Abstract
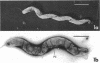
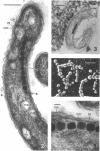
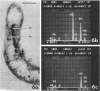
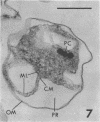
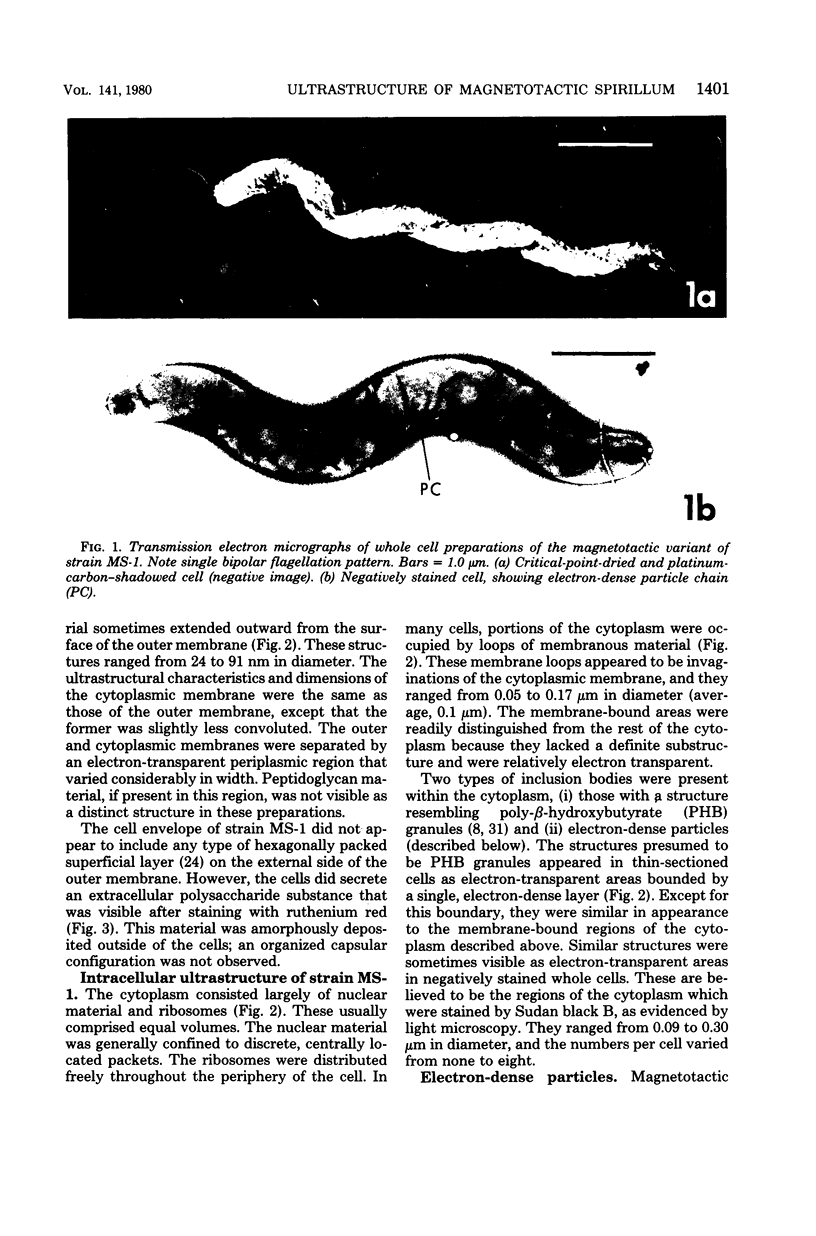
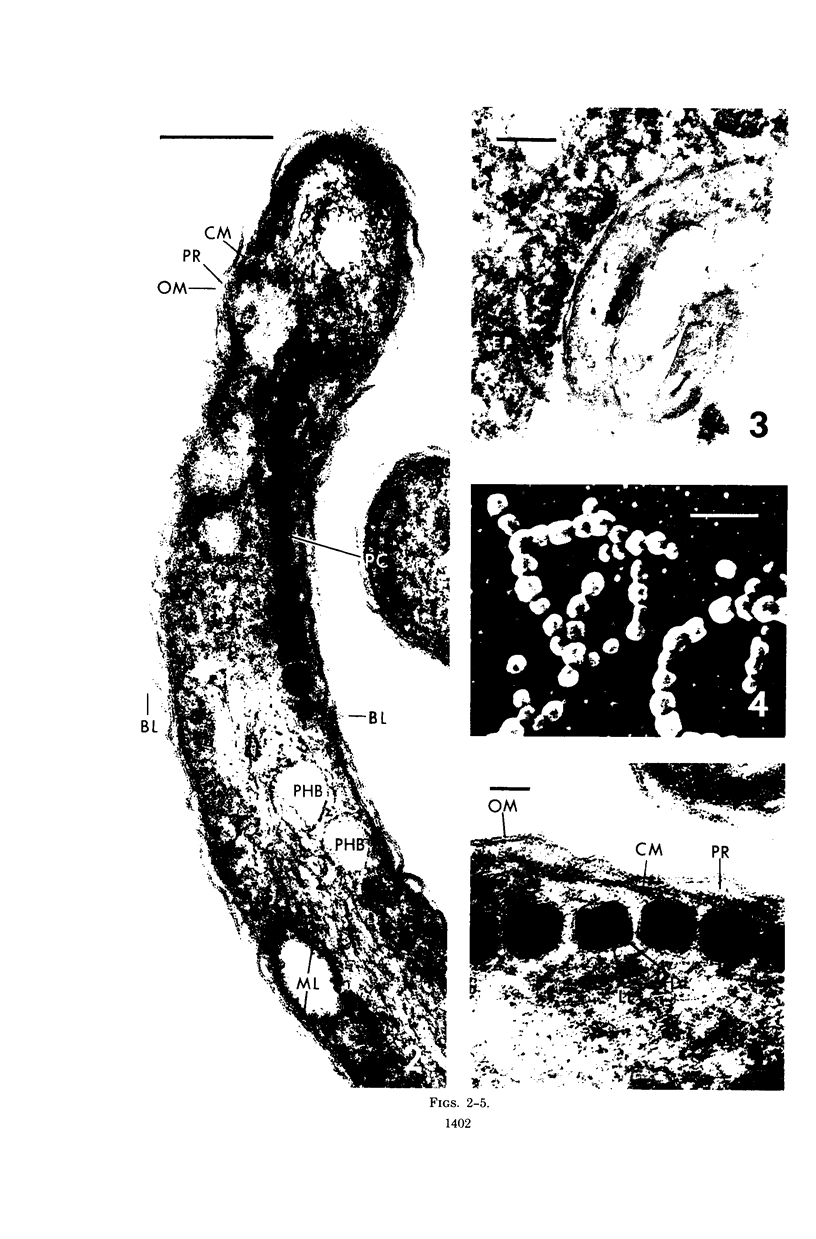
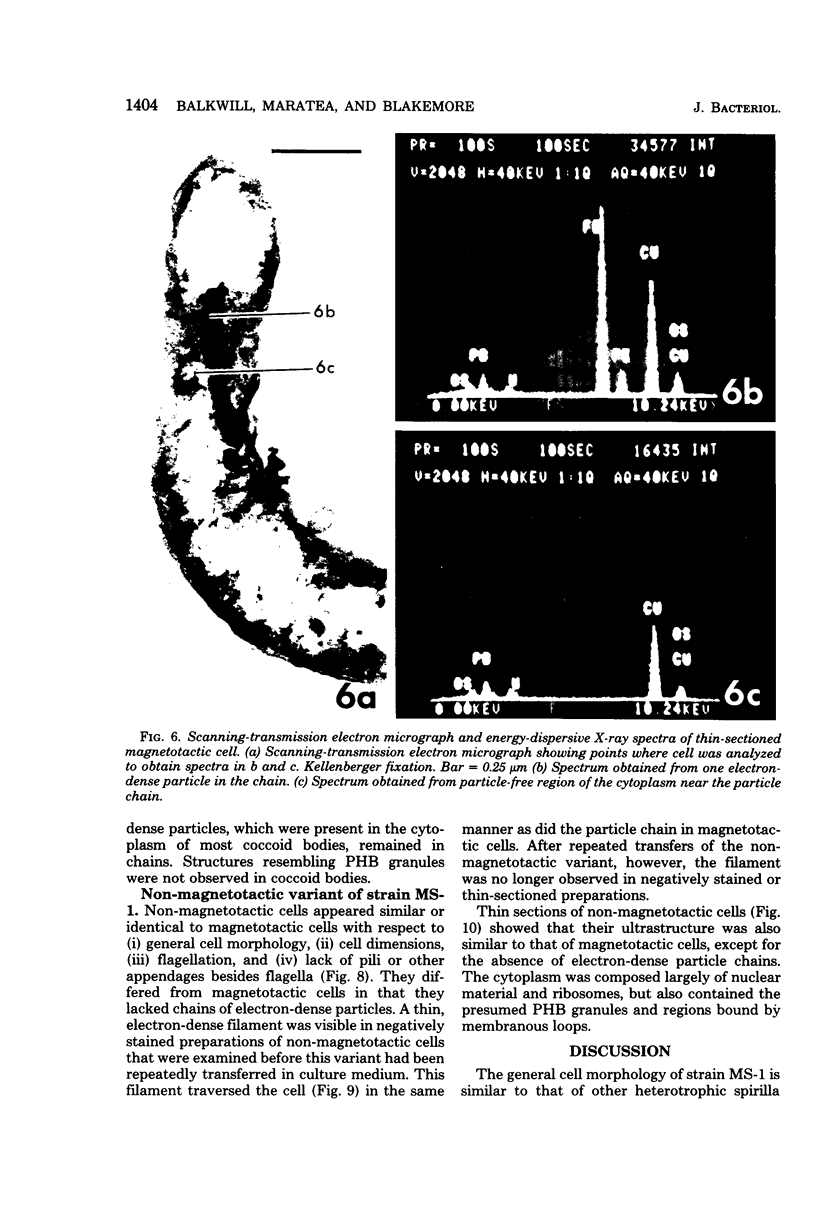
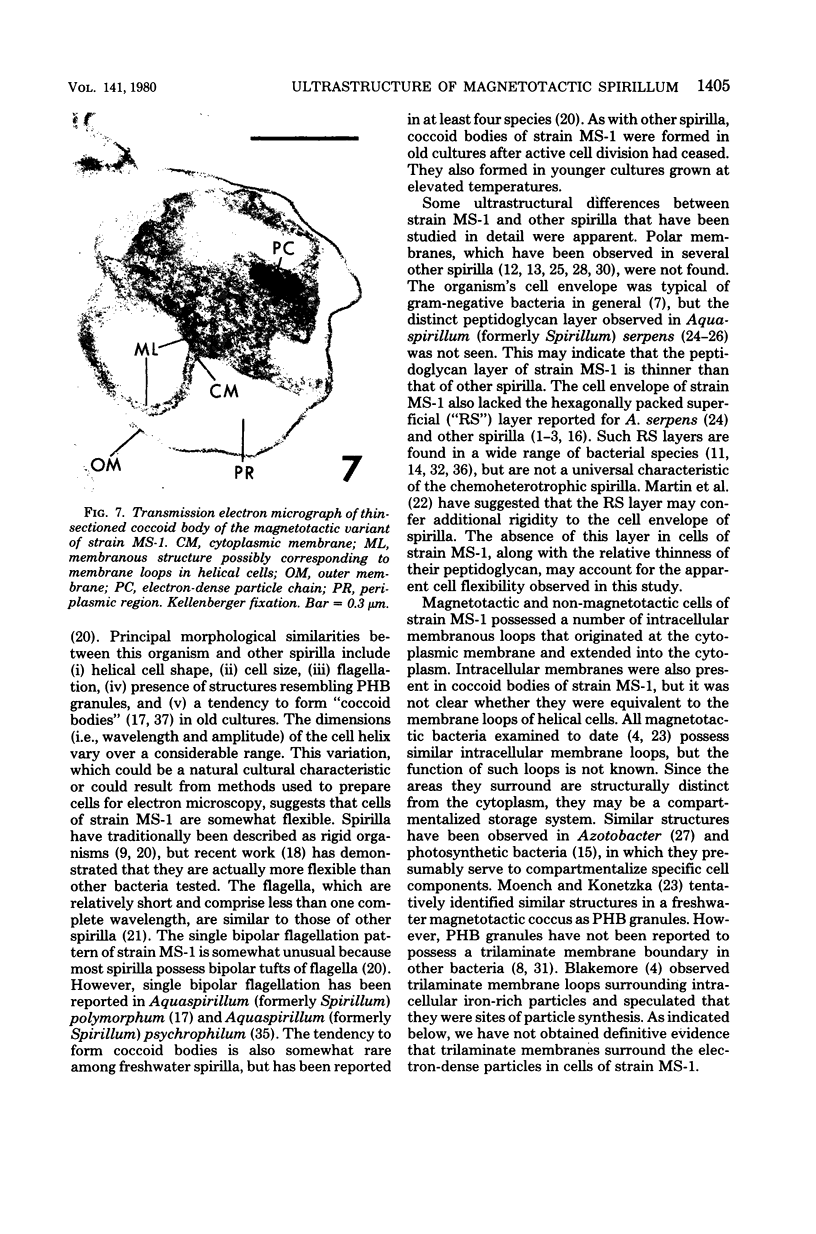
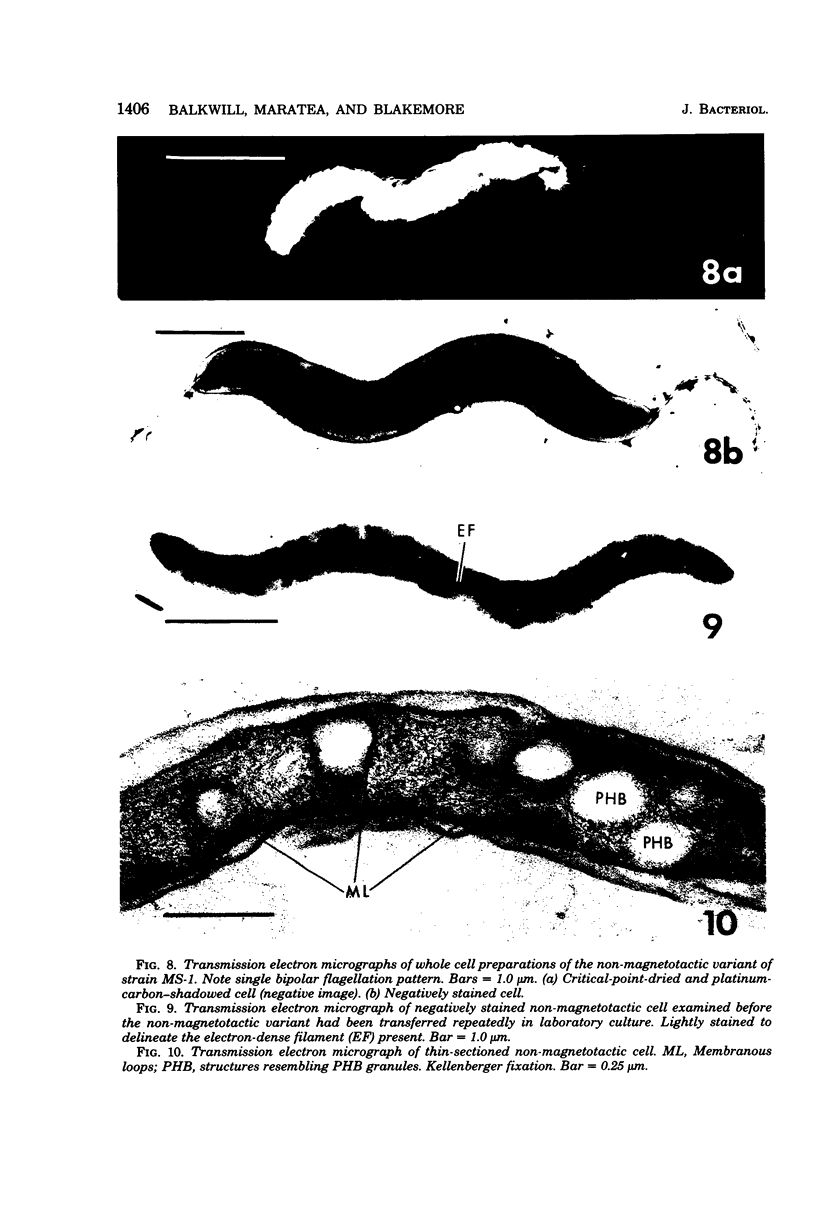
The ultrastructure of a magnetotactic bacterium (strain MS-1) was examined by transmission, scanning, and scanning-transmission electron microscopy. The organism resembled other spirilla in general cell morphology, although some differences were detected at the ultrastructural level. Electron-dense particles within magnetotactic cells were shown by energy-dispersive X-ray analysis to be localizations containing iron. A non-magnetotactic variant of strain MS-1 lacked these novel bacterial inclusion bodies. A chain of these particles traversed each magnetotactic cell in a specific arrangement that was consistent from cell to cell, seemingly associated with the inner surface of the cytoplasmic membrane. Each particle was surrounded by an electron-dense layer separated from the particle surface by an electron-transparent region. The term "magnetosome" is proposed for the electron-dense particles with their enveloping layer(s) as found in this and other magnetotactic bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J., Murray R. G. Dependence of the superficial layers of Spirillum putridiconchylium on Ca2+ or Sr2+. Can J Microbiol. 1976 Sep;22(9):1233–1244. doi: 10.1139/m76-183. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Superficial cell-wall layers on Spirillum "Ordal" and their in vitro reassembly. Can J Microbiol. 1976 Apr;22(4):567–582. doi: 10.1139/m76-085. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Superficial macromolecular arrays on the cell wall of Spirillum putridiconchylium. J Bacteriol. 1974 Sep;119(3):1019–1038. doi: 10.1128/jb.119.3.1019-1038.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. P., Maratea D., Wolfe R. S. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J Bacteriol. 1979 Nov;140(2):720–729. doi: 10.1128/jb.140.2.720-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. Magnetotactic bacteria. Science. 1975 Oct 24;190(4212):377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- Cagle G. D., Pfister R. M., Vela G. R. Improved staining of extracellular polymer for electron microscopy: examination of Azotobacter, Zoogloea, Leuconostoc, and Bacillus. Appl Microbiol. 1972 Sep;24(3):477–487. doi: 10.1128/am.24.3.477-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop W. F., Robards A. W. Ultrastructural study of poly- -hydroxybutyrate granules from Bacillus cereus. J Bacteriol. 1973 Jun;114(3):1271–1280. doi: 10.1128/jb.114.3.1271-1280.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel R. B., Blakemore R. P., Wolfe R. S. Magnetite in freshwater magnetotactic bacteria. Science. 1979 Mar 30;203(4387):1355–1356. doi: 10.1126/science.203.4387.1355. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- HICKMAN D. D., FRENKEL A. W. OBSERVATIONS ON THE STRUCTURE OF RHODOSPIRILLUM MOLISCHIANUM. J Cell Biol. 1965 May;25:261–278. doi: 10.1083/jcb.25.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICKMAN D. D., FRENKEL A. W. OBSERVATIONS ON THE STRUCTURE OF RHODOSPIRILLUM RUBUM. J Cell Biol. 1965 May;25:279–291. doi: 10.1083/jcb.25.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLT S. C., MARR A. G. LOCATION OF CHLOROPHYLL IN RHODOSPIRILLUM RUBRUM. J Bacteriol. 1965 May;89:1402–1412. doi: 10.1128/jb.89.5.1402-1412.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUWINK A. L. A macromolecular mono-layer in the cell wall of Spirillum spec. Biochim Biophys Acta. 1953 Mar;10(3):360–366. doi: 10.1016/0006-3002(53)90266-2. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac L., Ware G. C. The flexibility of bacterial cell walls. J Appl Bacteriol. 1974 Sep;37(3):335–339. doi: 10.1111/j.1365-2672.1974.tb00448.x. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg N. R. Biology of the chemoheterotrophic spirilla. Bacteriol Rev. 1976 Mar;40(1):55–115. doi: 10.1128/br.40.1.55-115.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Martin H. H., Heilmann H. D., Preusser H. J. State of the rigid-layer in celll walls of some gram-negative Bacteria. Arch Mikrobiol. 1972;83(4):332–346. doi: 10.1007/BF00425246. [DOI] [PubMed] [Google Scholar]
- Moench T. T., Konetzka W. A. A novel method for the isolation and study of a magnetotactic bacterium. Arch Microbiol. 1978 Nov 13;119(2):203–212. doi: 10.1007/BF00964274. [DOI] [PubMed] [Google Scholar]
- Oppenheim J., Marcus L. Correlation of ultrastructure in Azotobacter vinelandii with nitrogen source for growth. J Bacteriol. 1970 Jan;101(1):286–291. doi: 10.1128/jb.101.1.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITTENBERG S. C., WILLIAMS M. A. Microcyst formation and germination in Spirillum lunatum. J Gen Microbiol. 1956 Aug;15(1):205–209. doi: 10.1099/00221287-15-1-205. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. E., Keeler R. F., Bryner J. H. Anatomical features of Vibrio fetus: Electron microscopic survey. J Gen Microbiol. 1966 Jun;43(3):427–438. doi: 10.1099/00221287-43-3-427. [DOI] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Thorne K. J. Regularly arranged protein on the surfaces of Gram-negative bacteria. Biol Rev Camb Philos Soc. 1977 May;52(2):219–234. doi: 10.1111/j.1469-185x.1977.tb01351.x. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]