Glucose deficiency leads to the induction of eIF2α phosphorylation at serine 51, which results in a global inhibition of protein synthesis. Phosphorylation of eIF2α is an adaptive process that establishes a cytoprotective state in glucose-deficient cells, with possible implications in biological responses that interfere with glucose metabolism.
Abstract
Various forms of stress induce pathways that converge on the phosphorylation of the alpha (α) subunit of eukaryotic translation initiation factor eIF2 at serine 51 (S51), a modification that results in a global inhibition of protein synthesis. In many cases eIF2α phosphorylation is a biological response that facilitates cells to cope with stressful environments. Glucose deficiency, an important form of stress, is associated with an induction of apoptosis. Herein, we demonstrate that eIF2α phosphorylation is a key step in maintaining a balance between the life and death of a glucose-deficient cell. That is, eIF2α phosphorylation acts as a molecular switch that shifts cells from a proapoptotic to a cytoprotective state in response to prolonged glucose deficiency. This adaptation process is associated with the timely expression of proteins and activation of pathways with significant contributions to cell survival and adaptation including the X-linked inhibitor of apoptosis protein (XIAP). We also show that among the eIF2α kinases GCN2 plays a proapoptotic role whereas PERK and PKR play a cytoprotective one in response to glucose deficiency. Our data demonstrate that eIF2α phosphorylation is a significant determinant of survival and adaptation of glucose-deficient cells with possible important implications in biological processes that interfere with glucose metabolism.
INTRODUCTION
Glucose molecules are a fundamental source of fuel for physiological processes. Energy in the form of adenosine triphosphate (ATP) is extracted from glucose through glycolysis to produce two ATP molecules. Subsequently, in the presence of sufficient oxygen, the energy acquiring process continues to produce thirty-six ATP molecules through oxidative phosphorylation (Pelicano et al., 2006). When eukaryotic cells encounter conditions of limited oxygen supply, they are no longer capable of utilizing oxygen as the final electron acceptor during oxidative phosphorylation, and therefore cells resort to extracting energy through glycolysis (Pelicano et al., 2006). Otto Warburg discovered that cancer cells produce most of their ATP through glycolysis even in the presence of sufficient oxygen, a phenomenon commonly known as “aerobic glycolysis” or the “Warburg effect” (Warburg et al., 1927). This shift of glucose metabolism from oxidative phosphorylation to glycolysis, although inefficient in energy production, provides cancer cells with several growth advantages over the surrounding normal cells, such as the ability to survive conditions of fluctuating oxygen levels (Pouyssegur et al., 2006), to favor tumor invasion (Swietach et al., 2007), and to suppress anticancer immune factors (Fischer et al., 2007). Most importantly, proliferating cancer cells can utilize intermediates of the glycolytic pathway as precursors for synthesis of amino acids, nucleic acids, and lipids (Kroemer and Pouyssegur, 2008). Given the above-mentioned importance of glucose, decreased glucose uptake has been linked to the induction of apoptosis in most cells (Moley and Mueckler, 2000). The molecular mechanisms that underlie the proapoptotic effects of glucose deficiency are complex and in some cases cell type specific and involve an interplay between tumor suppressor and oncogenic pathways including p53, the hypoxia inducible factor 1α (HIF-1α) and AKT/protein kinase B (PKB; Kroemer and Pouyssegur, 2008).
Glucose deficiency is a strong inducer of the unfolded protein response (UPR), which was first characterized as a transcriptional up-regulation of a set of genes that encode glucose-regulated proteins (GRPs) in response to glucose/energy deprivation (Kozutsumi et al., 1988). As glucose levels decline, ATP supply decreases, so protein folding becomes less efficient in the lumen of endoplasmic reticulum (ER), leading to the accumulation of unfolded or misfolded proteins. UPR is now considered as an important signaling pathway evolved in the ER to cope with stress induced by protein misfolding. An important stress sensor of UPR is the PKR-like ER-associated kinase (PERK), which mediates the phosphorylation of the α subunit of the eukaryotic translation initiation factor eIF2 at serine 51 (S51; Harding et al., 1999). Phosphorylated eIF2α prevents the recycling of the eIF2-bound GDP to GTP by the guanine nucleotide exchange factor (GEF) eIF2B (Sonenberg and Hinnebusch, 2009). As such, formation of the eIF2-GTP-tRNAMet ternary complex is impeded resulting in the inhibition of translation initiation (Sonenberg and Hinnebusch, 2009). Although eIF2α phosphorylation leads to a global inhibition of protein synthesis, specific mRNAs can bypass this limitation and be efficiently translated under conditions of increased eIF2α phosphorylation. Translation of these mRNAs is facilitated by the presence of an internal ribosomal entry site (IRES) in their 5′ untranslated region (5′UTR; Komar and Hatzoglou, 2005), or the presence of small open reading frames (uORFs) in their 5′ UTRs as has been demonstrated for mRNAs encoding for the activating transcription factor 4 (ATF4) and ATF5 (Vattem and Wek, 2004; Zhou et al., 2008). Phosphorylation of eIF2α is mediated by a family of kinases each of which responds to distinct forms of environmental stress (Wek et al., 2006). In addition to PERK, the eIF2α kinase family includes the heme-regulated inhibitor (HRI), which is activated by iron or heme deficiency as well as oxidative stress, the general control nonderepressible-2 (GCN2), which is activated by uncharged tRNA caused by amino acid deficiency, and the RNA-dependent protein kinase (PKR), which is an interferon (IFN)-inducible protein that becomes activated by binding to double-stranded (ds) RNA.
Although eIF2α phosphorylation was previously shown to be induced in glucose-deficient cells (Scheuner et al., 2001; Gomez et al., 2008), the biological effects of eIF2α phosphorylation in response to this type of stress have not been understood. Also, it is not presently clear either whether PERK is the only kinase that responds to glucose deficiency and mediates the biological function of eIF2α phosphorylation (Gomez et al., 2008). Herein, we demonstrate that eIF2α phosphorylation is initially a proapoptotic response, which becomes cytoprotective under long-term glucose deficiency. Among the eIF2α kinases, PERK and PKR play a cytoprotective role as opposed to GCN2, which is mainly proapoptotic. At the molecular level, we show that adaptation to glucose deficiency is associated with an interplay between proapoptotic and cell survival pathways, all of which depend on eIF2α phosphorylation and converge on the regulation of caspase-3.
MATERIALS AND METHODS
Cell Culture Conditions and Treatments
HT1080 cells (CCL-121) were cultured in Dulbecco's modified Eagle medium (DMEM; Wisent, St.-Bruno, QC, Canada) plus 10% heat-inactivated calf serum (Invitrogen, Carlsbad, CA) and 100 of U/ml penicillin-streptomycin (Wisent). A549 cells (CCL-185), Hela cells (CCL-2), and HCT116 XIAP−/− cells (Cummins et al., 2004), and isogenic wild-type and XIAP−/− mouse embryonic fibroblasts (MEFs; Rumble et al., 2008), were maintained in DMEM plus 10% fetal bovine serum (Wisent) and 100 U/ml penicillin-streptomycin (Wisent). MEFs lacking PERK (Harding et al., 2000b), PKR (Durbin et al., 2002), GCN2 (Maurin et al., 2005) or that were deficient in the phosphorylation of eIF2α at S51 (i.e., eIF2αA/A MEFs; Scheuner et al., 2001) together with their isogenic wild-type counterparts were cultured as previously described (Krishnamoorthy et al., 2008). The DMEM used contains 50 mM glucose unless stated otherwise. For glucose deprivation, cells were maintained in glucose-deplete DMEM (Invitrogen) supplemented with serum and antibiotics as described above. 2-Deoxyglucose (2-DG; BioShop, Burlington, ON, Canada) was dissolved in sterile double-distilled water and used at a concentration of 50 mM in cells grown under glucose-replete DMEM conditions. Thapsigargin (TG; Sigma, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) and used at a concentration of 1 μM.
Protein Extraction and Immunoblot Analysis
Lysis of the cells, preparation of protein extracts, and quantification of proteins were performed as previously described (Baltzis et al., 2007). Preparation of protein extracts for detection of caspase-12 and caspase-3 was performed as reported (Cheong et al., 2003). Immunoblotting analysis was performed as previously described (Baltzis et al., 2007). The primary antibodies included anti-CHOP/GADD135 polyclonal antibody (sc-575, Santa Cruz Biotechnology, Santa Cruz, CA), anti-GRP78/BiP polyclonal antibody (sc-13968, Santa Cruz), anti-eIF2α mAb (L575A5, Cell Signaling, Beverly, MA), anti-eIF2α Ser(P)-51 polyclonal antibody (44728G, Invitrogen), anti-actin C4 mAb (MP Biomedicals, Solon, OH), anti-β-tubulin mAb (32–2600, Invitrogen), anti-ATF4 polyclonal antibody (10835, Proteintech, Chicago, IL), anti-XIAP and anti-cIAP1/2 polyclonal antibodies (Ungureanu et al., 2006), anti-AKTSer(P)-473 polyclonal antibody (9271, Cell Signaling), anti-AKT polyclonal antibody (9272, Cell Signaling), anti-TRAF2 polyclonal antibody (sc-7346, Santa Cruz), anti-Bcl-xL polyclonal antibody (2764, Cell Signaling), anti-S6 Ser(P)235/236 polyclonal antibody (2211, Cell Signaling), anti-S6 polyclonal antibody (Cell Signaling, 2212), anti-caspase-12 polyclonal antibody (2202, Cell Signaling), and anti-caspase-3 polyclonal antibody (9661, Cell Signaling). All antibodies were used at a final concentration of 0.1–1 μg/ml. After incubation with anti-mouse IgG or anti-rabbit IgG antibodies conjugated to horseradish peroxidase (HRP), proteins were visualized with enhanced chemiluminescence (ECL) reagent (Perkin Elmer Life Sciences, Waltham, CA) detection system according to the manufacturer's instructions. Quantification of protein bands was performed by densitometry using Scion Image (Frederick, MD) from NIH.
Polysome Profiles and mRNA Quantification
Polysome profiles were performed as previously described (Baltzis et al., 2007). RNA from each fraction of polysome analysis was isolated using Trizol reagent (Invitrogen). The relative distributions of specific mRNAs were monitored by reverse transcriptase (RT)-quantitative PCR (qPCR) as previously described (Li et al., 2008). Briefly cDNA was synthesized with the SuperScript III kit (Invitrogen), and the abundance of each cDNA was quantified by real-time PCR (qRT-PCR) using the Power SYBR Green PCR Master Mix (Applied Biosystem, Foster City, CA; Li et al., 2008). The primers that were used to visualize XIAP mRNA are as follows: XIAP forward primer 5′-CGAGCTGGGTTTCTTTATACCG-3′, XIAP reverse primer 5′-GCAATTTGGGGATATTCTCCTGT-3′, whereas those used to visualize Bcl-xL are as follows: Bcl-xL forward primer 5′-GACAAGGAGATGCAGGTATTGG-3′, Bcl-xL reverse primer 5′-TCCCGTAGAGATCCACAAAAGT-3′.
[35S]Methionine Labeling
[35S]Methionine labeling of cells was performed as previously described (Raven et al., 2008) with minor modifications. Briefly, cells were treated with 2-DG, or incubated in glucose-deplete media, or treated with 1 μM TG in glucose-replete media for various periods of time as specified in each experiment. EXPRESS 35S protein-labeling mix (PerkinElmer, Norwalk, CA; NEG-072007MC) was added to the cells at a final concentration of 30 μCi/ml for 30 min before protein extraction. The radioactivity was measured in 100 μg of protein extracts as described (Raven et al., 2008).
Flow Cytometry Analysis
Cells were prepared for flow cytometry as previously described (Kazemi et al., 2004). Data were analyzed using the WinMDI 2.8 software (Scripps Research Institute, San Diego, CA). Samples were gated on a dot-plot showing the forward scatter and side scatter to exclude debris not within normal cell size.
RNA Interference
Hela cells were transfected with nonspecific scrambled small interfering RNA (siRNA; Dharmacon, Boulder, CO) or human XIAP siRNA (Dharmacon; L-004098) by Lipofectamine 2000 (Invitrogen) according to manufacturer's protocol. Briefly, cells were plated in six-well plates, such that cells would be 50% confluent the following day. The next day cells are incubated with transfection reagent and 50 μM of siRNA in serum-free media for 4 h. Cells were then incubated in media containing 10% serum but lacking antibiotics for 24 h before being treated with glucose deprivation or 2-DG for an additional 24 h.
RESULTS
Human Tumor Cells Respond to Glucose Deficiency by Inducing eIF2α Phosphorylation
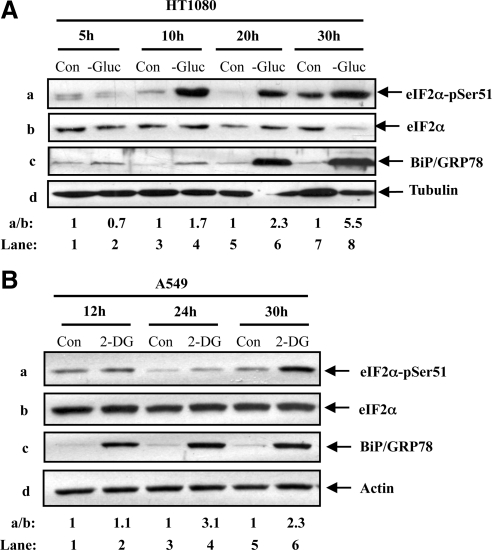
A previous study showed that eIF2α phosphorylation is induced in human pancreatic β-cells under conditions of glucose deprivation (Gomez et al., 2008). To determine whether this is applicable to other cell types, we used human fibrosarcoma HT1080 cells and the human lung cancer A549 cells to assess the phosphorylation of eIF2α at S51 under conditions of glucose deficiency. We observed that eIF2α phosphorylation was increased in HT1080 cells under conditions of short as well as prolonged glucose deprivation (Figure 1Aa). Induction of eIF2α phosphorylation was associated with an increased expression of the glucose-regulated protein 78/immunoglobulin binding protein (GRP78/BiP; Figure 1Ac), which is an indicator of the induction of the UPR. Phosphorylation of eIF2α and GRP78/BiP expression were also increased in A549 cells after treatment with 2-DG, a modified glucose molecule that acts as an inhibitor of glycolysis (Kang and Hwang, 2006; Figure 1B). These data indicated that tumor cells respond to glucose deficiency by increasing eIF2α phosphorylation and GRP78/BiP levels probably due to induction of UPR.
Figure 1.
Glucose deficiency induces eIF2α phosphorylation in human tumors. HT1080 cells (A) and A549 cells (B) were maintained in media containing glucose (Con; A, B), media lacking glucose (−Gluc; A) or in media containing glucose supplemented with 50 mM 2-deoxyglucose (2-DG; B) for the indicated times. (A and B) Whole cell extract (50 μg of protein) was used for immunoblot analysis with anti-eIF2α-pSer51 antibody (a), anti-eIF2α antibody (b), anti-BiP/GRP78 antibody (c), anti-tubulin antibody (Ad) or anti-actin antibody (Bd). The ratio of phosphorylated to total protein of eIF2α is indicated (a/b). Quantification of protein bands was performed by densitometry using Scion Image from NIH. The data represent one of three reproducible experiments. The ratio was set to 1 for each time point control (untreated).
Induction of eIF2α Phosphorylation Influences Cell Fate during Glucose Deficiency
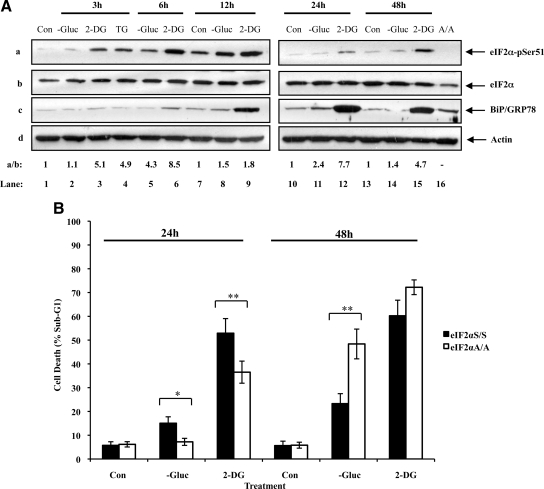
To address the significance of eIF2α phosphorylation, we assessed the effects of glucose deficiency on MEFs containing either a wild-type allele of eIF2α (eIF2αS/S) or a knockin eIF2α allele bearing the S51A mutation (eIF2αA/A). First, we verified that eIF2α phosphorylation and GRP78/BiP expression were both induced in wild-type MEFs under conditions of glucose deprivation or 2-DG treatment (Figure 2A). Then, we examined the susceptibility of eIF2αS/S and eIF2αA/A MEFs to glucose deficiency by measuring the population of cells in sub-G1 after treatment (Supplementary Figure 1). We found that glucose deprivation or 2-DG resulted in a higher amount of death in eIF2αS/S MEFs than in eIF2αA/A MEFs after 24 h of treatment (Figure 2B and Supplementary Figure 1). However, when cells were maintained without glucose or were treated with 2-DG for 48 h, we noticed that eIF2αA/A MEFs became more proapoptotic than eIF2αS/S MEFs (Figure 2B and Supplementary Figure 1). Between the two treatments of glucose deficiency, we noted that 2-DG was more efficient than glucose deprivation in inducing the death of eIF2αS/S and eIF2αA/A MEFs, possibly due to the strong inhibitory effects of 2-DG on glucose metabolism (Figure 2B). These data provided evidence for two different functions of eIF2α phosphorylation in glucose-deficient cells. That is, increased eIF2α phosphorylation in response to short-term glucose deficiency elicits a proapoptotic response that is counteracted by the induction of a cytoprotective response under prolonged stress.
Figure 2.
eIF2α phosphorylation determines cell fate during glucose deficiency. (A) eIF2αS/S MEFs were maintained in media containing glucose (Con), media deprived of glucose (−Gluc), or media containing glucose supplemented with 50 mM of 2-deoxyglucose (2-DG) for the indicated time points. MEFs were also treated with 1 μM thapsigargin (TG) for 3 h (lane 4). Whole cell extract (50 μg of protein) were subjected to immunoblot analysis for eIF2α-pSer51 (a), eIF2α (b), BiP/GRP78 (c), or actin (d). Extracts from eIF2αA/A cells were included as control (lane 16). The ratio of phosphorylated to total eIF2α protein for each lane is indicated (a/b). The ratio was set to 1 for each time point control (untreated). Quantification of protein bands was performed by densitometry using Scion Image from NIH. The data represent one of two reproducible experiments. (B) eIF2αS/S and eIF2αA/A MEFs were treated as in A for 24 or 48 h. The percentage of cells in sub-G1 phase, which represents cell death, was measured by propidium iodide staining and flow cytometry analysis (see also Supplementary Figure 1). Histograms show the average cell death ± SEM calculated from seven reproducible experiments. Two-tailed t test: *p < 0.05, **p < 0.01.
Distinct Roles of the eIF2α Kinases in Cells Maintained under Glucose Deficiency
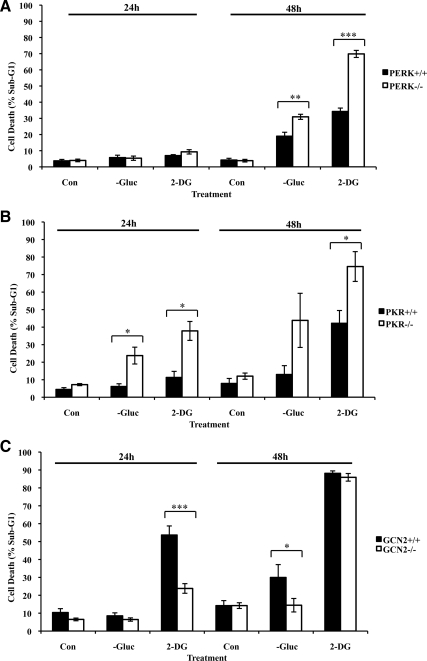
To identify the biological roles of the eIF2α kinases in glucose-deficient cells, we used MEFs that were devoid of each eIF2α kinase as well as wild-type isogenic MEFs (Figure 3). Because the function of HRI is restricted to erythroid cells, we focused our efforts on PKR, PERK, or GCN2. We found that both PKR and PERK played a cytoprotective effect given that PERK−/− and PKR−/− MEFs were more susceptible to the proapoptotic effects of glucose deprivation or 2-DG treatment than their corresponding wild-type isogenic MEFs (Figure 3, A and B, Supplementary Figures 2 and 3). On the other hand, GCN2 conveyed a proapoptotic effect because a higher number of GCN2+/+ than GCN2−/− MEFs was susceptible to death by glucose deprivation or 2-DG treatment (Figure 3C and Supplementary Figure 4). It is of interest that treatment with 2-DG for 48 h yielded a significant amount of death in both GCN2+/+ and GCN2−/− MEFs, making it difficult to conclude on the proapoptotic function of GCN2 for this particular time point (Figure 3C). From these experiments, we noticed that glucose deficiency did not produce an identical response in all wild-type MEFs. That is, although PERK+/+ (Figure 3A) and PKR+/+ MEFs (Figure 3B) were similarly susceptible to death by glucose deficiency, GCN2+/+ MEFs exhibited an increased sensitivity to glucose deficiency compared with PERK+/+ and PKR+/+ MEFs (Figure 3C). These different responses of the wild-type MEFs were most likely due to the differences in the genetic background of the cells. Nevertheless, the role of each eIF2α kinase in cell death induced by glucose deficiency is well supported by the fact that the data were obtained from the analysis of isogenic pairs of MEFs. The implication of all three eIF2α kinases in regulation of cell death by glucose deficiency implied a certain degree of redundancy for inducing eIF2α phosphorylation. This is consistent with our observations that induction of eIF2α phosphorylation was still detectable in MEFs deficient in each eIF2α kinase in response to glucose deprivation or 2-DG treatment (Supplementary Figure 5).
Figure 3.
Distinct roles of eIF2α kinases in response to glucose deprivation. PERK+/+ and PERK−/− MEFs (A), PKR+/+ and PKR−/− MEFs (B) or GCN2+/+ and GCN2−/− MEFs (C) were kept in media containing glucose (Con), media lacking glucose (−Gluc), or media containing glucose supplemented with 50 mM of 2-deoxyglucose (2-DG) for 24 or 48 h. The percentage of cells in sub-G1 phase, which represents cell death, was measured by propidium iodide staining and flow cytometry analysis (see also Supplementary Figures 2–4). Histograms show the average cell death ± SEM calculated from six, five, and seven reproducible experiments for PERK, PKR and GCN2 MEFs, respectively. Two-tailed t test: *p < 0.05, **p < 0.01, ***p < 0.001).
Glucose Deficiency Inhibits Global Protein Synthesis Independent of eIF2α Phosphorylation
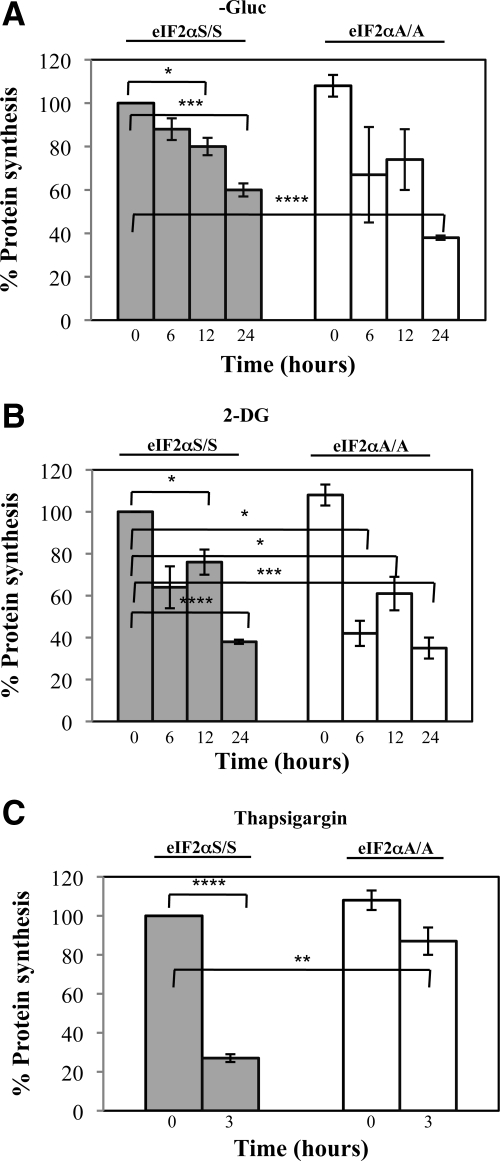
To address the mechanisms of regulation of apoptosis, we assessed the role of eIF2α phosphorylation in regulation of protein synthesis in cells maintained under glucose deficiency. To do so, eIF2αS/S and eIF2αA/A MEFs were subjected to metabolic labeling with [35S]methionine under conditions of glucose starvation or 2-DG treatment. As control, cells were subjected to ER stress after treatment with TG. We observed that the rates of protein synthesis were significantly reduced at comparable levels in both eIF2αS/S and eIF2αA/A MEFs during glucose deprivation (Figure 4A) or 2-DG treatment (Figure 4B). On the other hand, induction of ER stress by TG yielded a substantial inhibition of protein synthesis in eIF2αS/S MEFs but not in eIF2αA/A MEFs (Figure 4C) as previously documented (Harding et al., 2000b). These data indicated that inhibition of protein synthesis by glucose deficiency takes place independent of eIF2α phosphorylation. This is different from the regulation of protein synthesis in cells subjected to ER stress, suggesting that induction of eIF2α phosphorylation by glucose deficiency may not represent a typical ER stress response.
Figure 4.
Glucose deficiency impairs protein synthesis independent of eIF2α phosphorylation. eIF2αS/S and eIF2αA/A MEFs were maintained in media containing glucose, media deprived of glucose (−Gluc; A), or media containing glucose supplemented with 50 mM of 2-deoxyglucose (2-DG; B) for the indicated time points. The same cells were left untreated or treated with 1 μM of thapsigargin in media containing glucose for 3 h (C). (A–C) Cells were incubated in the presence of [35S]methionine for the last 30 min of each time point, and the amount of [35S] labeled protein was quantified as described in Materials and Methods. Histograms represent the mean ± SEM of two independent experiments performed in duplicates. Two-tailed t test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Induction of eIF2α Phosphorylation in Glucose-deficient Cells Affects Proteins with Distinct Roles in Cell Survival and Cell Death
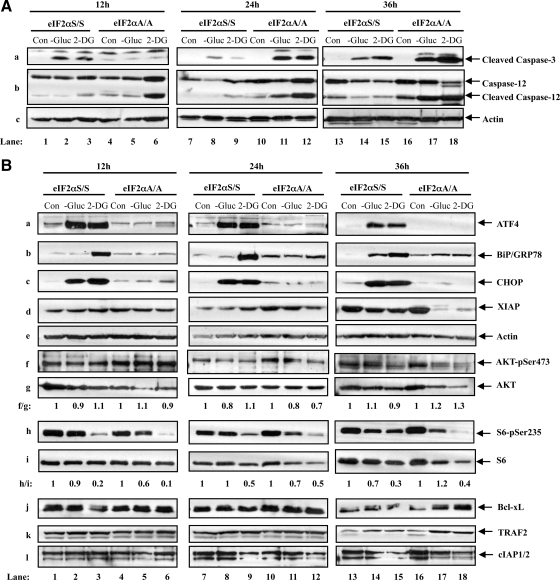
To identify the signaling pathways that determine the biological effects of eIF2α phosphorylation, first we looked at the regulation of caspase-3. We detected a higher amount of activated (i.e., cleaved) caspase-3 in eIF2αS/S than in eIF2αA/A MEFs at 12 h of glucose deprivation or 2-DG treatment (Figure 5Aa, cf. lanes 2 and 3 with 5 and 6). However, as cells were maintained under conditions of glucose deficiency for longer periods of time, we observed a progressive increase of cleaved caspase-3 in eIF2αA/A MEFs over eIF2αS/S MEFs, which became more evident at 36 h of treatment (Figure 5Aa, cf. lanes 14 and 15 with 17 and 18). We also looked at caspase-12, which is an ER-resident protein that is thought to promote the proapoptotic effects of caspase-9 and -3 (Masud et al., 2007). We detected a higher amount of cleaved caspase-12 in eIF2αA/A than in eIF2αS/S cells, which was further increased with the time of glucose deprivation or 2-DG treatment (Figure 5Ab). The levels of cleaved caspase-12 were proportional to the levels of cleaved caspase-3 in eIF2αA/A cells at 24 or 36 h of treatment, consistent with the notion that both enzymes contribute to the increased cell death in the eIF2αA/A cells at later time points.
Figure 5.
Induction of eIF2α phosphorylation in glucose-deficient cells affects proteins with distinct functions in cell survival and cell death. (A and B) eIF2αS/S and eIF2αA/A MEFs were maintained in media containing glucose (Con), glucose-free media (−Gluc), or media containing glucose supplemented with 50 mM of 2-deoxyglucose (2-DG) for the indicated times. Whole cell extract (50 μg of proteins) were subjected to immunoblot analysis for detection of cleaved caspase-3 (Aa), caspase-12 (Ab), or actin (Ac). The same amount of protein extracts was also used to detect ATF4 (Ba), BiP/GRP78 (Bb), CHOP (Bc), XIAP (Bd), actin (Be), AKTpSer473 (Bf), AKT (Bg), S6pSer235 (Bh), S6 (Bi), Bcl-xL (Bj), TRAF2 (Bk), and cIAP1/2 (Bl). The data represent one of three reproducible experiments. The ratio of phosphorylated to total AKT protein for each lane is indicated (f/g). The ratio of phosphorylated to total S6 protein for each lane is indicated (h/i). The ratio was set to 1 for each time point control (untreated).
It is well established that induction of eIF2α phosphorylation leads to the activation of the ATF4-CHOP proapoptotic pathway in response to ER stress (Harding et al., 2000a). We observed that glucose deficiency resulted in a substantial increase of ATF4 in eIF2αS/S MEFs but not in eIF2αA/A MEFs for the various periods of treatment (Figure 5Ba). Glucose deficiency also resulted in a substantial induction of CHOP in eIF2αS/S MEFs compared with eIF2αA/A MEFs (Figure 5Bc) most likely due to increased ATF4 (Figure 5Ba), which acts as a transcriptional inducer of the CHOP gene (Harding et al., 2000a). Taken together, these data suggested that induction of eIF2α phosphorylation in glucose-deficient cells results in the activation of the ATF4-CHOP pathway, which can account, at least in part, for the higher induction of death in eIF2αS/S than eIF2αA/A cells during the early times of glucose deficiency. Induction of ATF4 was associated with an up-regulation of GRP78/BiP in eIF2αS/S MEFs, which was more evident for 2-DG treatment than glucose deprivation (Figure 5Bb). This result indicated that glucose deprivation and 2-DG may not act through identical pathways, a notion that is also supported by previous studies (Kang and Hwang, 2006). This is further supported by our findings showing that caspase-12 cleavage did not occur similarly in cells subjected to 2-DG treatment or glucose withdrawal (Figure 5Ab). Moreover, cells treated with 2-DG were more susceptible to death than cells maintained under glucose deprivation.
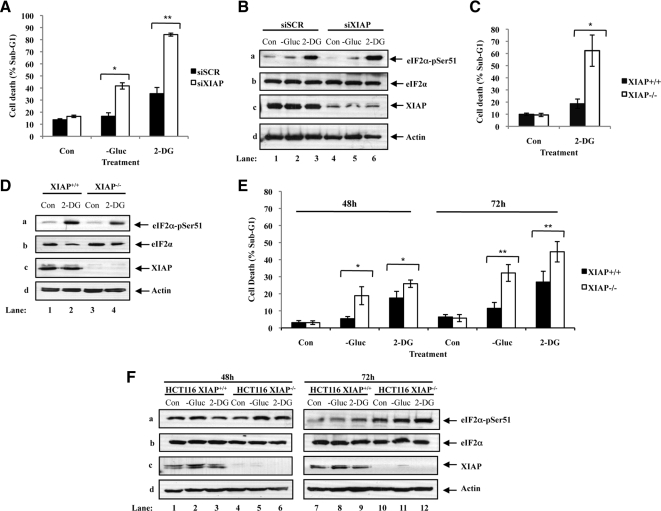
To determine the cause of accelerated death of eIF2αA/A MEFs in response to prolonged glucose deficiency, we examined the expression of proteins with key roles in regulation of apoptosis. We found that proteins like the cellular inhibitor of apoptosis protein 1/2 (cIAP1/2), Bcl-xL or TRAF2 were not substantially affected by the status of eIF2α phosphorylation in cells kept under glucose deficiency for a short or long period of time (Figure 5B, j–l). On the other hand, we saw that expression of X-linked inhibitor of apoptosis protein (XIAP) was substantially reduced in eIF2αA/A MEFs compared with eIF2αS/S MEFs after long-term glucose deficiency (Figure 5Bd). Inasmuch as XIAP functions as an inhibitor of caspase-9 and -3 (Suzuki et al., 2001; Morizane et al., 2005), its down-regulation can account for the increased amount of death in eIF2αA/A MEFs compared with eIF2αS/S MEFs after long-term glucose deficiency. The antiapoptotic role of XIAP in glucose-deficient cells was verified in HeLa cells in which XIAP was targeted by siRNA, in HCT116 cells containing an homozygous deletion of XIAP as well as in primary XIAP−/− MEFs (Figure 6 and Supplementary Figures 6-8).
Figure 6.
XIAP is required to promote cell survival during conditions of glucose deprivation. Hela cells (A and B) were treated with control scrambled siRNA or siRNA specific for XIAP for 24 h and maintained in media containing glucose (Con), media lacking glucose (−Gluc), or media containing glucose supplemented with 50 mM of 2-DG for additional 24 h. Primary XIAP+/+ and XIAP−/− MEFs (C and D) as well as HCT116 XIAP+/+ and HCT116 XIAP−/− cells (E and F) were then kept in media containing glucose (Con), media lacking glucose (−Gluc), or media containing glucose supplemented with 50 mM of 2-DG for 24 h. (A, C, and E) The percentage of cells in subG1 phase, which represents cell death, was measured by propidium iodide staining and flow cytometry analysis. Histograms show the average cell death ± SEM calculated from three independent experiments. Two-tailed t test: *p < 0.05, **p < 0.01. (B, D, and F) Whole cell extract (50 μg of protein) were subjected to immunoblot analysis for the indicated proteins.
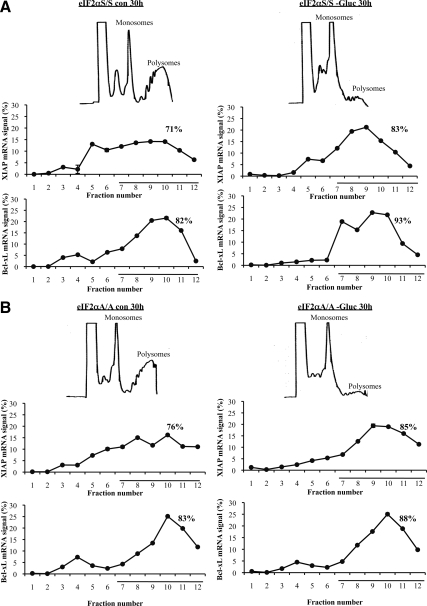
Previous work demonstrated that XIAP and Bcl-xL mRNAs contains an IRES that plays an essential role in the translation of each mRNA species under stress (Holcik et al., 1999; Yoon et al., 2006). Although glucose deficiency inhibits protein synthesis independent of eIF2α phosphorylation (Figure 4, A and B), we speculated that eIF2α phosphorylation might play a role in translational regulation of specific messages under conditions of glucose deficiency, and XIAP mRNA might be one of them. To test this hypothesis, we analyzed the efficiency of XIAP mRNA translation in glucose-deficient cells by polysome profiles. This is a technique that separates monosomes from polyribosomes (i.e., polysomes) according to their densities on a sucrose gradient. Efficiently translated mRNAs are bound to polyribosomes, whereas mRNAs that are poorly translated are found with monosomes or disomes. We observed that glucose starvation for 30 h resulted in a substantial reduction of the polyribosome fractions in both eIF2αS/S and eIF2αA/A MEFs (Figure 7). This observation was in line with the inhibition of global protein synthesis in both cell types that was measured by metabolic labeling with [35S]methionine (Figure 4A). Despite the translational blockade, we observed that a significant amount of XIAP as well as Bcl-xL mRNAs remained bound to polyribosomes in glucose-deprived cells, which was similar between eIF2αS/S and eIF2αA/A MEFs (Figure 7, A and B). Glucose deprivation enhanced ATF4 mRNA translation and reduced ribosomal protein (rp) L27 mRNA translation in eIF2αS/S compared with eIF2αA/A MEFs indicating the functional role of eIF2α phosphorylation in translational control under the experimental conditions (Supplementary Figure 9).
Figure 7.
Induction of eIF2α phosphorylation facilitates XIAP expression in glucose-deficient cells at a posttranslational level. eIF2αS/S (A) and eIF2αA/A MEFs (B) were kept in media containing glucose (Con) or in glucose-free media (−Gluc) for 30 h. Lysates were subjected to polysome profile analysis as described in Materials and Method. Gradients were fractionated and absorbance at 254 nm was recorded (top panels). The distribution of XIAP and Bcl-xL mRNAs in the fractions of the sucrose gradients was quantified by qRT-PCR (bottom panels), where the underlined fractions in the graphs signify the polysomes. The graphs represent the mean ± SEM of two independent experiments.
The above data indicated that the mRNA of both, XIAP and Bcl-xL can bypass the inhibitory effect of glucose deprivation on mRNA translation and be efficiently translated under this form of stress. Given that expression of XIAP mRNA did not differ between eIF2αS/S and eIF2αA/A MEFs in the absence or presence of glucose deficiency (data not shown), we conclude that down-regulation of XIAP in eIF2αA/A MEFs occurred at a posttranslational level. Because eIF2α phosphorylation can act as an inducer of PI3K-Akt/PKB pathway under certain forms of stress (Kazemi et al., 2007), and Akt/PKB was shown to stabilize XIAP by phosphorylation (Dan et al., 2004), we sought to examine a possible link between Akt/PKB and XIAP posttranslational regulation in glucose-deficient cells. We found that glucose deprivation or 2-DG treatment did not result in a substantial change of Akt/PKB phosphorylation at S473 or ribosomal S6 protein at S235 in both eIF2αS/S and eIF2αA/A MEFs when normalized to AKT or S6 total levels, respectively (Figure 5B, f and h). These data indicated that down-regulation of XIAP is not dependent on Akt/PKB in glucose-deficient cells. These findings also suggested that the adaptation process to glucose deficiency mediated by eIF2α phosphorylation does not rely on the PI3K-Akt/PKB pathway.
DISCUSSION
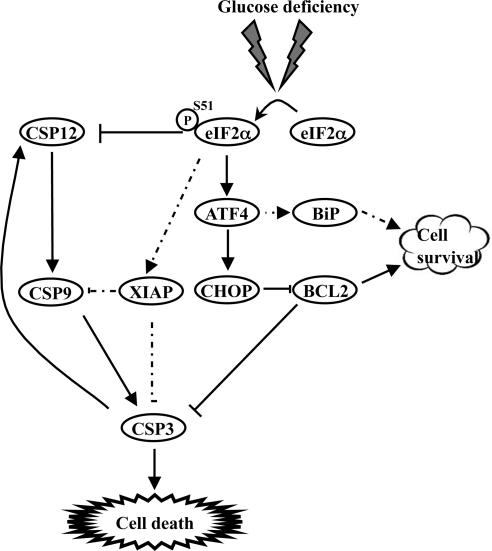
Glucose deficiency plays a key role in apoptosis through various mechanisms including the induction of the mitochondrial death cascade, the expression of proapoptotic Bax, activation of the JNK/MAPK pathway, and induction of p53-dependent apoptosis (Moley and Mueckler, 2000). Despite the induction of the proapoptotic pathways, cells are capable of adapting to prolonged glucose deficiency through mechanisms that are not well understood. Here, we provide strong evidence that eIF2α phosphorylation is a molecular switch that controls the balance between the death and survival of cells in response to glucose deficiency (Figure 8). From our work and work of others (Scheuner et al., 2001; Gomez et al., 2008), it appears that induction of eIF2α phosphorylation is not a cell type–specific effect but rather a general response of cells to glucose deficiency.
Figure 8.
Effect of eIF2α phosphorylation during conditions of glucose deficiency. The model displays the short (solid arrows) and prolonged effects (dashed arrows) of eIF2α phosphorylation on cells during glucose deficiency. Initially, eIF2α phosphorylation induces cells death through the induction of ATF4-CHOP pathway, which leads to activation of caspase-3 (CSP3). This proapoptotic pathway is counteracted by the up-regulation of GRP78/BiP, which in itself may not be sufficient to block the induction of cell death by CHOP. Under prolonged conditions of glucose deficiency, however, eIF2α phosphorylation is required to maintain the levels of XIAP in stressed cells, which functions as an inhibitor of caspase-9 (CSP9) and caspase-3 (CSP3). In addition, eIF2α phosphorylation acts as an inhibitor of caspase-12 (CSP12), which was previously shown to enhance the activation of caspase-9 and -3. These opposing effects of eIF2α phosphorylation on XIAP and caspase-12 may account for the induction of cytoprotective state, which overcomes the proapoptotic effects of ATF4-CHOP.
In line with our findings, previous work demonstrated that eIF2α phosphorylation is an important decision maker between cell survival and death in response to various forms of stress (Wek et al., 2006). Specifically, the survival properties of eIF2α phosphorylation are mediated by the activation of the phosphoinositide 3-kinase (PI3K; Kazemi et al., 2007), the induction of transcription factor NF-κB (Wek et al., 2006) or the proteasomal degradation of p53 (Baltzis et al., 2007). The proapoptotic effects of phosphorylated eIF2α are due to the induction of the ATF4-CHOP pathway (Wek et al., 2006) as well as the translational down-regulation of survival proteins such as Bcl-xL (Mounir et al., 2009). Consistent with a proapoptotic role, other studies showed that eIF2α dephosphorylation during various forms of stress correlates with better survival (Boyce et al., 2005). The ability of phosphorylated eIF2α to control cell survival or death depends on the type of stimuli and the specificity of the kinase that mediates the phosphorylation of eIF2α. That is, although PKR is mainly proapoptotic (Scheuner et al., 2006; Mounir et al., 2009), PERK is largely cytoprotective in response to various types of stress (Harding et al., 2000b). However, unlike other forms of stress, our data clearly show that PERK and PKR play a cytoprotective role as opposed to GCN2, which acts in a proapoptotic manner in response to glucose deficiency. This proapoptotic role of GCN2 was unexpected given that this eIF2α kinase is required for adaptation to amino acid deprivation in mice (Zhang et al., 2002). As such, glucose deficiency and amino acid starvation may utilize distinct pathways that converge on eIF2α phosphorylation with opposing biological outcomes. PKR, PERK, and GCN2 respond to glucose deficiency, but as yet each eIF2α kinase determines a distinct biological outcome in cells (Figure 3). Although the kinases have evolutionary conserved catalytic domains, their regulatory domains and their localization within the cells differs (Wek et al., 2006). Consequently, their interactions and proximity to diverse survival and apoptotic factors could partially account for the functional differences of the kinases in response to glucose deficiency. Furthermore, each eIF2α kinase may undergo distinct posttranslational modifications that modulate their specificity toward eIF2α and other possible substrates in response to various forms of stress. For example, our group demonstrated an important role of tyrosine phosphorylated PKR in the inhibition of protein synthesis through eIF2α phosphorylation-dependent and -independent mechanisms (Su et al., 2006).
Inhibition of protein synthesis by eIF2α phosphorylation is considered as a major cause of apoptosis (Holcik and Sonenberg, 2005). However, from [35S]methionine metabolic labeling (Figure 4) and polysome profile analyses (Figure 7), we found that inhibition of protein synthesis caused by glucose deficiency is independent of eIF2α phosphorylation. This is different from a typical ER stress response in which translational inhibition by eIF2α phosphorylation is essential for cytoprotection (Scheuner et al., 2001). During glucose deficiency we observed that proteins involved in UPR such as ATF4, CHOP, and BiP were up-regulated in manner that was dependent on eIF2α phosphorylation (Figure 5).
The stimulation of apoptosis involves a delicate balance between the inducers (e.g., CHOP) and the inhibitors of apoptosis (e.g., XIAP). The induction of CHOP in the eIF2αS/S cells explains the cleavage of caspase-3 observed at early time points in the eIF2αS/S (Ma and Hendershot, 2004; Kim et al., 2008). Although the eIF2αS/S cells remain apoptotic, there is a decrease in the execution of apoptosis after prolonged glucose deficiency, this may be explained by the induction of BiP, which confers cells protection during stressful conditions (Kim et al., 2008). In addition, XIAP protein levels were higher in the eIF2αS/S cells compared with the eIF2αA/A cells, a finding that can account for the protection against apoptosis during stress from glucose deficiency (Figures 5 and 6). XIAP is an important regulator of cell survival because it interferes with the activity of caspase-3 and -9 (Dubrez-Daloz et al., 2008). The eIF2αA/A cells were not capable of maintaining the levels of XIAP protein under prolonged glucose deficiency. Therefore, caspase-3 activation and hence accelerated death observed after prolonged stress in the eIF2αA/A cells was consistent with the decrease in XIAP protein levels. Although XIAP was demonstrated to be under IRES-mediated translational control (Holcik et al., 1999), our study shows that translational control of XIAP takes place efficiently in glucose-deficient cells in a manner that is independent of eIF2α phosphorylation (Figure 7). This is different from a recently described regulation of XIAP mRNA translation by phosphorylated eIF2α in response to osmotic stress (Bevilacqua et al., 2010), indicating the functional relationship between XIAP translation and eIF2α phosphorylation is dependent on the type of stress. Additionally, eIF2αS/S and eIF2αA/A cells contained similar amounts of cellular XIAP mRNA (data not shown), as such, XIAP down-regulation in eIF2αA/A cells is a posttranslational event through mechanisms that are not immediately known. For example, interaction of XIAP with other proteins such as survivin (Dohi et al., 2004), HS1-associated protein X1 (HAX-1; Kang et al., 2010), BMP receptor 2 (Liu et al., 2009), or Notch (Liu et al., 2007) have been shown to lead to XIAP stabilization by preventing its ubiquitination and proteasomal degradation. As such, the possibility remains that phosphorylated eIF2α regulates the expression and/or stability of one or more of XIAP interacting proteins under glucose deficiency. Although in theory treatment of eIF2αA/A MEFs with proteasome inhibitors should prevent the down-regulation of XIAP under glucose deficiency, in practice this experiment has led to inconclusive results due to high susceptibility of glucose-starved cells to deleterious side effects of proteasome inhibitors (data not shown). Another possibility is that phosphorylated eIF2α regulates expression and/or activity of a kinase other than Akt that controls XIAP stability. For example, protein kinase A (PKA) has been implicated in XIAP destabilization via the phosphorylation of survivin and disruption of the survivin-XIAP complex (Dohi et al., 2007). However, to date there is no evidence to suggest a role of PKA in glucose-deficient cells.
Previous work showed that during ER stress PERK elicits a cytoprotective response through the induction of the transcription and translation of cIAP1 and cIAP2 in a manner that relies on eIF2α phosphorylation (Hamanaka et al., 2009). Contrary to ER stress, however, we observed a down-regulation in cIAP1 and cIAP2 levels in glucose-deficient cells independent of eIF2α phosphorylation (Figure 5). This is another indication that glucose deficiency does not represent a typical ER stress response but rather a response that can integrate various proapoptotic and survival pathways all of which depend on eIF2α phosphorylation.
There has been an established link between eIF2α phosphorylation and regulation of glucose metabolism. That is, eIF2αA/A mice develop hypoglycemia that results in their death 18 h after birth (Scheuner et al., 2001). Further analysis demonstrated that these mice develop hepatic failure where eIF2α phosphorylation is required for the expression of enzymes necessary for gluconeogenesis: the making of glucose molecules from simpler carbon sugars (Scheuner et al., 2001). Interestingly, PERK−/− mice gradually develop diabetes 2–4 wk after birth because of the progressive loss of β-cells (Harding et al., 2001). Further work established that eIF2α phosphorylation is necessary to prevent oxidative damage and ER stress by regulating the expression of genes that maintain β-cell function and limit oxidative stress (Back et al., 2009). These findings together with our data provide further evidence that eIF2α phosphorylation plays an essential role in the regulation of glucose homeostasis.
In light of our findings, it is conceivable to speculate that eIF2α phosphorylation plays an important role in cancer development under conditions of low levels of glucose. This may be an essential process because cancer cells are frequently found in such stressful microenvironments and they appear to survive better than normal cells under these conditions. The cytoprotective effects of eIF2α phosphorylation under prolonged glucose deficiency might be sufficient to allow tumor progression and metastasis. Anticancer treatments have recently focused on targeting the glycolytic pathways of tumor (Pan and Mak, 2007), such that it was previously shown that 2-DG could be used alone or in combination with other tumor therapies to increase the efficiency of conventional treatment (Maschek et al., 2004; Kang and Hwang, 2006). As such, further elucidating the role of eIF2α phosphorylation in tumor development under conditions of limited glucose may prove critical for the development of strategies to bypass the cytoprotective effects of eIF2α phosphorylation and impair tumor growth.
Supplementary Material
ACKNOWLEDGMENTS
We thank F. Bunz and B. Vogelstein (John Hopkins University) for the HCT116 XIAP−/− cells; P. Baker (McGill University) for XIAP−/− MEFs; and Koromilas lab members for helpful comments. This work was supported by grants form the Canadian Cancer Society Research Institute (CCRSI 17285 to A.E.K.), Canadian Institutes of Health Research (CIHR MOP 89737 to H.M.), and the National Institutes of Health (DK60596 and DK53307 to M.H.; DK42394, HL52173, and PO1 HL057346 to R.J.K.). H.M. is a recipient of the Fonds de la recherche en santé Quebec (FRSQ) Master's Training Award. P.P. is a recipient of a postdoctoral award from the Montreal Center of Experimental Therapeutics in Cancer (MCETC). A.I.P. is a recipient the Doctoral Frederick Banting Charles Best Canadian Graduate Scholarship from the Canadian Institutes of Health Research (CIHR). M.H. is the CHEO Volunteer Association Endowed Scholar.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-01-0023) on July 21, 2010.
REFERENCES
- Back S. H., Scheuner D., Han J., Song B., Ribick M., Wang J., Gildersleeve R. D., Pennathur S., Kaufman R. J. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltzis D., Pluquet O., Papadakis A. I., Kazemi S., Qu L. K., Koromilas A. E. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J. Biol. Chem. 2007;282:31675–31687. doi: 10.1074/jbc.M704491200. [DOI] [PubMed] [Google Scholar]
- Bevilacqua E., et al. eIF2alpha phosphorylation tips the balance to apoptosis during osmotic stress. J. Biol. Chem. 2010;285:17098–17111. doi: 10.1074/jbc.M110.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M., et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Cheong J. W., Chong S. Y., Kim J. Y., Eom J. I., Jeung H. K., Maeng H. Y., Lee S. T., Min Y. H. Induction of apoptosis by apicidin, a histone deacetylase inhibitor, via the activation of mitochondria-dependent caspase cascades in human Bcr-Abl-positive leukemia cells. Clin. Cancer Res. 2003;9:5018–5027. [PubMed] [Google Scholar]
- Cummins J. M., Kohli M., Rago C., Kinzler K. W., Vogelstein B., Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004;64:3006–3008. doi: 10.1158/0008-5472.can-04-0046. [DOI] [PubMed] [Google Scholar]
- Dan H. C., Sun M., Kaneko S., Feldman R. I., Nicosia S. V., Wang H. G., Tsang B. K., Cheng J. Q. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J. Biol. Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- Dohi T., et al. An IAP-IAP complex inhibits apoptosis. J. Biol. Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- Dohi T., Xia F., Altieri D. C. Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol. Cell. 2007;27:17–28. doi: 10.1016/j.molcel.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrez-Daloz L., Dupoux A., Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7:1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- Durbin R. K., Mertz S. E., Koromilas A. E., Durbin J. E. PKR protection against intranasal vesicular stomatitis virus infection is mouse strain dependent. Viral Immunol. 2002;15:41–51. doi: 10.1089/088282402317340224. [DOI] [PubMed] [Google Scholar]
- Fischer K., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- Gomez E., Powell M. L., Bevington A., Herbert T. P. A decrease in cellular energy status stimulates PERK-dependent eIF2alpha phosphorylation and regulates protein synthesis in pancreatic beta-cells. Biochem. J. 2008;410:485–493. doi: 10.1042/BJ20071367. [DOI] [PubMed] [Google Scholar]
- Hamanaka R. B., Bobrovnikova-Marjon E., Ji X., Liebhaber S. A., Diehl J. A. PERK-dependent regulation of IAP translation during ER stress. Oncogene. 2009;28:910–920. doi: 10.1038/onc.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000a;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding H. P., Zeng H., Zhang Y., Jungries R., Chung P., Plesken H., Sabatini D. D., Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol. Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Harding H. P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000b;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding H. P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Holcik M., Lefebvre C., Yeh C., Chow T., Korneluk R. G. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat. Cell Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- Holcik M., Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- Kang H. T., Hwang E. S. 2-Deoxyglucose: an anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life Sci. 2006;78:1392–1399. doi: 10.1016/j.lfs.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kang Y. J., Jang M., Park Y. K., Kang S., Bae K. H., Cho S., Lee C. K., Park B. C., Chi S. W., Park S. G. Molecular interaction between HAX-1 and XIAP inhibits apoptosis. Biochem. Biophys. Res. Commun. 2010;393:794–799. doi: 10.1016/j.bbrc.2010.02.084. [DOI] [PubMed] [Google Scholar]
- Kazemi S., Mounir Z., Baltzis D., Raven J. F., Wang S., Krishnamoorthy J. L., Pluquet O., Pelletier J., Koromilas A. E. A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway. Mol. Biol. Cell. 2007;18:3635–3644. doi: 10.1091/mbc.E07-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi S., Papadopoulou S., Li S., Su Q., Wang S., Yoshimura A., Matlashewski G., Dever T. E., Koromilas A. E. Control of alpha subunit of eukaryotic translation initiation factor 2 (eIF2 alpha) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2 alpha-dependent gene expression and cell death. Mol. Cell. Biol. 2004;24:3415–3429. doi: 10.1128/MCB.24.8.3415-3429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Xu W., Reed J. C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Komar A. A., Hatzoglou M. Internal ribosome entry sites in cellular mRNAs: mystery of their existence. J. Biol. Chem. 2005;280:23425–23428. doi: 10.1074/jbc.R400041200. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy J., Mounir Z., Raven J. F., Koromilas A. E. The eIF2alpha kinases inhibit vesicular stomatitis virus replication independently of eIF2alpha phosphorylation. Cell Cycle. 2008;7:2346–2351. doi: 10.4161/cc.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Li Y., Bevilacqua E., Chiribau C. B., Majumder M., Wang C., Croniger C. M., Snider M. D., Johnson P. F., Hatzoglou M. Differential control of the CCAAT/enhancer-binding protein beta (C/EBPbeta) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J. Biol. Chem. 2008;283:22443–22456. doi: 10.1074/jbc.M801046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. H., Hsiao H. W., Tsou W. I., Lai M. Z. Notch inhibits apoptosis by direct interference with XIAP ubiquitination and degradation. EMBO J. 2007;26:1660–1669. doi: 10.1038/sj.emboj.7601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Shen J., Pu K., Katus H. A., Ploger F., Tiefenbacher C. P., Chen X., Braun T. GDF5 and BMP2 inhibit apoptosis via activation of BMPR2 and subsequent stabilization of XIAP. Biochim. Biophys. Acta. 2009;1793:1819–1827. doi: 10.1016/j.bbamcr.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Ma Y., Hendershot L. M. The role of the unfolded protein response in tumour development: friend or foe? Nat. Rev. Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- Maschek G., Savaraj N., Priebe W., Braunschweiger P., Hamilton K., Tidmarsh G. F., De Young L. R., Lampidis T. J. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- Masud A., Mohapatra A., Lakhani S. A., Ferrandino A., Hakem R., Flavell R. A. Endoplasmic reticulum stress-induced death of mouse embryonic fibroblasts requires the intrinsic pathway of apoptosis. J. Biol. Chem. 2007;282:14132–14139. doi: 10.1074/jbc.M700077200. [DOI] [PubMed] [Google Scholar]
- Maurin A. C., Jousse C., Averous J., Parry L., Bruhat A., Cherasse Y., Zeng H., Zhang Y., Harding H. P., Ron D., Fafournoux P. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–277. doi: 10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Moley K. H., Mueckler M. M. Glucose transport and apoptosis. Apoptosis. 2000;5:99–105. doi: 10.1023/a:1009697908332. [DOI] [PubMed] [Google Scholar]
- Morizane Y., Honda R., Fukami K., Yasuda H. X-linked inhibitor of apoptosis functions as ubiquitin ligase toward mature caspase-9 and cytosolic Smac/DIABLO. J. Biochem. 2005;137:125–132. doi: 10.1093/jb/mvi029. [DOI] [PubMed] [Google Scholar]
- Mounir Z., Krishnamoorthy J. L., Robertson G. P., Scheuner D., Kaufman R. J., Georgescu M. M., Koromilas A. E. Tumor suppression by PTEN requires the activation of the PKR-eIF2alpha phosphorylation pathway. Sci. Signal. 2009;2:ra85. doi: 10.1126/scisignal.2000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J. G., Mak T. W. Metabolic targeting as an anticancer strategy: dawn of a new era? Sci. STKE. 2007;2007:pe14. doi: 10.1126/stke.3812007pe14. [DOI] [PubMed] [Google Scholar]
- Pelicano H., Martin D. S., Xu R. H., Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J., Dayan F., Mazure N. M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Raven J. F., Baltzis D., Wang S., Mounir Z., Papadakis A. I., Gao H. Q., Koromilas A. E. PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2alpha phosphorylation. J. Biol. Chem. 2008;283:3097–3108. doi: 10.1074/jbc.M709677200. [DOI] [PubMed] [Google Scholar]
- Rumble J. M., Bertrand M. J., Csomos R. A., Wright C. W., Albert L., Mak T. W., Barker P. A., Duckett C. S. Apoptotic sensitivity of murine IAP-deficient cells. Biochem. J. 2008;415:21–25. doi: 10.1042/BJ20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Patel R., Wang F., Lee K., Kumar K., Wu J., Nilsson A., Karin M., Kaufman R. J. Double-stranded RNA-dependent protein kinase phosphorylation of the alpha-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J. Biol. Chem. 2006;281:21458–21468. doi: 10.1074/jbc.M603784200. [DOI] [PubMed] [Google Scholar]
- Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Hinnebusch A. G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q., Wang S., Baltzis D., Qu L. K., Wong A. H., Koromilas A. E. Tyrosine phosphorylation acts as a molecular switch to full-scale activation of the eIF2alpha RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 2006;103:63–68. doi: 10.1073/pnas.0508207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Nakabayashi Y., Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. USA. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P., Vaughan-Jones R. D., Harris A. L. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- Ungureanu N. H., Cloutier M., Lewis S. M., de Silva N., Blais J. D., Bell J. C., Holcik M. Internal ribosome entry site-mediated translation of Apaf-1, but not XIAP, is regulated during UV-induced cell death. J. Biol. Chem. 2006;281:15155–15163. doi: 10.1074/jbc.M511319200. [DOI] [PubMed] [Google Scholar]
- Vattem K. M., Wek R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Jiang H. Y., Anthony T. G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Yoon A., Peng G., Brandenburger Y., Zollo O., Xu W., Rego E., Ruggero D. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- Zhang P., et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Palam L. R., Jiang L., Narasimhan J., Staschke K. A., Wek R. C. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J. Biol. Chem. 2008;283:7064–7073. doi: 10.1074/jbc.M708530200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.