PDZRN3, a member of the PDZ domain–containing RING finger family of proteins plays an important role in negative feedback control of BMP-2–induced osteoblast differentiation in C2C12 mouse mesenchymal progenitor cells through inhibition of Wnt–β-catenin signaling.
Abstract
PDZRN3 is a member of the PDZ domain–containing RING finger family of proteins. We previously showed that PDZRN3 is essential for the differentiation of C2C12 mouse mesenchymal progenitor cells into myotubes. Mesenchymal progenitor cells differentiate into osteoblasts, chondrocytes, and adipocytes in addition to myotubes, and we have now examined the potential role of PDZRN3 in the differentiation of C2C12 cells into osteoblasts. The abundance of PDZRN3 in C2C12 cells was increased after the induction of osteoblast differentiation by exposure to bone morphogenetic protein (BMP)-2 in low-serum medium. Depletion of PDZRN3 in C2C12 cells by RNA interference resulted in marked enhancement of the BMP-2–induced up-regulation of alkaline phosphatase (ALP) activity. Dkk1, an inhibitor of Wnt signaling, markedly attenuated the enhancement of the BMP-2–induced increase in ALP activity by PDZRN3 depletion. The up-regulation of ALP activity by Wnta3a was also promoted by depletion of PDZRN3. Furthermore, the expression and Wnt3a-induced phosphorylation of LRP6 as well as the increase in the cytosolic abundance of β-catenin induced by Wnt3a were potentiated in PDZRN3-depleted cells. These results indicate that PDZRN3 plays an important role in negative feedback control of BMP-2–induced osteoblast differentiation in C2C12 cells through inhibition of Wnt–β-catenin signaling.
INTRODUCTION
PDZ (PSD-95/Discs-large/ZO-1) domains function as protein-protein recognition modules and mediate clustering of signaling molecules and organization of protein networks. More than 250 PDZ domains in more than 150 proteins are encoded in the human genome. Among PDZ domain–containing proteins, members of the PDZRN (PDZ domain–containing RING finger) family contain an NH2-terminal RING finger, two or four PDZ domains, and a COOH-terminal consensus PDZ domain–binding motif (Katoh, 2004). PDZRN3 and PDZRN4 contain two PDZ domains, but the functions of this subfamily of PDZRN proteins are largely unknown.
We previously cloned PDZRN3 cDNA from a human heart library with the use of a yeast two-hybrid screen for proteins that bind to the PDZ domains of PSD-95 (Ko et al., 2006). The PDZRN3 protein was found to be expressed in a variety of human tissues including heart, skeletal muscle, liver, and brain. We showed that PDZRN3 is essential for myogenic differentiation from myoblasts to myotubes with the use of C2C12 mouse mesenchymal progenitor cells and that its expression is up-regulated in vivo during regeneration of mouse skeletal muscle from satellite cells (Ko et al., 2006). In addition, PDZRN3 was shown by others to regulate surface expression of muscle-specific receptor tyrosine kinase (MuSK) by functioning as a synapse-associated E3 ubiquitin ligase (Lu et al., 2007). PDZRN3 thus plays important roles in muscle differentiation and development.
Mesenchymal progenitor cells differentiate into osteoblasts, chondrocytes, and adipocytes in addition to myotubes (Prockop, 1997). Bone morphogenetic proteins (BMPs) are members of the transforming growth factor β superfamily of proteins. BMP-2 inhibits myotube formation by C2C12 cells and shifts the differentiation pathway to the osteoblastic lineage (Katagiri et al., 1994). Ablation of BMP signaling has been shown to result in various skeletal and extraskeletal developmental abnormalities in vivo (Zhao, 2003). BMP-2 interacts with BMP receptor 1A (BMPR1A) or BMPR1B, both of which are serine-threonine kinases, as well as with their coreceptor BMPR2. Signaling events downstream of BMP receptor activation include phosphorylation of Smad1, Smad5, and Smad8, interaction of these phosphorylated Smad proteins with the comediator Smad4, nuclear translocation of the Smad complexes, and activation of gene transcription (Canalis et al., 2003; Miyazono et al., 2010). In addition to this Smad pathway, non-Smad pathways involving activation of the mitogen-activated protein kinases (MAPKs) c-Jun NH2-terminal kinase (JNK), p38, and extracellular signal–regulated kinase (ERK) also play important roles in BMP signaling (Moustakas and Heldin, 2005; Zhang, 2009). Furthermore, BMP-2 induces expression of Wnt ligands, and Wnt–β-catenin signaling is required for osteoblast differentiation (Chen et al., 2007). We have now investigated the possible role of PDZRN3 in BMP-2–induced differentiation of C2C12 cells into osteoblasts.
MATERIALS AND METHODS
Materials
Recombinant human BMP-2, mouse Wnt3a, and human Dkk1 were obtained from R&D Systems (Minneapolis, MN). Murine Wnt3a–containing conditioned medium was prepared from L Wnt-3A cells (CRL-2647; American Type Culture Collection, Manassas, VA). Protease inhibitor cocktail and phosphatase inhibitor cocktail were obtained from Roche (Branchburg, NJ), and p-nitrophenyl phosphate (pNPP), naphthol AS-MX phosphate, and fast blue BB salt were from Sigma-Aldrich (St. Louis, MO). Polyclonal antibodies to PDZRN3 were generated in rabbits with the use of a histidine-tagged NH2-terminal fragment of human PDZRN3 (amino acids 4-84) as antigen; the antibodies were purified from serum by affinity chromatography with a glutathione S-transferase fusion protein of the antigen. Rabbit polyclonal antibodies to ERK1/2 (KAP-MA001) or to β-catenin (RB-1491) were obtained from Assay Designs (Ann Arbor, MI) and Thermo Scientific (Fremont, CA), respectively. Rabbit polyclonal antibodies to phosphorylated Smad1 linker (Ser206, 9553), to dishevelled2 (3216), to phosphorylated β-catenin (Ser33/37, Thr41; 9561), or to phosphorylated low-density lipoprotein receptor-related protein 6 (LRP6) (Ser1490, 2568) as well as rabbit monoclonal antibodies to phosphorylated Smad1/5 (Ser463/465; 9516) or to Axin1 (2087) were from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal antibodies to Smad1/5/8 (sc-6031-R), to JNK (sc-571), or to adenomatous polyposis coli (APC; sc-896); goat polyclonal antibodies to frizzled1 (sc-30428), to frizzled4 (sc-66450), to frizzled6 (sc-32148), or to LRP5 (sc-21389); as well as mouse monoclonal antibodies to GSK3α/β (sc-7291) or to LRP6 (sc-25317) were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit monoclonal antibodies to phosphorylated JNK1 (Tyr185)/JNK2 (Tyr185)/JNK3 (Tyr223) (EP1597Y) were from Epitomics (Burlingame, CA). Mouse monoclonal antibodies to phosphorylated ERK1/2 (Thr202, Tyr204; 612358), to p38 MAPK (612168), or to phosphorylated p38 MAPK (Thr180, Tyr182; 612280) were from BD Biosciences (San Jose, CA). Mouse monoclonal antibodies to α-tubulin (T9026) were from Sigma-Aldrich.
Cell Culture
C2C12 cells were obtained from American Type Culture Collection and were maintained under 5% CO2 at 37°C in growth medium, consisting of DMEM supplemented with 10% fetal bovine serum. Differentiation of the cells into osteoblasts was induced at 90–95% confluence by replacement of growth medium with low-serum medium, consisting of DMEM supplemented with 2% horse serum (Invitrogen, Carlsbad, CA), and exposure of the cells to various concentrations of BMP-2. Differentiation of C2C12 cells into myotubes was induced by exposure to the low-serum medium without BMP-2.
RNA Interference
Recombinant adenoviral vectors encoding short hairpin RNAs (shRNAs) specific for mouse PDZRN3 mRNA were constructed with the use of the pENTR/U6 shRNA plasmid and the Gateway-based pAd-BLOCK-iT DEST vector (Invitrogen). Two targeting sequences for PDZRN3 (KD-1 and KD-2) and a scrambled version of KD-1 as a control (Scramb) were as follows: 5′-GCTCAGAACAGGAGAATAACG-3′ (KD-1), 5′-GGACACCTCGAACCAAGATGT-3′ (KD-2), and 5′-GCAAGGACAGACACGGAATAT-3′ (Scramb). For adenovirus-mediated expression of shRNA, C2C12 cells were seeded at a density of 2.5 × 104 cells per well in a 24-well plate and cultured in growth medium for 12 h. The cells were then infected with adenoviruses at a multiplicity of infection of 10 for 48 h, after which differentiation was induced by replacement of the medium with low-serum medium with or without BMP-2. An adenoviral vector encoding a form of mouse PDZRN3 mRNA that is resistant to KD-1 was prepared by silent mutation of the target sequence to 5′-GCAGTGAGCAAGAAAACAATG-3′.
Measurement of ALP Activity and ALP Staining
C2C12 cells were washed with phosphate-buffered saline and then lysed in a solution containing 150 mM NaCl, 20 mM Tris-HCl, pH 7.5, 0.3% Triton X-100, and protease inhibitor cocktail. Alkaline phosphatase (ALP) activity in cell lysates was determined with pNPP (50 mg/ml) as substrate in an assay buffer containing 50 mM Na2CO3 and 5 mM MgCl2. For histochemical analysis of ALP activity, cells were fixed with 4% formaldehyde for 10 min at room temperature, washed with phosphate-buffered saline, and incubated for 20 min at 37°C with a mixture of naphthol AS-MX phosphate (0.1 mg/ml), 0.5% N,N-dimethylformamide, 2 mM MgCl2, fast blue BB salt (0.6 mg/ml), and 0.1 M Tris-HCl, pH 8.5. The stained cells were observed by phase-contrast microscopy.
Immunoblot Analysis
C2C12 cells were washed twice with phosphate-buffered saline and then lysed in a solution containing 150 mM NaCl, 20 mM Tris-HCl, pH 7.5, 20 mM Na3VO4, 20 mM NaF, 5 mM EDTA, 0.3% Triton X-100, phosphatase inhibitor cocktail, and protease inhibitor cocktail. The lysates were analyzed for protein content with the use of a DC Protein Assay Kit (Bio-Rad, Hercules, CA), and equal amounts of total lysate protein were subjected to SDS-PAGE. The separated proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad), nonspecific sites of which were blocked before its consecutive exposure to primary antibodies and horseradish peroxidase–conjugated secondary antibodies (Promega, Madison, WI). Immune complexes were detected with the use of a Chemi-Lumi One detection kit (Nacalai Tesque, Kyoto, Japan) and Amersham Hyperfilm ECL (GE Healthcare/Amersham Biosciences, Piscataway, NJ). The signals were quantified densitometrically with an ATTO Lane & Spot Analyzer (ATTO, Tokyo, Japan).
RT-PCR Analysis
Total RNA was prepared from C2C12 cells with the use of an SV total RNA isolation system (Promega). Reverse transcription (RT) and PCR analysis was performed with 100 ng of total RNA and with the use of a Superscript One-Step RT-PCR system (Invitrogen) under conditions that maintain amplification in the exponential phase. The sequences of the PCR primers (sense and antisense, respectively) were 5′-CTGACTCTTGTCCTGCATCGGGACTC-3′ and 5′-ATGGGCTCCTTGGCTGTCTTGAAAGC-3′ for PDZRN3, 5′-CAGGAAGACTGCAAGAAGGCTCTGG-3′ and 5′-ACACGGTGTCACTGCGCTGAAGA-3′ for Runx2, 5′-GACCAGTACAAGCCACTGGA-3′ and 5′-ATCTGTGGTTGTTGAGTAGG-3′ for Smad6, 5′-CAGATTCCCAACTTCTTCTG-3′ and 5′-GTTGAAGATGACCTCCAGCC-3′ for Smad7, 5′-TCCTGCAGCATGTAATCGAC-3′ and 5′-GAGAGGGTGAGGCTCTGTTG-3′ for Id1, 5′-ACCTCAATGCGATTTGTTCC-3′ and 5′-GGTGTCAAAGAAGCCTCTGC-3′ for LRP6, and 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for glyceraldehyde-3-phosphate dehydrogenase (G3PDH).
Analysis of Cytosolic β-Catenin Level
C2C12 cells were washed twice with a solution containing 150 mM NaCl, 2 mM CaCl2, and 20 mM Tris-HCl, pH 7.5, and were then scraped into a solution containing 150 mM NaCl, 2 mM dithiothreitol, 20 mM Tris-HCl, pH 7.5, and protease inhibitor cocktail. The cells were homogenized with the use of a Polytron disrupter, and the homogenate was centrifuged at 500 × g for 10 min at 4°C. The resulting supernatant was centrifuged at 100,000 × g for 30 min at 4°C to yield a cytosolic fraction (supernatant). The fraction was assayed for protein content, and equal amounts of protein were subjected to immunoblot analysis with antibodies to β-catenin as described above.
Luciferase Reporter Assay
C2C12 cells in a 24-well plate were transfected for 16 h with 0.1 μg of the TCF/LEF reporter plasmid TOPflash or FOPflash as a negative control (Upstate Biotechnology, Lake Placid, NY) and with 0.1 μg of a β-galactosidase expression plasmid with the use of the Lipofectamine 2000 reagent (Invitrogen). The cells were then exposed to Wnt3a (200 ng/ml) for 24 h, harvested, lysed, and assayed for luciferase activity with the use of a Luciferase Assay System (Promega) and a Perkin Elmer-Cetus 2030 Multilabel Reader ARVO X4 (Waltham, MA). For each cell lysate, β-galactosidase activity was also measured with o-nitrophenyl-β-d-galactoside (Wako, Osaka, Japan) as a substrate to account for differences in transfection efficiency. The relative luciferase activity (TOPflash/FOPflash) was normalized by β-galactosidase activity.
Statistical Analysis
Data are presented as means ± SEM and were analyzed by Student's t test. p < 0.05 was considered statistically significant.
RESULTS
Expression of PDZRN3 during Osteoblast Differentiation
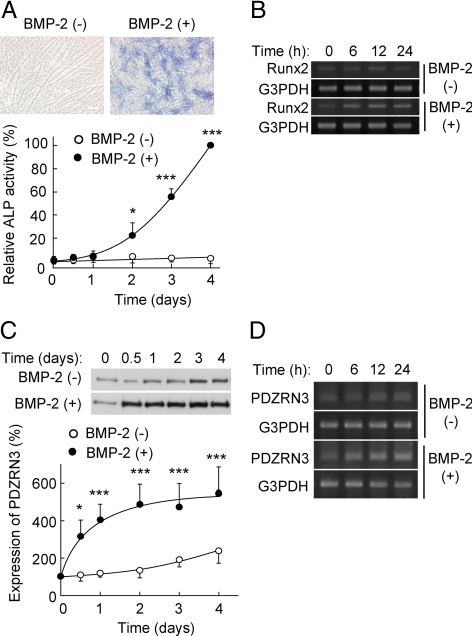
Whereas culture of C2C12 cells in low-serum medium containing 2% horse serum induces their differentiation into myotubes (Blau et al., 1985), culture in the additional presence of BMP-2 results in their differentiation into osteoblasts (Katagiri et al., 1994), which is accompanied by increases in both ALP activity (Figure 1A) and the abundance of Runx2 mRNA (Figure 1B). BMP-2 also markedly and rapidly increased the expression of PDZRN3 at both the protein (Figure 1C) and mRNA (Figure 1D) levels. Although a small increase in the abundance of PDZRN3 was apparent at 3 d after the induction of myotube differentiation, a much larger increase was apparent as early as 12 h after the induction of osteoblast differentiation (Figure 1C). These results thus suggested that PDZRN3 might play a role in the early phase of C2C12 cell differentiation into osteoblasts.
Figure 1.
Expression of PDZRN3 and Runx2 during BMP-2–induced differentiation of C2C12 cells into osteoblasts. (A) C2C12 cells were cultured in low-serum medium with or without BMP-2 (100 ng/ml). After 3 d, the cells were stained for ALP and observed by phase-contrast microscopy (top panels); bar, 50 μm. Cell lysates prepared at the indicated times were assayed for ALP activity with pNPP as substrate (bottom panel). Data are expressed as a percentage of the value for cells exposed to BMP-2 for 4 d and are means ± SEM from five independent experiments. *p < 0.05, ***p < 0.001 versus the corresponding value for cells cultured in the absence of BMP-2. (B) Total RNA prepared from cells cultured as in A was subjected to RT-PCR analysis of Runx2 and G3PDH (control) mRNAs. (C) Lysates of cells cultured as in A were subjected to immunoblot analysis with antibodies to PDZRN3 (top panel). The intensity of the PDZRN3 band was measured by image analysis and expressed as a percentage of the value at time 0 (bottom panel); data are means ± SEM from six independent experiments. *p < 0.05, ***p < 0.001 versus the corresponding value for cells cultured in the absence of BMP-2. (D) Total RNA prepared from cells cultured as in A was subjected to RT-PCR analysis of PDZRN3 and G3PDH mRNAs.
Up-Regulation of ALP and Runx2 by RNA Interference–mediated Depletion of PDZRN3
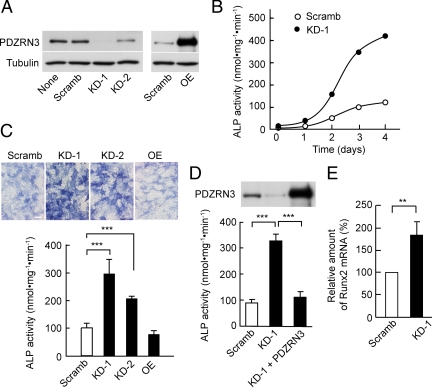
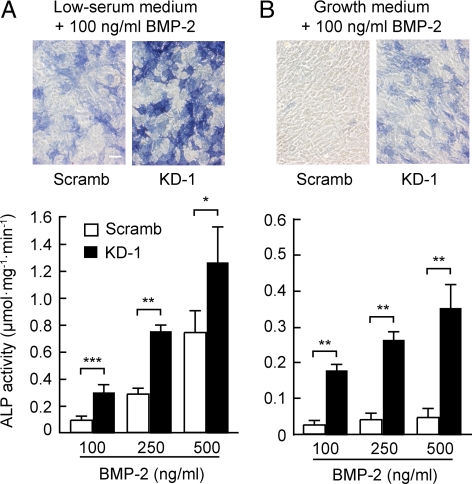
To clarify the potential role of PDZRN3 in osteoblast differentiation of C2C12 cells, we examined the effect of PDZRN3 depletion by adenovirus-mediated expression of a shRNA specific for PDZRN3 mRNA on ALP activity. Expression of two different such shRNAs (KD-1 or -2) but not that of a scrambled version of KD-1 (Scramb) resulted in a marked decrease in the basal abundance of PDZRN3 in C2C12 cells (Figure 2A). Depletion of PDZRN3 was associated with a marked enhancement of the BMP-2–induced increase in ALP activity compared with that apparent in cells expressing the scrambled shRNA (Figure 2, B and C). This effect of PDZRN3 depletion by the KD-1 shRNA was eliminated by adenovirus-mediated expression of PDZRN3 from an mRNA that is resistant to KD-1 (Figure 2D). Depletion of PDZRN3 also enhanced the BMP-2–induced increase in Runx2 gene expression (Figure 2E). Under our experimental conditions, the differentiation of C2C12 cells into chondrocytes and adipocytes was negligible and was not enhanced by depletion of PDZRN3 (data not shown). These results thus suggested that PDZRN3 might function as a negative regulator in osteoblast differentiation. The BMP-2–induced increase in ALP activity in PDZRN3-depleted C2C12 cells was apparent not only in low-serum medium but also in growth medium containing 10% fetal bovine serum (Figure 3). BMP-2 had little effect on ALP activity in C2C12 cells expressing the scrambled shRNA and maintained in growth medium. Adenovirus-mediated overexpression of PDZRN3 (Figure 2A) had no substantial effect on the BMP-2–induced increase in ALP activity in cells cultured in low-serum medium (Figure 2C).
Figure 2.
Effect of RNA interference–mediated PDZRN3 depletion on the BMP-2–induced up-regulation of ALP activity and Runx2 expression in C2C12 cells. (A) C2C12 cells were infected with adenoviral vectors encoding either a shRNA specific for PDZRN3 mRNA (KD-1 or -2), a scrambled version of KD-1 (Scramb), or PDZRN3 (OE) for 2 d in growth medium. Cell lysates were then prepared and subjected to immunoblot analysis with antibodies to PDZRN3 or to α-tubulin (control). (B) C2C12 cells were infected with adenoviral vectors for the PDZRN3 KD-1 or Scramb shRNAs for 2 d in growth medium, after which osteoblast differentiation was induced by replacement of the medium with low-serum medium containing BMP-2 (100 ng/ml). Cell lysates prepared at the indicated times thereafter were assayed for ALP activity. Data are expressed as nanomoles of pNPP hydrolyzed per milligram of protein per minute and are from a representative experiment. (C) Cells infected with adenoviral vectors as in A were stained for ALP (top panels; bar, 50 μm) and assayed for ALP activity (bottom panel) 3 d after the switch from growth medium to low-serum medium containing BMP-2 (100 ng/ml). Quantitative data are means ± SEM from four independent experiments. ***p < 0.001. (D) Cells infected with adenoviral vectors for PDZRN3 KD-1 or Scramb shRNAs, or for PDZRN3 KD-1 shRNA plus a form of PDZRN3 mRNA resistant to KD-1 were induced to differentiate into osteoblasts by culture for 3 d in low-serum medium containing BMP-2 (100 ng/ml). Lysates of the cells were then subjected to immunoblot analysis with antibodies to PDZRN3 (top panel) and assayed for ALP activity (bottom panel). Quantitative data are means ± SEM from five independent experiments. ***p < 0.001. (E) Cells were infected with adenoviral vectors and induced to differentiate into osteoblasts as in B. Total RNA prepared from the cells at 6 h after the induction of differentiation was subjected to RT-PCR analysis of Runx2 and G3PDH mRNAs. The amount of Runx2 mRNA was normalized by that of G3PDH mRNA. Data are means ± SEM from five independent experiments. **p < 0.01.
Figure 3.
BMP-2–induced osteoblast differentiation of PDZRN3-depleted cells in low-serum or growth medium. C2C12 cells infected with adenoviral vectors encoding PDZRN3 KD-1 or Scramb shRNAs were exposed to BMP-2 at the indicated concentrations in either low-serum (A) or growth (B) medium for 3 d, after which the cells were stained for ALP (top panels; bar, 50 μm) and assayed for ALP activity (bottom panels). Quantitative data are means ± SEM from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Effects of PDZRN3 Depletion on BMP Signaling
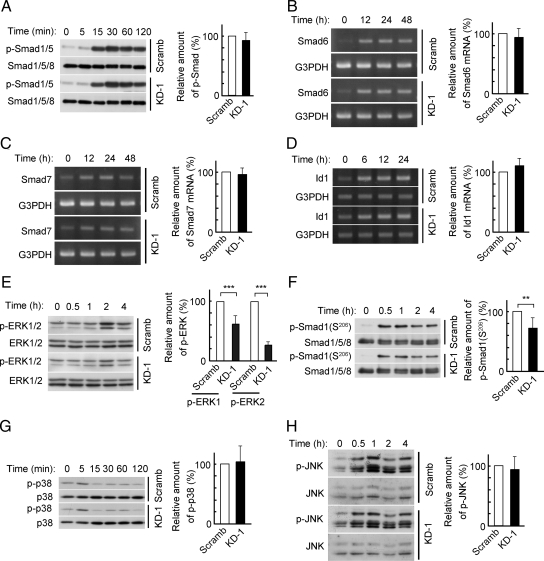
We next examined the effects of PDZRN3 depletion on BMP signaling in C2C12 cells. With regard to the Smad pathway, the BMP-2 induced increases in the amounts of phosphorylated Smads 1 or 5 (Smad1/5) or of the mRNAs for the inhibitory Smads (Smad6 and Smad7) were not affected by depletion of PDZRN3 (Figure 4, A–C). Depletion of PDZRN3 also had no substantial effect on the expression of a representative BMP-responsive gene, that for Id1 (Figure 4D). With regard to the non-Smad pathways, the BMP-2–induced increase in the amount of phosphorylated ERK1/2 was inhibited by depletion of PDZRN3 (Figure 4E), whereas the increases in the amounts of phosphorylated forms of JNK or p38 MAPK were not affected by PDZRN3 depletion (Figure 4, G and H). The BMP-2–induced phosphorylation of the linker region of Smad1, which is catalyzed by ERK, was also inhibited by depletion of PDZRN3 (Figure 4F).
Figure 4.
Effects of PDZRN3 depletion on BMP-2 signaling in C2C12 cells. C2C12 cells infected with adenoviral vectors for PDZRN3 KD-1 or Scramb shRNAs were induced to differentiate into osteoblasts by culture in low-serum medium containing BMP-2 (100 ng/ml). Cell lysates prepared at the indicated times thereafter were subjected to immunoblot analysis with antibodies to Smad1/5/8 or to phosphorylated (p-) Smad1/5 (A), to ERK1/2 or to phosphorylated ERK1/2 (E), to Smad1/5/8 or to phosphorylated Smad1 linker (Ser206) (F), to p38 MAPK or to phosphorylated p38 (G), or to JNK or to phosphorylated JNK (H). Total RNA also prepared from the cells at the indicated times was subjected to RT-PCR analysis of Smad6 (B), Smad7 (C), or Id1 (D) mRNAs. The amounts of phosphorylated Smad1/5 at 30 min (A), Smad6 mRNA at 24 h (B), Smad7 mRNA at 24 h (C), Id1 mRNA at 24 h (D), phosphorylated ERK1/2 at 2 h (E), phosphorylated Smad1 linker at 2 h (F), phosphorylated p38 at 60 min (G), and phosphorylated JNK at 1 h (H) were normalized by the abundance of the corresponding control protein or mRNA and are presented as means ± SEM from six to 10 independent experiments. **p < 0.01, ***p < 0.001.
Effects of PDZRN3 Depletion on Wnt Signaling
Various studies have indicated the existence of cross talk between BMP and Wnt signaling pathways (Rawadi et al., 2003; Nakashima et al., 2005; Vaes et al., 2005; Chen et al., 2007; Fuentealba et al., 2007; de Jesus Perez et al., 2009; Sato et al., 2009). We therefore examined whether Wnt signaling might contribute to the observed potentiation of the BMP-2–induced increase in ALP activity by PDZRN3 depletion. We found that Dkk1, an inhibitor of Wnt signaling, reduced ALP activity by ∼70% in BMP-2–treated cells expressing PDZRN3 shRNA but by only ∼20% in those expressing the scrambled shRNA (Figure 5). These results thus suggested that Wnt signaling was enhanced in the PDZRN3-depleted cells. This enhancement was likely not attributable to potentiation of BMP-2–induced secretion of Wnt from the cells, given that the abundance of mRNAs for Wnts (Wnt1, Wnt2, and Wnt3a) that induce the expression of ALP (Milat and Ng, 2009) was not increased by PDZRN3 depletion. The amount of Wnt1 mRNA was actually lower in PDZRN3-depleted cells, whereas that of Wnt2b mRNA did not differ between the cells expressing PDZRN3 shRNA and those expressing the scrambled shRNA and that of Wnt3a mRNA was too low to detect (data not shown).
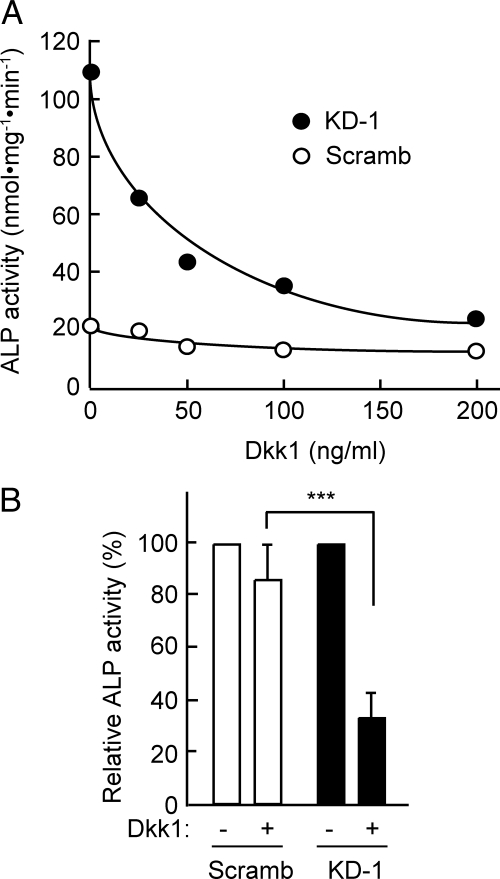
Figure 5.
Effect of Dkk1 on the BMP-2–induced increase in ALP activity in PDZRN3-depleted cells. (A) C2C12 cells infected with adenoviral vectors for PDZRN3 KD-1 or Scramb shRNAs were induced to differentiate into osteoblasts by culture for 3 d in low-serum medium containing BMP-2 (50 ng/ml) and the indicated concentrations of Dkk1. Cell lysates were then prepared and assayed for ALP activity. Data are from a representative experiment. (B) ALP activities of cells treated as in A and exposed to Dkk1 at 200 ng/ml. Data are expressed relative to the corresponding value for cells cultured in the absence of Dkk1 and are means ± SEM from nine independent experiments. ***p < 0.001.
Wnt signaling plays an important role in osteoblast development (Baron and Rawadi, 2007), and overexpression of Wnt3a was previously shown to increase ALP activity in C2C12 cells through canonical Wnt–β-catenin signaling not only in low-serum medium (Rawadi et al., 2003) but also in growth medium (Nakashima et al., 2005). We therefore examined whether the Wnt3a-induced increase in ALP activity is potentiated by PDZRN3 depletion. The Wnt3a-induced increase in ALP activity was indeed markedly greater in cells expressing PDZRN3 shRNA than in those expressing the scrambled shRNA, regardless of whether the cells were cultured in low-serum or growth medium (Figure 6). On the other hand, overexpression of PDZRN3 had no substantial effect on the Wnt3a-induced increase in ALP activity (data not shown), because it failed to affect BMP-2–induced ALP activity (Figure 2C). For cells cultured in growth medium, the Wnt3a-induced increase in the cytosolic abundance of β-catenin, which results in an increase in the amount of β-catenin in the nucleus, was significantly greater in cells depleted of PDZRN3 than in those expressing the scrambled shRNA (Figure 7A), indicating that canonical Wnt–β-catenin signaling was enhanced by PDZRN3 depletion. To confirm the participation of the canonical Wnt–β-catenin pathway in the enhancement of BMP-2–induced ALP activity apparent in PDZRN3-depleted cells, we determined the amounts of cytosolic β-catenin after induction of osteoblast differentiation by BMP-2. The BMP-2–induced increase in the abundance of β-catenin in the cytosol was indeed more pronounced in cells expressing PDZRN3 shRNA than in those expressing the scrambled shRNA, and this difference was apparent in both low-serum and growth media (Figure 7, B and C).
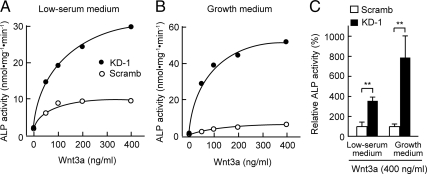
Figure 6.
Effect of PDZRN3 depletion on the Wnt3a-induced increase in ALP activity in C2C12 cells. (A and B) C2C12 cells infected with adenoviral vectors for PDZRN3 KD-1 or Scramb shRNAs were induced to differentiate into osteoblasts by exposure for 3 d to various concentrations of Wnt3a in low-serum (A) or growth (B) medium. Cell lysates were then prepared and assayed for ALP activity. Data are from a representative experiment. (C) ALP activities of cells treated as in A and B, and exposed to Wnt3a at 400 ng/ml. Data are expressed relative to the corresponding value for cells expressing the Scramb shRNA and are means ± SEM from five independent experiments. **p < 0.01.
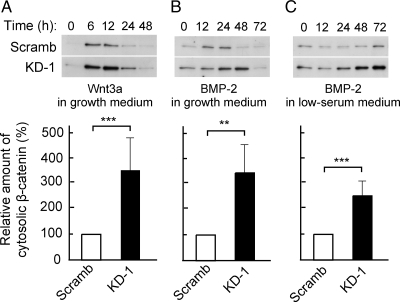
Figure 7.
Effects of PDZRN3 depletion on the cytosolic abundance of β-catenin during BMP-2– or Wnt3a-induced osteoblast differentiation. C2C12 cells infected with adenoviral vectors for PDZRN3 KD-1 or Scramb shRNAs were induced to differentiate into osteoblasts by culture for the indicated times in growth medium supplemented with either murine Wnt3a–containing conditioned medium (A) or BMP-2 (100 ng/ml; B) or in low-serum medium containing BMP-2 (100 ng/ml; C). A cytosolic fraction was then prepared from the cells and subjected to immunoblot analysis with antibodies to β-catenin (top panels). The levels of cytosolic β-catenin at 12 h (A), 24 h (B), or 48 h (C) were determined by densitometry; data are expressed relative to the corresponding value for cells expressing the Scramb shRNA and are means ± SEM from six or seven independent experiments (bottom panels). **p < 0.01, ***p < 0.001 versus the corresponding value for cells expressing the Scramb shRNA.
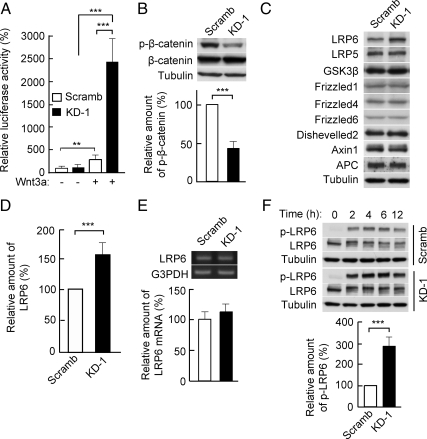
Inhibition of canonical Wnt–β-catenin signaling by PDZRN3 was also confirmed with a luciferase reporter assay in C2C12 cells transfected with the plasmid TOPflash, whose expression reflects that of Wnt–β-catenin target genes. Depletion of PDZRN3 resulted in significant enhancement of the Wnt3a-induced increase in luciferase activity (Figure 8A). Consistent with the increased abundance of β-catenin in the cytosol (Figure 7A), the extent of phosphorylation of β-catenin by GSK3β was decreased in Wnt3a-stimulated C2C12 cells that had been depleted of PDZRN3 (Figure 8B). We then examined the expression of proteins that participate in Wnt–β-catenin signaling, including that of APC, Axin1, dishevelled2, frizzled1, frizzled4, frizzled6, GSK3β, LRP5, and LRP6. The abundance of LRP6 was increased, whereas that of the other proteins was unaffected, in PDZRN3-depleted cells (Figure 8, C and D). In contrast, the amount of LRP6 mRNA did not differ between cells expressing PZDRN3 shRNA or the scrambled shRNA (Figure 8E). Finally, the Wnt3a-induced phosphorylation of LRP6 was also enhanced in PDZRN3-depleted cells (Figure 8F), suggesting that PDZRN3 negatively regulates Wnt–β-catenin signaling by reducing both the expression and Wnt3a-induced phosphorylation of LRP6.
Figure 8.
Enhancement of Wnt–β-catenin signaling by PDZRN3 depletion. (A) C2C12 cells infected with adenoviral vectors for PDZRN3 KD-1 or Scramb shRNAs were transfected with TOPflash or FOPflash luciferase plasmids and a β-galactosidase expression plasmid, cultured for 24 h in growth medium with or without Wnt3a (200 ng/ml), and then harvested for determination of luciferase activity. The relative luciferase activity (TOPflash/FOPflash) was normalized by β-galactosidase activity. Data are means ± SEM from six independent experiments. ***p < 0.001. (B and F) C2C12 cells infected with adenoviral vectors for PDZRN3 KD-1 or Scramb shRNAs were induced to differentiate into osteoblasts by culture for 6 h (B) or the indicated times (F) in growth medium supplemented with murine Wnt3a–containing conditioned medium. Cell lysates were then prepared and subjected to immunoblot analysis (top panels) with antibodies to phosphorylated (p-) β-catenin or to β-catenin (B) or with those to phosphorylated LRP6 or to LRP6 (F). The levels of phosphorylated β-catenin (B) and phosphorylated LRP6 (F) at 6 h were determined by densitometry and normalized by the corresponding amounts of β-catenin and α-tubulin, respectively; data are expressed relative to the corresponding value for cells expressing the Scramb shRNA and are means ± SEM from six to nine independent experiments (bottom panels). ***p < 0.001 versus the corresponding value for cells expressing the Scramb shRNA. (C–E) Cell lysates prepared from C2C12 cells infected with adenoviral vectors for PDZRN3 KD-1 or Scramb shRNAs were subjected to immunoblot analysis with antibodies to LRP6, LRP5, GSK3β, frizzled1, frizzled4, frizzled6, dishevelled2, Axin1, APC, and α-tubulin (C). The abundance of LRP6 was determined by densitometry and normalized by that of α-tubulin (D); data are expressed relative to the value for cells expressing the Scramb shRNA and are means ± SEM from 17 independent experiments. ***p < 0.001 versus the value for cells expressing the Scramb shRNA. Total RNA also prepared from the cells was subjected to RT-PCR analysis of LRP6 and G3PDH mRNAs (top panel in E). The levels of LRP6 mRNA were determined by densitometry and normalized by the amount of G3PDH mRNA (bottom panel in E); data are expressed relative to the value for cells expressing the Scramb shRNA and are means ± SEM from five independent experiments.
DISCUSSION
The differentiation of C2C12 cells into osteoblasts induced by BMP-2 was found to be accompanied by early up-regulation of PDZRN3. Depletion of PDZRN3 by RNA interference revealed that PDZRN3 inhibits the up-regulation of ALP activity, a marker of osteoblast differentiation, as well as that of the mRNA for Runx2, a key regulator of osteoblast differentiation (Komori et al., 1997). PDZRN3 was also shown to influence Wnt–β-catenin signaling but not to affect Smad or non-Smad pathways of BMP signaling with the exception of that mediated by ERK1/2 (see below). Our results thus suggest that the up-regulation of PDZRN3 during BMP-2–induced differentiation of C2C12 cells into osteoblasts results in inhibition of Wnt–β-catenin signaling and therefore contributes to negative feedback control of the differentiation process.
Wnt and BMP act synergistically to promote osteoblast differentiation and the formation of new bone (Mbalaviele et al., 2005; Chen et al., 2007). Both BMP-2 and Wnt3a induce the expression of ALP in and extracellular matrix mineralization by mesenchymal cells (Rawadi et al., 2003). Whereas the induction of ALP by Wnt3a is independent of BMP signaling, that by BMP-2 relies at least in part on Wnt–β-catenin signaling and is associated with increased Wnt expression (Rawadi et al., 2003). Several observations in the present study now suggest that PDZRN3 regulates Wnt–β-catenin signaling during BMP-2–induced differentiation of C2C12 cells into osteoblasts: 1) Depletion of PDZRN3 in C2C12 cells by RNA interference did not affect the activation of Smad1/5 or the expression of Smad6 or Smad7 genes. 2) Dkk1, an inhibitor of Wnt signaling, attenuated the enhancement of the BMP-2–induced increase in ALP activity apparent in PDZRN3-depleted cells. 3) Depletion of PDZRN3 enhanced not only the increase in ALP activity induced by BMP-2 but also that induced by Wnt3a. 4) Depletion of PDZRN3 conferred on BMP-2 the ability to increase ALP activity in cells maintained in growth medium as well as in those cultured in low-serum medium. The induction of ALP in C2C12 cells by Wnt3a was previously observed not only in low-serum medium (Rawadi et al., 2003; Nakashima et al., 2005) but also in growth medium (Lu et al., 2008), whereas that by BMP-2 is evident only in low-serum medium. 5) The BMP-2–induced increase in the cytosolic abundance of β-catenin was enhanced in cells depleted of PDZRN3.
We have shown that PDZRN3 is up-regulated by BMP-2 and that it plays an important role in negative feedback control of BMP-2–induced osteoblast differentiation in mesenchymal cells through inhibition of Wnt–β-catenin signaling. Sclerostin, a secreted cysteine-knot protein expressed primarily in bone, seems to have a similar mode of action in negative regulation of BMP-2–induced osteoblast differentiation and bone formation. It is up-regulated by BMP (Kamiya et al., 2008) and inhibits Wnt–β-catenin signaling but not the Smad pathway of BMP signaling, with the inhibition of Wnt–β-catenin signaling being mediated by binding of sclerostin to LRP5 or LRP6 (Li et al., 2005; Semenov et al., 2005). However, it is not known whether sclerostin suppresses the expression of LRP6, an effect demonstrated for PDZRN3 in the present study.
The BMP-2–induced phosphorylation (activation) of ERK1/2 was attenuated in PDZRN3-depleted cells. ERK1/2 phosphorylates Smad1 in its linker region and thereby inhibits the nuclear accumulation of Smad1 (Kretzschmar et al., 1997). Indeed, the BMP-2–induced phosphorylation of Smad1 in the linker region was also inhibited in cells depleted of PDZRN3. The enhancement of BMP-2–induced up-regulation of ALP activity by PDZRN3 depletion might thus result in part from enhancement of BMP signaling. The observed effect of Dkk1 (Figure 5A), however, indicated that ∼90% of the enhancement of the BMP-2–induced increase in ALP activity by depletion of PDZRN3 was due to activation of Wnt signaling. ERK might therefore not play a critical role in inhibition of the BMP-2–induced increase in ALP activity by PDZRN3.
We previously showed that PDZRN3 is required for myogenic differentiation of C2C12 cells (Ko et al., 2006). We have now shown that expression of PDZRN3 increases earlier and to a greater extent during the differentiation of C2C12 cells into osteoblasts than it does during their differentiation into myotubes. Although up-regulation of PDZRN3 was apparent at 12 h during osteoblast differentiation, it was not observed until 3 d into myotube differentiation, after that of myogenin and before that of myosin heavy chain (Ko et al., 2006). Although we found that PDZRN3 inhibits Wnt–β-catenin signaling during osteoblast differentiation, it might not inhibit such signaling during myogenic differentiation, given that our previous study showed that depletion of PDZRN3 inhibited myogenic differentiation without affecting the expression of myogenin (Ko et al., 2006). Wnt–β-catenin signaling is thought to promote the differentiation of myoblasts into myotubes in part by increasing the expression of myogenin (Rochat et al., 2004; Descamps et al., 2008). PDZRN3 might thus contribute to different signaling pathways during osteoblast and myogenic differentiation.
Overexpression of PDZRN3 in cultured myotubes was previously shown to attenuate agrin-induced clustering of acetylcholine receptors, whereas depletion of endogenous PDZRN3 by RNA interference enhanced such agrin-induced receptor clustering (Lu et al., 2007). The interaction of muscle-specific receptor tyrosine kinase and PDZRN3 was suggested to underlie these observations (Lu et al., 2007), although this kinase does not possess a PDZ domain or a consensus-binding motif for a PDZ domain in its COOH-terminus. Wnt–β-catenin signaling was found to promote agrin-induced clustering of acetylcholine receptors in cultured myotubes in one study (Henriquez et al., 2008), whereas it inhibited this effect in another study (Wang et al., 2008). It thus remains unclear whether negative regulation of Wnt–β-catenin signaling by PDZRN3 is responsible for the effect of PDZRN3 on agrin-induced clustering of acetylcholine receptors in myotubes.
Although depletion of PDZRN3 enhanced the BMP-2–induced increase in ALP activity, overexpression of PDZRN3 had no substantial effect on this up-regulation of ALP activity. The reason for this apparent discrepancy is unknown. Overexpression of PDZRN3 in C2C12 cells depleted of PDZRN3 abolished the effect of such depletion on ALP activity but did not result in a further decrease in this activity. The amount of endogenous PDZRN3 might thus not be limiting for inhibition of the BMP-2–induced increase in ALP activity.
Our analysis of Wnt–β-catenin signaling proteins revealed that PDZRN3 suppressed both the expression and Wnt3a-induced phosphorylation of LRP6 in C2C12 cells. The Wnt3a-induced phosphorylation of LRP6 results in inhibition of β-catenin phosphorylation and degradation and the consequent activation of Wnt-responsive genes (Tamai et al., 2004). LRP6 thus appears to be critical in the negative regulation of Wnt-induced osteoblastic differentiation by PDZRN3. Although the mechanism by which LRP6 is regulated by PDZRN3 remains to be determined, our observation that the amount of LRP6 mRNA was not increased in PDZRN3-depleted cells suggests that PDZRN3 might regulate the stability of LRP6.
We have shown that PDZRN3 negatively regulates BMP-2–induced osteoblastic differentiation of mesenchymal cells through inhibition of Wnt–β-catenin signaling. PDZRN3 likely promotes mesenchymal cell differentiation into myotubes by a distinct mechanism. Given that it is expressed in a variety of tissues including heart, skeletal muscle, liver, and brain (Ko et al., 2006) and that it possesses multiple sites for interaction with other proteins, PDZRN3 might perform different functions by interacting with different binding proteins in different cell types or in response to different stimuli.
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Scientific Research to M.I. and for Young Scientists (B) Grant 22790252 to T.H. from the Japan Society for the Promotion of Science and by a research promotion grant from Yamaguchi University to M.I.
Abbreviations used:
- ALP
alkaline phosphatase
- APC
adenomatous polyposis coli
- BMP
bone morphogenetic protein
- ERK
extracellular signal–regulated kinase
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- GSK
glycogen synthase kinase
- JNK
c-Jun NH2-terminal kinase
- LRP
low density lipoprotein receptor–related protein
- MAPK
mitogen-activated protein kinase
- PCR
polymerase chain reaction
- PDZ
PSD-95/Discs-large/ZO-1
- PDZRN
PDZ domain–containing RING finger
- pNPP
p-nitrophenyl phosphate
- RT
reverse transcription
- shRNA
short hairpin RNA.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-02-0117) on July 28, 2010.
REFERENCES
- Baron R., Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Canalis E., Economides A. N., Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- Chen Y., Whetstone H. C., Youn A., Nadesan P., Chow E. C., Lin A. C., Alman B. A. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J. Biol. Chem. 2007;282:526–533. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- de Jesus Perez V. A., Alastalo T. P., Wu J. C., Axelrod J. D., Cooke J. P., Amieva M., Rabinovitch M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J. Cell Biol. 2009;184:83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps S., Arzouk H., Bacou F., Bernardi H., Fedon Y., Gay S., Reyne Y., Rossano B., Levin J. Inhibition of myoblast differentiation by Sfrp1 and Sfrp2. Cell Tissue Res. 2008;332:299–306. doi: 10.1007/s00441-008-0574-z. [DOI] [PubMed] [Google Scholar]
- Fuentealba L. C., Eivers E., Ikeda A., Hurtado C., Kuroda H., Pera E. M., De Robertis E. M. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez J. P., Webb A., Bence M., Bildsoe H., Sahores M., Hughes S. M., Salinas P. C. Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc. Natl. Acad. Sci. USA. 2008;105:18812–18817. doi: 10.1073/pnas.0806300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N., Ye L., Kobayashi T., Mochida Y., Yamauchi M., Kronenberg H. M., Feng J. Q., Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801–3811. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T., Yamaguchi A., Komaki M., Abe E., Takahashi N., Ikeda T., Rosen V., Wozney J. M., Fujisawa-Sehara A., Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M., Katoh M. Identification and characterization of PDZRN3 and PDZRN4 genes in silico. Int. J. Mol. Med. 2004;13:607–613. [PubMed] [Google Scholar]
- Ko J. A., Kimura Y., Matsuura K., Yamamoto H., Gondo T., Inui M. PDZRN3 (LNX3, SEMCAP3) is required for the differentiation of C2C12 myoblasts into myotubes. J. Cell Sci. 2006;119:5106–5113. doi: 10.1242/jcs.03290. [DOI] [PubMed] [Google Scholar]
- Komori T., et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Doody J., Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., Harris S. E., Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Lu W., Kim K. A., Liu J., Abo A., Feng X., Cao X., Li Y. R-spondin1 synergizes with Wnt3A in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett. 2008;582:643–650. doi: 10.1016/j.febslet.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Lu Z., Je H. S., Young P., Gross J., Lu B., Feng G. Regulation of synaptic growth and maturation by a synapse-associated E3 ubiquitin ligase at the neuromuscular junction. J. Cell Biol. 2007;177:1077–1089. doi: 10.1083/jcb.200610060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbalaviele G., Sheikh S., Stains J. P., Salazar V. S., Cheng S. L., Chen D., Civitelli R. Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J. Cell Biochem. 2005;94:403–418. doi: 10.1002/jcb.20253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milat F., Ng K. W. Is Wnt signalling the final common pathway leading to bone formation? Mol. Cell Endocrinol. 2009;310:52–62. doi: 10.1016/j.mce.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Kamiya Y., Morikawa M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Heldin C. H. Non-Smad TGF-beta signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Nakashima A., Katagiri T., Tamura M. Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. J. Biol. Chem. 2005;280:37660–37668. doi: 10.1074/jbc.M504612200. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rawadi G., Vayssiere B., Dunn F., Baron R., Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- Rochat A., Fernandez A., Vandromme M., Moles J. P., Bouschet T., Carnac G., Lamb N. J. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol. Biol. Cell. 2004;15:4544–4555. doi: 10.1091/mbc.E03-11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M. M., Nakashima A., Nashimoto M., Yawaka Y., Tamura M. Bone morphogenetic protein-2 enhances Wnt/beta-catenin signaling-induced osteoprotegerin expression. Genes Cells. 2009;14:141–153. doi: 10.1111/j.1365-2443.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Semenov M., Tamai K., He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. A mechanism for Wnt coreceptor activation. Mol. Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Vaes B. L., et al. Microarray analysis reveals expression regulation of Wnt antagonists in differentiating osteoblasts. Bone. 2005;36:803–811. doi: 10.1016/j.bone.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wang J., Ruan N. J., Qian L., Lei W. L., Chen F., Luo Z. G. Wnt/beta-catenin signaling suppresses Rapsyn expression and inhibits acetylcholine receptor clustering at the neuromuscular junction. J. Biol. Chem. 2008;283:21668–21675. doi: 10.1074/jbc.M709939200. [DOI] [PubMed] [Google Scholar]
- Zhang Y. E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G. Q. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]