Abstract
During the past decades, a large body of information concerning the effects of endocrine disrupting compounds (EDCs) on animals and humans has been accumulated. EDCs are of synthetic or natural origin and certain groups are known to disrupt the action of androgens and to impair the development of the male reproductive tract and external genitalia. The present overview describes the effects of the different classes of EDCs, such as pesticides, phthalates, dioxins, and phytoestrogens, including newly synthesized resveratrol analogs on steroidogenesis in Leydig cells. The potential impact of these compounds on androgen production by Leydig cells during fetal development and in the adult age is discussed. In addition, the possible role of EDCs in connection with the increasing frequency of abnormalities in reproductive development in animals and humans is discussed.
1. Introduction
Androgens play a critical role in the development of the normal male phenotype. Disruption of androgen biosynthesis and actions by environmental endocrine disrupting compounds (EDCs), of synthetic or natural origin chemicals (Table 1), inhibiting critical cellular processes controlling steroidogenesis in Leydig cells (e.g., transport and delivery of cholesterol into mitochondria, steroidogenic enzyme expression or activity) and androgen binding to the androgens receptor (AR) may lead to incomplete masculinization and malformations in the male reproductive tract of both humans and animals. Androgen deficiency during prenatal development is thought to disturb differentiation of the Wolffian duct, a process crucial to the proper development of male internal reproductive organs. An increase in a number of human male reproductive developmental disorders such as cryptorchidism, hypospadias, testicular cancer, as well as decreased quality of semen all have all been proposed to be due to the action of EDCs. It has been hypothesized that EDCs play a role in the etiology of these abnormalities, collectively termed testicular dysgenesis syndrome (TDS) [1]. Reliable data on the prevalence of TDS are scarse but >5% of the Danish male population was reported to suffer from undescended testes, hypospadia, or testicular cancer [2]. Moreover, the recent finding of an age-independent decrease in testosterone and sex hormone-binding globulin (SHBG) levels in US and Danish populations over the past 20 years indicate an environmental influence such as a growing negative effect of EDCs on androgen production by Leydig cells. In addition, recent findings have demonstrated the association between exposures to EDCs and the development of obesity [3], a factor that may cause increased estrogenic effects on the humans and attenuate Leydig cell function and fertility [4].
Table 1.
Natural and synthetic chemicals affecting Leydig cell function.
| Chemical | Proposed target | Application or source |
|---|---|---|
| Procymidone | Androgen receptor antagonist | Fungicide, control of plant diseases |
| Linuron | Androgen receptor antagonist | Herbicide, postemergence control of weeds in crops |
| Vinclozolin | Androgen receptor antagonist | Fungicide |
| p,p′DDT | Androgen receptor antagonist | Pesticide |
| Dioxins | Aryl hydrocarbon receptor agonist | By-product of chlorinated hydrocarbons |
| Phthalates | Peroxisome proliferator-activated receptors (PPARs)? | Plasticizers |
| Genistein | ERs stimulator | Soy-derived food |
| Resveratrol | ERs stimulator | Red wine, red grape |
| Bisphenol A | ERs stimulator | Synthesis of polycarbonate plastics |
Corresponding references are present in the text.
The aim of this paper is to give an overview of the published literature on the effects of EDCs on Leydig cell function and steroidogenesis with particular focus on male reproduction and fertility.
2. Leydig Cell Populations
Two distinct generations of Leydig cells have been identified: fetal Leydig cells (FLCs) and adult Leydig cells. Despite their differences in morphological and biochemical properties, fetal and adult Leydig cells share the same principal function to produce androgens.
2.1. Fetal Leydig Cells
During the prenatal period, the (FLCs) are the primary source of androgens. These cells then secrete testosterone and other androgens, which regulate not only the masculinization of internal and external male genitalia, but also neuroendocrine functions. FLCs start to appear in the mesenchyme of the developing prenatal testis at E12.5 in mice, E14.5 in rats, and 7-8 weeks of gestation in human. FLCs undergo differentiation from precursor cells and their numbers increase significantly. In rats, FLC numbers increase from 2.5 × 104 per testis at E17 to about 1 × 105 cells per testis at E21 [5]. They then markedly decrease during several weeks after birth [6].
In the male fetus, FLCs seem to arise from multiple sources including the coelomic epithelium, gonadal ridge mesenchyme, and migrating mesonephric cells [7, 8]. Endothelial cells migrating from the mesonephros contribute to precursors of the peritubular myoid and vascular cell lineages [9, 10] and may also serve as the origin of fetal Leydig cells. There is also opinion that FLCs due to their expression of neuronal proteins may have origin from neural crest cells [11]. Moreover, it has been suggested that the FLCs may have first evolved from slight modifications of fetal adrenal cells [8]. This hypothesis is supported by the finding that adrenal cortex and gonads are both derived from the common adrenal-gonadal primordium before they separate into separate organs [8]. FLCs possess a well-developed steroidogenic machinery expressing the key steroidogenic enzymes, such as steroidogenic acute regulatory (StAR) protein, cytochrome P450scc, 3β-hydroxysteroid dehydrogenase-isomerase (3βHSD), cytochrome P450c17, and 5α-reductase (5α-R) required for androgen synthesis. Testosterone secreted by FLCs is required for masculinization and proper development of male reproductive organs. This steroid produced by FLCs is converted to 5α-dihydrotestosterone (DHT) by the enzyme 5α-R. DHT is a more potent androgen than testosterone and controls proper development of male external genitalia (e.g., the penis and scrotum) and the release of the testis from its urogenital ridge location [12]. Experiments on animals have shown that disruption of FLCs development results in feminization of external genitalia due to lack of androgens required for their masculinization [13]. Moreover, FLCs also produce insulin-like factor 3 (Insl3), a protein that plays a critical role in the regulation of the early phase of testicular descent [14]. Thus, FLC dysfunction may lead to incomplete masculinization and various malformations in the male reproductive tract of humans and animals (e.g., cryptorchidism and hypospadia).
2.2. Adult Leydig Cell Population
Adult Leydig cells originate postnatally and produce testosterone required for the pubertal development of external genitalia and onset of spermatogenesis, the important endocrine functions apparent at the onset of puberty. Adult Leydig cells are not derived from preexisting fetal Leydig cells, but most likely from undifferentiated peritubular-like stem cells, which express both stem and peritubular cell markers [15]. The Leydig stem cells proliferate neonatally and transform to progenitor cells by day 14 postpartum in rodents. Leydig cell progenitors are intermediates in the Leydig cell lineage. They have spindle shape and classify as Leydig cells because they express luteinizing hormone (LH) receptor and low levels of steroidogenic enzymes (e.g., 3β-hydroxysteroid dehydrogenase (3β-HSD), cytochrome P45017α-hydroxylase/C17-20 lyase (P450c17), 17β-hydroxysteroid dehydrogenase (17β-HSD)) and produce low but detectable levels of androgens [16, 17]. This type of Leydig cell exists for only a brief interval, postnatal days 14–28 in rats. During that time the cells proliferate, differentiate and give rise to immature Leydig cells. Immature Leydig cells function during day 28 to 56 and express high levels of steroidogenic enzymes involved in the biosynthesis and metabolism of testosterone (e.g., 5α-reductase and 3α-HSD), and therefore secrete more 5α-reduced androgens than testosterone [18]. A distinguishing characteristic of immature Leydig cells is their numerous cytoplasmic lipid droplets [19]. Immature Leydig cells divide once before differentiating into adult Leydig cells. Adult Leydig cells have low numbers of lipid drops, higher number of LH receptors, and the major androgen produced by the cells is testosterone due to enhanced expression of 17-KSR and suppression in the activities of 5α-reductase and 3α-HSD.
3. Steroidogenesis
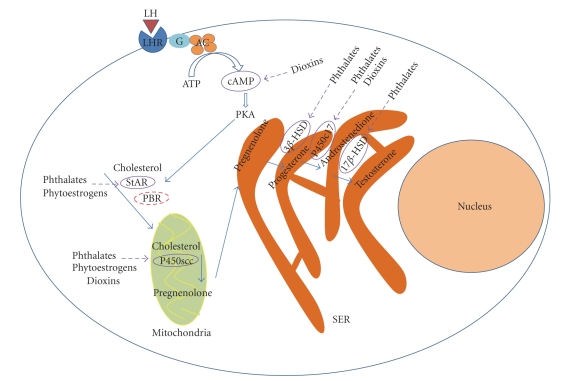
Steroidogenesis in Leydig cells is regulated mainly by LH, a glycoprotein produced by the anterior pituitary. Binding of LH to its 7-transmembrane G-protein coupled receptor stimulates adenylate cyclase activity, resulting in increased cyclic adenosine monophosphate (cAMP) formation [20]. cAMP stimulates the cAMP-dependent protein kinase (PKA), which phosphorylates serine and threonine residues on specific protein substrates [21]. cAMP activates steroidogenesis by temporally distinct manners; acutely (minutes) or chronically (hours). The delivery of cholesterol molecules into the inner mitochondrial membrane is generally accepted to be the rate-determining step in steroidogenesis [22]. The cholesterol transport mechanism is a complex process, involving an interaction between the steroidogenic acute regulatory (StAR) protein and the peripheral-type benzodiazepine receptor (PBR) [23, 24]. StAR expression is upregulated by trophic hormones, and after synthesis as a 37-kDa precursor protein, it needs to be processed to a 30-kDa mature protein to become functionally active [23]. PBR has high affinity to bind cholesterol from cytosolic donors and transfers it from the outer into the inner mitochondrial membrane [24]. StAR and PBR are suggested to work coordinately in the process of transporting cholesterol into mitochondria, where StAR is functioning as an initiator of cholesterol transport and PBR as a gate for cholesterol entry into mitochondria [25]. Once cholesterol reaches the inner mitochondrial membrane, it is immediately converted into pregnenolone. This reaction is one of the rate-limiting in steroidogenesis [14] and catalysed by the cytochrome P450 enzyme CYP11A1 and specific electron transferring proteins, localized at the inner mitochondrial membrane [14]. Pregnenolone then leaves the mitochondria to the smooth endoplasmic reticulum, where it is converted by 3β-HSD to progesterone. This steroid is then metabolised by P450c17 to androstenedione, which is converted to testosterone by 17β-HSD [14].
4. Effects of Environmental Antiandrogens on Hormonal Function of Leydig Cells
Environmental anthropogenic compounds may exert anti-androgenic effects. The pesticides procymidone, linuron, vinclozolin, p,p′-DDT and its derivatives, are all antagonists of AR and inhibit androgen-dependent tissue growth in vivo [26]. None of these pesticides or their metabolites appears to display significant affinity for the estrogen receptor or to inhibit 5α-reductase activity in vitro [27].
4.1. Procymidone
Procymidone (N-(3,5-dichlorophenyl)-1,2-dimethyl-cyclopropane-1,2-dicarboximide) is widely used as a fungicide for the control of plant diseases. This compound possesses weak anti-androgenic activity and may induce hypergonadotropism [28]. High mg quantities of procymidone were found in unhusked rice (1.4–2.3 mg/kg), tomatoes (about 5 mg/kg), grapes, and wine (US Environmental Protection Agency annual report, 1994) suggesting that an intake of significant quantities of this compound in the human is possible.
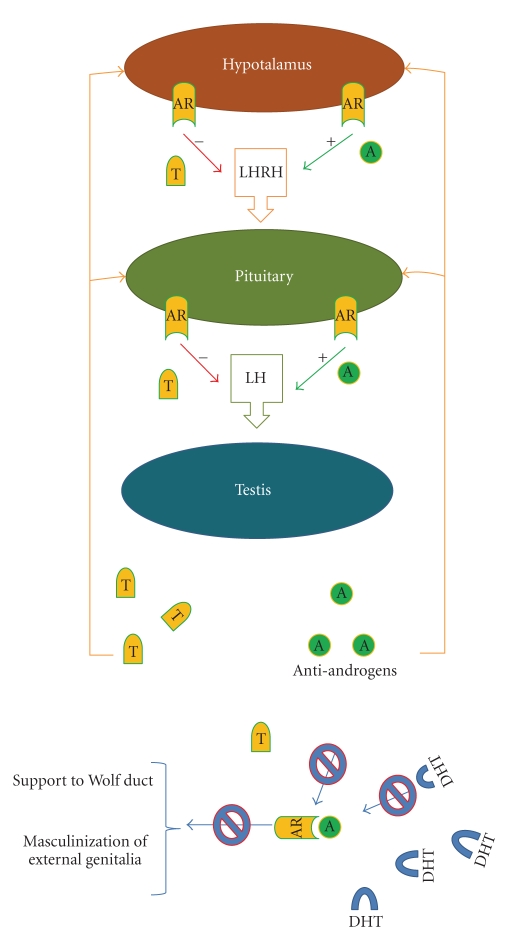
Long-term administration of procymidone is suggested to inhibit the negative feedback exerted by androgens on the hypothalamus and/or the pituitary, thereby causing hypergonadotropism in rats. The lesser ability of testosterone to bind AR in this situation suppresses the inhibitory feedback of testosterone on LH production by preventing the hypothalamus and anterior pituitary from recognizing the presence of testosterone. This results in hypersecretion of LH with concomitant elevation of serum testosterone (Figure 1). Long-term hypergonadotropism and hyperstimulation of Leydig cells induced by procymidone give rise to Leydig cell tumors in male rats [29]. Recent findings from our own laboratory reveal that dietary administration of procymidone to rats for three months not only enhances serum levels of LH and testosterone, but that the Leydig cells isolated from these animals display an enhanced capacity for producing testosterone in response to stimulation by hCG or (Bu)2cAMP, as well as elevated levels of StAR, P450scc, and P450c17 [30].
Figure 1.
Hypothesized effects of environmental antiandrogens on the hypothalamic-pituitary-testis axis. (A) In normal condition androgens as testosterone (T) and dihydrotestosterone (DHT) exert negative influence on secretion of LHRH and LH by the hypothalamus and the pituitary, respectively. (B) Antiandrogens compete with T and DHT for binding to the androgen receptor (AR). The impaired ability of testosterone (T) to bind AR weakens the inhibitory feedback of T on LHRH and LH production by preventing the hypothalamus and anterior pituitary from recognizing the presence of T. This results in hypersecretion of LH and increased production of T by Leydig cells. Blocking peripheral AR by antiandrogens inhibits androgen-mediated effects on target organs potentially resulting in incomplete masculinization and malformations of the male reproductive tract.
4.2. Linuron
Linuron (3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea) is marketed as a selective urea-based herbicide for pre- and/or postemergence control of weeds in crops and potatoes. This compound binds to both rat prostatic and human AR (hAR) and inhibits DHT-induced gene expression in vitro [31]. The anti-androgenic effect of linuron is observed clearly upon administration during gestation [32, 33] or in a Hershberger assay [33]. Administration of linuron (200 mg/kg daily) to sexually immature and mature rats for 2 weeks results in decreased weights of accessory sex organs and increased serum estradiol and LH levels, without altering the serum concentration of testosterone [31]. In contrast, prenatal exposure to linuron (75 mg/kg/day) during gestational days 14–18 in rats has recently been shown to decrease testosterone production by fetal Leydig cells [34]. This latter study suggests that linuron disrupts the development of androgen-dependent tissues primarily by acting as an AR antagonist and by inhibiting testosterone synthesis by the fetal testis. This suggestion agrees well with the evidence that in utero exposure to potent pharmaceutical antagonist of AR, flutamide decreases the expression levels of SF-1, Insl3, and 3βHSD in the developing fetal rat testis, suggesting impaired differentiation of the fetal Leydig cells [35].
4.3. Vinclozolin
Vinclozolin is a dicarboximide fungicide that has two active metabolites, M1 and M2, which both have anti-androgenic properties. These metabolites compete for androgen binding to AR and inhibit DHT-induced transcriptional activation by blocking AR binding to androgen response elements in DNA [36].
Oral administration of vinclozolin delayed pubertal maturation, decreased sex accessory gland growth and increased serum levels of LH, testosterone and 5α-androstane, 3α,17β-diol [37]. Studies in vivo have also revealed that vinclozolin-treated male offspring displayed female-like anogenital distance (AGD) at birth, retained nipples, undescended testes, and small sex accessory glands [26]. However, experiments in vitro revealed that vinclozolin did not affect basal and hCG-stimulated testosterone production by rat Leydig cells in primary culture [38]. Furthermore, the most sensitive period of rat fetal development to anti-androgenic effects of vinclozolin was found to be gestational days 16-17 [39].
4.4. p,p′-DDT
p,p′-DDE, a metabolite of the environmentally persistent pesticide DDT, acts as an antagonist of AR both in vivo and in vitro [27]. When p,p′-DDE (100 mg/kg daily) was administered to rats during fetal development on days 14–18 of gestation, the AGD was reduced and hypospadia, retention of nipples, and weight reduction of androgen-dependent tissues occurred [26]. Moreover, long-term administration of p,p′-DDE to rat pups from the time of weaning until approximately 50 days of age did not significantly alter the weights of accessory sex organs or serum hormone levels [27], but resulted in pronounced reduction of the weights of androgen-dependent tissues in the Hershberger assay [32]. As with rats, administration of p,p′-DDT or p,p′-DDE to rabbits during gestation resulted in reproductive abnormalities, including cryptorchidism, in the male offspring [32].
It has also been suggested that p,p′-DDE causes abnormalities in the reproductive development of wildlife [40]. For instance, high concentrations of p,p′-DDE, together with enhanced susceptibility of the developing reproductive system to this environmental anti-androgen due to a loss of genetic diversity, has been suggested to contribute to the high incidence of cryptorchidism in the Florida panther [40].
5. Phthalates and Leydig Cell Function
Phthalates are widely used as plasticizers in the production of plastics, as well as solvents in inks, and are present, among other places, in children's plastics toys, food packaging, and certain cosmetics [41]. Although diethylhexyl phthalate (DEHP) and monoethylhexyl phthalate (MEHP) disturb reproductive development in an anti-androgenic fashion, neither of these compounds binds to AR [42]. However, it is now established that these phthalates inhibit Leydig cell steroidogenesis at different ages of fetal development. For example, prenatal exposure of rats to DEHP attenuates serum levels of both LH and testosterone in male offspring at 21 and 35 days of postnatal age [43]. In contrast, treatment with DEHP for two weeks postnatally (beginning at 21 or 35 days of age) results in a significant decrease in the activity of 17βHSD and reduces testosterone production by Leydig cells by 50%. On the other hand, treatment of adult rats with DEHP does not influence their Leydig cell steroidogenesis [43]. From these findings it has been concluded that the effects of DEHP on Leydig cell steroidogenesis are dependent on the stage of development at the time of exposure and may involve modulation of the activities of steroidogenic enzymes and/or serum levels of LH. It has recently been demonstrated that chronic exposures to low environmentally relevant DEHP levels increased plasma levels of LH, testosterone, and 17β-estradiol [44]. These metabolic events coincided with high proliferative activity of Leydig cells, likely to be induced by the high levels of LH. Moreover, this study pointed out that DEHP might be indirectly estrogenic because it increased 17β-estradiol plasma levels, presumably due to induction of aromatase activity in Leydig cells by LH [44]. We have recently demonstrated that MEHP inhibits androgen production activated by human chorionic gonadotropin (hCG) in primary cultures of Leydig cells. This process was associated with decreased expression of steroidogenic acute regulatory (StAR) protein and reduced transport of cholesterol into mitochondria but no detectable adverse effect on steroidogenic enzymes was observed [45]. Moreover, upon exposure to MEHP alone, 5α-reductase activity was decreased in immature, but not in adult Leydig cells [45], indicating higher susceptibility to adverse effects of MEHP in young animals.
In line with the above observations and possibly contributing to undermasculinization of male fetuses, prenatal exposure of rats to di(n-butyl)phthalate (DBP) during days 12–21 of gestation gives rise to Leydig cell hyperplasia and adenomas, as well as agenesis of the epididymis. Despite the increased numbers of Leydig cells present after such exposure, testicular levels of testosterone are reduced at 18 and 21 days of gestation age. It has been suggested that the proliferation of Leydig cells observed in rat fetuses exposed to DBP may be a compensatory mechanism designed to increase testicular steroidogenesis in response to levels of testosterone that are insufficient to promote normal differentiation of the male reproductive tract [46]. In addition to reducing testicular levels of testosterone, inhibition of fetal Leydig cell steroidogenesis by phthalates causes numerous malformations of androgen-dependent tissues in male rats, including a decrease of AGD, hypospadia, epididymal agenesis, and testicular atrophy [47]. These abnormalities indicate a suboptimal masculinization of the Wolffian duct and urogenital sinus. In addition to suppression of androgen production by fetal Leydig cells, fetal exposure to phthalates has been shown to reduce Insl3 expression and to inhibit the transabdominal descent of the testis, most likely due to an altered maturation of the fetal Leydig cells [48].
It should be noted that there are very large species differences in the response to phthalate exposure. Mice and marmoset monkeys are almost insensitive to phthalates, while rats are very sensitive [49]. This is supported by the observation that administration of high doses of MBP to pregnant marmosets found no evidence for masculinization disorders [50], suggesting normal functioning of fetal Leydig cells. This finding is consistent with the absence of effect of MBP on steroidogenesis in vitro by fetal human testis explants [51]. Similarly, administration of high doses of DBP or MEHP to pregnant mice did not reduce testicular testosterone levels or affect the expression of the steroidogenic enzyme genes, as seen in the rat [52].
6. Effects of Dioxin on Leydig Cell Function
Polychlorinated dibenzo-p-dioxins such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are released into the environment as a by-product of the manufacture of chlorinated hydrocarbons and during other industrial processes, and are recognized as potent developmental and reproductive toxins. Upon binding of TCDD to an aryl hydrocarbon receptor (AhR), the receptor complex translocates into the nucleus, where AhR heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT), binds to dioxin-responsive enhancer elements and regulates the transcription of target genes [53]. It has been shown that the mechanisms of TCDD-induced disorders in the reproductive system of mammals are multiple and could involve (i) altered steroidogenesis [54, 55], (ii) reduced expression of sex steroid and LH receptors [56, 57], and (iii) induction of cytochrome P450 1 (CYP1) family enzymes resulting in inactivation of steroid hormones [58]. It is likely that the adverse effects following a TCDD exposure are result of a combination of the mechanisms described. The effects of TCDD on prenatal testis functioning is a dose-dependent process. It has been recently demonstrated that prenatal treatment of pregnant rats with low (0.3 μg/kg) dose of TCDD significantly reduced intratesticular testosterone of fetal males, while high (1 μg/kg) dose attenuated LH production by the pituitary of exposed male fetuses [59]. One possible explanation for TCDD-induced decrease in testicular steroidogenesis could be the reduction in Leydig cell number. In adult male rats exposed to TCDD, the size and the number of Leydig cells has been decreased [60]. Interestingly, pharmacological treatment of TCDD-exposed rats with human chorionic gonadotropin (hCG) preserves Leydig cell morphology and function [61]. TCDD treatment is also associated with inhibition of the mobilization of cholesterol and of the activities of cytochromes P450scc and P450c17 in Leydig cells [62, 63]. Many if not all of these alterations in steroidogenesis might be explained by the recent finding that TCDD inhibits the formation of cAMP by cultured Leydig cells [64].
7. Effect of Estrogenic Compounds on Hormonal Functions of Leydig Cells
Estrogen plays an important role in the modulation of testicular functions [4]. The inhibitory effect of estrogens on male reproductive function appears, at least in part, to be mediated by suppression of LH release by the pituitary. A direct effect of estrogen on Leydig cell steroidogenesis has also been demonstrated. In vivo experiments have shown that exogenously administered estradiol can inhibit steroidogenesis via suppression P450c17 activity in Leydig cells [65]. These findings were supported by experiments in vitro, showing that estradiol may interact directly with P450c17 as a noncompetitive inhibitor, by binding to the enzyme and inhibiting its activity [66].
Estrogen receptor α (ERα) is expressed by Leydig cells [67] and ERα null mice were shown to have lower sperm counts and elevated serum testosterone levels [68]. Moreover, the expression of several steroidogenic enzymes and factors such as P450c17, 17β-HSD, and StAR was higher in ERα deleted mice than in wild-type mice [69], suggesting that ERα-signalling can suppress androgen production in Leydig cells. Since exogenous compounds of anthropogenic or natural origin with estrogenic functions are widely distributed in food, air, and water, it is suggested that such environmental factors may negatively influence reproductive functions in humans.
Phytoestrogens are a various group of naturally occurring plant compounds. They are structurally similar to 17β-estradiol and have therefore the ability to cause estrogenic or/and antiestrogenic effects [70]. Chemically, the phytoestrogens can be divided into four main classes: flavonoids (e.g., genistein, daidzein, naringenin, and kaempferol), coumestans (coumestrol), lignans (matairesinol and secoisolariciresinol), and stilbene (resveratrol). The main sources of phytoestrogens are fruits, vegetables, and legumes [71]. It is important to note that humans can intake of high mg quantities of flavonoids (e.g., genistein, daidzein) via food consumption. Low μM levels of genistein are found in serum of the Japanese population [72] and in infants who consume large amounts of food products derived from soy beans [73]. Moreover, resveratrol is now popular drug widely used for prophylactic of cardiovascular diseases and its consumption may reach several grams per day [74].
The isoflavones genistein and daidzein are widely distributed in human and animal diet and were found to have a structural similarity to 17β-estradiol (E2) [75]. The highest amount of flavonoids has been found in soybeans and soy food (1,2-3,3 mg isoflavones/g dry weight) as well as in other types of beans and legumes [71]. The presence of genistein and daidzein in soy-based infant formula at high-μM concentrations and the fact that blood concentrations of the isoflavones are 1000 times higher than endogenous E2 [73] indicate a possibility for interaction with Leydig cell function and development of the reproductive system in male infants. Several studies have reported on an influence by isoflavones on testicular morphology and Leydig cell development. Treatment of mice with genistein was found to induce hyperplasia of Leydig cells [76]. Similarly, marmoset fed with soy formula milk developed large testes and morphological studies demonstrated an increased number of Leydig and Sertoli cells when compared with controls [77]. However, lower concentrations of serum testosterone associated with an increased number of Leydig cells have been observed in neonatal marmosets fed with soy milk formula when compared with animals fed with cow milk formula [78]. We have demonstrated recently that long-term dietary administration of genistein to rats suppressed the steroidogenic response of Leydig cells to hCG and (Bu)2cAMP by downregulating the expression of P450scc [30], suggesting that genistein and/or its metabolites have direct effects on Leydig cell function. The exact cellular and molecular mechanism behind these effects of genistein remains to be defined, but may involve activation of the estrogen receptor by the phytoestrogen. This suggestion agrees well with the evidence that high estrogen exposure reduces expression of SF-1, StAR, and CYP17 and attenuates fetal Leydig cell steroidogenesis in rodents [79, 80].
It has also been reported that long-term dietary administration of genistein reduces serum levels of testosterone and androstenedione without affecting the expression of StAR in rats [81]. Furthermore, this compound also suppresses both basal and LH-stimulated androgen production by rooster Leydig cells in vitro [82]. However, treatment of MA-10 cells with genistein and resveratrol was shown to suppress StAR gene expression whereas quercetin enhanced StAR promoter activity and expression, resulting in increased progesterone synthesis by the same cells [83]. Moreover, we have recently demonstrated that resveratrol and certain analogs structure dependently attenuated steroidogenesis in Leydig cells through suppression of the expression of StAR and cytochrome P450c17 [84]. These in vitro effects are similar to those observed in infant rats after exposure to potent ERs agonist diethylstilbestrol (DES), where significant suppression of testicular StAR expression associated with decline in plasma testosterone level was demonstrated [85], suggesting a role for ERs in the regulation of StAR gene expression and functioning.
Another synthetic compound with estrogenic activity, which is widely used in industry is bisphenol A (BPA). It stimulates estrogen dependent cellular proliferation and induces progesterone receptors [86]. The chemical structure of BPA is quite similar to that of a very potent estrogenic compound DES but it is a much weaker estrogen, approximately 1000- to 2000-fold less potent than estradiol [86]. It was demonstrated that exposure to environmentally relevant BPA levels has adverse effects on testicular function by decreasing pituitary LH secretion and reducing Leydig cell steroidogenesis [87]. BPA was found to act directly in Leydig cells because it decreased testosterone production after treatment of Leydig cells in vitro. Inhibition of steroidogenesis was ER mediated and was associated with inhibition of enzyme activity [87]. Analysis of steroidogenic enzyme gene expression by RT-PCR indicated that BPA caused specific inhibition of the P450c17 enzyme, which is known to be inhibited by estradiol [69]. Although BPA suppresses testosterone production via decreased LH secretion, there is also evidence that BPA interferes with LH receptor-ligand binding [88] and uncoupling of LH from the LH receptor potentially contributes to diminished LH stimulation of steroidogenesis. BPA also suppressed aromatase gene expression and Leydig cell estradiol biosynthesis in vivo [87]. However, BPA was recently demonstrated to increase aromatase expression but reduced testosterone synthesis in rat Leydig R2C cell line in vitro [89].
Thus, phytoestrogens and BPA may negatively influence Leydig cell function resulting in androgen insufficiency. This may contribute to undermasculinization of the male urogenital tract resulting in malformations such as hypospadia, cryptorchidism, and poor development of external genitalia in the male.
8. Concluding Remarks
EDCs of natural or anthropogenic origin may cause deleterious effects on male reproduction and fertility. The mechanisms of their adverse actions may be diverse but one important end point is reduced capacity of Leydig cells to produce androgens. Dysfunction of fetal Leydig cells induced by EDCs may cause incomplete masculinization and development of various malformations in the male reproductive tract of humans and animals. Attenuation of adult Leydig cell development and function may manifest by suppressed spermatogenesis and libido. The impaired Leydig cell function is displayed by a decrease in testosterone production as a consequence of reduced expression of several important steroidogenic factors, including StAR, CYP17A1, CYP11A1, and 3β-HSD. All these factors being important components of the steroidogenic machinery may serve as susceptible targets of EDC actions. However, our knowledge on the intracellular signalling cascades triggered by EDCs is still limited. Little is also known about the mechanisms that determine the selectivity of different EDCs for disruption of particular target molecules involved in steroidogenesis. Increasing evidence indicate that multiple mechanisms are causing endocrine disruption including (1) direct effects on the expression of genes of steroidogenic factors, (2) suppression of upstream signalling pathways controlling phosphorylation and activation of target proteins involved in transport of cholesterol into mitochondria of steroidogenic cells, and (3) direct interference with AR function blocking androgen action. A scheme describing potential sites of action of EDCs attenuating androgen action is presented in Figure 2.
Figure 2.
Scheme showing potential sites of action of EDCs on the steroidogenic machinery of Leydig cells. Inhibitory actions of EDCs on androgen biosynthesis by Leydig cells can be exerted via attenuation cAMP-PKA signaling, inhibition of cholesterol transport by the StAR/PBR complex, and suppression of expression and/or activity of different steroidogenic enzymes (e.g., 3βHSD, P450scc, P450c17, 17βHSD). Attenuation of functioning of one or several such target proteins by EDCs may lead to suppression of androgen production by Leydig cells and impairment of androgen-dependent physiological processes.
The action of EDCs on Leydig cell function and the reproductive potential is a complex process that depends on the exposure route, dose, the developmental stage of the exposed target organism, and many other factors. Together, these factors determine the potential risk for adverse consequences with long-lasting effects on male reproductive function.
Acknowledgments
The authors own studies cited were supported by grants from the Swedish Research Council, Children's Cancer Fund, EU, and Frimurare Barnhuset Foundation.
References
- 1.Skakkebæk NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Human Reproduction. 2001;16(5):972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 2.Boisen KA, Kaleva M, Main KM, et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363(9417):1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- 3.Hatch EE, Nelson JW, Stahlhut RW, Webster TF. Association of endocrine disruptors and obesity: perspectives from epidemiological studies. International Journal of Andrology. 2010;33(2):324–331. doi: 10.1111/j.1365-2605.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpe RM. The ’oestrogen hypothesis’—where do we stand now? International Journal of Andrology. 2003;26(1):2–15. doi: 10.1046/j.1365-2605.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 5.Kerr JB, Knell CM. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development. 1988;103(3):535–544. doi: 10.1242/dev.103.3.535. [DOI] [PubMed] [Google Scholar]
- 6.Habert R, Picon R. Control of testicular steroidogenesis in foetal rat: effect of decapitation on testosterone and plasma luteinizing hormone-like activity. Acta Endocrinologica. 1982;99(3):466–473. doi: 10.1530/acta.0.0990466. [DOI] [PubMed] [Google Scholar]
- 7.Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Molecular and Cellular Endocrinology. 2001;179(1-2):47–74. doi: 10.1016/s0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- 8.O’Shaughnessy PJ, Baker PJ, Johnston H, et al. The foetal Leydig cell—differentiation, function and regulation. International Journal of Andrology. 2006;29(1):90–95. doi: 10.1111/j.1365-2605.2005.00555.x. [DOI] [PubMed] [Google Scholar]
- 9.Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Current Biology. 1997;7(12):958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- 10.Capel B, Albrecht KH, Washburn LL, Eicher EM. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mechanisms of Development. 1999;84(1-2):127–131. doi: 10.1016/s0925-4773(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 11.Davidoff MS, Schulze W, Middendorff R, Holstein A-F. The Leydig cell of the human testis—a new member of the diffuse neuroendocrine system. Cell and Tissue Research. 1993;271(3):429–439. doi: 10.1007/BF02913725. [DOI] [PubMed] [Google Scholar]
- 12.Hughes IA, Acerini CL. Factors controlling testis descent. European Journal of Endocrinology. 2008;159:S75–82. doi: 10.1530/EJE-08-0458. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Tong M, Jameson JL. Distinct roles for Steroidogenic factor 1 and Desert hedgehog pathways in fetal and adult leydig cell development. Endocrinology. 2007;148(8):3704–3710. doi: 10.1210/en.2006-1731. [DOI] [PubMed] [Google Scholar]
- 14.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine Reviews. 2004;25(6):947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 15.Ge R-S, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(8):2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewing LL, Zirkin B. Leydig cell structure and steroidogenic function. Recent Progress in Hormone Research. 1983;39:599–635. doi: 10.1016/b978-0-12-571139-5.50019-7. [DOI] [PubMed] [Google Scholar]
- 17.Hardy MP, Kelce WR, Klinefelter GR, Ewing LL. Differentiation of Leydig cell precursors in vitro: a role for androgen. Endocrinology. 1990;127(1):488–490. doi: 10.1210/endo-127-1-488. [DOI] [PubMed] [Google Scholar]
- 18.Murono EP, Washburn AL. Regulation of 5α-reductase activity in cultured immature Leydig cells by human chorionic gonadotropin. Journal of Steroid Biochemistry. 1990;35(6):715–721. doi: 10.1016/0022-4731(90)90313-h. [DOI] [PubMed] [Google Scholar]
- 19.Shan L-X, Hardy MP. Developmental changes in levels of luteinizing hormone receptor and androgen receptor in rat Leydig cells. Endocrinology. 1992;131(3):1107–1114. doi: 10.1210/endo.131.3.1505454. [DOI] [PubMed] [Google Scholar]
- 20.Dufau ML. The luteinizing hormone receptor. Annual Review of Physiology. 1998;60:461–496. doi: 10.1146/annurev.physiol.60.1.461. [DOI] [PubMed] [Google Scholar]
- 21.Hansson V, Skålhegg BS, Taskén K. Cyclic-AMP-dependent protein kinase (PKA) in testicular cells. Cell specific expression, differential regulation and targeting of subunits of PKA. Journal of Steroid Biochemistry and Molecular Biology. 1999;69(1–6):367–378. doi: 10.1016/s0960-0760(99)00077-1. [DOI] [PubMed] [Google Scholar]
- 22.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocrine Reviews. 1996;17(3):221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 23.Stocco DM. The role of the StAR protein in steroidogenesis: challenges for the future. Journal of Endocrinology. 2000;164(3):247–253. doi: 10.1677/joe.0.1640247. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos V. In search of the function of the peripheral-type benzodiazepine receptor. Endocrine Research. 2004;30(4):677–684. doi: 10.1081/erc-200043971. [DOI] [PubMed] [Google Scholar]
- 25.Hauet T, Yao Z-X, Bose HS, et al. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into Leydig cell mitochondria. Molecular Endocrinology. 2005;19(2):540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- 26.Gray LE, Jr., Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicology and Industrial Health. 1999;15(1-2):48–64. doi: 10.1177/074823379901500106. [DOI] [PubMed] [Google Scholar]
- 27.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature. 1995;375(6532):581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 28.Hosokawa S, Murakami M, Ineyama M, et al. Effects of procymidone on reproductive organs and serum gonadotropins in male rats. Journal of Toxicological Sciences. 1993;18(2):111–124. doi: 10.2131/jts.18.111. [DOI] [PubMed] [Google Scholar]
- 29.Murakami M, Hosokawa S, Yamada T, et al. Species-specific mechanism in rat Leydig cell tumorigenesis by procymidone. Toxicology and Applied Pharmacology. 1995;131(2):244–252. doi: 10.1006/taap.1995.1067. [DOI] [PubMed] [Google Scholar]
- 30.Svechnikov K, Supornsilchai V, Strand M-L, et al. Influence of long-term dietary administration of procymidone, a fungicide with anti-androgenic effects, or the phytoestrogen genistein to rats on the pituitary-gonadal axis and Leydig cell steroidogenesis. Journal of Endocrinology. 2005;187(1):117–124. doi: 10.1677/joe.1.06192. [DOI] [PubMed] [Google Scholar]
- 31.Cook JC, Mullin LS, Frame SR, Biegel LB. Investigation of a mechanism for Leydig cell tumorigenesis by linuron in rats. Toxicology and Applied Pharmacology. 1993;119(2):195–204. doi: 10.1006/taap.1993.1060. [DOI] [PubMed] [Google Scholar]
- 32.Gray LE, Jr., Wolf C, Lambright C, et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p’-DDE, and ketoconazole) and toxic substances and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicology and Industrial Health. 1999;15(1-2):94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- 33.Lambright C, Ostby J, Bobseine K, et al. Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicological Sciences. 2000;56(2):389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss AK, Parks-Saldutti LG, Ostby JS, et al. A mixture of the "antiandrogens" linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biology of Reproduction. 2004;71(6):1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- 35.Brokken LJS, Adamsson A, Paranko J, Toppari J. Antiandrogen exposure in utero disrupts expression of desert hedgehog and insulin-like factor 3 in the developing fetal rat testis. Endocrinology. 2009;150(1):445–451. doi: 10.1210/en.2008-0230. [DOI] [PubMed] [Google Scholar]
- 36.Wong C-I, Kelce WR, Sar M, Wilson EM. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. Journal of Biological Chemistry. 1995;270(34):19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- 37.Monosson E, Kelce WR, Lambright C, Ostby J, Gray LE., Jr. Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicology and Industrial Health. 1999;15(1-2):65–79. doi: 10.1177/074823379901500107. [DOI] [PubMed] [Google Scholar]
- 38.Murono EP, Derk RC. The effects of the reported active metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, on testosterone formation by cultured Leydig cells from young adult rats. Reproductive Toxicology. 2004;19(1):135–146. doi: 10.1016/j.reprotox.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Wolf CJ, LeBlanc GA, Ostby JS, Gray LE., Jr. Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicological Sciences. 2000;55(1):152–161. doi: 10.1093/toxsci/55.1.152. [DOI] [PubMed] [Google Scholar]
- 40.Facemire CF, Gross TS, Guillette LJ., Jr. Reproductive impairment in the Florida panther: nature or nurture? Environmental Health Perspectives. 1995;103(4):79–86. doi: 10.1289/ehp.103-1519283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas JA, Thomas MJ. Biological effects of di-(2-ethylhexyl)phthalate and other phthalic acid esters. Critical Reviews in Toxicology. 1984;13(4):283–317. doi: 10.3109/10408448409023761. [DOI] [PubMed] [Google Scholar]
- 42.Parks LG, Ostby JS, Lambright CR, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences. 2000;58(2):339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 43.Akingbemi BT, Youker RT, Sottas CM, et al. Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biology of Reproduction. 2001;65(4):1252–1259. doi: 10.1095/biolreprod65.4.1252. [DOI] [PubMed] [Google Scholar]
- 44.Akingbemi BT, Ge R, Klinefelter GR, Zirkin BR, Hardy MP. Phthalate-induced Leydig cell hyperplasia is associated with multiple endocrine disturbances. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(3):775–780. doi: 10.1073/pnas.0305977101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svechnikov K, Svechnikova I, Söder O. Inhibitory effects of mono-ethylhexyl phthalate on steroidogenesis in immature and adult rat Leydig cells in vitro. Reproductive Toxicology. 2008;25(4):485–490. doi: 10.1016/j.reprotox.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 46.Mylchreest E, Sar M, Wallace DG, Foster PMD. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reproductive Toxicology. 2002;16(1):19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 47.Gray LE, Jr., Ostby J, Furr J, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Human Reproduction Update. 2001;7(3):248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 48.Wilson VS, Lambright C, Furr J, et al. Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicology Letters. 2004;146(3):207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocrine Reviews. 2009;30(7):883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 50.McKinnell C, Mitchell RT, Walker M, et al. Effect of fetal or neonatal exposure to monobutyl phthalate (MBP) on testicular development and function in the marmoset. Human Reproduction. 2009;24(9):2244–2254. doi: 10.1093/humrep/dep200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hallmark N, Walker M, McKinnell C, et al. Effects of monobutyl and Di(n-dutyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environmental Health Perspectives. 2007;115(3):390–396. doi: 10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaido KW, Hensley JB, Liu D, et al. Fetal mouse phthalate exposure shows that gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicological Sciences. 2007;97(2):491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- 53.Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochimica et Biophysica Acta. 2003;1619(3):263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 54.Baba T, Mimura J, Nakamura N, et al. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Molecular and Cellular Biology. 2005;25(22):10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mutoh J, Taketoh J, Okamura K, et al. Fetal pituitary gonadotropin as an initial target of dioxin in its impairment of cholesterol transportation and steroidogenesis in rats. Endocrinology. 2006;147(2):927–936. doi: 10.1210/en.2005-1125. [DOI] [PubMed] [Google Scholar]
- 56.Fukuzawa NH, Ohsako S, Wu Q, et al. Testicular cytochrome P450scc and LHR as possible targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the mouse. Molecular and Cellular Endocrinology. 2004;221(1-2):87–96. doi: 10.1016/j.mce.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Ohsako S, Miyabara Y, Nishimura N, et al. Maternal exposure to a low dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppressed the development of reproductive organs of male rats: dose-dependent increase of mRNA levels of 5α-reductase type 2 in contrast to decrease of androgen receptor in the pubertal ventral prostate. Toxicological Sciences. 2001;60(1):132–143. doi: 10.1093/toxsci/60.1.132. [DOI] [PubMed] [Google Scholar]
- 58.Badawi AF, Cavalieri EL, Rogan EG. Effect of chlorinated hydrocarbons on expression of cytochrome P450 1A1, 1A2 and 1B1 and 2- and 4-hydroxylation of 17β-estradiol in female Sprague-Dawley rats. Carcinogenesis. 2000;21(8):1593–1599. [PubMed] [Google Scholar]
- 59.Adamsson A, Simanainen U, Viluksela M, Paranko J, Toppari J. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on foetal male rat steroidogenesis. International Journal of Andrology. 2009;32(5):575–585. doi: 10.1111/j.1365-2605.2008.00900.x. [DOI] [PubMed] [Google Scholar]
- 60.Johnson L, Wilker CE, Safe SH, Scott B, Dean DD, White PH. 2,3,7,8-Tetrachlorodibenzo-p-dioxin reduces the number, size, and organelle content of Leydig cells in adult rat testes. Toxicology. 1994;89(1):49–65. doi: 10.1016/0300-483x(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 61.Wilker CE, Welsh TH, Jr., Safe SH, Narasimhan TR, Johnson L. Human chorionic gonadotropin protects Leydig cell function against 2,3,7,8-tetrachlorodibenzo-p-dioxin in adult rats: role of Leydig cell cytoplasmic volume. Toxicology. 1995;95(1–3):93–102. doi: 10.1016/0300-483x(94)02888-2. [DOI] [PubMed] [Google Scholar]
- 62.Mebus CA, Reddy VR, Piper WN. Depression of rat testicular 17-hydroxylase and 17,20-lyase after administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Biochemical Pharmacology. 1987;36(5):727–731. doi: 10.1016/0006-2952(87)90726-x. [DOI] [PubMed] [Google Scholar]
- 63.Moore RW, Jefcoate CR, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits steroidogenesis in the rat testis by inhibiting the mobilization of cholesterol to cytochrome P450(scc) Toxicology and Applied Pharmacology. 1991;109(1):85–97. doi: 10.1016/0041-008x(91)90193-i. [DOI] [PubMed] [Google Scholar]
- 64.Lai KP, Wong MH, Wong CKC. Inhibition of CYP450scc expression in dioxin-exposed rat Leydig cells. Journal of Endocrinology. 2005;185(3):519–527. doi: 10.1677/joe.1.06054. [DOI] [PubMed] [Google Scholar]
- 65.Kalla NR, Nisula BC, Menard R, Loriaux DL. The effect of estradiol on testicular testosterone biosynthesis. Endocrinology. 1980;106(1):35–39. doi: 10.1210/endo-106-1-35. [DOI] [PubMed] [Google Scholar]
- 66.Onoda M, Hall PF. Inhibition of testicular microsomal cytochrome P-450 (17α-hydroxylase/C-17,20-lyase) by estrogens. Endocrinology. 1981;109(3):763–767. doi: 10.1210/endo-109-3-763. [DOI] [PubMed] [Google Scholar]
- 67.Pelletier C, Labrie C, Labrie F. Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. Journal of Endocrinology. 2000;165(2):359–370. doi: 10.1677/joe.0.1650359. [DOI] [PubMed] [Google Scholar]
- 68.Eddy EM, Washburn TF, Bunch DO, et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137(11):4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 69.Akingbemi BT, Ge R, Rosenfeld CS, et al. Estrogen receptor-α gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144(1):84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- 70.Kuiper GGJM, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 71.Reinli K, Block G. Phytoestrogen content of foods—a compendium of literature values. Nutrition and Cancer. 1996;26(2):123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 72.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342(8881):1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 73.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350(9070):23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 74.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature Reviews Drug Discovery. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 75.Akingbemi BT, Braden TD, Kemppainen BW, et al. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular leydig cells in the adult rat. Endocrinology. 2007;148(9):4475–4488. doi: 10.1210/en.2007-0327. [DOI] [PubMed] [Google Scholar]
- 76.Lee B-J, Jung E-Y, Yun Y-W, et al. Effects of exposure to genistein during pubertal development on the reproductive system of male mice. Journal of Reproduction and Development. 2004;50(4):399–409. doi: 10.1262/jrd.50.399. [DOI] [PubMed] [Google Scholar]
- 77.Tan KAL, Walker M, Morris K, Greig I, Mason JI, Sharpe RM. Infant feeding with soy formula milk: effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Human Reproduction. 2006;21(4):896–904. doi: 10.1093/humrep/dei421. [DOI] [PubMed] [Google Scholar]
- 78.Sharpe RM, Martin B, Morris K, et al. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Human Reproduction. 2002;17(7):1692–1703. doi: 10.1093/humrep/17.7.1692. [DOI] [PubMed] [Google Scholar]
- 79.Guyot R, Odet F, Leduque P, Forest MG, Magueresse-Battistoni BL. Diethylstilbestrol inhibits the expression of the steroidogenic acute regulatory protein in mouse fetal testis. Molecular and Cellular Endocrinology. 2004;220(1-2):67–75. doi: 10.1016/j.mce.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 80.Saunders PTK, Majdic G, Parte P, et al. Fetal and perinatal influence of xenoestrogens on testis gene expression. Advances in Experimental Medicine and Biology. 1997;424:99–110. doi: 10.1007/978-1-4615-5913-9_19. [DOI] [PubMed] [Google Scholar]
- 81.Weber KS, Setchell KDR, Stocco DM, Lephart ED. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5α-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. Journal of Endocrinology. 2001;170(3):591–599. doi: 10.1677/joe.0.1700591. [DOI] [PubMed] [Google Scholar]
- 82.Opałka M, Kamińska B, Ciereszko R, Dusza L. Genistein affects testosterone secretion by Leydig cells in roosters (Gallus gallus domesticus) Reproductive Biology. 2004;4(2):185–193. [PubMed] [Google Scholar]
- 83.Chen Y-C, Nagpal ML, Stocco DM, Lin T. Effects of genistein, resveratrol, and quercetin on steroidogenesis and proliferation of MA-10 mouse Leydig tumor cells. Journal of Endocrinology. 2007;192(3):527–537. doi: 10.1677/JOE-06-0087. [DOI] [PubMed] [Google Scholar]
- 84.Svechnikov K, Spatafora C, Svechnikova I, Tringali C, Söder O. Effects of resveratrol analogs on steroidogenesis and mitochondrial function in rat Leydig cells in vitro. Journal of Applied Toxicology. 2009;29(8):673–680. doi: 10.1002/jat.1456. [DOI] [PubMed] [Google Scholar]
- 85.Mikkilä TFM, Toppari J, Paranko J. Effects of neonatal exposure to 4-tert-octylphenol, diethylstilbestrol, and flutamide on steroidogenesis in infantile rat testis. Toxicological Sciences. 2006;91(2):456–466. doi: 10.1093/toxsci/kfj156. [DOI] [PubMed] [Google Scholar]
- 86.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 87.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145(2):592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 88.Nikula H, Talonpoika T, Kaleva M, Toppari J. Inhibition of hCG-stimulated steroidogenesis in cultured mouse Leydig tumor cells by bisphenol A and octylphenols. Toxicology and Applied Pharmacology. 1999;157(3):166–173. doi: 10.1006/taap.1999.8674. [DOI] [PubMed] [Google Scholar]
- 89.Kim JY, Han EH, Kim HG, et al. Bisphenol A-induced aromatase activation is mediated by cyclooxygenase-2 up-regulation in rat testicular Leydig cells. Toxicology Letters. 2010;193(2):200–208. doi: 10.1016/j.toxlet.2010.01.011. [DOI] [PubMed] [Google Scholar]