This paper presents evidence that chromatin condensation, like nuclear envelope breakdown, is brought about through the combined effects of cyclins A2 and B1, and that cyclins B1 and B2 are largely responsible for maintenance of a spindle assembly checkpoint arrest.
Abstract
Here we have used siRNAs and time-lapse epifluorescence microscopy to examine the roles of various candidate mitotic cyclins in chromatin condensation in HeLa cells. Knocking down cyclin A2 resulted in a substantial (∼7 h) delay in chromatin condensation and histone H3 phosphorylation, and expressing an siRNA-resistant form of cyclin A2 partially rescued chromatin condensation. There was no detectable delay in DNA replication in the cyclin A2 knockdowns, arguing that the delay in chromatin condensation is not secondary to a delay in S-phase completion. Cyclin A2 is required for the activation and nuclear accumulation of cyclin B1-Cdk1, raising the possibility that cyclin B1-Cdk1 mediates the effects of cyclin A2. Consistent with this possibility, we found that chromatin condensation was tightly associated temporally with the redistribution of cyclin B1 to the nucleus. Moreover, a constitutively nuclear cyclin B1 rescued chromatin condensation in cyclin A2 knockdown cells. On the other hand, knocking down cyclin B1 delayed chromatin condensation by only about one hour. Our working hypothesis is that active, nuclear cyclin B1-Cdk1 normally cooperates with cyclin A2 to bring about early mitotic events. Because cyclin A2 is present only during the early stages of mitosis, we asked whether cyclin B knockdown might have more dramatic defects on late mitotic events. Consistent with this possibility, we found that cyclin B1- and cyclin B1/B2-knockdown cells had difficulty in maintaining a mitotic arrest in the presence of nocodazole. Taken together, these data suggest that cyclin A2 helps initiate mitosis, in part through its effects on cyclin B1, and that cyclins B1 and B2 are particularly critical for the maintenance of the mitotic state.
INTRODUCTION
Mitosis is one of the most spectacular events in cell biology. In animal cells it begins with a nuclear event, the condensation of the chromosomes, and a cytoplasmic event, the separation of the centrosomes, during prophase. This is followed by the breakdown of the nuclear envelope, which marks the onset of prometaphase. The condensed chromosomes migrate to the center of the cell by metaphase, and then the sister chromatids separate and move to opposite sides of the cell during anaphase. Finally cytokinesis occurs, the nuclear envelope reforms, the chromatin decondenses, and the daughter cells progress into interphase (Morgan, 2007).
Mitosis is initiated by abrupt changes in the activity of cyclin-dependent kinase (CDK) complexes. In their simplest forms, these complexes are heterodimers made up of a regulatory cyclin subunit and a catalytic CDK subunit. In the fission yeast Schizosaccharomyces pombe, a single CDK (Cdc2) and a single cyclin (Cdc13) are sufficient to drive the entire cell cycle (Fisher and Nurse, 1996). In most other organisms, multiple CDKs and cyclins are involved. For example, in humans there are ∼30 cyclin genes and 21 CDK-like genes. Eleven of the human cyclins (A1-2, B1-3, D1-3, E1-2, and H) and five of the CDKs (CDK1, 2, 4, 6, 7) have well-established roles as cell cycle regulators. Many of the remaining cyclins and CDKs appear to be regulators of transcription.
It still remains uncertain as to which of these many cyclins regulate mitosis, whether different cyclins are dedicated to different mitotic events, how much redundancy there is between cyclin-CDKs, and how the functions of the mitotic cyclin-CDKs are coordinated. Diverse lines of evidence now support the hypothesis that cyclins A and B are the principal mitotic cyclins in animals (Morgan, 2007), with a third class of cyclin (B3 cyclins) being important for mitosis in some species (Lehner and O'Farrell, 1990; Jacobs et al., 1998). The CDK partners of the A-, B-, and B3 cyclins are CDK1 and CDK2; the A-type cyclins can bind both CDK1 and CDK2, the B-type cyclins partner with CDK1, and cyclin B3 binds CDK2 (Nguyen et al., 2002).
In mammalian cell lines, cyclin A2 is first detectable during S-phase and is degraded during prometaphase (Pines and Hunter, 1990, 1991; den Elzen and Pines, 2001; Geley et al., 2001). Cyclin A2 shuttles dynamically between the cytoplasm and nucleus, with its steady-state concentration favoring the nucleus (Pines and Hunter, 1991; Jackman et al., 2002). Antibody injection experiments indicate that cyclin A2 is required for DNA replication and, once replication is complete, for mitotic entry (Pagano et al., 1992). Cyclins B1 and B2 become detectable slightly later and are degraded during metaphase (Minshull et al., 1990; Pines and Hunter, 1991; Jackman et al., 1995; Clute and Pines, 1999). Cyclin B1 shuttles between the cytoplasm and the nucleus and is primarily cytoplasmic during interphase, concentrates at the centrosome before mitosis, and then abruptly translocates into the nucleus ∼5 min before nuclear envelope breakdown (NEB) (Pines and Hunter, 1991; Ookata et al., 1992; Hagting et al., 1999). This translocation is tightly correlated with cyclin B1-Cdk1 activation (Gavet and Pines, 2010a, b). Cyclin B2 is localized to the Golgi apparatus (Jackman et al., 1995; Draviam et al., 2001). These localizations suggest that cyclin B2 mediates Golgi disassembly and that cyclin A2, B1, or both may mediate chromatin condensation and NEB.
Studies of knockout mice have shown that cyclins A2 and B1 are both required for viability, but cyclin A1, whose expression is confined to germ cells, is required only for male fertility, and the ubiquitously-expressed cyclin B2 is dispensable (Murphy et al., 1997; Brandeis et al., 1998; Liu et al., 1998). A mammalian cyclin B3 knockout has not been reported yet, but because its expression is confined to germ cells (Nguyen et al., 2002) it seems unlikely to be a general mitotic regulator in mammals. These findings are consistent with the idea that cyclin A2, cyclin B1, or both, are the main mitotic cyclins.
The advent of RNA interference (RNAi) approaches provided a way of testing this hypothesis and exploring the redundancies and relationships among the cyclins. In HeLa cells, a model system for the study of mitotic control that is particularly amenable to RNAi, knocking down cyclin A2 causes a substantial delay in NEB (Fung et al., 2007; Gong et al., 2007). The delay can be quantitatively accounted for by a delay in the activation of cyclin B1-Cdk1 complexes (Fung et al., 2007; De Boer et al., 2008) and in the translocation of cyclin B1 to the nucleus (Gong et al., 2007; De Boer et al., 2008). These findings suggest that NEB is promoted by cyclin A2 through the intermediacy of nuclear cyclin B1. This is reminiscent of the role cyclin A plays in mitosis in Drosophila, where cyclin A acts by promoting the accumulation and activation of cyclin B complexes (Jacobs et al., 1998; Dienemann and Sprenger, 2004; Reber et al., 2006), and it suggests that in both of these systems cyclin A-CDK, like the CDK-activating kinase (CAK) cyclin H-CDK7, may regulate mitosis mainly or exclusively through its effects on other cyclin-CDKs.
This hypothesis presupposes that cyclin B1 is required for mitosis in HeLa cells, and conflicting evidence has been presented on this point. One group, using a short hairpin RNA construct to knockdown cyclin B1 and assessing the consequences by immunoblotting, kinase assays, and examination of fixed cells, reported that cyclin B1 knockdown prevents mitotic entry (Fung et al., 2007), indicating that cyclin B1 is an essential regulator of mitosis. Our group, using diced siRNA pools (d-siRNAs) and live-cell video microscopy, found that knocking down cyclin B1, B2, or both delays mitosis only slightly (Gong et al., 2007). This suggests that even if cyclin B1 normally mediates NEB, its function can be covered by cyclin A2. This interpretation is consistent with the fact that a cyclin B1/A2 double knockdown showed a longer delay in the onset of NEB than either single knockdown showed (Gong et al., 2007). Qualitative defects in late mitotic events (especially abnormalities in cytokinesis) were common in cyclin B1/B2-double knockdown cells, suggesting that even if cyclins B1 and B2 are not essential for NEB, they are important in the later stages of mitosis (Gong et al., 2007).
Here we have addressed the question of which cyclins are required for another early mitotic event, chromatin condensation. We also examined whether cyclins B1 and B2 might be more critical during a spindle assembly checkpoint arrest, where the mitotic state must be maintained long after cyclin A2 has been degraded. We approached these questions in synchronized HeLa cells, using diced siRNA pools to knock down cyclins and time lapse fluorescence microscopy to assess the consequences. Our findings underscore the importance of cyclin A2 in the initiation of mitosis, suggest that cyclin A2's effects on chromatin condensation are at least partly mediated by cyclin B1, and indicate that cyclin B1 and cyclin B2 are important for the maintenance of a mitotic arrest in the presence of nocodazole.
MATERIALS AND METHODS
DNA Constructs and d-siRNA Pools
Cyclin expression constructs and the tdimer2-RFP mitotic biosensor (Jones et al., 2004) were generated as described (Gong et al., 2007). A fluorescently-tagged histone H2B was constructed by amplifying the open reading frame of human histone H2B from a HeLa cDNA library and cloning it into pEYFP-N1 or pECFP-N1 (Clontech, Palo Alto, CA) using XhoI and SalI. Diced siRNA pools were generated from ∼600 base pairs dsRNAs derived from a cyclin coding region or 3′-UTR, or from an irrelevant firefly luciferase (GL3) coding region, using recombinant human Dicer as described (Myers and Ferrell, 2005; Gong et al., 2007), or using TurboDicer (Genlantis, San Diego, CA).
Cell Culture, Transfection, and UV/Drug Treatments
HeLa cells (ATCC, Manassas, VA) were cultured at 37°C and 5% CO2 in DMEM, supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 4 mM glutamine (all from Invitrogen, Carlsbad, CA). Cells were synchronized by double thymidine block (2 mM thymidine for 18 h, release for 9 h, 2 mM thymidine for 17 h). Cells were cotransfected with a total of 25 nM d-siRNAs plus 10 ng of a histone H2B expression construct alone, or 5 ng of the histone H2B expression construct with 10 ng of the WT- or NLS-cyclin B1-YFP. For the flow cytometry and DNA damage experiments, cells were transfected with 25 nM d-siRNAs only. All transfections were carried out using GeneSilencer (Genlantis) during the first release from thymidine treatment. For DNA damage experiments, cells were released from thymidine block into growth media for 4 h, washed once with PBS, subject to UV irradiation at 50 μJ/cm2 in a UV Stratalinker 1800 (Stratagene/Agilent, Santa Clara, CA), and then replaced with growth media for 4 h before collection for immunoblotting. For nocodazole treatments, cells were released into growth media containing 100–200 ng/ml nocodazole (Sigma Aldrich, St. Louis, MO).
Flow Cytometry
Trypsinized cells were fixed in 2% paraformaldehyde followed by ice-cold 100% methanol for at least 20 min. Cells were incubated with anti–phospho-histone H3 antibody (mouse monoclonal, 1:200 dilution, Upstate Biotech/Millipore, Billenica, MA), followed by goat anti-mouse Alexa 647 secondary antibody (1:100 dilution, Invitrogen), both in PBS containing 1% BSA, 0.1% Triton X-100. Cells underwent a final staining with a solution containing 50 μg/μL propidium iodide and 0.1 mg/ml RNase A in 1% BSA in PBS. At least 1.4 × 104 cells were analyzed using the LSR flow cytometer (Becton Dickinson, Franklin Lakes, NJ), and data were collected using CellQuest software (Becton Dickinson) and analyzed using FlowJo software.
Microscopy and Image Analysis
Cells were imaged using the Axon ImageXpress 5000A or the ImageXpress Micro (Molecular Devices, Sunnyvale, CA). Images were captured at one frame per 3–5 min for 14–18 h with a ×10, ×20, or ×40 objective lens. Image analysis was performed using sequences of images compiled by the ImageXpress Console (Molecular Devices), ImageJ, and CellProfiler. Image analysis and two-line curve fitting scripts were written in MatLab.
Western Blot Analysis
Cells were resuspended in lysis buffer (100 mM Tris [pH 7.9], 0.42 M NaCl, 0.5% Triton X100, 1 mM EDTA, 1 mM EGTA), vortexed for 10 s every 10 min for 1 h, and microcentrifuged at maximum speed. Total protein in the supernatants were quantified using the BCA Assay kit (Pierce/Thermo, Rockford, IL). At least 8 μg of total protein was subject to 8% SDS-PAGE and transferred to PVDF membrane (Millipore). Dry membranes were probed using primary antibodies specific for cyclin A2, cyclin B1 (Santa Cruz Biotechnology, Santa Cruz, CA, sc-751, sc-752), phospho-histone H3 (Upstate Biotech), all at 1:1000 dilutions. Anti–phospho-Chk1 and anti-Chk1 antibodies were used according to manufacturer's instructions (Cell Signaling Technology, Danvers, MA). Membranes were then probed with horseradish peroxidase (HRP)-conjugated secondary antibodies at 1:2500 dilutions (Amersham/GE, Piscataway, NJ) in 0.1% Tween-20, 5% nonfat dry milk in PBS, followed by incubation with Immun-Star HRP substrate (Bio-Rad, Hercules, CA) or SuperSignal West Dura or Femto Maximum Sensitivity Substrate (Pierce). Chemiluminescence was detected using GelDoc EQ (Bio-Rad) and quantified using QuantityOne 4.5.0 (Bio-Rad).
RESULTS
Knocking Down Cyclin A2 Delays Chromatin Condensation
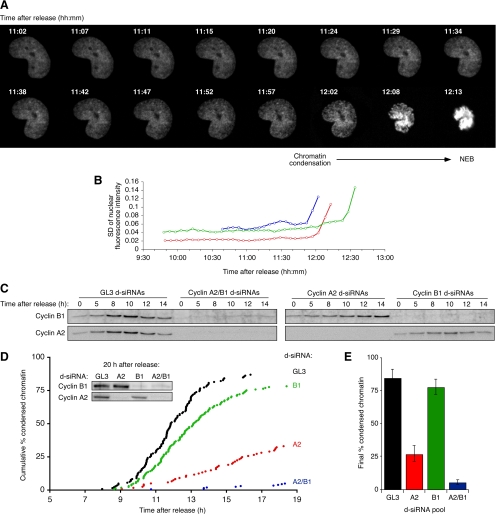
We developed a quantitative live-cell assay for chromatin condensation, using HeLa cells transiently expressing a fluorescent histone (H2B-YFP) (Kanda et al., 1998) and taking advantage of the fact that when chromatin condensation begins, the nuclear H2B-YFP fluorescence changes from uniform to variable (Figure 1A). The SD of the nuclear H2B-YFP fluorescence per pixel over the nucleus can be taken as a quantitative measure of how condensed the chromatin is; the higher the SD, the more condensed the chromatin. As shown in Figure 1B, the fluorescence SD typically took a sharp turn upward ∼10 min before NEB. We estimated the time at which chromatin condensation began by fitting two straight lines to the fluorescence variability data and interpolating the time at which the two lines intersected. The timings calculated by this method remained consistent between time-lapse images generated using ×10, ×20, or ×40 objective lenses and agreed well with subjective estimates of the onset of chromatin condensation.
Figure 1.
The effects cyclin A2 and cyclin B1 d-siRNAs on the timing of chromatin condensation in HeLa cells. (A–D) HeLa cells were synchronized by double thymidine block and transfected with histone H2B-YFP and GL3 or cyclin d-siRNAs during the first release from thymidine. (A) Sixteen frames from a time-lapse movie of a mitotic HeLa cell expressing histone H2B-YFP. Time stamps (hh:mm) indicate time after release from double thymidine block. (B) Scoring the onset of mitotic chromatin condensation for three cells. The red points correspond to the cell pictured in A. (C) Time course of cyclin levels in d-siRNA–treated synchronized HeLa cells as assessed by immunoblotting. (D) Timing of chromatin condensation in d-siRNA–treated HeLa cells. The immunoblot (inset) shows cyclin levels in d-siRNA treated cells at 20 h after release from double thymidine block. (E) Final cumulative % chromatin condensation events 20 h after release from double thymidine block. Data are expressed as means ± SE from 4 movies from two experiments.
To study the effects of cyclin knockdown on the timing of chromatin condensation, we used diced siRNA pools (d-siRNAs) generated against ∼600-bp stretches of the cyclin coding sequence. Diced siRNAs were chosen because of their low propensity for off target effects (Myers et al., 2006), their lack of interference with endogenous miRNA processing pathways (Grimm et al., 2006), and their record of specificity and efficacy in previous screens and studies (Myers et al., 2003; Liou et al., 2005; Myers and Ferrell, 2005; Brandman et al., 2007; Gong et al., 2007). HeLa cells were partially synchronized by thymidine treatment, released from the first thymidine block and transfected with control or cyclin d-siRNA pools, and then blocked again in S-phase by thymidine treatment. Five hours after the release from the second thymidine block (∼24 h post transfection), we began monitoring chromatin condensation by automated epifluorescence microscopy.
To assess the extent of cyclin knockdown we collected cells at various times after release from the thymidine block, lysed them, and subjected the lysates to immunoblotting for cyclins A2 and B1. In the GL3-transfected control cells, the levels of cyclin A2 and cyclin B1 rose steadily after release from the thymidine block, peaked at 10 h, and then fell (Figure 1C). The d-siRNA pools effectively and specifically knocked down the intended cyclin target(s) for at least 14 h and typically until at least 20 h after release from thymidine, without any detectable cross-silencing (Figure 1, C and D). The extent of silencing was high; residual cyclin levels were typically 0–25% of basal levels. Given that some of the residual cyclin was undoubtedly contributed by nontransfected cells, the residual cyclin levels in the transfected cells must have been smaller still.
Knocking down cyclin A2 delayed the onset of chromatin condensation by several hours (Figure 1D, red points); more than half of the control GL3 knockdown cells had undergone chromatin condensation by 12 h post-release, whereas <10% of the cyclin A2 knockdown cells had undergone chromatin condensation at that time and only a third of the cyclin A2 knockdown cells had undergone chromatin condensation by 19 h. Cyclin B1 knockdown resulted in a delay of about one hour (Figure 1D, green points), and knocking down both cyclin A2 and cyclin B1 virtually abolished chromatin condensation (Figure 1D, blue points). The delay in chromatin condensation seen in the cyclin A2 knockdown cells, and the further delay in the cyclin A2/B1 double knockdown cells, were reproducible from well to well and from experiment to experiment (Figure 1E). These results suggest that cyclin A2 is required for the initiation of mitotic chromatin condensation and that there is redundancy between cyclin A2 and cyclin B1. These findings agree well with previous functional studies which showed that microinjection of cyclin A2 antibodies blocks mitosis (Pagano et al., 1992), microinjection of a cyclin A2 function-blocking peptide (derived from p21 Cip1, which is a high-affinity inhibitor of cyclin A2-CDK2 and cyclin E-CDK2, a moderate affinity inhibitor of cyclin B-CDK1, and a putative activator of cyclin d-CDK4/6 complexes [Gu et al., 1993; Cheng et al., 1999; Sherr and Roberts, 1999]) into prophase cells results in the decondensation of previously condensed chromatin (Furuno et al., 1999), and that a similar inhibitor blocks mitosis in Xenopus egg extracts (Guadagno and Newport, 1996).
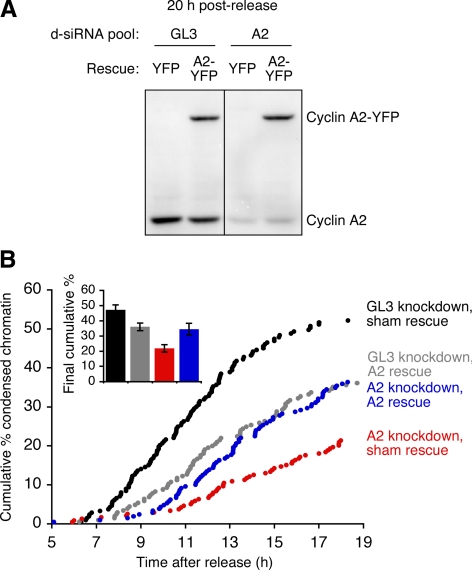
To determine whether the delay in chromatin condensation in cyclin A2 knockdown cells might be due to off-target effects rather than cyclin A2 knockdown per se, we generated d-siRNAs from a different region in the cyclin A2 cDNA, the 3′ untranslated region (3′-UTR). The cyclin A2 3′-UTR d-siRNAs knocked down the endogenous cyclin A2 (Figure 2A) and delayed chromatin condensation (Figure 2B, red points). Expression of cyclin A2 from a construct that lacked the 3′-UTR and hence was unaffected by the 3′-UTR d-siRNA, partially reversed the delay, restoring the timing of chromatin condensation to that seen in cells transfected with a control (GL3) d-siRNA plus the same 3′-UTR-less cyclin A2 (Figure 2B, blue points vs. gray points), although not to that seen in GL3 d-siRNA cells cotransfected with a sham rescue construct (Figure 2B, gray points vs. black points). These findings argue that the delay in the initiation of chromatin condensation seen in the cyclin A2 knockdown cells is not due to off-target effects.
Figure 2.
An RNAi-resistant form of cyclin A2 rescues chromatin condensation in cyclin A2 knockdown cells. (A–B) HeLa cells were synchronized by double thymidine block and transfected with histone H2B-YFP, one of two diced siRNA pools (GL3 d-siRNAs or d-siRNAs targeted to the 3′UTR of cyclin A2), and one of two rescue constructs (a sham YFP rescue construct or a cyclin A2-YFP rescue construct lacking the 3′UTR). (A) Cyclin levels of d-siRNA–treated HeLa cells at 20 h after release from double thymidine block, as assessed by immunoblotting. (B) Timing of chromatin condensation in cells treated with d-siRNAs plus a cyclin A2 or YFP rescue construct. Chromatin condensation was assessed by automated time-lapse epifluorescence microscopy. The bar graph (inset) shows the final cumulative % chromatin condensation events at 19 h after release from double thymidine block. Data are expressed as means ± SE from 6 movies from two experiments.
Histone H3 Phosphorylation Is Delayed in Cyclin A2 Knockdown Cells
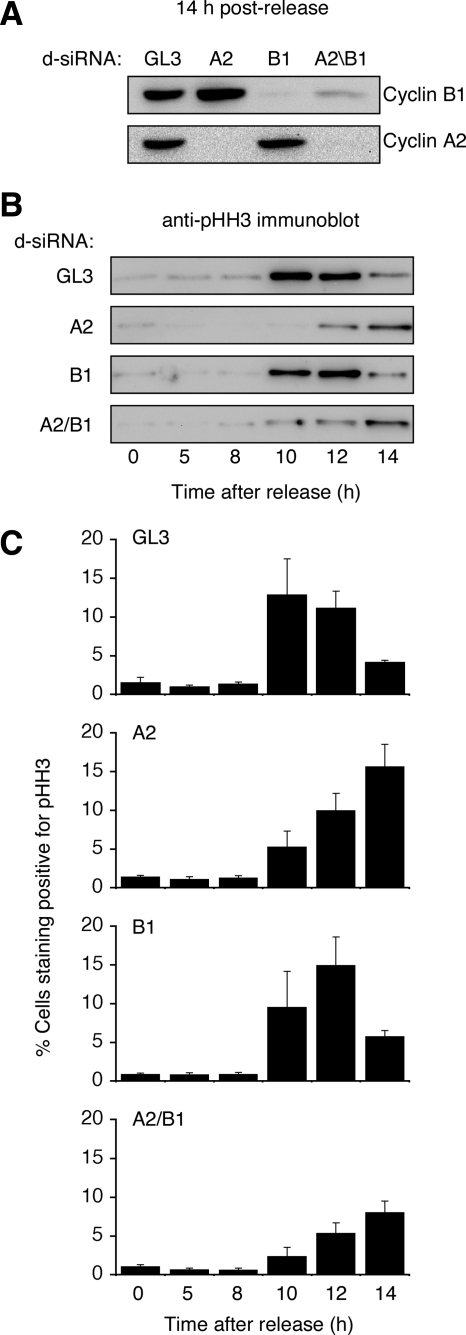
Fung and coworkers reported that cyclin B1 knockdown was just as effective as cyclin A2 knockdown in suppressing the mitotic phosphorylation of histone H3 at Ser 10 (Fung et al., 2007). Because histone H3 phosphorylation generally correlates well with chromatin condensation (Hendzel et al., 1997), and because we saw little effect of cyclin B1 knockdown on the timing of chromatin condensation (Figure 1), we set out to determine what effects our cyclin knockdowns had on histone H3 phosphorylation.
We synchronized HeLa cells, transfected them with cyclin d-siRNAs, and collected them at various times after release from thymidine for analysis by flow cytometry and immunoblotting. Cyclin knockdown was verified by Western blot analysis (Figure 3A). There was a small delay in the accumulation of phospho-histone H3 in cyclin B1 knockdown cells (Figure 3, B and C). There was a more dramatic delay in the appearance of phospho-histone H3 in the cyclin A2 knockdown cells and a further delay in the cyclin A2/B1 double knockdown cells (Figure 3, B and C). Thus the histone H3 phosphorylation data are consistent with the microscopy data shown in Figure 1; cyclin B1 knockdown had a small effect, cyclin A2 knockdown had a larger effect, and cyclin A2/B1 double knockdown had the largest effect.
Figure 3.
Cyclin A2 knockdown delays histone H3 phosphorylation. (A–C) HeLa cells were synchronized by double thymidine block and transfected with GL3 or cyclin d-siRNAs. (A) At 14 h after release from thymidine block, cyclin knockdown was verified by immunoblotting. Levels of histone H3 phosphorylation were assessed by immunoblotting (B) and flow cytometry (C). For C, data are expressed as means + SE from three independent experiments.
Knocking Down Cyclin A2 Has No Detectable Effect on DNA Replication or on Replication-Associated Activation of the DNA Checkpoint Response
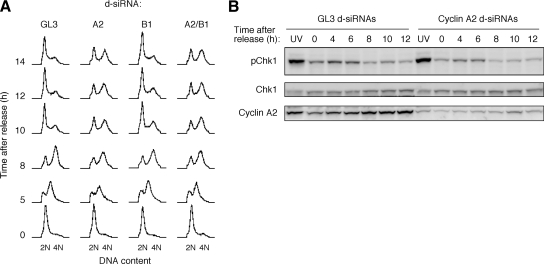
Previous work has demonstrated that cyclin A2 regulates S-phase events (Pagano et al., 1992). Thus one possible explanation for the delay in chromatin condensation seen in cyclin A2 knockdown cells could be slowed DNA replication, with delay in chromatin condensation being secondary to the activation of the DNA replication checkpoint. However, recent studies found no gross delay in DNA replication, as judged by propidium iodide staining and flow cytometry in cyclin A2 knockdown cells released from double-thymidine arrest (Fung et al., 2007; Gong et al., 2007). We corroborated this result. As shown in Figure 4A, DNA profiles at the time of release were normal in all of the knockdown cells, indicating that the siRNA treatments did not affect synchronization. In all of the control and cyclin knockdown cells, DNA replication was evident by 5 h post-release, with the majority of the cells present in a peak with an intermediate S-phase DNA content and a smaller fraction of the cells present in a G1-phase DNA-content peak. By 8 h post-release, the S-phase peak had shifted to a G2/M DNA content in both the control and cyclin knockdown cells. By 10 h post-release, most or the control and cyclin B1 knockdown cells had completed mitosis, with their DNA content returning to G1-phase levels, whereas most of the cyclin A2-knockdown and cyclin A2/B1 double-knockdown cells still had a G2/M DNA content, presumably because they were delayed in completing mitosis. Thus, in agreement with previous reports (Fung et al., 2007; Gong et al., 2007), DNA replication was not grossly slowed in the cyclin A2 knockdown cells, suggesting that the delay in chromatin condensation was not secondary to activation of the DNA replication checkpoint.
Figure 4.
Cyclin A2 knockdown does not delay DNA replication or activate the DNA replication/damage checkpoint. (A) Propidium iodide flow cytometry of cells knocked down with control (GL3) or cyclin d-siRNAs, at various times after release from double thymidine block. (B) Immunoblots of cells collected at various times after release from double thymidine block and probed for phospho-Chk1 (pChk1), total Chk1, and cyclin A2. UV denotes cells irradiated with UV light (50 μJ/cm2) at four hours after release and then collected at eight hours.
A more direct way to assess whether the DNA replication/damage checkpoint was activated is phospho-Chk1 (pChk1) immunoblotting; if checkpoint activation were responsible for the delay in mitotic entry seen in cyclin A2 knockdown cells, we would expect the pChk1 signal to be stronger or more sustained in the cyclin A2 knockdown cells than in control cells. To see whether this was the case, HeLa cells were synchronized by thymidine block, released and transfected with control (GL3) or cyclin A2 d-siRNAs, blocked again by thymidine treatment, and then released from the second thymidine block. At various times after release, we examined the cells by microscopy to gauge whether they were progressing into mitosis and then lysed the cells and subjected the lysates for immunoblotting. The knockdown of cyclin A2 was ∼70% (Figure 4B). This extent of knockdown was sufficient to almost completely prevent the cyclin A2 knockdown cells from entering mitosis during the first 12 h post-release.
At the time of release, both the GL3 and cyclin A2 knockdown cells showed low levels of pChk1 immunoreactivity, ∼25% of the levels seen in the positive-control UV-treated cells (Figure 4B). The pChk1 signal increased by 4–6 h post-release, presumably due to activation of the checkpoint during DNA replication, and then fell over the next several hours (Figure 4B). The pChk1 signal did not appear to be elevated or prolonged in the cyclin A2 knockdown cells (Figure 4B). These findings argue against the possibility that activation of the DNA replication/damage checkpoint is responsible for the delay in mitotic entry seen in cyclin A2 knockdown cells.
Chromatin Condensation Coincides with the Translocation of Cyclin B1 to the Nucleus
There are at least two plausible interpretations of the knockdown data. It is possible that cyclin A2 is directly responsible for chromatin condensation and that cyclin B1 can provide a backup function (although after a substantial delay, perhaps because cyclin B1's accumulation in the nucleus is delayed in cyclin A2 knockdown cells [Gong et al., 2007; De Boer et al., 2008]). Alternatively, it is possible that cyclin B1 is normally responsible for chromatin condensation, with cyclin A2 providing a backup function. To further explore the possibility that cyclin B1 might normally trigger chromatin condensation, we looked at the timing of chromatin condensation versus cyclin B1 translocation.
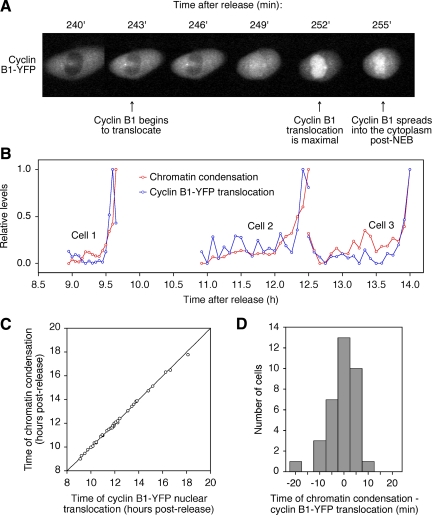
We cotransfected H2B-CFP and cyclin B1-YFP into synchronized HeLa cells and followed chromatin condensation and cyclin B1 nuclear accumulation by epifluorescence video microscopy. Typically cyclin B1-YFP redistributed from the cytoplasm to the nucleus over the course of ∼10 min (Figure 5A). NEB occurred within a few minutes of the completion of this redistribution, allowing the cyclin B1 to spread back into the cytoplasm, causing the intensity of the fluorescence in the region of the nucleus to fall by about half (Figure 5A).
Figure 5.
Chromatin condensation is temporally associated with the translocation of cyclin B1-YFP to the nucleus. HeLa cells were synchronized by double thymidine block and transfected with histone H2B-CFP and cyclin B1-YFP. (A) Six frames from a time lapse movie showing cyclin B1-YFP translocation just before nuclear envelope breakdown (NEB). (B) Chromatin condensation (red) and cyclin B1-YFP translocation for three typical cells. Chromatin condensation is inferred from the SD of the nuclear histone H2B-CFP fluorescence. Cyclin B1-YFP translocation is inferred from the integrated intensity of the nuclear cyclin B1-YFP. Both quantities are expressed relative to their maximum and minimum values for each cell. (C) Correlation between the timing of cyclin B1-YFP nuclear translocation and chromatin condensation. (D) Histogram of the time differences between chromatin condensation and cyclin-YFP nuclear translocation.
For each cell expressing both H2B-CFP and cyclin B1-YFP, we calculated the time when chromatin condensation commenced as described in Figure 1B and the time at which cyclin B1-YFP began to accumulate in the nucleus by a similar automated curve-fitting procedure. Figure 5B shows chromatin condensation and nuclear translocation data for three typical cells. The absolute timing of mitotic entry was highly variable from cell to cell, but chromatin condensation and cyclin B1-YFP nuclear translocation were always nearly coincident (Figure 5C), with no significant systematic lag between the two events (Figure 5D). These findings are consistent with the hypothesis that the entry of active cyclin B1-Cdk1 complexes into the nucleus rapidly brings about chromatin condensation. Alternatively, the two events could be independent of each other but tightly dependent upon some other event (such as the activation of cyclin A2-Cdk2 or cyclin A2-Cdk1).
Nuclear Cyclin B1 Can Substitute for Cyclin A2
To further explore the possibility that cyclin A2 brings about chromatin condensation through its effects on cyclin B1, we examined whether the expression of nuclear cyclin B1 could rescue the delay in chromatin condensation seen in cyclin A2 knockdown cells. We expressed cyclin A2 d-siRNAs plus either wild-type cyclin B1-YFP (WT-B1), a constitutively nuclear cyclin B1-YFP (NLS-B1), or a sham YFP rescue construct in synchronized HeLa cells, and monitored chromatin condensation with histone H2B-CFP and other mitotic events with a mitotic biosensor (MBS) (Jones et al., 2004). Cyclin immunoblots showed that cyclin A2 was knocked down in all of the cyclin A2 d-siRNA-transfected cells (Figure 6A). The transfected WT-cyclin B1-YFP construct was expressed at slightly higher levels than the endogenous cyclin B1 protein, and transfected NLS-cyclin B1-YFP protein was expressed at lower levels (Figure 6A). The cyclin A2 knockdown cells exhibited a substantial delay in chromatin condensation (Figure 6B, green points). Expression of WT-cyclin B1-YFP partially rescued chromatin condensation (Figure 6B, yellow points), and NLS-cyclin B1-YFP completely rescued it (Figure 6B, red points). Neither the WT-cyclin B1-YFP nor the NLS-cyclin B1-YFP protein altered the timing of chromatin condensation in the cells treated with GL3 d-siRNAs (Figure 6B, black, blue, and gray points).
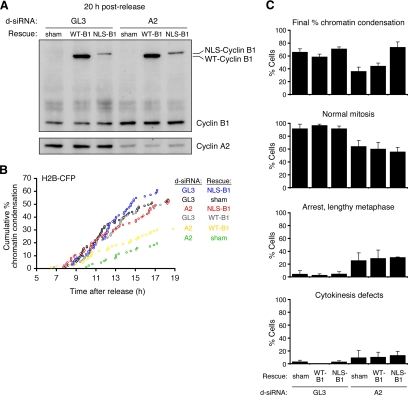
Figure 6.
Constitutively-nuclear cyclin B1 rescues the chromatin condensation delay in cyclin A2 knockdown cells. (A-C) HeLa cells were synchronized by double thymidine block and cotransfected with both the histone H2B-CFP and the mitotic biosensor (MBS), one d-siRNA pool (GL3 or cyclin A2 d-siRNAs), and one rescue construct (sham YFP, wild type [WT] cyclin B1-YFP, or a constitutively-nuclear version of cyclin B1-YFP [NLS-cyclin B1-YFP]). (A) Cyclin levels as assessed by immunoblotting. (B) The timing of chromatin condensation in cells expressing histone H2B-YFP using automated time-lapse epifluorescence microscopy. (C) Final % mitosis chromatin condensation and assessment of mitotic progression phenotypes using the MBS.
Knocking down cyclin A2 causes delays in early mitotic events—NEB (Gong et al., 2007) and chromatin condensation (Figures 1, 2, and 6B)—and it also causes defects in mitotic progression (Gong et al., 2007). This raises the question of whether NLS-cyclin B1-YFP rescues the defects in mitotic progression as well as it rescues chromatin condensation. Approximately 60% of the cells transfected with GL3 d-siRNAs underwent mitosis during the period of the microscopy (5–19 h after release from the second thymidine block), and mitosis was qualitatively and quantitatively normal in ∼90% of the cells that underwent mitosis (Figure 6). In the cyclin A2 knockdown cells subjected to a sham rescue, the number of mitotic cells dropped, and the proportion of the mitotic cells that underwent normal mitosis fell from ∼90–60%. However, although the number of cells entering mitosis was restored to normal in the NLS-cyclin B1-YFP-transfected cells, the proportion of the mitoses that were normal was not restored (Figure 6C). These data suggest that although NLS-cyclin B1-YFP can rescue the timing of chromatin condensation, it cannot completely replace cyclin A2 function for normal mitotic progression. This could be because of some required function of cyclin A2 per se, or because the tempo of cyclin B1-Cdk1 nuclear import is different in NLS-cyclin B1-YFP–expressing cyclin A2-knockdown cells compared with control cells.
Cyclins B1 and B2 Are Required to Maintain Mitosis in the Presence of Nocodazole
The present study and our previous work (Gong et al., 2007) focus on early mitotic events—chromatin condensation and NEB, respectively—and found relatively subtle phenotypes in cyclin B1 knockdown cells. The previous study also hinted that both cyclins B1 and B2 might be more important for later mitotic events, because cyclin B1/B2 double knockdown cells displayed a high incidence of late mitotic abnormalities (Gong et al., 2007). This hypothesis is also suggested by the fact that cyclin A2 is degraded before cyclins B1 and B2 (Pines and Hunter, 1991; Clute and Pines, 1999), and so the phosphorylations carried out by cyclin A2-Cdks may have turned over by the later parts of mitosis. If so, then lengthening the duration of mitosis might increase the severity of the cyclin B1/B2 knockdown phenotypes.
To test this hypothesis, we treated cells with the microtubule depolymerizing drug nocodazole. Nocodazole-treated cells progress through interphase with near-normal timing and undergo NEB normally, but then arrest in prometaphase due to the inability of the cell to satisfy the spindle assembly checkpoint. Nocodazole-arrested cells degrade their cyclin A2 normally but do not degrade cyclin B1 or B2. They can maintain a spindle assembly checkpoint arrest for many hours. Eventually nocodazole-treated cells sometimes exit mitosis, a process thought to be due to the gradual degradation of B-type cyclins (Brito and Rieder, 2006). Thus, cyclin B1 and/or B2 knockdown cells might have difficulty maintaining the mitotic state in the presence of nocodazole. Alternatively, other kinases active during a spindle assembly checkpoint arrest, such as p38 MAP kinase and Erk1/2 (Minshull et al., 1994; Wang et al., 1997; Takenaka et al., 1998; Eves et al., 2006; Rosner, 2007), might be able to maintain the arrest in the absence of the B-type cyclins.
To examine these possibilities, we synchronized HeLa cells, transfected them with the MBS plus control (GL3), cyclin B1, and/or cyclin B2 d-siRNAs, and released the cells from thymidine block into normal growth medium or growth medium containing 100 ng/ml nocodazole. We then determined the timing of NEB (Figure 8A) and how long the mitotic state was maintained (Figures 7 and 8). Figure 7A shows an example of a GL3 transfected cell in the absence of nocodazole. The cell broke down its nuclear envelope, as indicated by the dispersal of the MBS from the nucleus to the whole of the cell, and began reforming its nucleus, as indicated by the reaccumulation of the MBS near the center of the cell, within 40–45 min (Figure 7A). Cyclin B1, cyclin B2, and cyclin B1/B2 double knockdown cells also generally completed mitosis in the absence of nocodazole, with cyclin B1 and cyclin B1/B2 knockdown cells taking slightly longer to complete mitosis (data not shown).
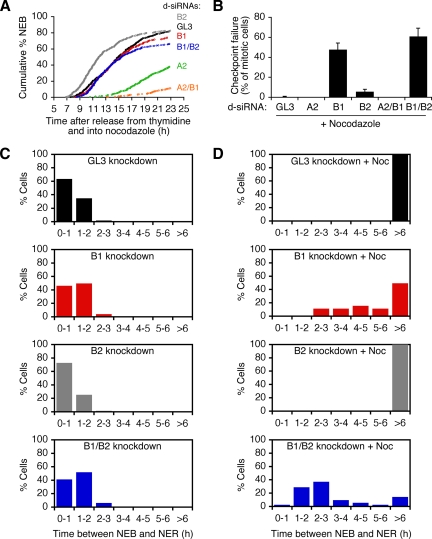
Figure 8.
The spindle assembly checkpoint arrest is compromised in cyclin B1 and cyclin B1/B2 knockdown cells. (A–D) HeLa cells were synchronized by double thymidine block, transfected with the mitotic biosensor (MBS) plus various d-siRNAs, and released into growth media containing 100–200 ng/ml nocodazole. (A) Timing of nuclear envelope breakdown (NEB) in GL3 or cyclin knockdown cells in the presence of nocodazole. (B) Final % of mitotic cells that exited mitosis and reformed their nuclear envelopes. (C and D) The duration of mitosis in control and cyclin knockdown cells treated without (C) or with (D) nocodazole in the growth media.
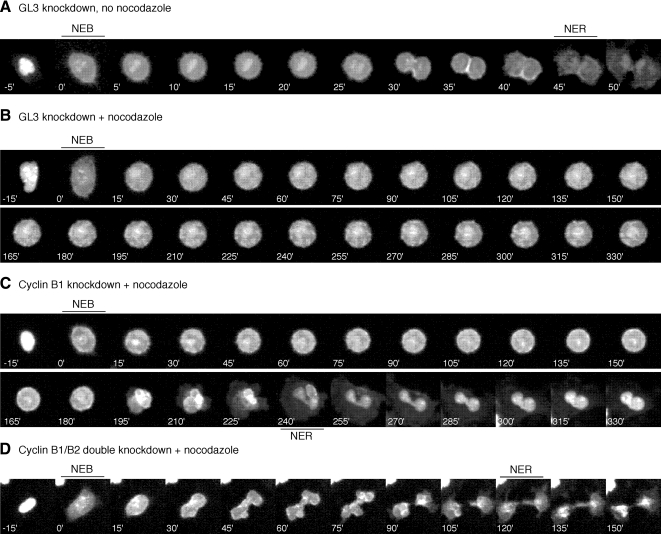
Figure 7.
Defects in the spindle assembly checkpoint in cyclin B1/B2 knockdown cells. (A–D) HeLa cells were synchronized by double thymidine block, transfected with the mitotic biosensor (MBS) plus GL3 or cyclin d-siRNAs, and released into growth media containing the microtubule poison, nocodazole. (A) A control GL3-transfected cell undergoes a qualitatively normal mitosis in ∼40 min, whereas (B) GL3 cells arrest in mitosis in the presence of nocodazole. (C) Cyclin B1 knockdown cells round up in the presence of nocodazole but eventually flatten out and reform their nuclear envelopes. (D) Cyclin B1/B2 double knockdown cells flatten sooner than cyclin B1 single knockdown cells. Times indicate minutes relative to NEB.
In the presence of nocodazole, the control GL3 cells and cyclin B2 knockdown cells underwent NEB and maintained a round mitotic morphology throughout the duration of the time-lapse movie (Figure 7B and data not shown). In contrast, many of the cyclin B1 and cyclin B1/B2 double knockdown cells underwent NEB, stayed round for a short period of time, and then flattened back out, with the nuclear envelope reforming and the MBS relocalizing to the nucleus (Figure 7, C and D). Some of these cells appeared to have attempted cytokinesis and failed, resulting in abnormal multi-lobed interphase nuclei (Figure 7C).
About half (48%) of the cyclin B1 knockdown cells and 61% of the cyclin B1/B2 double knockdown cells exited mitosis before the end of the video, whereas mitotic exit was rarely seen in GL3 cells (0.5%) and cyclin B2 knockdown cells (6%) (Figure 8B). Spindle assembly checkpoint failure was not seen in cyclin A2 knockdown cells (Figure 8B). The median duration of the nocodazole-induced mitotic arrest was more than 6 h in the GL3 and cyclin B2 knockdown cells, 4.3 h in the cyclin B1 knockdown cells, and 2.2 h in the cyclin B1/B2 double knockdown cells. Thus, the ability to maintain a mitotic arrest was compromised in the cyclin B1 cells, and was further compromised in the cyclin B1/B2 double knockdown cells. This implies that both cyclin B1 and cyclin B2 help maintain mitosis in nocodazole-treated cells, with the role of cyclin B2 being most apparent when cyclin B1 is knocked down.
DISCUSSION
Here we began by addressing the question of which cyclins are required for an early mitotic event, chromatin condensation. Using diced siRNA pools and fluorescence video microscopy, we found that knocking down cyclin A2 delays chromatin condensation (Figure 1), indicating that cyclin A2 directly or indirectly regulates the process and corroborating previous microinjection studies with cyclin A2 antibodies and p21-derived cyclin A2 inhibitors (Pagano et al., 1992; Furuno et al., 1999). The delay in chromatin condensation was also seen with a second d-siRNA pool, derived from the cyclin A2 3′-UTR, and was partially rescued by expressing an RNAi-resistant cyclin A2 construct (Figure 2), arguing that the delay is due to cyclin A2 knockdown rather than an off-target effect. Cyclin A2 knockdown also delayed the onset of histone H3 phosphorylation, an event that correlates with chromatin condensation.
In principle the delay in chromatin condensation seen in cyclin A2 knockdown cells could be due to a delay in DNA replication and a consequent activation of the DNA replication/damage checkpoint response. However, we found no evidence for a delay in DNA replication or for prolonged activation of the DNA replication checkpoint in cyclin A2 knockdown cells (Figure 4). The simplest interpretation of these results is that cyclin A2 is a mitotic regulator, as previously proposed (Swenson et al., 1986; Pagano et al., 1992; Guadagno and Newport, 1996; Furuno et al., 1999; Fung et al., 2007; Gong et al., 2007; Deibler and Kirschner, 2010). The alternative possibility is that even though cyclin A2 is dispensable for the replication of most of the DNA, a small proportion of the DNA remains unreplicated and brings about enough activation of the DNA replication checkpoint to block mitotic entry but not enough checkpoint activation to detect by phospho-Chk1 immunoblotting.
We then assessed whether cyclin A2's role in chromatin condensation might be mediated by cyclin B1-Cdk1, whose nuclear accumulation and activation is regulated by cyclin A2 (Fung et al., 2007; Gong et al., 2007; De Boer et al., 2008). Knocking down cyclin B1 alone caused a small delay in chromatin condensation, and it caused a marked delay in a cyclin A2-knockdown background (Figure 1), consistent with the idea that both cyclin A2 and cyclin B1 can support chromatin condensation. By examining individual cells we found that chromatin condensation commences when cyclin B1 begins to accumulate in the nucleus (Figure 5), putting cyclin B1 in the proper location to bring about chromatin condensation and demonstrating that the cyclin B1 nuclear translocation and chromatin condensation are tightly linked. This finding agrees with recent results from Gavet and Pines showing that cyclin B1-Cdk1 activation, as monitored with a FRET reporter, is tightly associated with cyclin B1-Cdk1 nuclear translocation and chromatin condensation (Gavet and Pines, 2010a, b). Expression of a constitutively-nuclear form of cyclin B1 rescues chromatin condensation in cyclin A2 knockdown cells (Figure 6), indicating that nuclear cyclin B1-Cdk1 complexes can support chromatin condensation in the absence or near-absence of cyclin A2.
There are at least three plausible models consistent with these observations. The first is that cyclin B1-Cdk1 is the normal initiator of chromatin condensation, with cyclin A2 being required because it is necessary for the activation and nuclear accumulation of cyclin B1-Cdk1 complexes. That is, cyclin A2-Cdk is exclusively a primer kinase, and cyclin B1-Cdk1 is an effector kinase. This model is compatible with recent live cell imaging studies of Cdk1 activation that emphasize the tight temporal correlation between cyclin B1-Cdk1 activation and chromatin condensation (Gavet and Pines, 2010a, b) and with the evidence shown here of the tight correlation between cyclin B1-Cdk1 translocation and chromatin condensation. It also fits well with the biochemical identification of cyclin B1-Cdk1 as M-phase promoting factor in frog eggs and marine invertebrate eggs (Dunphy et al., 1988; Lohka et al., 1988; Labbe et al., 1989). The main evidence against this model is the relatively modest delay in chromatin condensation seen in cyclin B1 knockdown cells. However, there are several possible ways to rationalize this modest delay: the small residual levels of cyclin B1 present in knockdown cells could be sufficient to mediate chromatin condensation, or the cyclin A2-Cdk complexes could provide a back-up function.
The second model is that cyclin A2 normally triggers both cyclin B1-Cdk1 activation/translocation and chromatin condensation, but the former is not required for the latter. In this model, cyclin A2 is both a priming kinase and an effector kinase for early mitotic events, whereas cyclin B1 is an effector kinase only for late mitotic events. Consistent with this model, we did see some cells where chromatin condensation began before any detectable cyclin B1 translocation (Figure 5), but there was too much uncertainty in the experimental data to rule out the possibility that some small amount of cyclin B1 was always present in the nucleus at the time of chromatin condensation.
The third model, which we favor (Figure 9), is a hybrid of the first two. Cyclin A2 triggers cyclin B1-Cdk1 activation/translocation, and the nuclear cyclin B1-Cdk1 and cyclin A2 complexes cooperate to bring about early mitotic events like chromatin condensation and NEB. This model accounts for all of our experimental observations, including the relatively mild delays in chromatin condensation (this work) and NEB (Gong et al., 2007) seen in cyclin B1-knockdown cells, and the near-abolition of both events in cyclin A2/B1-double knockdown cells. This model is also supported by recent studies of HeLa cell extracts (Deibler and Kirschner, 2010). These extracts carry out Cdk activation and NEB in vitro (Deibler and Kirschner, 2010) and are more amenable to biochemical dissection than intact cells. Deibler and Kirschner have found that in HeLa cell extracts, cyclin A2 synergizes with fully-active cyclin B1-Cdk1 to promote NEB (Deibler and Kirschner, 2010), in strong support of the hybrid model.
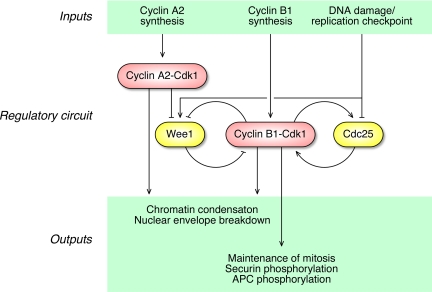
Figure 9.
Schematic view of the hypothesized roles of cyclin A2 and cyclin B1 in the regulation of early and late mitotic events. Based on recent studies of HeLa cell extracts (Deibler and Kirschner, 2010), we assume that Cdk1 is the relevant cyclin A2-associated Cdk.
Notable motif-level features of this hybrid model (Figure 9) include feed-forward regulation (cyclin A2 promotes chromatin condensation and NEB both directly and through the intermediacy of cyclin B1-Cdk1), interlinked feedback loops, and reciprocal regulation (Cdk1 activates Cdc25 and inactivates Wee1; DNA damage activates Wee1 and inactivates Cdc25). Modeling studies and quantitative experimental studies in Xenopus egg extracts argue that these features help generate a robust, bistable mitotic trigger (Novak and Tyson, 1993; Pomerening et al., 2003; Sha et al., 2003; Ferrell, 2008). In many ways, the triggering of mitosis in HeLa cells is more complicated than it is in Xenopus extract. For example, the timing of mitosis is determined by the rate of cyclin B synthesis in Xenopus extracts and embryo (Hartley et al., 1996), but not in somatic cells (Jin et al., 1996; Jin et al., 1998). In addition, Cdk1 appears to increase in activity more gradually in HeLa cell extracts than it does in Xenopus extracts (Deibler and Kirschner, 2010). Finally, there are checkpoints present in somatic cells that are inoperative in Xenopus extracts and embryos (Dasso and Newport, 1990; Minshull et al., 1994). The systems-level motifs shown in Figure 9 may contribute to the robustness of the more complicated somatic cell mitotic trigger, or, alternatively, they may be vestiges of the simpler embryonic cell cycle oscillator circuit.
We also addressed the question of whether cyclins B1 and B2 might be more critical for later steps in mitosis. Knocking down cyclin B1 and B2 has been reported to cause qualitative defects in mitotic progression (Gong et al., 2007). However, mitosis is normally fairly brief in HeLa cells, and there is only a short time between when cyclin A2 is degraded (in prometaphase) and when cyclins B1 and B2 are degraded (in metaphase). This may explain why cyclin A2 can cover for cyclins B1 and B2 fairly well in an unperturbed mitosis. Mitosis can be lengthened or arrested by adding microtubule-disrupting agents like nocodazole. It seemed plausible that a requirement for B1/B2 function might be more apparent in the spindle assembly checkpoint arrest than it is in an unperturbed mitosis. Indeed, this was the case: nocodazole-treated cyclin B1 knockdown cells were defective in maintaining the mitotic state, and cyclin B1/B2 double knockdown cells were more severely defective (Figures 7 and 8). This result was not unexpected; nevertheless, given how unexpectedly subtle the early mitotic phenotypes were in cyclin B1/B2 knockdown cells, it was nice to obtain a result that conformed to expectations.
The cyclin B1 knockdown phenotypes seen here and in our previous work (Gong et al., 2007) are weaker than those reported by Fung and coworkers (Fung et al., 2007). There are several possible explanations for this discrepancy. It is possible that Fung et al. achieved a more complete cyclin B1 knockdown than we did, although from the cyclin B1 blots shown in the three papers this did not appear to be the case. It is possible that the shRNA constructs used by Fung and coworkers produced off-target effects or caused a general saturation of normal miRNA processing and export pathways (Grimm et al., 2006), and that this contributed to the stronger phenotype they reported. It is also possible that cyclin A2 can cover the functions of cyclin B1 and B2 to differing extents in different cell lines (even in different HeLa cell lines). Whatever the explanation, our work shows that cyclin A2 is the single most vulnerable cyclin target for the disruption of M-phase, and that knocking down cyclins A2 and B1 together synergistically disrupts M-phase. These findings could be of interest in developing antimitotic therapeutic agents.
The present studies point toward the importance of cyclin A2 in early mitotic events and underscore the many unanswered questions about the regulation of cyclin A2 complexes. When, exactly, are the cyclin A2 complexes activated? And what brings about their activation? Is their activation triggered simply by the accumulation of cyclin A2 to a threshold level (probably not, because cyclin A2 overexpression does not accelerate mitotic entry [Figure 2]), or the release of the CDK regulators Wee1 and Cdc25 from control by the DNA replication checkpoint, or something else? Answers to these questions will be important for understanding how the initiation of mitosis is tied into the earlier events of the cell cycle.
ACKNOWLEDGMENTS
We thank Ari Firestone, Xuedong Liu, Sam Pearlman, Joe Pomerening, Silvia Santos, Tony Tsai, Jeff Ubersax, Qiong Yang, other members of the Ferrell lab, Josh Jones and members of the Meyer lab, and D. Solow-Cordero and J. Wu from the Stanford High-Throughput BioScience Center for advice and assistance with these studies and for help with this manuscript. This work was supported by a grant from the National Institutes of Health (GM046383).
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-05-0393) on July 21, 2010.
REFERENCES
- Brandeis M., Rosewell I., Carrington M., Crompton T., Jacobs M. A., Kirk J., Gannon J., Hunt T. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc. Natl. Acad. Sci. USA. 1998;95:4344–4349. doi: 10.1073/pnas.95.8.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O., Liou J., Park W. S., Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito D. A., Rieder C. L. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M., Olivier P., Diehl J. A., Fero M., Roussel M. F., Roberts J. M., Sherr C. J. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute P., Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1999;1:82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- Dasso M., Newport J. W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- De Boer L., Oakes V., Beamish H., Giles N., Stevens F., Somodevilla-Torres M., Desouza C., Gabrielli B. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene. 2008;27:4261–4268. doi: 10.1038/onc.2008.74. [DOI] [PubMed] [Google Scholar]
- Deibler R. W., Kirschner M. W. Quantitative reconstitution of mitotic CDK1 activation in somatic cell extracts. Mol. Cell. 2010;37:753–767. doi: 10.1016/j.molcel.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen N., Pines J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 2001;153:121–136. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienemann A., Sprenger F. Requirements of cyclin a for mitosis are independent of its subcellular localization. Curr. Biol. 2004;14:1117–1123. doi: 10.1016/j.cub.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Draviam V. M., Orrechia S., Lowe M., Pardi R., Pines J. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J. Cell Biol. 2001;152:945–958. doi: 10.1083/jcb.152.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988;54:423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Eves E. M., Shapiro P., Naik K., Klein U. R., Trakul N., Rosner M. R. Raf kinase inhibitory protein regulates aurora B kinase and the spindle checkpoint. Mol. Cell. 2006;23:561–574. doi: 10.1016/j.molcel.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr. Feedback regulation of opposing enzymes generates robust, all-or-none bistable responses. Curr. Biol. 2008;18:R244–245. doi: 10.1016/j.cub.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. L., Nurse P. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 1996;15:850–860. [PMC free article] [PubMed] [Google Scholar]
- Fung T. K., Ma H. T., Poon R. Y. Specialized roles of the two mitotic cyclins in somatic cells: cyclin A as an activator of M phase-promoting factor. Mol. Biol. Cell. 2007;18:1861–1873. doi: 10.1091/mbc.E06-12-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno N., den Elzen N., Pines J. Human cyclin A is required for mitosis until mid prophase. J. Cell Biol. 1999;147:295–306. doi: 10.1083/jcb.147.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavet O., Pines J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J. Cell Biol. 2010a;189:247–259. doi: 10.1083/jcb.200909144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavet O., Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell. 2010b;18:533–543. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley S., Kramer E., Gieffers C., Gannon J., Peters J. M., Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D., Pomerening J. R., Myers J. W., Gustavsson C., Jones J. T., Hahn A. T., Meyer T., Ferrell J. E., Jr. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr. Biol. 2007;17:85–91. doi: 10.1016/j.cub.2006.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D., Streetz K. L., Jopling C. L., Storm T. A., Pandey K., Davis C. R., Marion P., Salazar F., Kay M. A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Gu Y., Turck C. W., Morgan D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Guadagno T. M., Newport J. W. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Hagting A., Jackman M., Simpson K., Pines J. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol. 1999;9:680–689. doi: 10.1016/s0960-9822(99)80308-x. [DOI] [PubMed] [Google Scholar]
- Hartley R. S., Rempel R. E., Maller J. L. In vivo regulation of the early embryonic cell cycle in Xenopus. Dev. Biol. 1996;173:408–419. doi: 10.1006/dbio.1996.0036. [DOI] [PubMed] [Google Scholar]
- Hendzel M. J., Wei Y., Mancini M. A., Van Hooser A., Ranalli T., Brinkley B. R., Bazett-Jones D. P., Allis C. D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Jackman M., Firth M., Pines J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J. 1995;14:1646–1654. doi: 10.1002/j.1460-2075.1995.tb07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M., Kubota Y., den Elzen N., Hagting A., Pines J. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol. Biol. Cell. 2002;13:1030–1045. doi: 10.1091/mbc.01-07-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. W., Knoblich J. A., Lehner C. F. Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 1998;12:3741–3751. doi: 10.1101/gad.12.23.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P., Gu Y., Morgan D. O. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J. Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P., Hardy S., Morgan D. O. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J. Cell Biol. 1998;141:875–885. doi: 10.1083/jcb.141.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. T., Myers J. W., Ferrell J. E., Meyer T. Probing the precision of the mitotic clock with a live-cell fluorescent biosensor. Nat. Biotechnol. 2004;22:306–312. doi: 10.1038/nbt941. [DOI] [PubMed] [Google Scholar]
- Kanda T., Sullivan K. F., Wahl G. M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 1998;8:377–385. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- Labbe J.-C., Capony J.-P., Caput D., Cavadore J.-C., Derancourt J., Kaghad M., Lelias J.-M., Picard A., Doree M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989;8:3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Matzuk M. M., Sung W. K., Guo Q., Wang P., Wolgemuth D. J. Cyclin A1 is required for meiosis in the male mouse. Nat. Genet. 1998;20:377–380. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Hayes M. K., Maller J. L. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc. Natl. Acad. Sci. USA. 1988;85:3009–3013. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990;9:2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Sun H., Tonks N. K., Murray A. W. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Morgan D. O. The Cell Cycle: Principles of Control. London, UK: New Science Press Ltd.; 2007. [Google Scholar]
- Murphy M., Stinnakre M. G., Senamaud-Beaufort C., Winston N. J., Sweeney C., Kubelka M., Carrington M., Brechot C., Sobczak-Thepot J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat. Genet. 1997;15:83–86. doi: 10.1038/ng0197-83. [DOI] [PubMed] [Google Scholar]
- Myers J. W., Chi J. T., Gong D., Schaner M. E., Brown P. O., Ferrell J.E.J. Minimizing off-target effects by usiing diced siRNAs for RNA interference. J. RNAi Gene Silencing. 2006;17:181–194. [PMC free article] [PubMed] [Google Scholar]
- Myers J. W., Ferrell J. E., Jr. Silencing gene expression with Dicer-generated siRNA pools. In: Carmichael G. G., editor. RNA Silencing: Methods and Protocols. vol. 309. Totowa NJ: Humana Press Inc.; 2005. pp. 93–196. [DOI] [PubMed] [Google Scholar]
- Myers J. W., Jones J. T., Meyer T., Ferrell J. E., Jr. Recombinant Dicer efficiently converts large dsRNAs into siRNAs suitable for gene silencing. Nature Biotechnology. 2003;21:324–328. doi: 10.1038/nbt792. [DOI] [PubMed] [Google Scholar]
- Nguyen T. B., Manova K., Capodieci P., Lindon C., Bottega S., Wang X. Y., Refik-Rogers J., Pines J., Wolgemuth D. J., Koff A. Characterization and expression of mammalian cyclin b3, a prepachytene meiotic cyclin. J. Biol. Chem. 2002;277:41960–41969. doi: 10.1074/jbc.M203951200. [DOI] [PubMed] [Google Scholar]
- Novak B., Tyson J. J. Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J. Cell Sci. 1993;106:1153–1168. doi: 10.1242/jcs.106.4.1153. [DOI] [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Okano T., Tachibana K., Kishimoto T. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO J. 1992;11:1763–1772. doi: 10.1002/j.1460-2075.1992.tb05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J. Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerening J. R., Sontag E. D., Ferrell J. E., Jr. Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat. Cell Biol. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- Reber A., Lehner C. F., Jacobs H. W. Terminal mitoses require negative regulation of Fzr/Cdh1 by Cyclin A, preventing premature degradation of mitotic cyclins and String/Cdc25. Development. 2006;133:3201–3211. doi: 10.1242/dev.02488. [DOI] [PubMed] [Google Scholar]
- Rosner M. R. MAP kinase meets mitosis: a role for Raf Kinase Inhibitory Protein in spindle checkpoint regulation. Cell Div. 2007;2:1. doi: 10.1186/1747-1028-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W., Moore J., Chen K., Lassaletta A. D., Yi C. S., Tyson J. J., Sible J. C. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc. Natl. Acad. Sci. USA. 2003;100:975–980. doi: 10.1073/pnas.0235349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986;47:861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Takenaka K., Moriguchi T., Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- Wang X. M., Zhai Y., Ferrell J. E., Jr. A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J. Cell Biol. 1997;137:433–443. doi: 10.1083/jcb.137.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]