Abstract
Heavy alcohol use is commonplace among HIV-infected individuals; however, the extent that alcohol use adversely impacts HIV disease progression has not been fully elucidated. Fairly strong evidence suggests that heavy alcohol consumption results in behavioral and biological processes that likely increase HIV disease progression, and experimental evidence of the biological effect of heavy alcohol on simian immunodeficiency virus in macaques is quite suggestive. However, several observational studies of the effect of heavy alcohol consumption on HIV progression conducted in the 1990s found no association of heavy alcohol consumption with time to AIDS diagnosis, while some more recent studies showed associations of heavy alcohol consumption with declines of CD4 cell counts and nonsuppression of HIV viral load. We discuss several plausible biological and behavioral mechanisms by which alcohol may cause HIV disease progression, evidence from prospective observational human studies, and suggest future research to further illuminate this important issue.
Keywords: Alcohol, HIV disease progression, CD4, HIV viral load, Adherence, Prospective studies, Nutrition, Immune activation, Bacterial translocation
Introduction
Heavy alcohol use is commonplace among HIV-infected individuals and its impact upon behaviors and the course of disease has been examined over the past two decades [1–3]. However, the extent that alcohol use results in deleterious effects on the progression of HIV disease has not been fully elucidated. Although alcohol may directly affect HIV disease progression in multiple ways, obtaining data to document its overall impact and contributions associated with specific mechanisms is difficult. Alcohol consumption has direct effects on several aspects of the immune system; yet, experimental studies to assess more directly its impact on HIV disease have been limited to the related model of simian immunodeficiency virus (SIV) in primates. In humans, observational studies may be hampered by measurement error and confounding. Incomplete assessment of behavioral (eg, other substance use, medication adherence, nutritional deficiencies) and psychosocial (eg, depressive symptoms) factors associated with HIV disease progression impedes rigorous determination of alcohol’s direct effects. In addition, traditional analytic methods may fail to account for the potential feedback loop between alcohol consumption and health status (ie, that alcohol consumption tends to decrease as health declines). In this article we review the empirical studies and the major mechanisms by which alcohol may affect HIV disease progression (Fig. 1) based on the literature available as of early 2010.
Fig. 1.
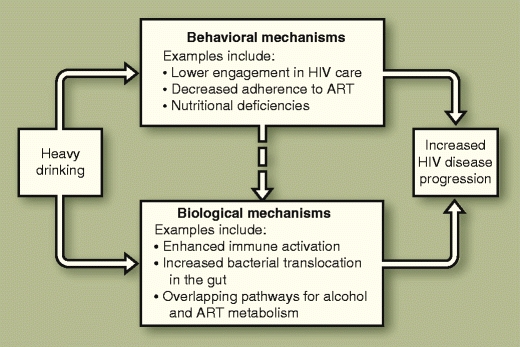
Potential mechanisms of HIV disease progression. ART—antiretroviral therapy
HIV Disease Progression: Definitions
Progression of HIV infection in humans or SIV infection in the HIV primate model has been defined most typically in terms of viral load, CD4 cell count, AIDS-defining clinical end points (eg, opportunistic infections), and mortality. More recent evidence of the association of the level of immune activation with clinical outcomes is increasingly recognized; however, few data exist at present to consider this marker of HIV disease progression. In this literature review we sought published research that provided measurement of alcohol consumption as well as one of the following measures of HIV disease progression: HIV viral load, CD4 cell count, opportunistic infections, or death.
Alcohol Use: Definitions
Alcohol use can be defined by the amount consumed (eg, at-risk, heavy) or by the consequences of its use (eg, abuse, dependence). The consumption threshold for at-risk use as defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) is >4 drinks on an occasion or >14 drinks in a week for men, >3 and >7 respectively, for women [4]. This level of drinking has been used to define “heavy” drinking for those with HIV infection [5••], although its use was chosen based on NIAAA’s use of the at-risk levels in the general population and was not specific for HIV-infected individuals. The category of “moderate” alcohol consumption is used for one whose alcohol use falls between abstinence (ie, no use of alcohol) and heavy use.
Another dimension to recognize when defining alcohol use is the variable nature of its use, in that for almost half of an HIV-infected cohort of persons with a history of alcohol problems, use was observed to increase or decline over a median period of 3.4 years [6]. Studies that only measure drinking at baseline fail to account for the dynamic nature of alcohol consumption, which may adversely affect study results and conclusions.
Biological Basis for Alcohol Affecting HIV Disease Progression
The Effects of Alcohol on the Immune System
Chronic alcohol consumption has been shown to be associated with increased susceptibility to infectious diseases (eg, tuberculosis and bacterial pneumonia), increased severity of diseases (eg, viral hepatitis), and increased risk of cancers (eg, hepatocellular carcinoma) [7]. These illnesses may also be accelerated by vitamin deficiencies, malnutrition, or other substance use. Chronic alcohol consumption can lead to liver disease and cirrhosis, which can impact immunocompetence. Recent animal and human studies have shown that alcohol consumption has deleterious effects on both the innate and the acquired immune responses. Impaired innate immune responses may cause susceptibility to infection, while impaired acquired immune responses such as impaired B lymphocyte function, altered cytokine balance, and chronic T-cell activation may accelerate disease progression, including that caused by HIV. In addition, alcohol may play a role in translocation of bacteria and bacterial products from the gut to cause HIV immune activation, resulting in increased HIV disease progression [8].
Alcohol and Nutrition
Micro-nutrient (ie, vitamin and mineral) deficiencies, including selenium and vitamins D, A, B-12, and E, zinc, and iron, have been associated with more rapid HIV disease progression [9–11]. At the same time, antiretroviral therapy (ART) is thought to decrease some and restore other micronutrient levels [12, 13]. Overall nutritional status has been associated with HIV progression in several prospective studies [14–16], while there is also an effect of HIV infection on nutritional status [17]. Alcohol consumption is associated with nutritional deficiencies, due to a high percentage of caloric intake from alcohol, decreased absorption of nutrients, and interference with the metabolism of nutrients [18]. Therefore, it is plausible that nutritional deficiency is a mechanism by which alcohol might result in more rapid HIV disease progression. The link between alcohol use, decreased nutrition, and immune markers has been demonstrated experimentally in the SIV model [19]; however, it is not clear for HIV infection in humans.
Alcohol and ART Effectiveness
Some ART, including non-nucleoside reverse transcriptase inhibitors and protease inhibitors, is metabolized by the human cytochrome P450 system. There is evidence that alcohol may impact the metabolism of these medications by two different mechanisms, enzymatic induction [20], associated with chronic alcohol use, and enzymatic inhibition due to competition of ethanol for various cytochrome P450 isozymes, associated with acute ethanol use [21]. Because numerous drugs are metabolized by the P450 pathway, chronic alcohol users may be at higher risk for drug toxicities and ineffective therapy due to inadequate plasma drug concentration. Chronic alcohol consumption may also alter drug protein binding. We examine the evidence as to whether heavy alcohol consumption reduces the effectiveness of ART as measured by failure to achieve viral suppression, even with good adherence.
Behavioral/Psychosocial Basis for Alcohol Affecting HIV Disease Progression
Several behavioral and psychosocial factors that are associated with heavy alcohol consumption are also associated with HIV disease progression, and therefore should be considered when conducting multivariate modeling. We summarize these associations in the following sections.
Access to and Retention in HIV Care and Receipt of ART
Researchers in the United States and internationally have found that heavy drinkers are less likely than others to be receiving ART [22•, 23, 24••]. This may be a consequence of barriers to consistent medical treatment or due to physicians’ impressions that heavy drinkers are unable to competently use ART. Indeed, heavy alcohol use was independently associated with lower retention in care among indigent patients [25]. Because early and consistent HIV care and receipt of opportunistic infection (OI) prophylaxis and ART are key factors in slowing the progression of HIV [26], these findings suggest one mechanism by which heavy alcohol consumption may accelerate HIV disease progression.
ART Adherence
Alcohol consumption has been consistently associated with poorer ART adherence. A recent meta-analysis found that those who used alcohol or drank relatively more were 50%–60% as likely to be classified as adherent compared with those who abstained or drank relatively less [27•]. Some studies have found a dose-response relationship between alcohol consumption and ART adherence [24••, 28], in addition to a temporal association between drinking episodes and missed doses [28]. As ART adherence is a known predictor of HIV outcome, this strongly suggests that decreased adherence is one mechanism by which alcohol may increase HIV disease progression. These findings elevate the importance of attempting to control for ART adherence in examining the biological impact of alcohol on HIV disease progression apart from its impact on adherence. However, adherence is challenging to accurately measure and therefore may pose problems even if included in statistical models. Alternatively and likely more effectively, studies of persons infected with HIV who are not yet on ART may reveal key insights about this important issue.
Alcohol and Comorbidities
Comorbidities may complicate the issue of alcohol’s impact on HIV disease progression and make it difficult to determine the association. For example, depression, which can be exacerbated by the effects of alcohol [29], has been shown to reduce adherence to ART [30], and depression, stress, and trauma may have worsening effects on HIV progression [30, 31].
Illicit substance use is strongly associated with heavy alcohol consumption, especially in minority and inner-city populations. Several studies have shown associations between illicit substance use, especially crack cocaine, and HIV progression [32•, 33]. Substance use other than alcohol has also been associated with lower rates of receipt of ART [24••]. Hence, controlling for other non-alcohol substance use is important in seeking an understanding of the impact of alcohol consumption on HIV disease progression.
Evidence of a Causal Association between Alcohol Use and HIV Disease Progression
Experimental Studies
The direct impact of alcohol consumption on HIV disease progression has been studied using animal models. The strengths of these studies include the absence of ART adherence as a possible mediator of disease progression, and the capacity to control the quantity of alcohol consumed as well as behavioral factors (eg, nutrition), which are not easily accounted for in human studies. Evidence from studies in macaques suggests that heavy alcohol consumption has consequences for increased SIV progression. Experimental administration of the equivalent of heavy doses of alcohol as compared to sucrose to macaques infected with SIV was associated with early plasma CD4 cell loss in some studies [34, 35] but not others [36, 37], while several studies found greater SIV viral load at various times post-infection [19, 34, 36, 37]. Bagby et al. [36] found a significantly more rapid onset of end-stage disease in eight alcohol-administered macaques compared with eight controls. Higher viral load in alcohol-exposed macaques was associated with a higher percentage of SIV target cells (CD4) in the gut coupled with lower percentages of CD8 cells, creating a blunted mucosal immune response in early infection in one study [37]. The alcohol-exposed group consumed significantly fewer calories than the controls in another study [19]. Taken together, these findings suggest a biologically deleterious effect of heavy alcohol administration on disease progression in SIV-infected primates.
Human Observational Studies: Pre-Highly Active Antiretroviral Therapy (HAART)
A number of clinical studies assessed alcohol use in cross-sectional and prospective analyses. Associations between HIV outcomes such as CD4 cell counts and HIV viral load in cross-sectional studies may reflect differences in the time of study entry by alcohol consumption category, therefore we will focus in this review on prospective studies. Several prospective studies were published using data collected in the pre-HAART era, as described below.
An analysis conducted in the Multicenter AIDS Cohort Study (MACS) included 1706 HIV-infected men, and examined alcohol consumption by average number of drinks per day, ranging from zero to greater than two, the latter meeting the threshold for “heavy” drinking in men. There was no association between drinks per day both at enrollment and prospectively and the development of AIDS [38]. A later analysis of the MACS determined that decreasing alcohol consumption (ie, having a significantly negative slope in the average number of drinks per week in the prior 6 months) was associated with developing AIDS-related conditions, suggesting a decrease in alcohol consumption as HIV progressed [39]. Studies conducted in men in the Netherlands [40] and Norway [41] found no association between the number of drinks per day in the prior 6 months or daily drinking, respectively, with the development of AIDS. A study of vitamin deficiencies (n = 312) reported that in a multivariate model that included age, HIV-related symptoms, baseline CD4 cell count, and several vitamin concentrations, frequent alcohol consumption (>2 times/week) at study baseline was associated with increased time to CD4 cell count declines to 200 cells/mm3 and time to AIDS [42]. A study of 403 persons seroconverting during the Tricontinental Seroconverter Study found that any alcohol use during the first three quarters of the follow-up period, limited to avoid the feedback loop between symptoms and alcohol consumption, was not associated with time to AIDS or death [43]. The selection of “any alcohol use” as a main independent variable, as in the latter study, is a coarse measure to assess alcohol’s impact on HIV disease progression. All of the preceding studies were conducted among men who had sex with men.
Two early studies were conducted among injecting drug users. One study among 496 HIV-infected methadone maintenance patients found no association between daily alcohol consumption in the prior month and time to AIDS or death in a time-dependent multivariate model that included age, sex, CD4 cell count, zidovudine use, having two or more symptoms, and crack cocaine use, while crack cocaine use was independently associated with progression to AIDS [44]. A study conducted among 188 injection drug users found that very heavy alcohol consumption (>21 drinks per week) at baseline was associated with increased %CD8 cells 2–5 years after seroconversion; no impact on CD4 cell count or %CD4 was found [45].
The only study of the issue from a developing country was a cohort study of 105 HIV clinic patients who were not on ART, conducted in Zimbabwe. This study showed no association between any alcohol consumption at baseline and successive CD4 cell count and HIV viral loads over a period of 6 months; however, follow-up was quite limited and changes in these outcomes were not examined [46].
In summary, in the pre-HAART era, no association of alcohol use with more rapid HIV disease progression was identified; however, some studies’ measurement of alcohol consumption was limited in detail or only measured at baseline, and the studies largely examined men.
Human Observational Studies: Post-HAART Studies
Studies conducted after the introduction of HAART differed from the earlier studies in that most used CD4 cell count and/or HIV viral load as the study outcomes, an understandable strategy given the reduction in OI and death in the HAART era. Notably, these studies either stratified by or controlled for ART use, and the measurement of alcohol use was more detailed than in the previous studies.
Chander et al. [24••] reported observations among 1711 persons enrolled in an urban HIV clinic cohort from 1998–2003. They found that heavy alcohol use in the prior 6 months alone and combined with injection drug use was associated with decreased viral load suppression after adjusting for age, race, nadir CD4 cell count, and years on ART. Controlling for self-reported adherence in these analyses attenuated the effect of heavy drinking, providing evidence for the causal chain between alcohol and HIV outcomes via ART adherence. Because the attenuation of the effect from a 24% to a 14% reduction in odds of viral suppression was accompanied by somewhat wider confidence intervals and due to the imprecise nature of ART adherence measurement, these data are inconclusive as to whether there is an effect of alcohol on viral suppression beyond that attributed to poorer adherence.
An analysis of participants in two cohorts of a total of 595 HIV-infected persons with a history of alcohol problems examined CD4 cell counts and HIV viral loads at 6-month intervals for up to 7 years [5••]. Upon regression analysis, among subjects not on ART, heavy alcohol consumption was associated with a lower CD4 cell count, on average a difference of 49 cells/mm3. There was no association between heavy alcohol consumption and CD4 cell count among those on ART, in analyses that adjusted for baseline CD4, adherence to ART, homelessness, depressive symptoms, and several other variables. Heavy alcohol use was not associated with HIV viral load in those on ART and those not on ART. All analyses among those on ART adjusted for 3-day self-reported adherence, suggesting that there is no detectable alcohol effect beyond the effect on adherence among those on ART. However, the CD4 cell count difference suggests that there might be an effect of heavy alcohol consumption on HIV progression among those not on ART.
A recent publication by Baum et al. [47••] examined the association between alcohol consumption and HIV outcomes in a cohort of active alcohol or illicit drug users. In this study, frequent alcohol consumption (defined as ≥ 2 drinks/day on average) compared to moderate alcohol use and abstention was associated with a decline of CD4 cell count to less than 200 cells/mm3, among those who had a baseline CD4 cell count of greater than 200 cells/mm3. The model in this study controlled for baseline CD4 cell count, HIV viral load, ART status, years since tested HIV positive, age, and gender. A similar model that examined the same factors but was restricted to those not on ART showed a stronger association. The effect size was also larger when the predictor variable was the combination of frequent alcohol use and crack cocaine use; however, the independent effects of alcohol and crack cocaine use were not shown. In addition, frequent alcohol consumption was associated with increased HIV viral load in a multivariate model controlling for the same variables as above except viral load. However, when stratifying by ART the association was significant only among those on ART, and the authors suggested that the association was mediated by adherence. These analyses are in contrast to a recent analysis of the same cohort, which found that crack cocaine use but not alcohol use, coded only as current yes versus no, was associated with HIV progression [33]. This illustrates the importance of using a more detailed alcohol consumption history to ascertain the relationship of alcohol use and HIV disease progression.
Two studies in women in the post-HAART era failed to find an association between alcohol consumption and HIV outcomes. A recent study of 516 women in the HIV Epidemiologic Research Study (HERS) cohort examined the effects of alcohol consumption (ie, none, moderate, and heavy) on both depressive symptoms and CD4 cell count [48••]. The analysis showed significant associations between both moderate and heavy alcohol consumption and depressive symptoms and between depressive symptoms and CD4 cell count. The direct association between alcohol consumption and CD4 cell count was not statistically significant. The indirect effects of alcohol consumption on CD4 cell count via depression were not reported; therefore, we cannot comment on effect of alcohol on CD4 cell count via the effect on depression. In addition, a large study of 1686 HIV-positive women in the Women’s Interagency HIV Study (WIHS) found that there was no positive association between heavy alcohol consumption and time to newly acquired AIDS-defining illnesses or AIDS-related death, in repeated measures models that adjusted for crack use, ART use and adherence, CD4 cell count at baseline, HIV viral load at baseline, year of HIV diagnosis, and demographic variables [32•]. This study found a strong association of persistent crack use and AIDS-related mortality and both persistent and intermittent crack use and newly acquired AIDS illnesses. This result is consistent with other studies that included crack use in multivariate models [33, 34].
Lastly, a study conducted multivariate modeling of the effect of drink types on HIV viral load suppression, CD4 cell count, and thymus volume in 165 patients after 24 weeks of ART [49••]. In models that controlled for demographics, baseline Centers for Disease Control and Prevention HIV stage, and adherence, heavy alcohol consumption was not associated with the outcomes while consuming predominantly liquor compared to beer or wine was associated with lack of HIV viral suppression, decreased thymus size, and change in CD4 cell count. This study highlights a potential future area of interest, that is, impact of alcohol beverage type.
In summary, we identified six studies in the post-HAART era, and three demonstrated an association between heavy alcohol use and at least one measure of HIV disease progression [5••, 24••, 47••].
Discussion
Overall, we found that there is strong biological plausibility that heavy alcohol consumption might hasten HIV disease progression. We touched on several biological mechanisms by which this might occur, including direct immunological effects of alcohol, interactions and competitions with drugs, nutritional intake, and metabolic deficiencies. In addition, we reviewed behavioral factors by which alcohol consumption could affect HIV progression, such as reduced/poor retention in HIV care, adherence, nutrition, and mental health. Given this strong evidence, one would expect that simple bivariate analyses would show strong associations between heavy alcohol consumption and HIV outcomes, and these associations would be attenuated when causal pathway variables are included in the model. However, this is not the case in most of the analyses reviewed above. Instead, pre-HAART studies showed no associations between heavy alcohol consumption and HIV outcomes, while some but not all of the later studies did find such an association. There are several possible explanations for these findings.
First, several of the early studies measured alcohol consumption at baseline, yet alcohol consumption changes over the course of HIV infection [6]. Such a misclassification of the exposure could cause an association to be obscured. However, for the studies that included time-dependent measures of heavy alcohol consumption, the feedback loop between declining health and subsequent declining alcohol consumption might have counteracted any deleterious effects of alcohol on disease progression. While some studies attempted to address that issue, by measuring changes in alcohol consumption or only measuring alcohol consumption in the first several years after diagnosis, current statistical methods such as marginal structural models may be more powerful in detecting associations [50]. This feedback loop may not have been an issue in the later studies, because ART is usually started before patients develop any outward signs of disease progression. Another issue is that some of the earlier studies focused on any or current alcohol consumption, rather than heavy alcohol consumption, which may explain the lack of associations in these studies if heavy alcohol consumption is needed to accelerate HIV disease progression.
Another possible issue is that the risk profile of the populations studied changed over time, with a shift from predominantly men who had sex with men, to inner-city clinic patients and poly-substance users. The latter groups of patients may have engaged in heavier levels of alcohol consumption or other illicit drug use which was associated with HIV disease progression.
Another possible explanation for the lack of association in the early studies is that the outcome measures shifted over time from AIDS-defining illnesses to biological markers of immunological decline (CD4) or viral replication. If alcohol has a direct effect on immune function, then it is more likely that there will be a significant association when CD4 cell count is used as the outcome variable.
Lastly, it is important to consider the possibility that publication bias became a more pervasive issue as the AIDS epidemic wore on. It seems quite conceivable that researchers evaluated associations between alcohol consumption and HIV disease outcomes in their cohorts, but did not pursue these analyses to the stage of publication if the findings were not statistically significant. Because this question is still unresolved, we suggest an analysis of existing cohort study datasets, taking into account the measurement and analysis issues raised above.
Given the ubiquitous nature of alcohol use among the people of the world who are infected with HIV, quantifying the impact of alcohol consumption on HIV disease progression has major implications on the AIDS epidemic if even only a modest effect is found. Hence, in addition to taking optimal advantage of existing data to further illuminate the relationship of HIV disease and alcohol use, identifying a cohort in which ART has not been initiated and in which alcohol is heavily consumed and can be measured would provide very valuable empirical data. Such a cohort could provide key insights into this issue, particularly if the data are collected in a manner that learns from past studies’ limitations.
Conclusions
The link between alcohol use and HIV disease progression is clearly complicated to disentangle, and the more recent empirical evidence is suggestive but not strong. Although alcohol-related behavior appears to impact HIV disease progression through ART adherence, biological mechanisms are also likely to be implicated. Future studies should continue to investigate this important topic in order to provide clearer evidence, ultimately with the goals of utilizing the most valid measurement and statistical techniques and furthering our understanding by carefully controlling for confounding and meanwhile examining mechanisms of action. These studies are crucial so that the true impact and cost-effectiveness of interventions designed to slow or prevent alcohol-associated HIV disease progression can be determined.
Acknowledgments
Disclosure
No potential conflicts of interest relevant to this article were reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Samet JH, Horton NJ, Meli S, et al. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.ALC.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 2.Conigliaro J, Gordon AJ, McGinnis KA, et al. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J Acquir Immune Defic Syndr. 2003;33:521–525. doi: 10.1097/00126334-200308010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Galvan FH, Bing EG, Fleishman JA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 4.National Institute on Alcohol Abuse and Alcoholism . Helping patients who drink too much: a clinician's guide. Updated 2005 edition. Bethesda, MD: National Institutes of Health; 2007. [Google Scholar]
- 5.••Samet JH, Cheng DM, Libman H, et al. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertholet N, Cheng DM, Samet JH, et al.: Alcohol consumption patterns in HIV-infected adults with alcohol problems. Drug Alcohol Depend 2010, In press. [DOI] [PMC free article] [PubMed]
- 7.Szabo G. Consequences of alcohol consumption on host defense. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- 8.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fawzi W, Msamanga G, Spiegelman D, Hunter DJ. Studies of vitamins and minerals and HIV transmission and disease progression. J Nutr. 2005;135:938–944. doi: 10.1093/jn/135.4.938. [DOI] [PubMed] [Google Scholar]
- 10.Mehta S, Fawzi W. Effects of vitamins, including vitamin A, on HIV/AIDS patients. Vitam Horm. 2007;75:355–383. doi: 10.1016/S0083-6729(06)75013-0. [DOI] [PubMed] [Google Scholar]
- 11.Lanzillotti JS, Tang AM. Micronutrients and HIV disease: a review pre- and post-HAART. Nutr Clin Care. 2005;8:16–23. [PubMed] [Google Scholar]
- 12.Drain PK, Kupka R, Mugusi F, Fawzi WW. Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. Am J Clin Nutr. 2007;85:333–345. doi: 10.1093/ajcn/85.2.333. [DOI] [PubMed] [Google Scholar]
- 13.Rousseau MC, Molines C, Moreau J, Delmont J. Influence of highly active antiretroviral therapy on micronutrient profiles in HIV-infected patients. Ann Nutr Metab. 2000;44:212–216. doi: 10.1159/000046686. [DOI] [PubMed] [Google Scholar]
- 14.Tang AM, Forrester J, Spiegelman D, et al. Weight loss and survival in HIV-positive patients in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:230–236. doi: 10.1097/00126334-200210010-00014. [DOI] [PubMed] [Google Scholar]
- 15.Tang AM, Jacobson DL, Spiegelman D, et al. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV-infected patients, 1995 to 2003. J Acquir Immune Defic Syndr. 2005;40:70–76. doi: 10.1097/01.qai.0000159627.54149.2e. [DOI] [PubMed] [Google Scholar]
- 16.Quach LA, Wanke CA, Schmid CH, et al. Drug use and other risk factors related to lower body mass index among HIV-infected individuals. Drug Alcohol Depend. 2008;95:30–36. doi: 10.1016/j.drugalcdep.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–477S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 18.Watzl B, Watson RR. Role of alcohol abuse in nutritional immunosuppression. J Nutr. 1992;122(3 Suppl):733–737. doi: 10.1093/jn/122.suppl_3.733. [DOI] [PubMed] [Google Scholar]
- 19.Molina PE, McNurlan M, Rathmacher J, et al. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res. 2006;30:2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 20.Lieber CS, DeCarli LM. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem. 1970;245:2505–2512. [PubMed] [Google Scholar]
- 21.Lieber CS. Medical and nutritional complications of alcoholism: mechanisms and management. New York: Plenum Press; 1992. [Google Scholar]
- 22.•Conen A, Fehr J, Glass TR, et al. Self-reported alcohol consumption and its association with adherence and outcome of antiretroviral therapy in the Swiss HIV Cohort Study. Antivir Ther. 2009;14:349–357. [PubMed] [Google Scholar]
- 23.Martinez P, Andia I, Emenyonu N, et al. Alcohol use, depressive symptoms and the receipt of antiretroviral therapy in southwest Uganda. AIDS Behav. 2008;12:605–612. doi: 10.1007/s10461-007-9312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.••Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43:411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham WE, Sohler NL, Tobias C, et al. Health services utilization for people with HIV infection: comparison of a population targeted for outreach with the U.S. population in care. Med Care. 2006;44:1038–1047. doi: 10.1097/01.mlr.0000242942.17968.69. [DOI] [PubMed] [Google Scholar]
- 26.Sabin CA, Smith CJ, Gumley H, et al. Late presenters in the era of highly active antiretroviral therapy: uptake of and responses to antiretroviral therapy. AIDS. 2004;18:2145–2151. doi: 10.1097/00002030-200411050-00006. [DOI] [PubMed] [Google Scholar]
- 27.•Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braithwaite RS, McGinnis KA, Conigliaro J, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.ALC.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan LE, Saitz R, Cheng DM, et al. The impact of alcohol use on depressive symptoms in human immunodeficiency virus-infected patients. Addiction. 2008;103:1461–1467. doi: 10.1111/j.1360-0443.2008.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 31.Gore-Felton C, Koopman C. Behavioral mediation of the relationship between psychosocial factors and HIV disease progression. Psychosom Med. 2008;70:569–574. doi: 10.1097/PSY.0b013e318177353e. [DOI] [PubMed] [Google Scholar]
- 32.•Cook JA, Burke-Miller JK, Cohen MH, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baum MK, Rafie C, Lai S, et al. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50:93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Perez-Casanova AE, Tirado G, et al. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr. 2005;39:386–390. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- 35.Marcondes MC, Watry D, Zandonatti M, et al. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2008;32:1583–1592. doi: 10.1111/j.1530-0277.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagby GJ, Zhang P, Purcell JE, et al. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 37.Poonia B, Nelson S, Bagby GJ, et al. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res Hum Retroviruses. 2006;22:589–594. doi: 10.1089/aid.2006.22.589. [DOI] [PubMed] [Google Scholar]
- 38.Kaslow RA, Blackwelder WC, Ostrow DG, et al. No evidence for a role of alcohol or other psychoactive drugs in accelerating immunodeficiency in HIV-1-positive individuals. A report from the Multicenter AIDS Cohort Study. JAMA. 1989;261:3424–3429. doi: 10.1001/jama.261.23.3424. [DOI] [PubMed] [Google Scholar]
- 39.Penkower L, Dew MA, Kingsley L, et al. Alcohol consumption as a cofactor in the progression of HIV infection and AIDS. Alcohol. 1995;12:547–552. doi: 10.1016/0741-8329(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 40.van Griensven GJ, de Vroome EM, de Wolf F, et al. Risk factors for progression of human immunodeficiency virus (HIV) infection among seroconverted and seropositive homosexual men. Am J Epidemiol. 1990;132:203–210. doi: 10.1093/oxfordjournals.aje.a115649. [DOI] [PubMed] [Google Scholar]
- 41.Eskild A, Petersen G. Cigarette smoking and drinking of alcohol are not associated with rapid progression to acquired immunodeficiency syndrome among homosexual men in Norway. Scand J Soc Med. 1994;22:209–212. doi: 10.1177/140349489402200309. [DOI] [PubMed] [Google Scholar]
- 42.Tang AM, Graham NM, Chandra RK, Saah AJ. Low serum vitamin B-12 concentrations are associated with faster human immunodeficiency virus type 1 (HIV-1) disease progression. J Nutr. 1997;127:345–351. doi: 10.1093/jn/127.2.345. [DOI] [PubMed] [Google Scholar]
- 43.Veugelers PJ, Page KA, Tindall B, et al. Determinants of HIV disease progression among homosexual men registered in the Tricontinental Seroconverter Study. Am J Epidemiol. 1994;140:747–758. doi: 10.1093/oxfordjournals.aje.a117322. [DOI] [PubMed] [Google Scholar]
- 44.Webber MP, Schoenbaum EE, Gourevitch MN, et al. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- 45.Crum RM, Galai N, Cohn S, et al. Alcohol use and T-lymphocyte subsets among injection drug users with HIV-1 infection: a prospective analysis. Alcohol Clin Exp Res. 1996;20:364–371. doi: 10.1111/j.1530-0277.1996.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 46.Chandiwana SK, Sebit MB, Latif AS, et al. Alcohol consumption in HIV-I infected persons: a study of immunological markers, Harare, Zimbabwe. Cent Afr J Med. 1999;45:303–308. doi: 10.4314/cajm.v45i11.8505. [DOI] [PubMed] [Google Scholar]
- 47.••Baum MK, Rafie C, Lai S, et al. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses. 2010;26:511–518. doi: 10.1089/aid.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.••Ghebremichael M, Paintsil E, Ickovics JR, et al. Longitudinal association of alcohol use with HIV disease progression and psychological health of women with HIV. AIDS Care. 2009;21:834–841. doi: 10.1080/09540120802537864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.••Miguez-Burbano MJ, Lewis JE, Fishman J, et al. The influence of different types of alcoholic beverages on disrupting highly active antiretroviral treatment (HAART) outcome. Alcohol Alcohol. 2009;44:366–371. doi: 10.1093/alcalc/agp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bryan J, Yu Z, Van Der Laan MJ. Analysis of longitudinal marginal structural models. Biostatistics. 2004;5:361–380. doi: 10.1093/biostatistics/kxg041. [DOI] [PubMed] [Google Scholar]


