Hypoxia-inducible factor-1 (HIF-1) is a key transcription factor for responses to low oxygen. Here we report that the generation of mitochondrial reactive oxygen species are essential for regulating HIF-1 in normal oxygen conditions in the vasculature.
Abstract
Hypoxia-inducible factor-1 (HIF-1) is a key transcription factor for responses to low oxygen. Different nonhypoxic stimuli, including hormones and growth factors, are also important HIF-1 activators in the vasculature. Angiotensin II (Ang II), the main effecter hormone in the renin-angiotensin system, is a potent HIF-1 activator in vascular smooth muscle cells (VSMCs). HIF-1 activation by Ang II involves intricate mechanisms of HIF-1α transcription, translation, and protein stabilization. Additionally, the generation of reactive oxygen species (ROS) is essential for HIF-1 activation during Ang II treatment. However, the role of the different VSMC ROS generators in HIF-1 activation by Ang II remains unclear. This work aims at elucidating this question. Surprisingly, repression of NADPH oxidase-generated ROS, using Vas2870, a specific inhibitor or a p22phox siRNA had no significant effect on HIF-1 accumulation by Ang II. In contrast, repression of mitochondrial-generated ROS, by complex III inhibition, by Rieske Fe-S protein siRNA, or by the mitochondrial-targeted antioxidant SkQ1, strikingly blocked HIF-1 accumulation. Furthermore, inhibition of mitochondrial-generated ROS abolished HIF-1α protein stability, HIF-1–dependent transcription and VSMC migration by Ang II. A large number of studies implicate NADPH oxidase–generated ROS in Ang II–mediated signaling pathways in VSMCs. However, our work points to mitochondrial-generated ROS as essential intermediates for HIF-1 activation in nonhypoxic conditions.
INTRODUCTION
Hypoxia-inducible factor-1 (HIF-1) is a key hypoxia-inducible transcription factor responsible for the adaptation of cells and tissues to low oxygen by regulating such responses as cell metabolism, proliferation and survival, erythropoiesis, and angiogenesis (Semenza, 2003). HIF-1 binds to hypoxia-response elements (HRE) found in promoter or enhancer DNA regions of target hypoxia-inducible genes that include vascular endothelial growth factor (VEGF), glucose transporter-1 (Glut-1), nitric oxide synthases, and likely between 100 and 200 others (Kaelin and Ratcliffe, 2008).
The active HIF-1 complex is a heterodimer consisting of an oxygen-sensitive HIF-1α subunit and a constitutively expressed HIF-1β subunit. HIF-1α possesses an oxygen-dependent degradation domain (ODDD) containing two key proline residues which are, in the presence of oxygen, hydroxylated by HIF prolyl-hydroxylases (PHDs; Kaelin and Ratcliffe, 2008). HIF-1α prolyl-hydroxylation allows for recognition by pVHL, the product of the von Hippel-Lindau tumor suppressor gene, the substrate recognition component of an E3 ubiquitin ligase complex that polyubiquitinates and targets HIF-1α for proteasomal degradation. In hypoxic conditions, low oxygen leads to HIF-1α stabilization due to inhibition of prolyl-hydroxylation and subsequent decreases in HIF-1α ubiquitination and degradation (Cockman et al., 2000; Epstein et al., 2001; Schofield and Ratcliffe, 2004).
In addition to hypoxia, there are also different nonhypoxic HIF activators that include growth factors, hormones, cytokines, and viral proteins (Dery et al., 2005). In vascular smooth muscle cells (VSMCs) we have shown that angiotensin II (Ang II), a vasoactive hormone linked to many cardiovascular functions and diseases, is a potent HIF-1 activator (Richard et al., 2000). Treatment of VSMCs with Ang II regulates HIF-1 by common as well as distinct mechanisms from hypoxia. Ang II increases HIF-1α stability (as in hypoxia), but additionally increases HIF-1α translation and transcription (Page et al., 2002, 2008; Lauzier et al., 2007).
Reactive oxygen species (ROS) are small, extremely reactive molecules due to their unpaired valence electrons. High ROS concentrations are very damaging to cells, because they lead to a free-radical–mediated chain reaction–targeting proteins, lipids, polysaccharides, and DNA (Turrens, 2003). However, low and intermediary levels of ROS are crucial to a number of cellular signaling events (Cai et al., 2002; Varela et al., 2004; Felty et al., 2005; Kimura et al., 2005b; Rhee, 2006; Zhang et al., 2007; Lassegue and Griendling, 2010). There are a number of intracellular ROS generators. Two of the most documented include the NADPH (reduced nicotinamide adenine dinucleotide phosphate) oxidase and the mitochondria. The NADPH oxidase is a complex comprised of membrane bound (Nox1-4 and p22phox) and cytoplasmic (p47phox, p67phox and Rac) subunits (Babior, 2004). Once activated, cytoplasmic subunits interact with their membrane-bound counterparts and create an active complex which oxidizes NADPH, leading to the generation of ROS. In VSMCs, a large body of literature indicates that NADPH oxidase–derived ROS (noxROS) are a primary source of ROS after Ang II treatment (Sorescu et al., 2001; Garrido and Griendling, 2009). On the other hand, mitochondria mainly produce ROS at complex I and complex III of the electron transport chain, whereas a total of nine other ROS-producing sites have been identified (Andreyev et al., 2005). Interestingly, complex III produces ROS in both the mitochondrial matrix and the intermembrane space, whereas the other sites produce superoxide solely in the mitochondrial matrix (Zhang et al., 2007). Mitochondrial-derived ROS (mtROS) have recently gained attention in vascular biology and were shown to be increased by Ang II (Kimura et al., 2005a; Doughan et al., 2008; Nozoe et al., 2008).
Our studies have shown that the generation of ROS during Ang II treatment is essential for both increased HIF-1α translation and stability. Ang II augments HIF-1α translation by noxROS-dependent increases in receptor tyrosine kinase transactivation and phosphatidylinositol 3-kinase (PI3K)/p70S6 kinase (p70S6K) pathway activation (Page et al., 2002; Lauzier et al., 2007). Additionally, our recent work indicated that ROS/H2O2 increased HIF-1α protein accumulation through a Fenton reaction where Fe2+, an essential PHD cofactor, is oxidized to Fe3+, leading to the inactivation of PHD, decreased HIF-1α hydroxylation, and increased HIF-1α protein stabilization (Page et al., 2008).
Here, we set out to identify the ROS generator responsible for HIF-1 stabilization during Ang II treatment. Surprisingly, we show that targeting noxROS generation did not play a major role in HIF-1α stabilization and accumulation in VSMCs treated with Ang II. Instead, the specific targeting of mtROS generation by inhibition of mitochondrial complex III and by using mitochondrial-targeted antioxidants completely abolishes HIF-1α stabilization and accumulation during the treatment of VSMCs with Ang II by reestablishing PHD activity and HIF-1α hydroxylation. Additionally, Ang II–stimulated HIF-1 activity in VSMCs, measured by HIF-1–mediated target gene expression and VSMC migration depends on mtROS generation. These results indicate that mtROS are essential intermediates for HIF-1 induction and activation by Ang II in VSMCs.
MATERIALS AND METHODS
Materials
Ang II, PDGF-BB, myxothiazol, and stigmatellin were all from Sigma-Aldrich (St. Louis, MO). MG132 was from EMD Chemicals (Gibbstown, NJ). VAS2870 was from Vasopharm (Wuerzburg, Germany). Anti-HIF-1α antibody was raised in our laboratory in rabbits immunized against the last 20 amino acids of the C terminal of human HIF-1α (Richard et al., 1999). Anti-hydroxylated HIF-1α antibodies, against either hydroxylated Pro402 or hydroxylated Pro564 of human HIF-1α, were obtained as previously described (Chan et al., 2002, 2005). Monoclonal anti-phospho-p42/p44 MAPK and anti-α-tubulin antibodies were from Sigma-Aldrich. Anti-phospho-p70S6 kinase (Thr389), p70S6 kinase, phospho-AKT, AKT, and monoclonal p38 MAP kinase antibodies were from Cell Signaling (Beverly, MA). p22phox and Rieske Fe-S protein monoclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-p38 (pTpY180/182) polyclonal antibody was from Invitrogen (Carlsbad, CA). Total polyclonal p42/p44 MAPK antibody was from Upstate/Millipore (Billerica, MA). Anti-glutathione S-transferase (GST) antibody was from Novus Biologicals (Littleton, CO). Monoclonal HA.11 antibody was from Covance (Emeryville, CA). Horseradish peroxidase-coupled anti-mouse and anti-rabbit antibodies were from Promega (Madison, WI). A GST-HIF-1α fusion protein, comprised of amino acids 344-582 from human HIF-1α, and pVHL-hemagglutinin (HA) constructs were kind gifts from Drs. Jacques Pouysségur and Peter Ratcliffe respectively. The mitochondrial antioxidant, SkQ1 was from Drs. Vladimir Skulachev and Oleg Fedorkin at the Institute of Mitoengineering, Moscow State University (Skulachev et al., 2009).
Cell Culture
Rat VSMCs were isolated from the thoracic aortas of 6 wk-old male Wistar rats by enzymatic dissociation (Owens et al., 1986). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin, 50 U/ml streptomycin (Invitrogen, Carlsbad, CA) and 2 mM glutamine in a humid atmosphere (5% CO2, 95% air). Cells were serially passaged once reaching confluence, and only cells between passages 4 and 12 were used for experiments. In all experiments, cells were deprived of serum overnight. For MitoSOX assays, cells were cultured in phenol red–free DMEM. Hypoxic conditions were obtained by placing cells in a sealed hypoxic workstation (Ruskinn Technology, Bridgend, United Kingdom). The oxygen level in this workstation was maintained at 1%, with the residual gas mixture containing 94% nitrogen and 5% CO2.
Western Blot Analysis
Confluent cells were lysed in a 2× Laemmli buffer. Protein concentration was determined by Lowry assay. Samples were resolved on SDS-polyacrylamide gels and electrophoretically transferred to polyvinylidene difluoride membranes (PVDF, Immobilon-P, Millipore). Proteins were analyzed using indicated antibodies and visualized with an enhanced chemiluminescence (ECL) system (GE Healthcare, Piscataway, NJ) or with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Western blots were quantified using Odyssey quantification software (LI-COR Biosciences) or ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/).
RNA Silencing
Cells were seeded in six-well plates at a density of 6 × 105 cells/well. Twenty-four hours after plating, small interfering RNA (siRNA) oligonucleotides were transfected by CaPO4 precipitation. Thirty-six hours after transfection, cells were then deprived of FBS for 16 h before stimulation. All siRNAs were obtained from Applied Biosystems/Invitrogen and the specific sequences used are as follows: rat HIF-1α (accession no. NM_024359): sense: 5′-AGGACAAGUCACCACAGGAuu-3′; rat p22phox (accession no. NM_024160): sense: 5′-AAGUACCUGACCGCUGUGGtg-3′; rat Rieske Fe-S (accession no. NM_001008888; predesigned siRNA (accession no. s145479): sense 5′-GCAUGAUUUAGAGCGUGUAtt-3′. As a control oligonucleotide, Silencer Negative Control 2 siRNA was used.
mtROS Assay
mtROS production was determined using MitoSOX Red mitochondrial superoxide indicator (Invitrogen), which is selectively targeted to the mitochondria and is fluorescent upon ROS oxidation. MitoSOX was used according to the manufacturer's protocol and published literature (Robinson et al., 2008). Briefly, VSMCs were seeded on glass-bottomed cell culture dishes and serum-deprived overnight in phenol red-free DMEM. VSMCs were then incubated with MitoSOX (1 μM) for a total of 1 h before analysis. Cells were then pretreated or not with inhibitors as indicated 20 min after MitoSOX addition. Ang II (100 nM) was added during the final 20 min. Cell imaging was performed with a FV1000 confocal microscope equipped with a live cell apparatus (60× oil, 1.4 NA) driven by FluoView software (Olympus, Tokyo, Japan). Fluorescence quantification was performed using the Measure Integrated Density function of the ImageJ software.
HIF-1α Half-Life Analysis
VSMCs were pretreated with stigmatellin or myxothiazol for 20 min and then treated with Ang II. After 4 h, cycloheximide (30 μg/ml) was added for different periods of time (up to 30 min). VSMCs were then lysed in a 2× Laemmli buffer. HIF-1α and α-tubulin protein levels were evaluated by Western blotting followed by quantification and the ratio of HIF-1α to α-tubulin was determined. HIF-1α half-life under experimental conditions was estimated by plotting data as the HIF-1–α-tubulin ratio versus time under cycloheximide treatment.
pVHL Capture Assay
pVHL capture assay was performed as previously described (Page et al., 2008). Briefly, VSMCs were grown to confluence, serum-deprived for 16 h, and stimulated as indicated for 4 h. Cells were washed once in PBS and twice in ice-cold HEB buffer (20 mM Tris, pH 7.5, 5 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol). Cells were then lysed using a Dounce homogenizer, and cytoplasmic extracts were isolated by centrifugation (20,000 × g). Cytoplasmic extracts (250 μg) were incubated with Sepharose-bound GST-HIF-1α (30 μg) for 1 h at room temperature. The Sepharose-bound GST-HIF-1α was then washed with NETN buffer (150 mM NaCl, 0.5 mM EDTA, 20 mM Tris, pH 8.0, 0.5% Igepal, 100 μM deferoxamine) and incubated overnight with in vitro–translated pVHL-HA in NETN at 4°C. Samples were then washed with NETN, denatured with 2× Laemmli buffer, resolved in SDS-polyacrylamide gels (12%), and revealed using anti-HA and anti-GST antibodies.
Intracellular Ascorbate Assay
VSMCs were grown to confluence on 100-mm plates in culture media supplemented with ascorbate (250 μM). Cells were serum-deprived for 16 h in ascorbate supplemented DMEM, and fresh DMEM without ascorbate was added 1 h before treatments. Cells were washed with PBS and lysed in a 90% methanol/1 mM EDTA solution. Samples were briefly sonicated and centrifuged at 20,000 × g. Ascorbate levels were then analyzed by spectrophotometry using a modified protocol (Queval and Noctor, 2007). Samples were diluted in 0.2 mM NaH2PO4, pH 5.6, and absorbance was measured at 265 nm. Ascorbate peroxidase (0.4 U) was added to samples for 5 min, and the absorbance was again measured at 265 nm. Ascorbate concentrations were determined as the difference in absorbance prior and after the addition of ascorbate peroxidase. A Lowry protein assay was used for normalization of the samples.
Northern Blot Analysis
Confluent VSMCs were lysed with TRIzol reagent (Invitrogen). RNA and protein were extracted according to the manufacturer's protocol. RNA concentration was then quantified using RiboGreen (Invitrogen). RNA (20 μg) was resolved on 1% agarose/6% formaldehyde gels and transferred to Hybond N+ nylon membranes (GE Healthcare) before hybridization with a radioactive cDNA probes comprising either the total coding sequence of the mouse VEGF gene and the human GLUT1 gene or the 900-base pair coding sequence of the human HIF-1α gene. An oligonucleotide probe against 18S rRNA was used as a loading control. Northern blots were quantified using a STORM phosphoimaging system equipped with ImageQuant software (GE Healthcare Life Sciences).
Cell Migration Assay
VSMCs were serum deprived for 16 h before determination of cell migration by Boyden chamber assay using a 48-well reusable chamber (Neuroprobe, Gaithersburg, MD). Polycarbonate PVPF membranes (8.0 μm; GE Water & Process Technologies, Trevose, PA), coated with rat tail type I collagen (VWR International, West Chester, PA), were used to separate the upper and lower chambers. VSMCs were added to the upper chamber at a density of 5 × 104 cells per well in DMEM containing 0.1% fatty-acid-free BSA. VSMCs were allowed to migrate toward the lower chamber containing media with or without 100 nM Ang II and inhibitors for 4 h at 37°C. After treatment, membranes were fixed with methanol and subjected to Giemsa staining (Thermo Fisher Scientific, Waltham, MA). Removal of nonmigrated cells was performed by wiping membranes with cotton swabs. Membranes were then air dried on a microscope slide. Cells were counted in three different fields. Results shown are means ± SD of four independent experiments performed in triplicate.
Statistical Analysis
For Western blot and imaging studies, results are representative of three independent experiments. Unless otherwise noted, quantification results are expressed as means ± SEM of three independent experiments. Statistical analyses of different experiments were performed using InStat 3 (GraphPad). Unless otherwise noted, Student's t tests were performed. Results were deemed significant if they attained a 95% confidence level (p < 0.05).
RESULTS
noxROS Do Not Play a Major Role in HIF-1α Accumulation by Ang II in VSMCs
A large body of evidence indicates that the NADPH oxidase is the principle ROS generator in Ang II–treated VSMCs (Lyle and Griendling, 2006; Garrido and Griendling, 2009). We therefore decided to first examine whether NADPH oxidase–derived ROS were implicated in HIF-1α accumulation under nonhypoxic conditions. VAS2870, a specific NADPH oxidase inhibitor, was first used to inhibit noxROS generation (ten Freyhaus et al., 2006). As seen in Figure 1A, when used at reported functional concentrations (10–50 μM), VAS2870 showed no effect on increased HIF-1α protein levels in VSMCs treated with Ang II. We also investigated the role of noxROS using a molecular approach. Inhibiting the p22phox subunit can abolish noxROS generation because this membrane bound protein is an essential member of the NADPH oxidase complex (Ushio-Fukai et al., 1996; Ambasta et al., 2004; Hanna et al., 2004; Modlinger et al., 2006). Targeting the p22phox subunit with a specific siRNA was able to efficiently decrease p22phox protein levels in VSMCs (Figure 1B). However, p22phox silencing was unable to modify HIF-1α protein levels after a treatment with Ang II. Activation of the PI3K pathway has been linked to NADPH oxidase activity and noxROS generation (Page et al., 2002; Baumer et al., 2008). We therefore determined if NADPH oxidase inhibition with VAS2870 or p22phox siRNA inhibited the PI3K pathway by evaluating p70S6 kinase (p70S6K) phosphorylation, a downstream target of this pathway. As seen in Figure 1, A and B, Ang II–induced p70S6K phosphorylation was indeed inhibited by pretreatment of VSMCs with both VAS2870 and p22phox siRNA. These results indicate that noxROS generation does not play a major role in regulating HIF-1α accumulation in VSMCs during Ang II stimulation.
Figure 1.
noxROS do not regulate HIF-1α accumulation. (A) Quiescent VSMCs were pretreated with VAS2870 (indicated concentrations) for 20 min and then treated or not with Ang II (100 nM) for 4 h. HIF-1α, phospho-p70S6K, total-p70S6K, and α-tubulin levels were then analyzed by Western blot. (B) VSMCs were transfected with a siRNA against p22phox or a control sequence. Quiescent VSMCs were treated or not with Ang II (100 nM) for 4 h. HIF-1α, p22phox, phospho-p70S6K, total-p70S6K, and α-tubulin levels were then analyzed by Western blot.
mtROS Are Essential for Increased HIF-1α Accumulation by Ang II in VSMCs
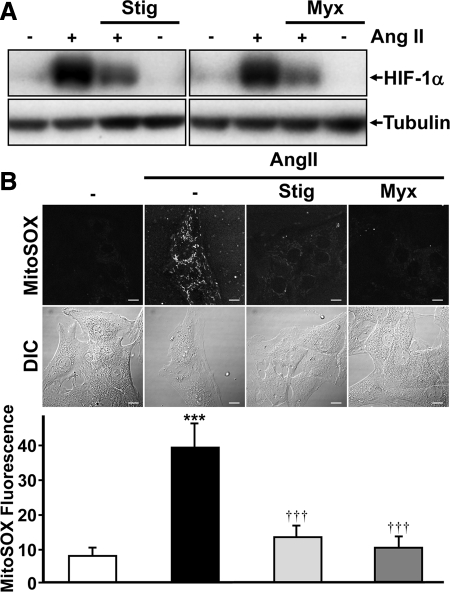
We next attempted to determine the role of mitochondrial-generated ROS in HIF-1α accumulation by Ang II. We first used a pharmacological approach, using stigmatellin and myxothiazol, two inhibitors of the mitochondrial electron transport chain (ETC), specifically complex III. Interestingly, both compounds strikingly inhibited HIF-1α accumulation by Ang II (Figure 2A). However, myxothiazol and stigmatellin were unable to inhibit HIF-1α accumulation by CoCl2 (Supplemental Figure 1), a HIF-1 inducer that potently inhibits HIF-1α hydroxylation and functions independently of oxygen levels and mtROS generation (Sanjuan-Pla et al., 2005). Using MitoSOX, a mitochondrial-targeted probe for detecting ROS, results show that treatment of VSMCs with Ang II does indeed increase mtROS generation (Figure 2B). As expected, stigmatellin and myxothiazol both inhibited mtROS generation in VSMCs by Ang II (Figure 2B), whereas VAS2870 was ineffective (Supplemental Figure 2A). Our previous studies have shown that diphenyleneiodonium (DPI), which blocks ROS generation through its activity as a flavoprotein-containing enzyme inhibitor, potently represses Ang II–induced HIF-1α accumulation in VSMCs (Richard et al., 2000; Page et al., 2002). In accordance with our past studies, DPI also strikingly decreased mtROS generation (Supplemental Figure 2B). Finally, our results show that pretreatment of VSMCs with stigmatellin or myxothiazol does not affect other Ang II/AT1-related cellular signaling because p70S6K, p38MAPK, AKT, and p42/44 MAPK activation/phosphorylation levels were not modified (Supplemental Figure 3).
Figure 2.
mtROS regulate HIF-1α accumulation. (A) Quiescent VSMCs were pretreated with stigmatellin (Stig, 1 μM) or myxothiazol (Myx, 1 μM) for 20 min and then treated or not with Ang II (100 nM) for 4 h. HIF-1α and α-tubulin levels were analyzed by Western blot. (B) VSMCs were seeded on 35-mm glass-bottomed cell culture dishes and loaded with MitoSOX (1 μM) for 1 h. During the final 40 min, cells were pretreated or not with stigmatellin (1 μM) or myxothiazol (1 μM). Ang II (100 nM) was then added 20 min before imaging as described in Materials and Methods. Differential interference contrast microscopy (DIC) was used for whole cell imaging. Scale bars, 10 μm. Bottom panel is the quantification of results from B. Data are expressed as arbitrary units of MitoSOX fluorescence and are an average ± SD of five independent experiments, n > 50 cells. ***p < 0.001 compared with untreated VSMCs. †††p < 0.001 compared with Ang II–treated VSMCs.
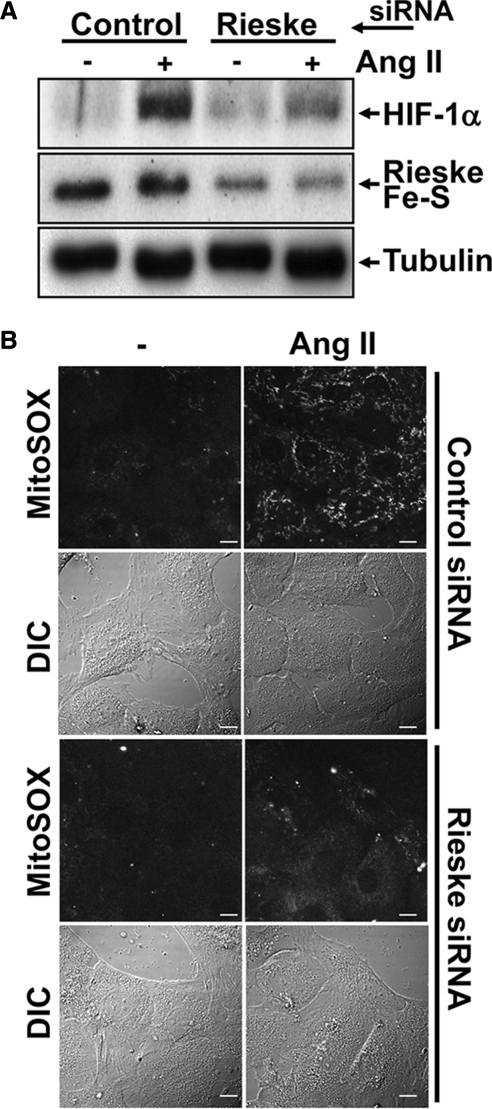
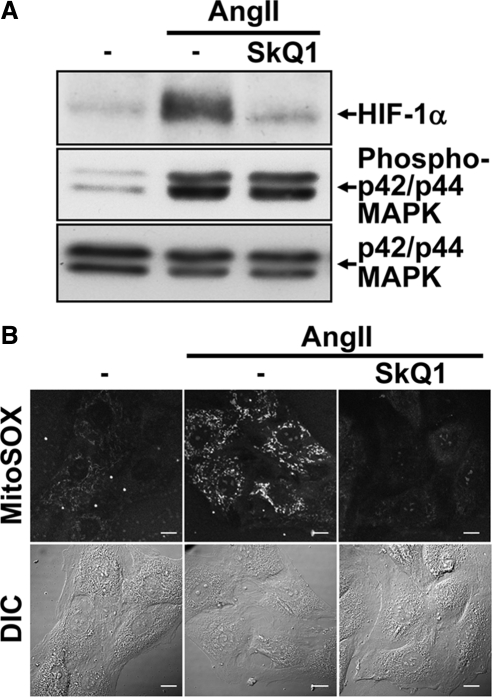
Two additional approaches were used to confirm the role played by mtROS in HIF-1α induction in VSMCs after Ang II treatment. First, we investigated the importance of the Rieske Fe-S protein, an essential member of complex III. Without Rieske Fe-S, mtROS are not generated because complex III cannot initiate the Q cycle (Brunelle et al., 2005). As seen with complex III inhibitors, targeting Rieske Fe-S with a specific siRNA led to an inhibition of HIF-1α accumulation after Ang II treatment (Figure 3A). Second, we utilized a mitochondrial-targeted antioxidant: SkQ1. Developed to investigate the role of mitochondrial ROS in ageing, this Skulachev (Sk) ion is strongly localized to the mitochondria. SkQ1 is a “rechargeable” antioxidant because it is reduced by the mitochondrial ETC (Skulachev et al., 2009). As seen in Figure 4A, the pretreatment of VSMCs with SkQ1 before Ang II treatment blocked HIF-1α protein accumulation. As expected, both the siRNA knockdown of Rieske Fe-S and the pretreatment of VSMCs with SkQ1 abolished mtROS generation by Ang II as determined by MitoSOX staining (Figures 3B and 4B and Supplemental Figure 4). Taken together, our results show that in VSMCs, mtROS generation is essential for Ang II–induced HIF-1α accumulation.
Figure 3.
mtROS regulate HIF-1α accumulation. (A) VSMCs were transfected with a siRNA against Rieske Fe-S protein (100 nM) or a control sequence. Cells were then serum-deprived overnight and treated or not with Ang II (100 nM) for 4 h. HIF-1α, Rieske Fe-S protein, and α-tubulin levels were analyzed by Western blot. (B) VSMCs seeded on glass-bottomed cell culture dishes were transfected and treated as in A. Cellular mtROS levels were analyzed by MitoSOX staining as described in Figure 2. Scale bars, 10 μm.
Figure 4.
mtROS regulate HIF-1α accumulation. (A) Quiescent VSMCs were pretreated with SkQ1 (500 nM) and then treated or not with Ang II (100 nM) for 4 h. HIF-1α, phospho p42/p44 MAPK, total p42/p44 MAPK and α-tubulin levels were analyzed by Western blot. (B) VSMCs seeded on glass-bottomed cell culture dishes were transfected and treated as in A. Cellular mtROS levels were analyzed by MitoSOX staining as described in Figure 2. Scale bars, 10 μm.
mtROS Are Involved in Increasing HIF-1α Stabilization
Our previous work demonstrated that ROS were essential in HIF stabilization during the treatment of VSMCs with Ang II (Page et al., 2008). We therefore attempted to determine the importance of mtROS on HIF-1α stabilization. We first examined the role of mtROS on HIF-1α half-life using the general protein synthesis inhibitor, cycloheximide (Laughner et al., 2001; Page et al., 2008; Michaud et al., 2009). Incubating cells with cycloheximide after HIF-1 induction blocks de novo protein synthesis. Thus, cellular HIF-1α protein levels are dependent on the maintenance of protein stabilization mechanisms. In these experimental conditions, changes in HIF-1α half-life would therefore reflect changes in HIF-1α stability. Here, HIF-1α half-life was determined by Western blotting and the quantification of HIF-1α levels over time in the presence of cycloheximide. When VSMCs were treated with Ang II, HIF-1α half-life was 20 ± 2.8 min (Figure 5). However, when VSMCs were pretreated with either stigmatellin or myxothiazol, HIF-1α half-life under Ang II treatment was significantly decreased (7.0 ± 0.5 and 5.5 ± 0.6 min, respectively). It is important to note that the inhibition of mtROS generation did not modify increased HIF-1α mRNA levels by Ang II (Supplemental Figure 5). This last result was expected because our studies have shown that Ang II–increased HIF-1α mRNA levels are ROS-independent in VSMCs (Page et al., 2002). Therefore, our results indicate that HIF-1α stability is increased through Ang II–dependent mtROS generation in VMSC.
Figure 5.
mtROS regulate HIF-1α half-life. (A) Quiescent VSMCs were pretreated with stigmatellin (1 μM) or myxothiazol (1 μM) for 20 min and then treated or not with Ang II (100 nM) for 4 h. HIF-1α half-life was measured by then treating cells with cycloheximide (30 μg/ml) for the indicated times to block all de novo protein synthesis. HIF-1α and α-tubulin levels were then analyzed by Western blot. (B) HIF-1α protein levels in A were quantified using the Odyssey Infrared Imaging System and normalized to α-tubulin. HIF-1α half-life was determined by plotting data as HIF-1α–α-tubulin ratio versus time under cycloheximide treatment. Data expressed are an average ± SEM of three independent experiments. **p < 0.01 compared with Ang II–treated VSMCs.
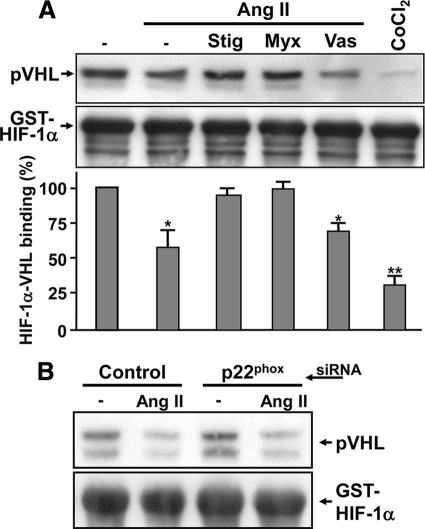
We then investigated whether mtROS could act on two main steps involved in HIF-1α protein degradation; HIF-1α prolyl hydroxylation and HIF-1α binding to pVHL. To analyze HIF-1α hydroxylation status, specific antibodies against hydroxylated HIF-1α on its two key ODDD proline residues were used (Chan et al., 2002; Chan et al., 2005). As seen in Figure 6, VSMCs treated with Ang II show decreased HIF-1α hydroxylation, most notably on the Pro402 residue (Page et al., 2008). More interestingly, the decrease in HIF-1α hydroxylation by Ang II was reversed when VSMCs were pretreated with either stigmatellin or myxothiazol. Additionally, VAS2870 had no significant effect on HIF-1α's hydroxylation status during Ang II treatment. A crucial event leading to HIF-1α protein degradation is its binding to the product of the von Hippel-Lindau tumor suppressor gene, pVHL. Binding to pVHL is a direct result of HIF-1α hydroxylation (Ivan et al., 2001). To determine the effect of Ang II–induced mtROS generation on pVHL binding, a pVHL capture assay was used. A GST-HIF-1α fusion protein, comprised of amino acids 344-582 from human HIF-1α, was subjected to modification by Ang II–treated VSMCs cell extracts followed by interaction with in vitro–translated pVHL. As we previously demonstrated, Ang II treatment led to decreased HIF-1α binding to pVHL (Figure 7). More interestingly, pretreatment of VSMC with stigmatellin or myxothiazol was able to restore HIF-1α binding to pVHL (Figure 7A). Finally, VAS2870 or siRNA against p22phox had no significant effect on HIF-1α's hydroxylation status or levels of pVHL binding during Ang II treatment (Figures 6 and 7). It is important to note that inhibitors or p22phox had no significant effect on HIF-1α hydroxylation or pVHL binding in cells not treated with Ang II (data not shown). Taken together, these results indicate that Ang II–generated mtROS are essential for HIF-1α prolyl-hydroxylase inactivation in VSMCs.
Figure 6.
mtROS regulate HIF-1α hydroxylation. Quiescent VSMCs were pretreated with stigmatellin (1 μM), myxothiazol (1 μM), or VAS2870 (10 μM) for 20 min. Cells were then treated with MG132 (20 μM) to increase HIF-1 levels and stimulated with Ang II (100 nM) or CoCl2 (200 μM) for 4 h. Nuclear extracts (100 μg) were analyzed by Western blot with anti-hydroxylated-Pro402-HIF-1α (P402-OH), anti-hydroxylated-Pro564-HIF-1α (P564-OH), anti-HIF-1α and anti-α-tubulin antibodies (top). Western blots in A were quantified with ImageJ or the Odyssey Infrared Imaging System using α-tubulin as a loading control (bottom panel). Results are expressed as a percentage of hydroxylated HIF-1 (normalized to total HIF-1α protein levels) on Pro402 (■) or Pro564 (▩) compared with untreated cells and are an average ± SEM of three independent experiments. **p < 0.01 and *p < 0.05 compared with untreated VSMCs.
Figure 7.
mtROS inhibit VHL-HIF-1α binding. (A) Quiescent VSMCs were pretreated with stigmatellin (1 μM), myxothiazol (1 μM), or VAS2870 (10 μM) for 20 min and subsequently treated or not with Ang II (100 nM) or CoCl2 for 4 h. Cytoplasmic extracts were incubated with GST-HIF-1α coupled to Sepharose beads for 1 h. Samples were then incubated in the presence of in vitro–translated pVHL and resolved by SDS-PAGE (12%). Immunoblotting was performed using anti-HA (pVHL) and anti-GST antibodies (A, top). Western blots were quantified with the Odyssey Infrared Imaging System using GST-HIF-1α as a loading control (A, bottom panel). Results are expressed as a percentage of pVHL binding normalized to GST-HIF-1α protein levels compared with untreated cells and are an average ± SEM of four independent experiments. **p < 0.01 and *p < 0.05 compared with untreated VSMCs. (B) VSMCs were transfected with a siRNA against p22phox or a control sequence. Quiescent VSMCs were treated or not with Ang II (100 nM) for 4 h. VHL-capture assay was then performed as in A.
Our previous studies showed that HIF-1α prolyl hydroxylase activity is inactivated during Ang II stimulation by a ROS-induced depletion of intracellular ascorbate (Page et al., 2008). To determine whether mtROS were implicated in depleting intracellular ascorbate, ascorbate levels were measured in Ang II–stimulated VSMCs that were pretreated with stigmatellin or myxothiazol. As seen in Supplemental Figure 6, Ang II treatment decreased intracellular ascorbate levels by 65.2 ± 6.2%. The inhibition of mtROS generation with stigmatellin or myxothiazol was able to completely reverse intracellular ascorbate depletion caused by the treatment of VSMCs with Ang II. These results indicate that mtROS generation by Ang II in VSMCs leads to reduced cellular ascorbate concentrations, decreased HIF-1α hydroxylation, decreased HIF-1α binding to pVHL and increased HIF-1α stability.
mtROS Are Required for Increased HIF-1 Transcriptional Activity by Ang II
The importance of mtROS for HIF-1 transcriptional activity was analyzed by evaluating the expression of two HIF-1 target genes: VEGF and Glut-1. As seen in Figure 8, Ang II increased the expression of both VEGF and Glut-1, an effect that was strongly inhibited by pretreating cells with either stigmatellin or myxothiazol. As with HIF-1α protein accumulation, a maximal inhibition was observed with 1 μM of either complex III inhibitor (Supplemental Figure 7). To confirm that VEGF and Glut-1 expression during Ang II treatment were indeed dependent on HIF-1 in VSMCs, we targeted HIF-1α expression with a specific siRNA. Our results show that the expression of these two transcripts is nearly exclusively dependent on HIF-1 activation (Supplemental Figure 8A). Effective silencing of HIF-1α can be observed in Supplemental Figure 8B. Taken together, these results indicate that Ang II regulates HIF-1–dependent genes such as VEGF and Glut-1 through mtROS generation in VSMCs.
Figure 8.
mtROS are essential for HIF-1–dependent activity. (A) Quiescent VSMCs were pretreated with stigmatellin (1 μM) or myxothiazol (1 μM) for 20 min and then treated or not with Ang II (100 nM) for 4 h. Total RNA was extracted and resolved on formaldehyde/agarose gels. Northern blotting was performed using specific VEGF- and Glut-1–radiolabeled probes. An 18S RNA probe was used as a control for gel loading. Northern blots were quantified by phosphoimaging using total ribosomal 18S RNA as a loading control (Bottom panels). Data expressed are an average ± SEM of four independent experiments. ***p < 0.001 compared with untreated VSMCs. †p < 0.05 compared with Ang II–treated VSMCs. ‡Nonsignificant compared with Ang II–treated VSMCs.
HIF-1 and mtROS Are Essential for Ang II–induced VSMC Migration
VSMC migration occurs during a number of different vascular pathologies, especially when vascular remodeling is involved (Gerthoffer, 2007). Ang II is a potent vascular remodeler and activator of VSMC migration (Xi et al., 1999; Guo et al., 2009). Additionally, HIF-1 was shown to be important for VSMC migration in hypoxic conditions (Corley et al., 2005; Osada-Oka et al., 2008). We wanted to determine whether HIF-1 and mtROS were involved in Ang II–mediated VSMC migration. For this assay, we used a Boyden chamber to analyze the potential of VSMCs to migrate across a porous membrane toward Ang II. HIF-1's role in VSMC migration was determined by targeting HIF-1α expression with a specific siRNA (Supplemental Figure 8). As seen in the top panel of Figure 9, Ang II significantly increased the migration of VSMCs transfected with a control siRNA that was nearly completely blocked in VSMCs transfected with a HIF-1α siRNA. To determine the role of mtROS in Ang II–mediated VSMC migration, VSMCs were treated with stigmatellin and myxothiazol during Boyden chamber assay. Both compounds potently inhibited VSMC migration toward Ang II (Figure 9, bottom panel). Interestingly, the effects of HIF-1 and mtROS on VSMC migration were specific to Ang II because VSMC migration toward PDGF-BB was unaffected by HIF-1α knockdown or complex III inhibition. Taken together, our results demonstrate the essential role of HIF-1 and mitochondrial ROS in Ang II–induced VSMC migration.
Figure 9.
mtROS are essential for Ang II–dependent VSMC migration. VSMCs were transfected with a siRNA against HIF-1 or a control sequence (top) or left untransfected (bottom). Cells were serum-starved 24 h after transfection. Quiescent VSMCs (5 × 105 cells/well) were placed in the upper level of a Boyden chamber and allowed to migrate through a collagen-coated microporous membrane for 4 h toward the lower chamber that contained 100 nM Ang II in DMEM only (top) or in DMEM supplemented or not with stigmatellin (1 μM) or myxothiazol (1 μM; bottom). Cells were then fixed, stained, and counted as described in Materials and Methods. Data expressed are an average ± SEM of three independent experiments performed in triplicate. ***p < 0.001 compared with untreated (−) VSMCs.
DISCUSSION
Treating VSMCs with Ang II leads to altered HIF-1α proline hydroxylation, decreased pVHL binding, reduced HIF-1α ubiquitination, and proteasomal degradation leading to increased HIF-1α stabilization (Page et al., 2008). ROS generation plays a pivotal role in these Ang II–mediated effects on the HIF-1 system. Our current work now identifies mitochondrial-derived ROS as essential intermediates leading to the stabilization of HIF-1α, the activation of HIF-1, increased in HIF-1 target gene expression, and associated cellular effects, such as VSMC migration.
It is well accepted that the NADPH oxidase plays a primary role in VSMC biology. noxROS have been implicated in various VSMC signaling mechanisms originating from AT1 receptor activation after Ang II stimulation (Garrido and Griendling, 2009). noxROS have been implicated in increasing HIF-1α translation (Page et al., 2002; Lauzier et al., 2007). Additionally, a recent study showed the involvement of the NADPH oxidase in HIF-2 stabilization (Diebold et al., 2010). Because Ang II–induced HIF-1α stability was ROS dependent, our first studies pushed us to investigate the role of the NADPH oxidase and noxROS in HIF-1α stabilization. Under our conditions, the NADPH oxidase does not play a major role in HIF-1α accumulation. This is shown here by targeting p22phox by RNAi and using the specific NADPH inhibitor, VAS2870. However, siRNA against other NADPH oxidase subunits, including Nox1, Nox4 and p47phox, were equally ineffective to block HIF-1α accumulation after Ang II treatment. (D. A. Patten, E. L. Pagé, and D. E. Richard, unpublished observations). These unexpected results led us to demonstrate here that mitochondrial/complex III–generated mtROS are indispensable for HIF-1 activation by Ang II. It is important to note that the NADPH oxidase and the mitochondria are not the only ROS-generating systems in VSMCs (San Martin and Griendling, 2010). It is possible that the activity of other oxidases and ROS generators may affect HIF-1 activation at different levels. Continued studies in this area will clarify the role of other oxidases and ROS generators on HIF-1 activation in nonhypoxic conditions.
Our previous work described that DPI, which blocks ROS generation through its inhibitory effects on flavoprotein-containing enzymes, blocked HIF-1α accumulation during Ang II treatment. We suggested this was mediated through the inhibition of the NADPH oxidase. Not surprisingly, it has been reported that DPI is not a specific NADPH oxidase inhibitor (Hutchinson et al., 2007; Aldieri et al., 2008). Moreover, DPI inhibits all flavin-containing enzymes, including those found in the mitochondria (Li and Trush, 1998). Here, our work shows that DPI does indeed block mtROS generation in VSMCs during Ang II treatment and indicates that DPI blocks HIF-1α stabilization by Ang II.
Our results show that mtROS are essential for blocking HIF-1α hydroxylation and pVHL binding during Ang II treatment. It is well accepted that hydroxylation of proline residues 564 and 402 (Pro402 and Pro564) of human HIF-1α regulates pVHL-mediated proteasomal degradation (Ivan et al., 2001; Masson et al., 2001). Further studies indicated that the modification of only one of these proline residues is sufficient to stabilize HIF-1α (Chan et al., 2005). Our previous work demonstrated that treating VSMCs with Ang II primarily suppressed the hydroxylation of Pro402 (Page et al., 2008). In agreement with these observations, here we show that mtROS are responsible for decreasing Pro402 hydroxylation while Pro564 hydroxylation is mostly unaffected. Taken together, our results show that increased mtROS results in an inactivation of PHD.
H2O2 is known to be an important mediator of the effects of Ang II on VSMC (Zafari et al., 1998). Our previous studies demonstrated that H2O2 led to the inhibition of PHD activity, as measured by reduced pVHL/HIF-1α binding (Page et al., 2008). This indicates that superoxide, produced in the mitochondria, is transformed into H2O2, which effectively inhibits PHD activity. This inhibition most probably occurs due to the Fenton reaction and the oxidation of Fe2+ to Fe3+, which decreases available ascorbate, leading to decreased hydroxylation of HIF-1α on Pro402, impaired binding of pVHL to HIF-1α, and HIF-1α stabilization under normal oxygen conditions. These results also suggest a crucial role of superoxide dismutase (SOD) in HIF-1 activation by Ang II. Preliminary evidence suggest that SOD activity is indeed involved because diethylthiocarbamate (DETC), a SOD inhibitor, led to a significant inhibition of HIF-1α accumulation under Ang II treatment in VSMCs (G. A. Robitaille and D. E. Richard, unpublished observations). Further studies are needed to clearly identify the role of different SOD enzymes in HIF-1 activation. Given the importance of mtROS in HIF-1 induction, mitochondrial SOD2 may be of particular interest.
Because Ang II can activate both noxROS and mtROS in VSMCs after Ang II treatment, our results indicate a specificity of mtROS in regulating HIF-1α stabilization. The reason for this specificity for mtROS remains to be elucidated. Theoretically, mtROS differ from noxROS by only the area in which it is produced and the local expression of SOD. We hypothesize that local mtROS/H2O2 gradients exist around the mitochondria which is responsible for inhibition of PHD activity and HIF-1α stabilization signaling.
Exactly how Ang II increases mtROS remains to be elucidated. By using two ETC complex III inhibitors and a siRNA against the Rieske Fe-S, our results indicate an essential role of complex III. However, the exact sequence of events leading to complex III activation and mtROS generation after AT1 receptor activation remains unclear. In contrast to our findings, Kimura et al. (2005b) have proposed that in cardiac tissue, mtROS are produced during Ang II treatment through NADPH oxidase-derived ROS-induced ROS release (RIRR). In other words, the initial burst of noxROS leads to increased mtROS. Our results indicate that in VSMCs, RIRR does not occur because inhibition of the NADPH oxidase does not block Ang II–induced mtROS generation as measured by MitoSOX nor does it impede HIF-1 induction. It is important to note that results from Kimura et al. were based on data obtained using the classical NADPH oxidase inhibitor, apocynin. However, recent results now indicate that apocynin is not a simple NADPH oxidase inhibitor in vascular cells and can also act as an antioxidant (Heumuller et al., 2008). Additionally, it was shown that the expression of a dominant negative form of Rac1 can suppress mtROS generation by Ang II (Nozoe et al., 2008) Because Rac1 is required for proper NADPH oxidase assembly, this suggested that the NADPH oxidase is required for increasing mtROS (Griendling et al., 2000). Although the role of Rac1 on HIF-1 induction and activation has been described (Hirota and Semenza, 2001), Rac1 also regulates other cellular processes in VSMCs including cytoskeletal organization, gene transcription, cell proliferation and membrane trafficking through interactions with PI3K, p21-activated kinase (PAK), Ras, and p70 S6 kinase (Chou and Blenis, 1996; Kaibuchi et al., 1999; Liliental et al., 2000; Sun et al., 2000). Additionally, Rac1 has been shown to regulate HIF-1α mRNA expression through activation of the NF-κB pathway in different cell systems including VSMCs (Gorlach et al., 2003; Diebold et al., 2008; Kim et al., 2008). Finally, previous studies have shown that Rac1 does not affect mtROS generation in TNF-treated endothelial cells (Deshpande et al., 2000). Therefore, the effect of Rac1 on HIF-1 induction could potentially be attributed to any number of cellular processes not directly linked to mtROS generation. Finally, a recent study shows that inversely, mtROS can lead to NADPH oxidase activation (Lee et al., 2006). Given these divergent studies and our results, further investigation is needed to clearly delineate the mechanisms by which mtROS generation is activated in VSMCs after Ang II treatment.
HIF-1 regulation by Ang II occurs through three independent mechanisms. Until now, we have been unable to determine which mechanism played a primordial role in HIF-1 induction and activation. Our work here partly defines the relative contribution of the increased translation, transcription, and stability of HIF-1α by Ang II. We can now speculate that increased HIF-1α stability is of primary importance for HIF-1 regulation by Ang II. Also, because the inhibition of the NADPH oxidase had little or no effect on HIF-1α accumulation, and we have previously demonstrated that noxROS are important for increased HIF-1α translation by Ang II; our results indicate that increased HIF-1α protein translation is not essential for the prolonged accumulation of HIF-1α levels under Ang II treatment (Page et al., 2002). However, increased HIF-1α translation may be important for the rapid accumulation of HIF-1α after Ang II receptor activation.
Because mitochondrial oxidative damage is thought to contribute in a number of human degenerative diseases, the development of antioxidants that are targeted to the mitochondria has gained significant interest. Generally, mitochondrial antioxidants include an antioxidant moiety (ubiquinone, tocopherol, nitroxide) and a covalently attached lipophilic triphenylphosphonium cation that serves for specific uptake by the mitochondria (Smith et al., 2008). MitoQ and SkQ1 are two compounds that have been developed to specifically suppress mtROS generation. These two compounds have an added advantage over other mitochondrial-targeted antioxidants because they can be regenerated by accepting electrons from the respiratory chain. MitoQ has been shown to inhibit HIF-1α accumulation during hypoxia (Bell et al., 2007). However, recent evidence suggests that the concentration window for MitoQ's antioxidant properties is very small before displaying prooxidant properties (Antonenko et al., 2008). Using a different antioxidant moiety (plastoquinone), SkQ1 demonstrated more potent antioxidant properties and a larger functional concentration window than MitoQ (Skulachev et al., 2009). Here, we successfully used SkQ1 to decrease mtROS generation during Ang II treatment, which effectively inhibited HIF-1α accumulation. Our studies therefore confirm the effectiveness of mitochondrial antioxidants as inhibitors of HIF-1 activation under nonhypoxic conditions and point to interesting therapeutic leads. Our recent in vivo studies have demonstrated a potential role for nonhypoxic HIF-1 induction in vascular remodeling diseases (Lambert et al. 2010). Mitochondrial antioxidants, such as SkQ1, will prove interesting tools for further investigation in Ang II–mediated pathophysiological effects which involve VSMC migration, such as vascular remodeling.
Mitochondrial-derived ROS have gained substantial interest in the regulation of HIF-1 induction and activation. The hypoxic activation of HIF-1α has been shown by two independent groups to be regulated by mtROS (Chandel et al., 2000; Guzy et al., 2005). Additionally, a nonhypoxic activator of HIF-1, thrombopoietin (TPO), has been shown to control HIF-1α levels through the generation of mtROS (Yoshida et al., 2008). Although the NADPH oxidase has classically been shown as the primary ROS generator in VSMCs and noxROS to be involved in different signaling pathways, our study now identifies mtROS as an essential intermediate for PHD inactivation, HIF-1α stabilization, and HIF-1 activation when VSMCs are stimulated with Ang II. Taken together, these studies suggest that mitochondrial ROS are a common intermediate signal transducer between hypoxic and nonhypoxic stimuli leading to the activation of HIF-1.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Sébastien Bonnet, Marie-Claude Lauzier, Maude Michaud, and Elisabeth Pagé for their perspective and insightful advice. This work was supported by Grants MOP-49609 and MOP-102760 from the Canadian Institutes of Health Research (CIHR) and the Heart and Stroke Foundations of Québec and Canada. D.E.R. is the recipient of a CIHR New Investigator Award. D.A.P. held a Graduate Scholarship from La Société Québécoise d'Hypertension Artérielle (SQHA). V.E.R. holds a Banting and Best Canada Graduate Scholarship from the CIHR.
Abbreviations used:
- Ang II
angiotensin II
- ETC
electron transport chain
- GLUT-1
glucose transporter-1
- HIF
hypoxia-inducible factor
- HRE
hypoxia-response element
- ODDD
oxygen-dependent degradation domain
- PHD
HIF prolyl-hydroxylase
- pVHL
von Hippel-Lindau tumor suppressor protein
- ROS
reactive oxygen species
- mtROS
mitochondrial-derived ROS
- noxROS
NADPH oxidase–derived ROS
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cells.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-01-0025) on July 21, 2010.
REFERENCES
- Aldieri E., Riganti C., Polimeni M., Gazzano E., Lussiana C., Campia I., Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr. Drug Metab. 2008;9:686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- Ambasta R. K., Kumar P., Griendling K. K., Schmidt H. H., Busse R., Brandes R. P. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- Andreyev A. Y., Kushnareva Y. E., Starkov A. A. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Antonenko Y. N., et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry. 2008;73:1273–1287. doi: 10.1134/s0006297908120018. [DOI] [PubMed] [Google Scholar]
- Babior B.M. NADPH oxidase. Curr. Opin. Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Baumer A. T., Ten Freyhaus H., Sauer H., Wartenberg M., Kappert K., Schnabel P., Konkol C., Hescheler J., Vantler M., Rosenkranz S. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J. Biol. Chem. 2008;283:7864–7876. doi: 10.1074/jbc.M704997200. [DOI] [PubMed] [Google Scholar]
- Bell E. L., Klimova T. A., Eisenbart J., Moraes C. T., Murphy M. P., Budinger G. R., Chandel N. S. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle J. K., Bell E. L., Quesada N. M., Vercauteren K., Tiranti V., Zeviani M., Scarpulla R. C., Chandel N. S. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Cai H., Li Z., Dikalov S., Holland S. M., Hwang J., Jo H., Dudley S. C., Jr, Harrison D. G. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J. Biol. Chem. 2002;277:48311–48317. doi: 10.1074/jbc.M208884200. [DOI] [PubMed] [Google Scholar]
- Chan D. A., Sutphin P. D., Denko N. C., Giaccia A. J. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. J. Biol. Chem. 2002;277:40112–40117. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- Chan D. A., Sutphin P. D., Yen S. E., Giaccia A. J. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol. Cell. Biol. 2005;25:6415–6426. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel N. S., McClintock D. S., Feliciano C. E., Wood T. M., Melendez J. A., Rodriguez A. M., Schumacker P. T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Chou M. M., Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- Cockman M. E., Masson N., Mole D. R., Jaakkola P., Chang G. W., Clifford S. C., Maher E. R., Pugh C. W., Ratcliffe P. J., Maxwell P. H. Hypoxia inducible factor-alpha binding and ubiquitylation by the von hippel-lindau tumor suppressor protein. J. Biol. Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- Corley K. M., Taylor C. J., Lilly B. Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells. J. Cell. Biochem. 2005;96:971–985. doi: 10.1002/jcb.20559. [DOI] [PubMed] [Google Scholar]
- Dery M. A., Michaud M. D., Richard D. E. Hypoxia-inducible factor 1, regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Deshpande S. S., Angkeow P., Huang J., Ozaki M., Irani K. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 2000;14:1705–1714. doi: 10.1096/fj.99-0910com. [DOI] [PubMed] [Google Scholar]
- Diebold I., Djordjevic T., Hess J., Gorlach A. Rac-1 promotes pulmonary artery smooth muscle cell proliferation by upregulation of plasminogen activator inhibitor-1, role of NFkappaB-dependent hypoxia-inducible factor-1alpha transcription. Thromb. Haemost. 2008;100:1021–1028. [PubMed] [Google Scholar]
- Diebold I., Flugel D., Becht S., Belaiba R. S., Bonello S., Hess J., Kietzmann T., Gorlach A. The hypoxia-inducible Factor-2alpha is stabilized by oxidative stress involving NOX4. Antioxid. Redox Signal. 2010;13:425–436. doi: 10.1089/ars.2009.3014. [DOI] [PubMed] [Google Scholar]
- Doughan A. K., Harrison D. G., Dikalov S. I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- Epstein A. C., et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Felty Q., Xiong W. C., Sun D., Sarkar S., Singh K. P., Parkash J., Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005;44:6900–6909. doi: 10.1021/bi047629p. [DOI] [PubMed] [Google Scholar]
- Garrido A. M., Griendling K. K. NADPH oxidases and angiotensin II receptor signaling. Mol. Cell. Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer W. T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- Gorlach A., Berchner-Pfannschmidt U., Wotzlaw C., Cool R. H., Fandrey J., Acker H., Jungermann K., Kietzmann T. Reactive oxygen species modulate HIF-1 mediated PAI-1 expression: involvement of the GTPase Rac1. Thromb. Haemost. 2003;89:926–935. [PubMed] [Google Scholar]
- Griendling K. K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Guo J., Chen H., Ho J., Mancini J., Sontag T., Laporte S. A., Richard D. E., Lebrun J. J. TGFbeta-induced GRK2 expression attenuates AngII-regulated vascular smooth muscle cell proliferation and migration. Cell Signal. 2009;21:899–905. doi: 10.1016/j.cellsig.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Guzy R. D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K. D., Simon M. C., Hammerling U., Schumacker P. T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Hanna I. R., Hilenski L. L., Dikalova A., Taniyama Y., Dikalov S., Lyle A., Quinn M. T., Lassegue B., Griendling K. K. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic. Biol. Med. 2004;37:1542–1549. doi: 10.1016/j.freeradbiomed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Heumuller S., Wind S., Barbosa-Sicard E., Schmidt H. H., Busse R., Schroder K., Brandes R. P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Hirota K., Semenza G. L. Rac1 activity is required for the activation of hypoxia-inducible factor 1. J. Biol. Chem. 2001;276:21166–21172. doi: 10.1074/jbc.M100677200. [DOI] [PubMed] [Google Scholar]
- Hutchinson D. S., Csikasz R. I., Yamamoto D. L., Shabalina I. G., Wikstrom P., Wilcke M., Bengtsson T. Diphenylene iodonium stimulates glucose uptake in skeletal muscle cells through mitochondrial complex I inhibition and activation of AMP-activated protein kinase. Cell Signal. 2007;19:1610–1620. doi: 10.1016/j.cellsig.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Ratcliffe P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Kuroda S., Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kim J., et al. Hypoxia-induced IL-18 increases hypoxia-inducible factor-1alpha expression through a Rac1-dependent NF-kappaB pathway. Mol. Biol. Cell. 2008;19:433–444. doi: 10.1091/mbc.E07-02-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Zhang G. X., Nishiyama A., Shokoji T., Yao L., Fan Y. Y., Rahman M., Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005a;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- Kimura S., Zhang G. X., Nishiyama A., Shokoji T., Yao L., Fan Y. Y., Rahman M., Suzuki T., Maeta H., Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005b;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- Lambert C. M., Roy M., Robitaille G. A., Richard D. E., Bonnet S. HIF-1 inhibition decreases systemic vascular remodeling diseases by promoting apoptosis through a hexokinase2-dependent mechanism. Cardiovasc. Res. 2010 doi: 10.1093/cvr/cvq152. (in press). Epub ahead of print. doi: 10.1093/cvr/cvq152. [DOI] [PubMed] [Google Scholar]
- Lassegue B., Griendling K.K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb. Vasc. Biol. 2010:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughner E., Taghavi P., Chiles K., Mahon P. C., Semenza G. L. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzier M. C., Page E. L., Michaud M. D., Richard D. E. Differential regulation of hypoxia-inducible factor-1 through receptor tyrosine kinase transactivation in vascular smooth muscle cells. Endocrinology. 2007;148:4023–4031. doi: 10.1210/en.2007-0285. [DOI] [PubMed] [Google Scholar]
- Lee S. B., Bae I. H., Bae Y. S., Um H. D. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J. Biol. Chem. 2006;281:36228–36235. doi: 10.1074/jbc.M606702200. [DOI] [PubMed] [Google Scholar]
- Li Y., Trush M. A. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem. Biophys. Res. Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- Liliental J., Moon S. Y., Lesche R., Mamillapalli R., Li D., Zheng Y., Sun H., Wu H. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 2000;10:401–404. doi: 10.1016/s0960-9822(00)00417-6. [DOI] [PubMed] [Google Scholar]
- Lyle A. N., Griendling K. K. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology. 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- Masson N., Willam C., Maxwell P. H., Pugh C. W., Ratcliffe P. J. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M. D., Robitaille G. A., Gratton J. P., Richard D. E. Sphingosine-1-phosphate: a novel nonhypoxic activator of hypoxia-inducible factor-1 in vascular cells. Arterioscler. Thromb. Vasc. Biol. 2009;29:902–908. doi: 10.1161/ATVBAHA.109.185280. [DOI] [PubMed] [Google Scholar]
- Modlinger P., et al. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension. 2006;47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- Nozoe M., Hirooka Y., Koga Y., Araki S., Konno S., Kishi T., Ide T., Sunagawa K. Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J. Hypertens. 2008;26:2176–2184. doi: 10.1097/HJH.0b013e32830dd5d3. [DOI] [PubMed] [Google Scholar]
- Osada-Oka M., Ikeda T., Akiba S., Sato T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via HIF-1alpha-dependent expression of thrombospondin-1. J. Cell. Biochem. 2008;104:1918–1926. doi: 10.1002/jcb.21759. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J. Cell Biol. 1986;102:343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E. L., Chan D. A., Giaccia A. J., Levine M., Richard D. E. Hypoxia-inducible factor-1alpha stabilization in nonhypoxic conditions: role of oxidation and intracellular ascorbate depletion. Mol. Biol. Cell. 2008;19:86–94. doi: 10.1091/mbc.E07-06-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E. L., Robitaille G. A., Pouyssegur J., Richard D. E. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J. Biol. Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- Queval G., Noctor G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal. Biochem. 2007;363:58–69. doi: 10.1016/j.ab.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Rhee S. G. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Richard D. E., Berra E., Gothie E., Roux D., Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 1999;274:32631–32638. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- Richard D. E., Berra E., Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J. Biol. Chem. 2000:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- Robinson K. M., Janes M. S., Beckman J. S. The selective detection of mitochondrial superoxide by live cell imaging. Nat. Protoc. 2008;3:941–947. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- San Martin A., Griendling K. K. Redox control of vascular smooth muscle migration. Antioxid. Redox Signal. 2010;12:625–640. doi: 10.1089/ars.2009.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan-Pla A., Cervera A. M., Apostolova N., Garcia-Bou R., Victor V. M., Murphy M. P., McCreath K. J. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. FEBS Lett. 2005;579:2669–2674. doi: 10.1016/j.febslet.2005.03.088. [DOI] [PubMed] [Google Scholar]
- Schofield C. J., Ratcliffe P. J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Semenza G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Skulachev V. P., et al. An attempt to prevent senescence: a mitochondrial approach. Biochim. Biophys. Acta. 2009;1787:437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Smith R. A., et al. Mitochondria-targeted antioxidants in the treatment of disease. Ann. NY Acad. Sci. 2008;1147:105–111. doi: 10.1196/annals.1427.003. [DOI] [PubMed] [Google Scholar]
- Sorescu D., Somers M. J., Lassegue B., Grant S., Harrison D. G., Griendling K. K. Electron spin resonance characterization of the NAD(P)H oxidase in vascular smooth muscle cells. Free Radic. Biol. Med. 2001;30:603–612. doi: 10.1016/s0891-5849(00)00507-4. [DOI] [PubMed] [Google Scholar]
- Sun H., King A. J., Diaz H. B., Marshall M. S. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr. Biol. 2000;10:281–284. doi: 10.1016/s0960-9822(00)00359-6. [DOI] [PubMed] [Google Scholar]
- ten Freyhaus H., Huntgeburth M., Wingler K., Schnitker J., Baumer A. T., Vantler M., Bekhite M. M., Wartenberg M., Sauer H., Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Turrens J. F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M., Zafari A. M., Fukui T., Ishizaka N., Griendling K. K. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- Varela D., Simon F., Riveros A., Jorgensen F., Stutzin A. NAD(P)H oxidase-derived H(2)O(2) signals chloride channel activation in cell volume regulation and cell proliferation. J. Biol. Chem. 2004;279:13301–13304. doi: 10.1074/jbc.C400020200. [DOI] [PubMed] [Google Scholar]
- Xi X. P., Graf K., Goetze S., Fleck E., Hsueh W. A., Law R. E. Central role of the MAPK pathway in ang II-mediated DNA synthesis and migration in rat vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999;19:73–82. doi: 10.1161/01.atv.19.1.73. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Kirito K., Yongzhen H., Ozawa K., Kaushansky K., Komatsu N. Thrombopoietin (TPO) regulates HIF-1alpha levels through generation of mitochondrial reactive oxygen species. Int. J. Hematol. 2008;88:43–51. doi: 10.1007/s12185-008-0091-6. [DOI] [PubMed] [Google Scholar]
- Zafari A. M., Ushio-Fukai M., Akers M., Yin Q., Shah A., Harrison D. G., Taylor W. R., Griendling K. K. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- Zhang G. X., Lu X. M., Kimura S., Nishiyama A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc. Res. 2007;76:204–212. doi: 10.1016/j.cardiores.2007.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.