (C) Fluorescence in situ hybridization (FISH) analysis of PB-modified CAR+ T cells. Number of copies of the CD19RCD28 transgene integrated on electroporation with PB vectors and propagation on CD19-specific K562-derived aAPCs was determined by FISH analysis as described in Materials and Methods. Data shown are a representation after analyzing 40–50 individual metaphase spreads. Twenty-three pairs of chromosomes are shown and the arrow indicates the integration sites. (D) Idiogram of a Giemsa-banded karyotype of PB-modified T cells, showing no apparent numerical or structural chromosome alterations.
Lack of long-term expression of PB transposase
The continued presence of transposase in PB-modified T cells may lead to genotoxicity. Therefore, we undertook genomic PCR analysis to evaluate for the continued presence of the codon-optimized PB transposase. Using T cells that had been electroporated with PB transposon and transposase and had undergone 5 weeks of coculture with K562-aAPCs, we could not detect the PB transposase gene (size, ∼1750 bp) (Fig. 5B). These results indicate that the PB transposase was not appreciably integrated into the genome of T cells expressing the CD19RCD28 CAR.
Number of copies of integrated transposon by fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was performed to assess the copy number of the integrated CAR transgene after electrotransfer of the PB system and numeric expansion of T cells for 4 weeks on K562-aAPCs. The PB-modified CAR+ T cells were observed to carry only one copy of the CD19RCD28 transgene per cell (Fig. 5C). These results are comparable to those observed with CAR+ T cells modified with the SB transposon/transposase system (our unpublished data).
Karyotype of genetically modified T cells
The overall integrity of the chromosome structure was evaluated as a measure of global genotoxicity associated with undesired and continued transposition. Giemsa-banding analysis of the PB-transfected T cells showed a normal male karyotype, 46 XY, with no apparent significant numerical or structural chromosome alterations (Fig. 5D). These data support the premise that PB transposition in human T cells is not associated with major translocations and chromosomal aberrations, although the possibility of chromosomal damage below the limit of detection of this technique cannot be excluded.
Discussion
To obtain preclinical data for nonviral gene transfer by PB transposon/transposase system in gene therapy trials we genetically modified primary human T cells with a codon-optimized CD19-specific second-generation CAR. Our data demonstrate for the first time that the PB system can be electrotransferred into human T cells to express a desired CAR. The efficient integration efficiency of PB was confirmed by the stable expression of CD19-specific CAR within 3 weeks of coculture on K562-aAPCs and IL-2 of human T cells coelectroporated with pPB-CAR (transposon) and pCMV-hpB (transposase), compared with cells electroporated with transposon alone. In the present study, we observed that the majority of CAR+ T cells were CD4+, raising a concern about their ability to participate in an antitumor response in vivo. However, published results indicate that in addition to a “helper” role, adoptively transferred CD4+ T cells can also eliminate cancer cells in vivo in the absence of CD8+ T cells (Mumberg et al., 1999; Lundin et al., 2003; Corthay et al., 2005; Liu et al., 2008).
Electrotransfer with PB plasmids and subsequent CAR-mediated propagation on aAPCs supported proliferation of memory T cells, in particular TCM and TEM with associated desired phenotypes, as subsets of the CAR+ T cells maintained expression of CD27, CD28, CD45RO, CD62L, and CCR7. These data have implications for improved in vivo efficacy as antigen-specific TCM cell subsets are associated with long-term persistence after adoptive transfer in macaques (Berger et al., 2008). It is not currently known whether adoptive transfer of CAR+ T cells enriched for TCM will provide superior protective immunity against human cancer.
There are at least two potential advantages for electrotransfer of the PB transposon system compared with virus-mediated transduction for generation of T cells for clinical application. One study found that the PB system had decreased integration frequency into or within 50 kb of the transcriptional start sites of known proto-oncogenes in comparison with what has been reported for gammaretroviral and human lentiviral vectors (Galvan et al., 2009). In addition, nonviral therapies using DNA are less expensive to produce and thus may be more widely applicable for gene transfer compared with the use of clinical-grade recombinant viruses. However, there are trade-offs to electrotransfer of plasmids compared with transduction, such as potentially reduced integration frequency.
There is also a choice of which transposon/transposase system to use for genetic modification of therapeutic T cells. It has been shown that an engineered PB transposon with minimal length 5′ and 3′ terminal repeats exhibited greater transposition activity in transfected cultured human cells compared with an SB system (Wilson et al., 2007) and creation of hyperactive PB transposase elements may further increase integration efficiency. The ability of PB to transpose large cassettes efficiently could be exploited in gene therapy trials in which expression of a large transgene, or coexpression of more than one therapeutic transgene, is necessary. The PB transposase might also be amenable to modification to improve targeted integration events, for it has been shown that PB transposase coupled to the GAL4 DNA-binding domain retains transposition activity whereas similarly manipulated transposases of Tol2 and SB11 were inactive (Wu et al., 2006). To improve the utility of a PB-derived transposase to recognize human sequences, the transposase may be modified to achieve targeted integration, such as by addition of a zinc finger DNA-binding domain (Maragathavally et al., 2006; Wu et al., 2006; Cadinanos and Bradley, 2007; Wilson et al., 2007). Regulation of PB activity by an inducible PB system may further provide safety in clinical trials (Cadinanos and Bradley, 2007).
There are also potential obstacles in using the PB transposon/transposase system for therapeutic gene transfer. Any integrating element carries with it the potential risk of genotoxicity. Like some other transposable elements, there are domesticated PB-like elements within the human genome (Sarkar et al., 2003; Newman et al., 2008). Although the protein and DNA sequences of these elements are different from those of the PB system used in this study, how these elements may affect PB activity or how PB may alter these elements within the human genome will need to be addressed regarding clinical utility. Our initial results in addressing the safety of the PB system for genetic modification of T cells for cancer therapy showed (1) no evidence of unwanted autonomous proliferation after gene transfer; (2) no expression of the PB transposase after propagation on aAPCs, which will limit potential ongoing transposition due to the continued presence of PB transposase; and (3) a normal karyotype after PB transposition. Ultimately, a suicide gene could be used in combination with the therapeutic gene(s) of interest in PB applications for conditional removal of cells that underwent gene transfer in vivo.
The simplicity of our gene therapy approach, namely, electroporating T cells with two DNA plasmids and selectively expanding CAR+ T cells, including TCM, on γ-irradiated K562 aAPCs, lends impetus to the development of clinical-grade CAR+ T cells using the PB system for application in human immunotherapy trials.
Supplementary Material
Acknowledgments
The authors thank the M.D. Anderson Cancer Center (MDACC) Flow Cytometry Core Laboratory (NIH grant 5P30CA016672-32) and the MDACC Molecular Cytogenetics Core. This work was supported by Cancer Center Support Grant CA16672, R01 (CA124782 and CA120956), R21 (CA129390 and CA116127), the Department of Defense (PR064229), Alex's Lemonade Stand Foundation, the Alliance for Cancer Gene Therapy, the Burroughs Wellcome Fund, a Department of Veterans Affairs career development award, the Gillson Longenbaugh Foundation, the Leukemia and Lymphoma Society, the Lymphoma Research Foundation, the Miller Foundation, the National Foundation for Cancer Research, the Pediatric Cancer Research Foundation, the National Marrow Donor Program, and the William Lawrence and Blanche Hughes Foundation.
Author Disclosure Statement
The authors state that no competing financial interests exist.
References
- Bachmann M.F. Wolint P. Schwarz K. Jager P. Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor α and CD62L. J. Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- Berger C. Jensen M.C. Lansdorp P.M. Gough M. Elliott C. Riddell S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E. Marin V. Giordano Attianese G.M. Dander E. D'Amico G. Biondi A. Chimeric T-cell receptors: New challenges for targeted immunotherapy in hematologic malignancies. Haematologica. 2007;92:381–388. doi: 10.3324/haematol.10873. [DOI] [PubMed] [Google Scholar]
- Cadinanos J. Bradley A. Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 2007;35:e87. doi: 10.1093/nar/gkm446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary L.C. Goebel M. Corsaro B.G. Wang H.G. Rosen E. Fraser M.J. Transposon mutagenesis of baculoviruses: Analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology. 1989;172:156–169. doi: 10.1016/0042-6822(89)90117-7. [DOI] [PubMed] [Google Scholar]
- Cooper L.J. Topp M.S. Serrano L.M. Gonzalez S. Chang W.C. Naranjo A. Wright C. Popplewell L. Raubitschek A. Forman S.J. Jensen M.C. T-cell clones can be rendered specific for CD19: Toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- Corthay A. Skovseth D.K. Lundin K.U. Rosjo E. Omholt H. Hofgaard P.O. Haraldsen G. Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ding S. Wu X. Li G. Han M. Zhuang Y. Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Elick T.A. Lobo N. Fraser M.J., Jr Analysis of the cis-acting DNA elements required for piggyBac transposable element excision. Mol. Gen. Genet. 1997;255:605–610. doi: 10.1007/s004380050534. [DOI] [PubMed] [Google Scholar]
- Fewell J.G. Matar M. Slobodkin G. Han S.O. Rice J. Hovanes B. Lewis D.H. Anwer K. Synthesis and application of a non-viral gene delivery system for immunogene therapy of cancer. J. Control. Release. 2005;109:288–298. doi: 10.1016/j.jconrel.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Fraser M.J. Cary L. Boonvisudhi K. Wang H.G. Assay for movement of lepidopteran transposon IFP2 in insect cells using a baculovirus genome as a target DNA. Virology. 1995;211:397–407. doi: 10.1006/viro.1995.1422. [DOI] [PubMed] [Google Scholar]
- Fraser M.J. Ciszczon T. Elick T. Bauser C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol. Biol. 1996;5:141–151. doi: 10.1111/j.1365-2583.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Galvan D.L. Nakazawa Y. Kaja A. Kettlun C. Cooper L.J. Rooney C.M. Wilson M.H. Genome-wide mapping of PiggyBac transposon integrations in primary human T cells. J. Immunother. 2009;32:837–844. doi: 10.1097/CJI.0b013e3181b2914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S. Naranjo A. Serrano L.M. Chang W.C. Wright C.L. Jensen M.C. Genetic engineering of cytolytic T lymphocytes for adoptive T-cell therapy of neuroblastoma. J. Gene Med. 2004;6:704–711. doi: 10.1002/jgm.489. [DOI] [PubMed] [Google Scholar]
- Guo G. Yang J. Nichols J. Hall J.S. Eyres I. Mansfield W. Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. Guo H. Kang J. Choi S. Zhou T.C. Tammana S. Lees C.J. Li Z.Z. Milone M. Levine B.L. Tolar J. June C.H. McIvor R.S. Wagner J.E. Blazar B.R. Zhou X. Sleeping Beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol. Ther. 2008;16:580–589. doi: 10.1038/sj.mt.6300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.C. Popplewell L. DiGiusto D.L. Kalos M. Cooper L.J.N. Raubitschek A. Forman S.J. A first-in-human clinical trial of adoptive therapy using CD19-specific chimeric antigen receptor re-directed T cells for recurrent/refractory follicular lymphoma. Mol. Ther. 2007;15:S142. [Google Scholar]
- Jones S. Peng P.D. Yang S. Hsu C. Cohen C.J. Zhao Y. Abad J. Zheng Z. Rosenberg S.A. Morgan R.A. Lentiviral vector design for optimal T cell receptor gene expression in the transduction of peripheral blood lymphocytes and tumor-infiltrating lymphocytes. Hum. Gene Ther. 2009;20:630–640. doi: 10.1089/hum.2008.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Lobo N. Bauser C.A. Fraser M.J., Jr The minimum internal and external sequence requirements for transposition of the eukaryotic transformation vector piggyBac. Mol. Genet. Genomics. 2001;266:190–198. doi: 10.1007/s004380100525. [DOI] [PubMed] [Google Scholar]
- Li X. Harrell R.A. Handler A.M. Beam T. Hennessy K. Fraser M.J., Jr piggyBac internal sequences are necessary for efficient transformation of target genomes. Insect Mol. Biol. 2005;14:17–30. doi: 10.1111/j.1365-2583.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- Liu Z. Noh H.S. Chen J. Kim J.H. Falo L.D., Jr. You Z. Potent tumor-specific protection ignited by adoptively transferred CD4+ T cells. J. Immunol. 2008;181:4363–4370. doi: 10.4049/jimmunol.181.6.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.Y. Condomines M. Tarte K. Nadal L. Delteil M.C. Rossi J.F. Ferrand C. Klein B. B7-1 and 4-1BB ligand expression on a myeloma cell line makes it possible to expand autologous tumor-specific cytotoxic T cells in vitro. Exp. Hematol. 2007;35:443–453. doi: 10.1016/j.exphem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin K.U. Hofgaard P.O. Omholt H. Munthe L.A. Corthay A. Bogen B. Therapeutic effect of idiotype-specific CD4+ T cells against B-cell lymphoma in the absence of anti-idiotypic antibodies. Blood. 2003;102:605–612. doi: 10.1182/blood-2002-11-3381. [DOI] [PubMed] [Google Scholar]
- Maragathavally K.J. Kaminski J.M. Coates C.J. Chimeric Mos1 and piggyBac transposases result in site-directed integration. FASEB J. 2006;20:1880–1882. doi: 10.1096/fj.05-5485fje. [DOI] [PubMed] [Google Scholar]
- Mitra R. Fain-Thornton J. Craig N.L. piggyBac can bypass DNA synthesis during cut and paste transposition. EMBO J. 2008;27:1097–1109. doi: 10.1038/emboj.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D. Monach P.A. Wanderling S. Philip M. Toledano A.Y. Schreiber R.D. Schreiber H. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-γ. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J.C. Bailey A.D. Fan H.Y. Pavelitz T. Weiner A.M. An abundant evolutionarily conserved CSB–PiggyBac fusion protein expressed in Cockayne syndrome. PLoS Genet. 2008;4:e1000031. doi: 10.1371/journal.pgen.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein A.F. Riddell S.R. Brown M. Corey L. Baerlocher G.M. Lansdorp P.M. Greenberg P.D. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. J. Exp. Med. 2004;200:1407–1417. doi: 10.1084/jem.20040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.R. DiGiusto D.L. Slovak M. Wright C. Naranjo A. Wagner J. Meechoovet H.B. Bautista C. Chang W.C. Ostberg J.R. Jensen M.C. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- Rolle C.E. Carrio R. Malek T.R. Modeling the CD8+ T effector to memory transition in adoptive T-cell antitumor immunotherapy. Cancer Res. 2008;68:2984–2992. doi: 10.1158/0008-5472.CAN-07-3040. [DOI] [PubMed] [Google Scholar]
- Rossig C. Brenner M.K. Chimeric T-cell receptors for the targeting of cancer cells. Acta Haematol. 2003;110:154–159. doi: 10.1159/000072465. [DOI] [PubMed] [Google Scholar]
- Sallusto F. Lenig D. Forster R. Lipp M. Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sarkar A. Sim C. Hong Y.S. Hogan J.R. Fraser M.J. Robertson H.M. Collins F.H. Molecular evolutionary analysis of the widespread piggyBac transposon family and related “domesticated” sequences. Mol. Genet. Genomics. 2003;270:173–180. doi: 10.1007/s00438-003-0909-0. [DOI] [PubMed] [Google Scholar]
- Schertzer J.D. Lynch G.S. Plasmid-based gene transfer in mouse skeletal muscle by electroporation. Methods Mol. Biol. 2008;433:115–125. doi: 10.1007/978-1-59745-237-3_7. [DOI] [PubMed] [Google Scholar]
- Schmieder A.H. Grabski L.E. Moore N.M. Dempsey L.A. Sakiyama-Elbert S.E. Development of novel poly(ethylene glycol)-based vehicles for gene delivery. Biotechnol. Bioeng. 2007;96:967–976. doi: 10.1002/bit.21199. [DOI] [PubMed] [Google Scholar]
- Serrano L.M. Pfeiffer T. Olivares S. Numbenjapon T. Bennitt J. Kim D. Smith D. McNamara G. Al-Kadhimi Z. Rosenthal J. Forman S.J. Jensen M.C. Cooper L.J. Differentiation of naive cord-blood T cells into CD19-specific cytolytic effectors for posttransplantation adoptive immunotherapy. Blood. 2006;107:2643–2652. doi: 10.1182/blood-2005-09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H. Manuri P.R. Olivares S. Dara N. Dawson M.J. Huls H. Hackett P.B. Kohn D.B. Shpall E.J. Champlin R.E. Cooper L.J. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits E. Ponsaerts P. Lenjou M. Nijs G. Van Bockstaele D.R. Berneman Z.N. Van Tendeloo V.F. RNA-based gene transfer for adult stem cells and T cells. Leukemia. 2004;18:1898–1902. doi: 10.1038/sj.leu.2403463. [DOI] [PubMed] [Google Scholar]
- Van Tendeloo V.F. Ponsaerts P. Berneman Z.N. mRNA-based gene transfer as a tool for gene and cell therapy. Curr. Opin. Mol. Ther. 2007;9:423–431. [PubMed] [Google Scholar]
- Wiehe J.M. Ponsaerts P. Rojewski M.T. Homann J.M. Greiner J. Kronawitter D. Schrezenmeier H. Hombach V. Wiesneth M. Zimmermann O. Torzewski J. mRNA-mediated gene delivery into human progenitor cells promotes highly efficient protein expression. J. Cell. Mol. Med. 2007;11:521–530. doi: 10.1111/j.1582-4934.2007.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.A. Sleeping beauty vector system moves toward human trials in the United States. Mol. Ther. 2008;16:1515–1516. doi: 10.1038/mt.2008.169. [DOI] [PubMed] [Google Scholar]
- Wilson M.H. Coates C.J. George A.L., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- Woltjen K. Michael I.P. Mohseni P. Desai R. Mileikovsky M. Hamalainen R. Cowling R. Wang W. Liu P. Gertsenstein M. Kaji K. Sung H.K. Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.C. Meir Y.J. Coates C.J. Handler A.M. Pelczar P. Moisyadi S. Kaminski J.M. piggyBac is a flexible and highly active transposon as compared to Sleeping Beauty, Tol2, and Mos1 in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15008–15013. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X. Huang X. Nodland S.E. Mates L. Ma L. Izsvak Z. Ivics Z. Lebien T.W. McIvor R.S. Wagner J.E. Zhou X. Stable gene transfer and expression in cord blood-derived CD34+ hematopoietic stem and progenitor cells by a hyperactive Sleeping Beauty transposon system. Blood. 2009;114:1319–1330. doi: 10.1182/blood-2009-03-210005. [DOI] [PubMed] [Google Scholar]
- Zanzonico P. Koehne G. Gallardo H.F. Doubrovin M. Doubrovina E. Finn R. Blasberg R.G. Riviere I. O'Reilly R.J. Sadelain M. Larson S.M. [131I]FIAU labeling of genetically transduced, tumor-reactive lymphocytes: Cell-level dosimetry and dose-dependent toxicity. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:988–997. doi: 10.1007/s00259-005-0057-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.