Abstract
The Mouse Metabolic Phenotyping Center (MMPC) Consortium was established to address the need to characterize the growing number of mouse models of metabolic diseases, particularly diabetes and obesity. A goal of the MMPC Consortium is to propose standard methods for assessing metabolic phenotypes in mice. In this article, we discuss issues pertaining to the design and performance of various tests of glucose metabolism. We also propose guidelines for the description of methods, presentation of data and interpretation of results. The recommendations presented in this article are based on the experience of the MMPC Consortium and other investigators.
Introduction
The miniaturization of metabolic techniques for use in the mouse has resulted in important advances in our understanding of the pathophysiology of diabetes and its associated complications. An important goal of the Mouse Metabolic Phenotyping Center (MMPC) Consortium (http://www.mmpc.org/shared/missionStatement.aspx) is to propose standard methodologies for these key metabolic techniques. To this end, guidelines must be established for performing tests of glucose metabolism. It is recognized that it will not be possible for all metabolic tests to be performed in the exact same manner across different laboratories, as the degree of technical expertise and the availability of equipment and reagents will never be consistent from one laboratory to another. However, certain parameters can be standardized, regardless of where tests are conducted. Guidelines must also be established for the description of methods and presentation of results from metabolic tests. This will provide authors and reviewers the necessary information for interpreting results that might differ as a result of differences in methodology. In this article, we discuss issues pertaining to the standardization of methods for assessing glucose metabolism in mice. These include basic considerations when designing and interpreting experiments, as well as consensus recommendations on choosing the appropriate test, performing the test(s), and describing methods and results.
Basic considerations
This section discusses parameters that investigators and reviewers should consider when designing and interpreting tests of glucose metabolism, regardless of the test being conducted. This includes factors that are inherent to the mouse model(s) being tested, as well as factors related to how tests are conducted. Differences in these parameters can affect the results obtained from metabolic tests. It is therefore crucial for these factors to be described accurately in methods sections.
Factors inherent to the mouse model
Strain
A comprehensive analysis of glucose metabolism in mice from four commonly used inbred strains (C57BL/6, 129X1/Sv, FVB/N and DBA/2) demonstrated strain-dependent differences in insulin action, the neuroendocrine response to hypoglycaemia, and insulin secretion (Berglund et al., 2008). Previous studies have also demonstrated strain-dependent differences in metabolic phenotypes in both wild-type and genetically modified mice (Coleman, 1992; Colombo et al., 2003; Kulkarni et al., 2003; Goren et al., 2004; Haluzik et al., 2004a; Biddinger et al., 2005; Burgess et al., 2005). Mice of the same background strain can also differ on the basis of the vendor from which they were obtained. For example, a naturally occurring deletion of exons 7–11 in the nicotinamide nucleotide transhydrogenase gene in C57BL/6 mice from the Jackson Laboratories (C57BL/6J) results in impaired glucose-stimulated insulin secretion (Freeman et al., 2006). Other genetic variations within C57BL/6 substrains have also been reported (Mekada et al., 2009). Because of these factors, authors should list the source as well as the strain of animals used for experiments and/or breeding in methods sections. There are several resources that provide investigators with phenotype information for various mouse strains (http://www.jax.org/phenome; http://www.informatics.jax.org; http://www.amdcc.org).
Husbandry
To ensure a common genetic background in comparisons, heterozygous mating pairs should be used to generate control and experimental animals. This also equalizes the environment in utero and during early life to a great extent. Control mice should be studied in parallel with experimental mice. It is also important to introduce a congenic breeder of the parent strain approximately every five generations to limit the effects of genetic drift or variation within a strain that can occur in an isolated colony (http://jaxmice.jax.org/genetichealth/drift.html). Experimental mice should be handled at least once every week after weaning. This will acclimatize the mice to being handled, potentially reducing this as a stressor during subsequent metabolic tests (Balcombe et al., 2004).
Age and sex
Glucose tolerance and insulin sensitivity deteriorate with age in mice (Grundleger et al., 1980; Bailey and Flatt, 1982; Carvalho et al., 1996). Therefore, metabolic experiments should be carried out on age-matched mice. As sex can also influence the phenotype resulting from genetic mutations (Tiraby et al., 2007; Shi et al., 2008; Macotela et al., 2009), it is recommended that studies be conducted on mice from the same sex. If mice from both sexes are used, investigators should attempt to include equal numbers of mice from each sex and make comparisons within same-sex groups, if sexual dimorphisms exist.
Factors related to procedures
Diet and body composition
Many investigators use diets labeled as ‘chow’, ‘low fat’ or ‘high fat’. However, for a given type of diet, the source of nutrients can vary among different diet manufacturers. Investigators should provide information on the manufacturer, product number, and percentage of calories from carbohydrate, fat and protein in the diets used. High-fat diets are regularly used to elicit changes in body composition (e.g. increased fat mass) and to precipitate insulin resistance in mice (Surwit et al., 1988; Collins et al., 2004). If differences in the source of nutrients are a concern, then complementary control and high-fat diets should be used.
When using high-fat diets, effects on body composition should be noted. Differences in body weight and composition not only affect glucose metabolism but also influence the manner in which metabolic tests are conducted and how results are normalized (discussed below). The phenotypic response to high-fat feeding can be heterogeneous within a strain of mice, as has been reported for C57BL/6J mice (Burcelin et al., 2002). Although comparing weight-matched mice is ideal, this is not always possible. Thus, factoring differences in body weight into the experimental design and interpretation of data is important.
Anesthesia
To minimize stress, some investigators perform tests on anesthetized mice. Anesthesia affects heart rate and blood flow and induces hyperglycemia in mice (Bailey and Flatt, 1980; Brown et al., 2004; Pomplun et al., 2004; Brown et al., 2005; Tanaka et al., 2009). Therefore, assessment of glucose metabolism in anesthetized mice yields results that are not physiological. Because there are procedural means to minimize stress other than anesthesia, tests of glucose metabolism should not be performed in anesthetized mice and should instead be performed in conscious mice.
Blood sampling
The choice of which method to use for blood acquisition depends on a variety of factors, including the skill set available to the investigator, the nature of the test being conducted, and the blood volume requirement. In conscious mice, blood is most commonly obtained from the tip of the tail or via a surgically implanted arterial catheter. Retro-orbital bleeding can also be used, although this can require anesthesia and is most useful when a single sample is needed rather than frequent, repeated samples. Sampling from the tail tip is a simple procedure that can be carried out in any laboratory: 1–2 mm of tissue is cut from the tail tip distal to the bone with sharp scissors or a scalpel, and then blood is obtained by direct flow or by gently massaging (‘milking’) the tail and collecting the blood in a capillary tube or other container. Following the initial cut, a 2-hour recovery period is recommended prior to obtaining the first test sample. Subsequent samples are obtained by gently removing the scab and repeating the massaging procedure. Sampling from the tail tip is appropriate when only small volumes of blood are needed. Larger samples often require prolonged massaging and hence cause more stress to the mouse, resulting in increased catecholamine levels, endogenous glucose production (EGP) and whole-body glucose disappearance (Rd) (Ayala et al., 2006). One distinct advantage of tail-tip sampling is that it does not require catheterization, in contrast to arterial sampling.
Sampling from an arterial catheter has the advantage of providing vascular access during an experiment without introducing handling stress (Balcombe et al., 2004; Ayala et al., 2006). This is especially important for metabolic tests that require the taking of large blood volumes. However, because catheterization of the carotid artery is invasive, it can result in the loss of animals prior to the study (e.g. from lower surgical survival and loss of catheter patency), and can result in stroke. Given the difficulty in performing the surgical procedure, it might not be possible to use arterial sampling as a standard method for blood acquisition in every laboratory.
Fasting
In a typical metabolic study, mice are fasted for either 14–18 hours (overnight fast) or for 5–6 hours (morning fast). Overnight fasting provokes a catabolic state in mice, as they primarily consume at night. In lean mice, overnight fasting reduces lean body mass by ∼15% (Ayala et al., 2006). This metabolic stress is compounded by the fact that mice are typically housed at around 23°C, well below their thermo-neutral temperature of 30°C (Lodhi and Semenkovich, 2009). Prolonged fasting of mice at subthermo-neutral temperatures can result in torpor, characterized by a decrease in the metabolic rate (Geiser, 2004; Swoap et al., 2006). Overnight fasting nearly depletes liver glycogen stores in the mouse. This has the advantage of reducing variability in baseline blood glucose. However, mice have a unique metabolic response to prolonged fasting that contrasts with the response seen in humans. Specifically, a prolonged fast impairs insulin-stimulated glucose utilization in humans, but enhances it in normal mice (Heijboer et al., 2005; Ayala et al., 2006) as well as in some strains of transgenic mice (Ren et al., 1995; Halseth et al., 1999). Therefore, overnight fasting is useful for studies where the focus is on glucose utilization (e.g. effects on muscle uptake of glucose). Otherwise, a 5- to 6-hour fast is sufficient to assess insulin action within a more physiological context.
Ambient lighting and time of day
Because mice are nocturnal, many investigators house their mice in rooms in which the light-dark cycle is altered to fit the needs of the experiment (e.g. a reversed light-dark cycle). This enables metabolic tests to be conducted during hours that are convenient for the investigators (daytime) and that also occur during the metabolically active period for mice (dark cycle). If this approach is used, investigators should indicate whether the metabolic tests are conducted under simulated dark cycle conditions (e.g. under red light) or under conditions of standard lighting. This is not a trivial point, as changes to circadian rhythms have been shown to affect glucose metabolism (Rudic et al., 2004; Turek et al., 2005; Kohsaka and Bass, 2007). Along these lines, the time of day (or night) during which any experiments are conducted is also an important factor and should be reported. Over a 24-hour period, mice, similarly to humans, experience variations in glucose and metabolic hormones, and these can affect interpretation of results. It is therefore important for all experiments in a given study to be carried out at the same time of the day (or night).
There are many other issues that could be added to this discussion. The message we wish to convey is that the outcome of metabolic tests depends not only on the model used, but also on the manner in which the animals are maintained and the means by which the experiments are conducted. Therefore, standardized procedures should be used whenever possible. Because it might not be possible to standardize all procedures, the ability to compare results among laboratories is crucially dependent on a detailed description of the methodology.
Considerations when performing phenotyping tests of glucose metabolism in mice
This section discusses issues that investigators should consider when performing specific phenotyping tests of glucose metabolism in mice. The discussion focuses on how to perform the tests, and how methodology and results should be presented.
Choosing a test
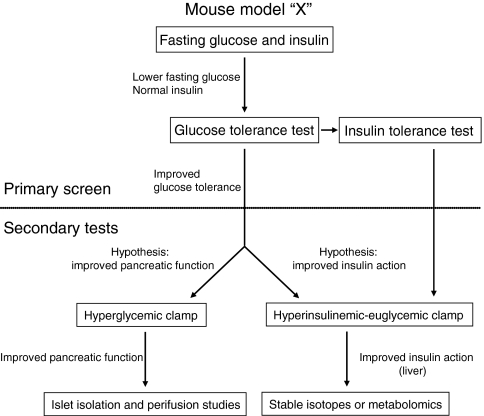
The process of determining whether a mouse model exhibits an alteration in glucose metabolism is illustrated in Fig. 1. The first step is to carry out primary screening tests. The simplest screening test is to measure fasting insulin and glucose. The example in Fig. 1 depicts a mouse model that exhibits low fasting glucose but normal fasting insulin compared with control mice. To further characterize a metabolic phenotype, glucose tolerance tests (GTTs) and/or insulin tolerance tests (ITTs) can then be performed. These tests measure the changes in blood glucose levels over a 1- to 2-hour interval following the administration of a bolus of glucose (for the GTT) or insulin (for the ITT). Measuring insulin levels should be a standard practice for these tests, particularly for GTTs. Differences in insulin levels can often explain observed differences in glucose tolerance and can suggest what further tests should be conducted. The mouse model in Fig. 1 exhibits enhanced glucose tolerance. If this is associated with unusually high insulin levels, it would suggest a phenotype associated with enhanced insulin secretion from β-cells. This can be further tested using more-sophisticated experiments, including hyperglycemic clamps and/or studies on isolated islets. If, however, insulin levels are normal for the observed levels of glucose, it can be hypothesized that the improved glucose tolerance is due to improved insulin action. A hyperinsulinemic-euglycemic clamp could then be conducted to test this hypothesis. This latter technique can include radioactive glucose isotopes to identify the tissue(s) where insulin action is improved. If a hepatic phenotype is expected or observed, stable isotope and metabolomic analyses could be used to further elucidate specific fluxes that might be affected.
Fig. 1.
Choosing a test of glucose metabolism in mice. For a given mouse model, the first step is to perform screening tests. The simplest test is to measure fasting insulin and glucose. In this example, mouse model “X” exhibits lower fasting glucose but normal insulin levels compared with control mice. The next screening test is to perform a GTT; in this example, mouse model “X” has improved glucose tolerance compared with control mice. ITTs can be used as an additional screening test if an effect on insulin action is suspected. If an improved glucose tolerance is thought to be due to enhanced pancreatic function, then a hyperglycemic clamp would be conducted. If the improvement is thought to be due to enhanced insulin action, then a hyperinsulinemic-euglycemic clamp would be conducted. Each of these tests can be followed up by more-specific analyses (isolated perifusion studies for islet function and/or stable isotope or metabolomic studies for glucose fluxes).
Surgical catheterization
Catheter implantation allows for all tests of glucose metabolism (or other parameters in the blood) to be done with little to no handling, thus minimizing stress. This is particularly useful in clamp studies, which require frequent blood sampling, often with large blood volumes. Other metabolic tests, such as fasting measurements and tolerance tests, can most easily be done without catheterization. It must be recognized that catheter implantation adds time, effort and cost to any procedure. If intravenous infusions are used, a catheter is surgically implanted into a jugular or femoral vein. Arterial catheters are used for blood sampling and are inserted into the carotid or femoral artery. A detailed description of the catheterization procedure for the jugular vein and the carotid artery is available at the Vanderbilt MMPC website (http://www.mc.vanderbilt.edu/mmpc). Briefly, using sterile techniques, catheters are inserted into the right jugular vein and left common carotid artery. The free ends of the catheters are externalized and affixed behind the head. After surgery, mice are housed individually and allowed to recover for at least 5–7 days. The general post-operative health of the mice (weight, activity, feeding, grooming, wound healing) is monitored daily. Failure to return to within 10% of presurgery weight (within 15% for obese mice) by post-surgery day 5 is indicative of a general health problem and is used as an exclusion criterion.
Measuring glucose
Measurements of glucose concentration can be obtained from whole-blood samples or from plasma (or serum) samples. Ideally, glucose levels should be measured from plasma samples. This is particularly necessary when calculating glucose fluxes using isotopic techniques. There are commercially available instruments that measure glucose directly from plasma samples (e.g. from YSI, Analox). The advantage of these instruments is that plasma glucose values can be obtained during an experiment. However, this requires the acquisition of larger blood volumes (10–20 μl) and the additional step of separating plasma from blood cells. Alternatively, if a plasma glucose analyzer is not available, glucose values can be obtained from plasma samples using an enzymatic spectrophotometric or fluorometric assay following completion of the experiments.
Hand-held whole-blood glucose monitors (e.g. from Bayer, Roche, BD) are more commonly used than plasma glucose analyzers. Because of their size, they are more practical than plasma analyzers, particularly when performing multiple experiments at once. Another advantage of whole-blood monitors is that they require small blood volumes – typically 5 μl or less. This is especially advantageous in tests that require multiple sampling (e.g. GTTs, ITTs, clamp experiments). As previously stated, if isotopic techniques are used to assess glucose flux, then glucose concentration should be measured from plasma samples. This can be done following completion of the experiment using an enzymatic assay.
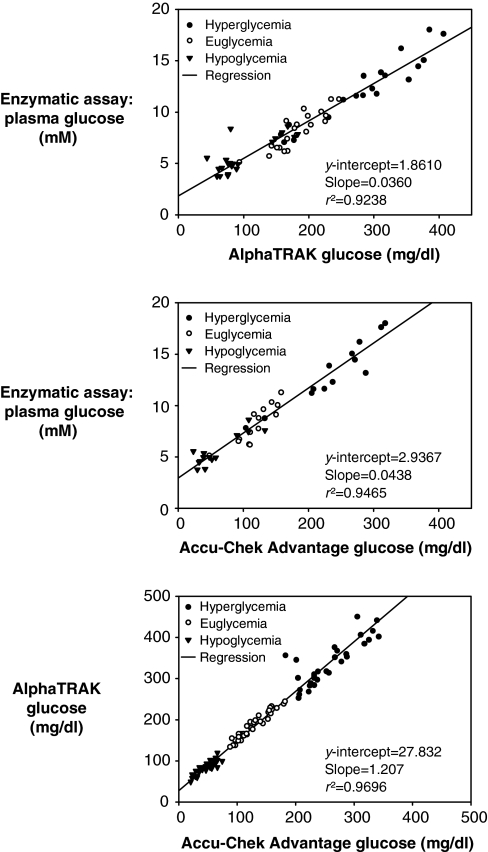
It is important to note that whole-blood glucose monitors are designed for measurement of glucose in human blood. The AlphaTRAK whole-blood glucose monitor by Abbott Laboratories is advertised as being calibrated for the unique properties of animal blood. One advantage of this monitor is that it has a much higher range (i.e. up to ∼1000 mg/dl) compared with other hand-held monitors. Notably, the regression line obtained when comparing whole-blood glucose values from the AlphaTRAK® glucose monitor versus plasma glucose values determined with an enzymatic assay does not intersect the y-axis at zero (Fig. 2). This is similar to what is observed with the Roche Accu-Chek Advantage® whole-blood glucose monitor (Fig. 2). The regression line comparing the two different whole-blood glucose monitors also does not intersect the y-axis at zero (Fig. 2). Given the differences between monitors, investigators should select the brand of monitor they deem most accurate (i.e. when whole-blood glucose values are compared with plasma glucose values) and only use that brand of monitor.
Fig. 2.
Comparison of whole-blood glucose meters and plasma glucose measurements. The regression line obtained when comparing glucose values obtained using the AlphaTRAK® (top panel) or the Accu-Chek Advantage (middle panel) whole-blood glucose monitors and plasma glucose values obtained using an enzymatic assay does not intersect the y-axis at zero. Comparison of glucose values obtained with each monitor (bottom panel) shows that the regression line also does not intersect the y-axis at zero.
Measuring fasting glucose and insulin
Measurement of fasting glucose and insulin is a simple screening method for detecting alterations in glucose metabolism. Typically, a single blood sample is taken from an unrestrained mouse by cutting off the tip of the tail or via an arterial catheter. Alternatively, retro-orbital bleeding can also be used to obtain samples. Methods for measuring glucose have been discussed. There are many commercially available kits for measuring plasma insulin levels, either by radioimmunoassay or by ELISA. Plasma requirements for these assays range from 5–25 μl.
Investigators must be careful not to over-interpret findings from fasting measurements in mice. Fasting measurements in humans are used as a surrogate index of insulin sensitivity. Humans typically fast overnight, and morning glucose and insulin levels represent a basal ‘steady state’. Mice consume food throughout the day and, as such, feeding patterns in mice do not mimic human feeding patterns. It is thus possible that fasting glucose and insulin levels do not represent a basal steady-state in mice. This might explain the different response to fasting between mice and humans with regard to insulin sensitivity (Heijboer et al., 2005; Ayala et al., 2006). An alternative approach is to measure hemoglobin A1c (HbA1c) levels, which are indicative of long-term glycemic trends. A recent publication reported a strong correlation between HbA1c and 6-hour fasting glucose levels compared with overnight fasting glucose levels in three mouse strains (C57BL/6J, DBA/2J and KK/HIJ) with and without diabetes (Han et al., 2008).
Glucose tolerance tests
GTTs assess the disposal of a glucose load administered via oral or intragastric dosing (OGTT), intraperitoneal injection (IPGTT) or intravenous injection (IVGTT). The results of a GTT are determined by insulin secretion, insulin action, and ‘glucose effectiveness’. The protocol for carrying out a GTT is simple. Following a fast, a glucose load is administered and blood glucose is measured over a span of 2 hours. Typically, a blood sample (5 μl or less) is taken prior to the glucose load (for baseline measurements) and then at 15- to 30-minute intervals following the glucose load for the duration of the experiment. Andrikopoulos and colleagues recently reported that differences in fasting duration, route of administration of glucose and amount of glucose administered all affect results obtained from GTTs in anesthetized mice (Andrikopoulos et al., 2008). The standard approach for fasting mice prior to a GTT is the overnight fast (Heikkinen et al., 2007; Muniyappa et al., 2008). This is likely a procedural remnant from GTTs performed in humans, which are typically conducted in overnight-fasted subjects. As discussed above, a shorter (5- to 6-hour) fast is more physiological for mice. The choice of the route of administration depends on a number of variables, including the specific hypothesis being tested and the level of expertise of the personnel performing the tests. OGTTs represent the most physiological route of entry of glucose. Typically, the glucose load is gavaged directly into the stomach via a gavage catheter or administered with a feeding needle. Mice can also be trained to consume a small volume of glucose solution in a short window of time. Whether administered orally or intragastrically, the clearance of glucose during an OGTT is affected by several factors, including the rate of gastric emptying and the incretin effect. If an investigator wishes to circumvent these processes, then an IPGTT or an IVGTT can be performed.
When administering a GTT to humans, a fixed (standard) dose of glucose is given, regardless of the weight of the patient. The standard approach in mice is to base the dose of glucose on the weight of the mouse, usually at 1 or 2 g/kg (Heikkinen et al., 2007; Muniyappa et al., 2008). This is reasonable as long as the weight and body composition for different cohorts are similar. However, in models with increased weight, which is common in many diabetic models, the increased body weight is typically due to a higher fat mass, without a proportionately higher lean mass. This is an important consideration, as lean mass (muscle, brain and liver) is the principal site of glucose disposal. If a glucose dose is administered based on total body weight, then the dose given to an obese mouse will be biased by the increase in fat mass. Therefore, the amount of glucose to which the lean tissue is exposed in an obese mouse will be disproportionately high compared with that in a non-obese mouse with similar lean mass. Obese mice could be misdiagnosed as being glucose intolerant simply because they receive more glucose for the same lean body mass. This bias towards glucose intolerance in obese mice will be greater with increasing glucose dose (e.g. 2 g/kg vs 1 g/kg) (Andrikopoulos et al., 2008). If body composition data are available, then it is more appropriate to base the dose of glucose for a GTT on the lean body mass (McGuinness et al., 2009).
The standard presentation of results from GTTs is a description of blood glucose levels over time after the glucose administration. Generally, a time course of absolute glucose levels is presented. This is valid as long as the groups being compared have equivalent fasting glucose levels. When fasting glucose levels differ, as is often the case with diabetic or insulin-resistant models, a time course of absolute glucose levels should still be presented along with a calculation of the area under the curve above baseline glucose. Accurate interpretation of a GTT can also benefit greatly from presentation of a time course of insulin levels. This requires sampling of larger blood volumes than those used for the measurement of glucose. Depending on the assay, up to 50 μl of blood can be required to measure insulin levels. Thus, the frequency of sample acquisition can be influenced by the sampling method (i.e. tail vs arterial catheter). Typically, samples are obtained at baseline and every 30 minutes following administration of the glucose bolus. A common over-interpretation associated with the GTT is that this test can be used to assess insulin action. Investigators should be aware that glucose tolerance and insulin action are not equivalent.
Insulin tolerance tests
Like GTTs, ITTs monitor glucose concentration over time, but in response to a bolus of insulin rather than of glucose. The convention is to conduct ITTs in mice following a short (5- to 6-hour) fast. Glucose concentration is monitored every 15 to 30 minutes for 60 to 90 minutes following a bolus of insulin administered via intraperitoneal or intravenous injection. The degree to which glucose falls following the insulin bolus is indicative of whole-body insulin action.
Some of the issues regarding how GTTs should be performed also apply to ITTs. Differences in body weight and composition influence the dose of the insulin bolus (McGuinness et al., 2009). An obese mouse (i.e. a mouse with increased fat mass) will receive a larger dose of insulin than a non-obese mouse even though the mass of insulin-sensitive tissue (lean mass) might not differ significantly, or at least proportionately, to the difference in total body mass. Thus, normalizing the insulin dose to lean body mass, if such information is available, is a more accurate means of determining the dose of insulin to be given. As for the duration of the fast, ITTs are typically conducted following short fasts in order to avoid the hypoglycemia that would likely occur in overnight-fasted animals.
As with GTTs, results from ITTs should be presented as a time course of glucose levels. In addition, results can be expressed as the inverse area under the curve below baseline glucose. A common method for presenting glucose levels during an ITT is as a percentage of basal glucose. This is a valid means of presenting results if the groups being compared have equivalent fasting glucose levels. However, if fasting glucose differs among groups, interpretation of a relative fall in glucose can lead to an erroneous conclusion. For the same absolute decrease in blood glucose following an insulin bolus, a mouse with higher fasting glucose will exhibit a smaller percentage fall in glucose. Therefore, if blood glucose is expressed only as a percentage of basal, the conclusion would be that the mouse with higher fasting glucose is insulin resistant. Although this might be the case, drawing such a conclusion from the ITT results would be an over-interpretation of the data. It is also important to note that the half-life of insulin is ∼10 minutes in mice (Cresto et al., 1977). Therefore, differences in the glucose concentration after the initial fall (i.e. beyond 30 minutes after the insulin bolus) might not reflect an effect on insulin action. Thus, if the only difference in glucose levels among groups occurs at the 120-minute time point, this is not indicative of an effect on insulin action. Interpretation of such data requires measurement of insulin levels.
In mice, when blood glucose levels fall below ∼80 mg/dl the counter-regulatory response to insulin is activated (Jacobson et al., 2006a). If a particular mouse model exhibits a defect in the counter-regulatory response, this could be misinterpreted as enhanced insulin action.
Hyperglycemic clamps
Hyperglycemic clamps are used to assess the pancreatic response to hyperglycemia. Following a 5- to 6-hour fast, blood samples are obtained for measurement of baseline glucose, insulin and C-peptide. The clamp begins with a priming dose of glucose followed by a variable glucose infusion. The objective of the priming dose is to quickly achieve target hyperglycemia, typically ∼100–150 mg/dl above fasting glucose values. The priming dose, which can vary depending on the mouse model, is calculated based on the target hyperglycemia and the volume of distribution of glucose. Glucose is then infused at a variable rate to maintain hyperglycemia for the duration of the experiment, which is typically 2 hours. Blood samples (∼50 μl) are taken every 5 minutes for the first 20 minutes of the clamp to assess first-phase insulin secretion. Small (5 μl or less) blood samples are then taken every 10 minutes for the remainder of the experiment to measure blood glucose, and the glucose infusion is adjusted accordingly. Larger (∼50 μl) blood samples are also taken every 20 minutes for the measurement of insulin and C-peptide levels. To prevent a fall in hematocrit as a result of the repeated blood sampling, saline-washed erythrocytes from a strain-matched donor mouse should be infused.
Performing a hyperglycemic clamp using cut-tail sampling can be difficult because of the frequency of sampling and the volume of blood required for insulin and C-peptide analysis. As previously discussed, massaging the tail to obtain larger blood volumes is stressful to the mouse. Therefore, sampling via an arterial catheter is preferred.
Results from hyperglycemic clamp experiments should indicate a time course of glucose, insulin and C-peptide levels. This gives the reader of studies using this technique an opportunity to determine how quickly hyperglycemia was achieved and how well it was maintained, as well as to assess any effects of hyperglycemia on first-phase and second-phase insulin secretion.
Hyperinsulinemic-euglycemic clamps
The hyperinsulinemic-euglycemic clamp, or insulin clamp, is widely considered the gold standard for assessing insulin action in vivo. The miniaturization of the insulin clamp for use in mice has greatly advanced research of diabetes and obesity. Several different permutations of this method are currently being used in mice. However, some of these variations are neither intuitive nor well described. On occasion, one might attempt to compare or interpret results from different laboratories when, in effect, meaningful comparisons or interpretations are impossible.
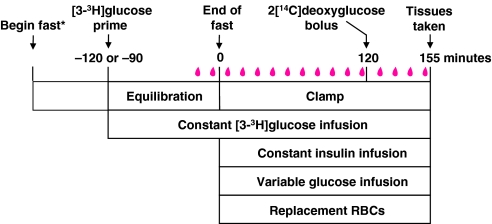
A detailed description of the approach used to perform insulin clamps in mice at the Vanderbilt MMPC can be found on its website (http://www.mc.vanderbilt.edu/mmpc). The Vanderbilt MMPC also offers a yearly course entitled ‘Glucose Clamping the Conscious Mouse: A Laboratory Course’, in which students learn techniques for performing and interpreting insulin clamps. The general protocol for an insulin clamp is straightforward (Fig. 3). Prior to the clamp, mice are fasted to prevent the appearance of meal-derived glucose. Issues regarding the appropriate duration of the fast were discussed above. Following the fast, mice receive a constant infusion of insulin to achieve a steady-state hyperinsulinemia above fasting insulin levels. The constant infusion can be preceded by a priming dose to more quickly achieve steady-state insulin levels. The increased insulin suppresses EGP and stimulates glucose uptake by skeletal muscle and adipose tissue. A variable glucose infusion is administered to maintain euglycemia. The glucose infusion rate is determined by measuring blood glucose at regular intervals, typically every 10 minutes, and adjusting the glucose infusion accordingly. This variable rate of glucose infusion indicates whole-body insulin action, as mice with enhanced insulin action require a greater infusion of glucose. Isotopic tracer infusions can be applied to the general clamp protocol to assess sites where insulin action is affected. The most commonly used isotopes are [3-3H]glucose for the assessment of rates of EGP and Rd, and 2[14C]deoxyglucose for the assessment of tissue-specific glucose uptake. To prevent a fall in hematocrit caused by blood sampling, infusion of saline-washed erythrocytes from a strain-matched donor mouse is recommended.
Fig. 3.
Protocol for hyperinsulinemic-euglycemic clamps in mice. *Mice are fasted either overnight (14 to18 hours) or for shorter intervals (5 to 6 hours) prior to insulin infusion. A primed-continuous infusion of [3-3H]glucose is administered beginning either 120 or 90 minutes prior to the clamp period. Blood samples (in red) are obtained at the end of the equilibration period for assessment of basal parameters, such as basal glucose turnover and insulin concentration. The clamp period consists of a constant insulin infusion and a variable glucose infusion. Blood samples are obtained every 10 minutes for the measurement of glucose concentration. The variable glucose infusion is adjusted accordingly to maintain euglycemia. Saline-washed erythrocytes (RBCs), or an equiosmolar solution, are administered to prevent a fall in hematocrit. From t=80 to 120 minutes, blood samples are obtained for the measurement of steady-state clamp glucose turnover and other hormones and metabolites. A bolus of 2[14C]deoxyglucose can then be administered for the measurement of tissue-specific glucose uptake.
Although the general protocol for an insulin clamp is straightforward, parameters such as the site of blood sampling, fasting duration and insulin infusion rates vary from one laboratory to another. Therefore, it is up to the investigator to recognize how these differences can influence the data obtained from a clamp and make decisions regarding how to perform the experiments accordingly (Ayala et al., 2006). Furthermore, the data are only as useful as the accuracy and completeness of the description of the methodology (Wasserman et al., 2009).
Blood sampling
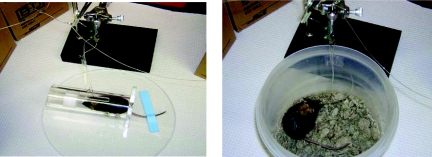
Sampling during insulin clamp studies is generally done via the cut tail or an arterial catheter. For cut-tail sampling during an insulin clamp, the mouse is placed in an over-sized restrainer (e.g. a rat restrainer), the tail is restrained with adhesive tape (Fig. 4), and the tip of the tail (∼1 mm) is cut off with sharp scissors or a surgical scalpel. This is typically done at least 2 hours before the first blood sample is taken to allow for acclimatization. Elevation of catecholamines as a result of obtaining large blood volumes from the cut tail was discussed above. Interestingly, catecholamine levels are typically not elevated when small volumes of blood (∼5 μl) are obtained from the cut tail for measurement of blood glucose (Ayala et al., 2006); therefore, as long as sample volumes are small, the cut tail is a viable sampling method during an insulin clamp. Some investigators might find that cut-tail sampling is more suitable to their needs and expertise and will perform insulin clamps on anesthetized mice to avoid the confounding effects of stress due to handling. As discussed above, however, anesthesia can affect the metabolism of glucose (Bailey and Flatt, 1980; Brown et al., 2004; Pomplun et al., 2004; Brown et al., 2005). At the very least, investigators performing clamps using the cut-tail sampling method should report indices of stress, such as catecholamine levels or corticosterone levels obtained at the end of a study. Alternatively, when sampling from an arterial catheter, neither restraint nor handling is required.
Fig. 4.
Methods for obtaining blood samples during hyperinsulinemic-euglycemic clamps in conscious mice. Blood can be obtained by cutting off the tip of the tail (left) or via a surgically implanted arterial catheter (right). Sampling from the cut tail requires restraint of both the mouse and the tail and handling of the tail throughout the experiment. When sampling from an arterial catheter, restraint is not necessary, and once the mouse is tethered to the swivel apparatus it is no longer handled.
Insulin infusion
The rate of the insulin infusion used in an insulin clamp depends on several factors, including the hypothesis being addressed and the particular mouse model being used. Typically, insulin infusions of 2.5–4.0 mU kg−1 minute−1 are sufficient to suppress EGP 80–100% and stimulate Rd two- to three-fold (Ayala et al., 2006). Insulin-resistant models (e.g. ob/ob or db/db mice) require a significantly greater infusion of insulin (e.g. 20 mU kg−1 minute−1) than more insulin-sensitive models to achieve the same suppression of EGP and stimulation of Rd. As discussed in the section above on ITTs, if body composition is known, insulin infusion rates can be normalized to lean body mass. Although the rate at which insulin is constantly infused during the clamp can vary depending on the experimental hypothesis, the priming dose of insulin is a calculated value. The purpose of a priming dose is to rapidly achieve steady-state levels of an infusate (i.e. insulin in this case), which are then maintained with the subsequent constant infusion. The priming dose of insulin for a clamp experiment can thus be calculated based on the target insulin concentration and the volume of distribution of insulin. For example, if the target insulin concentration during a clamp is 50 μU/ml, which is typical for a 2.5 mU kg−1 minute−1 insulin infusion, and assuming that the volume of distribution of insulin is 300 ml/kg (Ferrannini and Cobelli, 1987a; Ferrannini and Cobelli, 1987b), then an appropriate priming dose is 15 mU/kg. Initial priming doses as high as 300 mU/kg have been reported in the mouse clamp literature (Ayala et al., 2006). This is akin to a greatly exaggerated first-phase insulin release, which can have long-lasting metabolic effects (Luzi and DeFronzo, 1989; Del Prato et al., 2002). Thus, if an insulin prime is administered, the dose should be calculated based on standard principles for equilibrium analysis.
Use of isotopic tracers
When using [3-3H]glucose for the assessment of glucose turnover, a priming dose of this tracer is administered at the beginning of the equilibration period to quickly achieve a steady-state isotope specific activity (Fig. 2). Some investigators have modified this protocol either by adding a second tracer prime at the beginning of the clamp period (i.e. at the end of the equilibration period) or by completely eliminating the first priming dose and giving the priming dose only at the end of the equilibration period (Fernandez et al., 2001; Kim et al., 2001; Haluzik et al., 2002; Kim, H. et al., 2003; Kim, J. K. et al., 2003; Spurlin et al., 2003; Haluzik et al., 2004b; Kim, H. J. et al., 2004; Kim, J. K. et al., 2004; Zabolotny et al., 2004). Administering a second priming dose after the tracer has already equilibrated is reasonable if it is anticipated that the glucose volume of distribution is increased by the insulin clamp (i.e. increased intracellular glucose). However, the second priming dose that has been given in previous studies (10 μCi) is far in excess of any possible increase in intracellular glucose. It is better not to give the second prime, or at least to scale it down considerably. The result of such a large [3-3H]glucose prime is that it disrupts the glucose specific activity and it leads to a large influx of labeled glucose, resulting in artificially increased appearance of [3H]-labeled water and labeling of glycogen stores.
The isotopic 2-deoxyglucose method has been used to estimate a tissue-specific glucose metabolic index (Kraegen et al., 1985). When this method is used to assess muscle glucose metabolism, investigators should indicate the muscle type where measurements were made as different muscles have remarkably different sensitivities to insulin. This technique has been examined and discussed in detail for use in the rat (Kraegen et al., 1985). It is likely that all the same strengths and weaknesses of its use in the rat apply to the mouse.
Presentations of results
Glucose levels, glucose infusion rates (GIRs) and insulin levels should all be reported in any manuscript where insulin clamps are performed. With regard to glucose levels and GIRs, the convention in the mouse literature has been to present average values during the clamp period. This reductionist approach does not give the reader the ability to determine how long it took to achieve steady-state blood glucose and GIRs, and the variation of these parameters prior to that. Furthermore, the time points used to obtain average values of glucose and GIRs are often not defined. As values of glucose and GIRs are by definition collected in all clamp studies, these data should be provided. Clamp insulin concentrations are generally absent from the mouse clamp literature. The inherent problem is that insulin values often vary among different genotypes or in response to dietary modifications. For example, fasting insulin levels are higher in C57BL/6 mice fed a high-fat diet compared with counterparts fed normal chow. This often results in higher insulin levels in high-fat-fed mice at the end of a clamp, even if both groups receive the same insulin infusion (Ayala et al., 2008). Fasting and clamp insulin concentrations are required to interpret a clamp experiment.
Glucose turnover is often presented in relative terms, such as percentage suppression of EGP or stimulation of Rd. Although these data are useful, absolute turnover rates should also be presented. EGP is calculated by subtracting the GIR from the total rate of glucose appearance (Ra). Occasionally, negative values of EGP are calculated, especially when GIRs are high. Although it is impossible to have a negative EGP, a negative value for EGP should not be automatically set to zero or excluded from the data set when determining the average EGP for a group (Finegood et al., 1988). This can create an unintended bias in the data. High negative EGP rates can indicate a technical problem (e.g. non-steady-state error or a contaminant in the tracer), which might require further examination. One approach that limits the appearance of negative values of EGP is to add [3-3H]glucose to the exogenous glucose infusate – a technique known as the ‘hot GINF’ method (Finegood et al., 1987). When calculating the specific activity for glucose [i.e. the ratio of [3-3H]-labeled to non-labeled (‘cold’) glucose] for turnover calculations, values for ‘cold’ glucose should be obtained from plasma, not whole blood. Plasma glucose concentrations can be obtained during the experiment if a plasma glucose analyzer is used. These analyzers require 10–20 μl of whole blood, whereas whole-blood glucose meters typically require <5 μl. Plasma glucose values can be determined after the clamp experiment using standard enzymatic assays on deproteinized plasma samples.
Hyperinsulinemic-hypoglycemic clamps
Although not included in the tests depicted in Fig. 1, the hyperinsulinemic-hypoglycemic clamp, or hypoglycemic clamp, is a useful test of the counter-regulatory response to hypoglycemia (Jacobson et al., 2006a; Jacobson et al., 2006b; Berglund et al., 2008). The protocol for hypoglycemic clamps is identical to that of insulin clamps, except that a larger insulin infusion dose is used (typically 20 mU kg−1 minute−1) and blood glucose is maintained at ∼50 mg/dl. Blood samples are taken for the measurement of glucagon and counter-regulatory hormones, such as catecholamines and corticosterone. Given the effects of potential stress caused by sampling from the cut tail, it is best to perform this test on mice sampled via an arterial catheter.
Conclusions
The use of genetic mouse models is crucial to the study of glucose metabolism and insulin action. For investigators performing phenotypic studies on a new mouse model, it is crucial that tests are performed and interpreted correctly to guide subsequent mechanistic studies. Here, we have described methods, established a minimal set of reportable parameters and provided rationale for standardized practices for testing glucose metabolism in the conscious mouse (summarized in Tables 1 and 2). These recommendations comprise the best practices on the basis of the experiences of the MMPC Consortium and the experiences of other investigators. It is understandable that technical limitations of a laboratory or specific experimental objectives might not be compatible with all recommendations. Nevertheless, a complete description of methodologies used is essential and should be a requirement for publication of studies that involve metabolic testing of mice. A clear and fair presentation of the data is necessary to fully appreciate a given result, and to inform the productive scientific debates and discussions that are necessary to move this field forward.
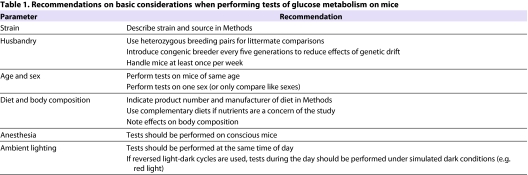
Table 1.
Recommendations on basic considerations when performing tests of glucose metabolism on mice
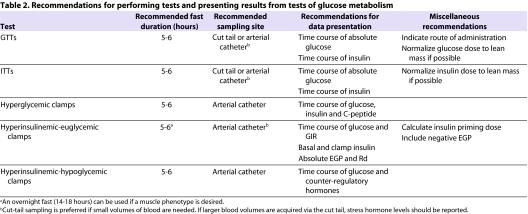
Table 2.
Recommendations for performing tests and presenting results from tests of glucose metabolism
Acknowledgments
We thank Stephen C. Woods for his editorial assistance in preparing this manuscript. This work was supported by the following NIH grants: 5-U24-DK059637-10 (Vanderbilt University MMPC), 5-U24-DK076169-05 (Yale University MMPC and Case Western Reserve University MMPC), 5-U24-DK076126-05 (University of Washington MMPC) and 5-U24-DK059630-10 (University of Cincinnati MMPC). Deposited in PMC for release after 12 months.
REFERENCES
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. (2008). Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 295, E1323–E1332 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. (2006). Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55, 390–397 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Hansotia T, Flock G, Seino Y, Wasserman DH, Drucker DJ. (2008). Insulin action in the double incretin receptor knockout mouse. Diabetes 57, 288–297 [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Flatt PR. (1980). Insulin and glucagon during pentobarbitone anaesthesia. Diabetes Metab. 6, 91–95 [PubMed] [Google Scholar]
- Bailey CJ, Flatt PR. (1982). Hormonal control of glucose homeostasis during development and ageing in mice. Metabolism. 31, 238–246 [DOI] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. (2004). Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 43, 42–51 [PubMed] [Google Scholar]
- Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, Jewell MM, Powers AC, Wasserman DH. (2008). Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 57, 1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. (2005). Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 54, 1314–1323 [DOI] [PubMed] [Google Scholar]
- Brown ET, Umino Y, Solessio E, Loi T, Quinn R, Barlow R. (2004). Anesthesia affects mouse ERG and blood glucose. Invest Ophthalmol Vis Sci. 45, B742–B745 [Google Scholar]
- Brown ET, Umino Y, Loi T, Solessio E, Barlow R. (2005). Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis Neurosci. 22, 615–618 [DOI] [PubMed] [Google Scholar]
- Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. (2002). Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab. 282, E834–E842 [DOI] [PubMed] [Google Scholar]
- Burgess SC, Jeffrey FM, Storey C, Milde A, Hausler N, Merritt ME, Mulder H, Holm C, Sherry AD, Malloy CR. (2005). Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am J Physiol Endocrinol Metab. 289, E53–E61 [DOI] [PubMed] [Google Scholar]
- Carvalho CR, Brenelli SL, Silva AC, Nunes AL, Velloso LA, Saad MJ. (1996). Effect of aging on insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of rats. Endocrinology 137, 151–159 [DOI] [PubMed] [Google Scholar]
- Coleman DL. (1992). The influence of genetic background on the expression of mutations at the diabetes (db) locus in the mouse. VI: Hepatic malic enzyme activity is associated with diabetes severity. Metabolism 41, 1134–1136 [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. (2004). Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 81, 243–248 [DOI] [PubMed] [Google Scholar]
- Colombo C, Haluzik M, Cutson JJ, Dietz KR, Marcus-Samuels B, Vinson C, Gavrilova O, Reitman ML. (2003). Opposite effects of background genotype on muscle and liver insulin sensitivity of lipoatrophic mice. Role of triglyceride clearance. J Biol Chem. 278, 3992–3999 [DOI] [PubMed] [Google Scholar]
- Cresto JC, Lavine RL, Buchly ML, Penhos JC, Bhathena SJ, Recant L. (1977). Half life of injected 125I-insulin in control and ob/ob mice. Acta Physiol Lat Am. 27, 7–15 [PubMed] [Google Scholar]
- Del Prato S, Marchetti P, Bonadonna RC. (2002). Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes. 51, S109–S116 [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Le Roith D. (2001). Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 15, 1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E, Cobelli C. (1987a). The kinetics of insulin in man. I. General aspects. Diabetes Metab Rev. 3, 335–363 [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Cobelli C. (1987b). The kinetics of insulin in man. II. Role of the liver. Diabetes Metab Rev. 3, 365–397 [DOI] [PubMed] [Google Scholar]
- Finegood DT, Bergman RN, Vranic M. (1987). Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36, 914–924 [DOI] [PubMed] [Google Scholar]
- Finegood DT, Bergman RN, Vranic M. (1988). Modeling error and apparent isotope discrimination confound estimation of endogenous glucose production during euglycemic glucose clamps. Diabetes 37, 1025–1034 [DOI] [PubMed] [Google Scholar]
- Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. (2006). Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 55, 2153–2156 [DOI] [PubMed] [Google Scholar]
- Geiser F. (2004). Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 66, 239–274 [DOI] [PubMed] [Google Scholar]
- Goren HJ, Kulkarni RN, Kahn CR. (2004). Glucose homeostasis and tissue transcript content of insulin signaling intermediates in four inbred strains of mice: C57BL/6, C57BLKS/6, DBA/2, and 129X1. Endocrinology 145, 3307–3323 [DOI] [PubMed] [Google Scholar]
- Grundleger ML, Godbole VY, Thenen SW. (1980). Age-dependent development of insulin resistance of soleus muscle in genetically obese (ob/ob) mice. Am J Physiol. 239, E363–E371 [DOI] [PubMed] [Google Scholar]
- Halseth AE, Bracy DP, Wasserman DH. (1999). Overexpression of hexokinase II increases insulinand exercise-stimulated muscle glucose uptake in vivo. Am J Physiol. 276, E70–E77 [DOI] [PubMed] [Google Scholar]
- Haluzik M, Dietz KR, Kim JK, Marcus-Samuels B, Shulman GI, Gavrilova O, Reitman ML. (2002). Adrenalectomy improves diabetes in A-ZIP/F-1 lipoatrophic mice by increasing both liver and muscle insulin sensitivity. Diabetes 51, 2113–2118 [DOI] [PubMed] [Google Scholar]
- Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, Stannard B, Dietz KR, Le Roith D, Reitman ML. (2004a). Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology 145, 3258–3264 [DOI] [PubMed] [Google Scholar]
- Haluzik M, Gavrilova O, LeRoith D. (2004b). Peroxisome proliferator-activated receptor-alpha deficiency does not alter insulin sensitivity in mice maintained on regular or high-fat diet: hyperinsulinemic-euglycemic clamp studies. Endocrinology 145, 1662–1667 [DOI] [PubMed] [Google Scholar]
- Han BG, Hao CM, Tchekneva EE, Wang YY, Lee CA, Ebrahim B, Harris RC, Kern TS, Wasserman DH, Breyer MD, et al. (2008). Markers of glycemic control in the mouse: comparisons of 6-hand overnight-fasted blood glucoses to Hb A1c. Am J Physiol Endocrinol Metab. 295, E981–E986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijboer AC, Donga E, Voshol PJ, Dang ZC, Havekes LM, Romijn JA, Corssmit EP. (2005). Sixteen hours of fasting differentially affects hepatic and muscle insulin sensitivity in mice. J Lipid Res. 46, 582–588 [DOI] [PubMed] [Google Scholar]
- Heikkinen S, Argmann CA, Champy MF, Auwerx J. (2007). Evaluation of glucose homeostasis. Curr. Protoc. Mol. Biol. Chapter 29, Unit 29B 23. [DOI] [PubMed]
- Jacobson L, Ansari T, McGuinness OP. (2006a). Counterregulatory deficits occur within 24 h of a single hypoglycemic episode in conscious, unrestrained, chronically cannulated mice. Am J Physiol Endocrinol Metab. 290, E678–E684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Ansari T, Potts J, McGuinness OP. (2006b). Glucocorticoid-deficient corticotropin-releasing hormone knockout mice maintain glucose requirements but not autonomic responses during repeated hypoglycemia. Am J Physiol Endocrinol Metab. 291, E15–E22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW, Fernandez AM, Reitman ML, Yakar S, Stannard B, Heron-Milhavet L, et al. (2003). Peroxisome proliferator-activated receptor-alpha agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes 52, 1770–1778 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, et al. (2004). Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53, 1060–1067 [DOI] [PubMed] [Google Scholar]
- Kim JK, Zisman A, Fillmore JJ, Peroni OD, Kotani K, Perret P, Zong H, Dong J, Kahn CR, Kahn BB, et al. (2001). Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest. 108, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Gavrilova O, Chao L, Higashimori T, Choi H, Kim HJ, Yu C, Chen Y, Qu X, et al. (2003). Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes 52, 1311–1318 [DOI] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, et al. (2004). PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 114, 823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Bass J. (2007). A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 18, 4–11 [DOI] [PubMed] [Google Scholar]
- Kraegen EW, James DE, Jenkins AB, Chisholm DJ. (1985). Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 248, E353–E362 [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Almind K, Goren HJ, Winnay JN, Ueki K, Okada T, Kahn CR. (2003). Impact of genetic background on development of hyperinsulinemia and diabetes in insulin receptor/insulin receptor substrate-1 double heterozygous mice. Diabetes 52, 1528–1534 [DOI] [PubMed] [Google Scholar]
- Lodhi IJ, Semenkovich CF. (2009). Why we should put clothes on mice. Cell Metab. 9, 111–112 [DOI] [PubMed] [Google Scholar]
- Luzi L, DeFronzo RA. (1989). Effect of loss of first-phase insulin secretion on hepatic glucose production and tissue glucose disposal in humans. Am J Physiol. 257, E241–E246 [DOI] [PubMed] [Google Scholar]
- Macotela Y, Boucher J, Tran TT, Kahn CR. (2009). Gender and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58, 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. (2009). NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 297, E849–E855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, Yoshiki A. (2009). Genetic differences among C57BL/6 substrains. Exp Anim. 58, 141–149 [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. (2008). Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 294, E15–E26 [DOI] [PubMed] [Google Scholar]
- Pomplun D, Mohlig M, Spranger J, Pfeiffer AF, Ristow M. (2004). Elevation of blood glucose following anaesthetic treatment in C57BL/6 mice. Horm Metab Res. 36, 67–69 [DOI] [PubMed] [Google Scholar]
- Ren JM, Marshall BA, Mueckler MM, McCaleb M, Amatruda JM, Shulman GI. (1995). Overexpression of Glut4 protein in muscle increases basal and insulin-stimulated whole body glucose disposal in conscious mice. J Clin Invest. 95, 429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. (2004). BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. (2008). Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab. 294, E630–E639 [DOI] [PubMed] [Google Scholar]
- Spurlin BA, Thomas RM, Nevins AK, Kim HJ, Kim YJ, Noh HL, Shulman GI, Kim JK, Thurmond DC. (2003). Insulin resistance in tetracycline-repressible Munc18c transgenic mice. Diabetes 52, 1910–1917 [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. (1988). Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37, 1163–1167 [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. (2006). The full expression of fasting-induced torpor requires beta 3-adrenergic receptor signaling. J Neurosci. 26, 241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kawano T, Tomino T, Kawano H, Okada T, Oshita S, Takahashi A, Nakaya Y. (2009). Mechanisms of impaired glucose tolerance and insulin secretion during isoflurane anesthesia. Anesthesiology 111, 1044–1051 [DOI] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Capel F, Mairal A, Crampes F, Rami J, Pujol C, Boutin JA, Langin D. (2007). Resistance to high-fat-diet-induced obesity and sexual dimorphism in the metabolic responses of transgenic mice with moderate uncoupling protein 3 overexpression in glycolytic skeletal muscles. Diabetologia 50, 2190–2199 [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. (2005). Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DH, Ayala JE, McGuinness OP. (2009). Lost in translation. Diabetes 58, 1947–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabolotny JM, Haj FG, Kim YB, Kim HJ, Shulman GI, Kim JK, Neel BG, Kahn BB. (2004). Transgenic overexpression of protein-tyrosine phosphatase 1B in muscle causes insulin resistance, but overexpression with leukocyte antigen-related phosphatase does not additively impair insulin action. J Biol Chem. 279, 24844–24851 [DOI] [PubMed] [Google Scholar]