Abstract
Aims
Automatic daily transmission of data from implantable cardioverter–defibrillators (ICDs) enables the remote monitoring of device status and leads function. We report on a 2-year experience with remote monitoring in 40 recipients of high-voltage ICD leads, prone to fracture and under advisory since October 2007.
Methods and results
The ICDs were remotely monitored as well as systematically interrogated in the ambulatory department every 3 months. The patients were also seen in case of abnormal lead impedance, or other manifestations consistent with lead dysfunction. Over a mean follow-up of 22 ± 4 months after ICD implantation, four lead dysfunctions were suspected because of remotely transmitted oversensing of noise artifacts, abrupt rise in pacing impedance, or both. A lead fracture needing lead replacement was confirmed in three patients (7.5%), two of them before any inappropriate therapy and one after the delivery of three inappropriate shocks. No lead failure was observed in the remaining 36 patients, either at the time of ambulatory visits or during remote monitoring.
Conclusion
Remote monitoring allowed the early and reliable detection of ICD leads failure without requiring any patient intervention.
Keywords: Telemedicine, Remote monitoring, Lead failure, Implantable cardioverter–defibrillator, Inappropriate shock
Introduction
The lead system is a critical component of all implantable cardioverter–defibrillators (ICDs). However, because of their complex structure, the leads are susceptible to defects and fractures,1 exposing patients to the potentially fatal risks of inappropriate therapy, failure to defibrillate, or loss of pacing function.2–4 Among several lead models that have been under advisory,5,6 the latest concerns the Sprint Fidelis high-voltage ICD leads (Medtronic Inc., Minneapolis, MN, USA),7 which have been withdrawn from distribution because of a high fracture rate.8 The prophylactic extraction of these implanted leads was not recommended,9 because of the risks of fatal and non-fatal complications.10,11 Adverse clinical events related to lead fractures may be preceded by asymptomatic anomalous lead characteristics, such as abnormal lead impedance, oversensing due to rapid, non-cardiac potentials, with or without undersensing, or inappropriate increase in pacing rate.12–16 A closer follow-up schedule, the use of special software that can detect abnormal lead function, and specific ICDs programming to prevent inappropriate therapy were recommended by the manufacturer and approved by the National Safety Agency in October 2007 (Table 1) for all recipients of Sprint Fidelis leads.9,17 However, the recommended follow-up at 3-month intervals, including measurements of lead impedance, and sensing and pacing characteristics, was found insufficient.13,18 Detection methods embedded in the implanted devices with sound alerts,12 and algorithms increasing the number of intervals for the detection of ventricular fibrillation (VF) were tested to prevent inappropriate therapies, and though helpful, were also imperfect.19 Given the unpredictability of lead fractures, a daily remote lead monitoring system seemed most likely to detect a lead fracture early and minimize the risk of adverse clinical events.
Table 1.
October 2007 decisions and recommendations regarding the management of Sprint Fidelis leads
| Decisions and recommendations from the manufacturer | Additional recommendations from AFSSAPS |
|---|---|
| Suspension of commercial distribution | Reminder to the physicians to inform the lead recipients, in compliance with article L.1111-2 of the Public Health Code |
| Recall of non-implanted leads | Schedule an ambulatory visit within a maximum of 3 months, followed by the 3-month follow-up schedule recommended by the manufacturer |
| It would be inappropriate to extract or replace the lead prophylactically (statement by an independent physician review committee). This recommendation is not applicable to patients presenting with special circumstances. The risk associated with an overt lead fracture must be weighed against the risk associated with extraction of the lead or its replacement | At each routine follow-up: Verify that the ICD software has been updated and, for recipients of ICD not manufactured by Medtronic, verify that the device includes similar software capable of triggering an alarm in case of lead fracture. |
| Follow-ups should include: Specific device programming and update of the ICD software in order to increase the likelihood of detecting a lead fracture and to lower the risk of inappropriate therapy delivery. |
AFSSAPS, the legal authority of the French Agency for the Sanitary Safety of Health Products; ICD, implantable cardioverter–defibrillator.
The ECOST trial
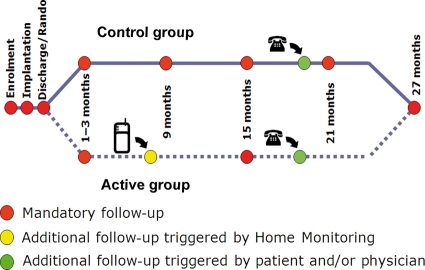
The Effectiveness and Cost of ICDs Follow-up Schedule with Telecardiology (ECOST) is an ongoing randomized trial, which compares a strategy of remote monitoring of ICDs with conventional follow-ups. The first aim of this study is to investigate, with a non-inferiority hypothesis, whether a daily remote monitoring system has an impact on the number of patients experiencing at least one serious adverse event (including all-cause mortality, cardiac- or device-related serious adverse events). Launched in January 2007 and planned to last until mid-2010, ECOST will enrol 400 patients at 45 French medical centres. Patients are enrolled after a first implant, or replacement, of a single or dual chamber ICD, with Home Monitoring™ (HM) function (Biotronik SE & Co. KG, Berlin, Germany),20 for primary or secondary prevention of sudden cardiac death. No specific recommendation was made initially regarding the type of lead to implant. Patients assigned to HM undergo automatic daily remote monitoring and are seen in the ambulatory department at yearly intervals, unless an anomalous ICD function or an event of clinical concern is reported by HM, requiring an ambulatory visit (Figure 1). Patients assigned to the control group are followed in the ambulatory department at 6-month intervals. The overall follow-up duration is 27 months.
Figure 1.
Follow-ups scheduled in the ECOST trial. After a first in-clinic follow-up, 1–3 months after ICD implantation, patients assigned to Home Monitoring (HM) undergo automatic daily remote monitoring. They are seen at yearly intervals unless an anomalous ICD function or an event of clinical concern is reported by HM. Patients assigned to the control group are seen in the ambulatory department every 6 months. Additional ambulatory visits can be requested by the patients or physicians at any time in both study groups. RANDO = randomization in a 1:1 ratio.
The protocol of ECOST complies with the French law on the protection of persons undergoing biomedical research (Huriet law) and was approved by appropriate authorities, including the legal authority of the French Agency for the Sanitary Safety of Health Products (AFSSAPS) and ethics committees. The study complies with the Declaration of Helsinki. Informed consent has been obtained from the subjects.
Reaction of the ECOST safety committee to the Sprint Fidelis advisory
Following the recommendations issued by the National Safety Agency, the Executive, Safety, Serious Adverse Events, and the Electrogram (EGM) Analysis Committees of ECOST met in November 2007 to discuss the follow-up of the recipients of Sprint Fidelis leads enrolled in the trial. To comply with the health authorities' recommendations, access to HM was offered to all recipients of Sprint Fidelis lead, regardless of the random study assignment, along with ambulatory ICDs follow-ups at 3-month intervals, constituting a parallel registry within the ECOST trial.
Objectives of this analysis
Home Monitoring enables daily automatic transmission of information, including leads data.21,22 How the remote monitoring of ICDs may be used to improve the quality of care remains uncertain.23,24 There is a paucity of prospective data supporting the safety and efficacy of this surveillance technology applied to leads under advisory.14,15 This report describes our 2-year observations with HM in recipients of Sprint Fidelis lead.
Methods
Home monitoring system and remote event settings
Home Monitoring uses mobile phone links to transmit ICDs data automatically on a daily basis, as well as instantly upon the occurrence of a potentially clinically relevant event. Variables that are typically monitored include battery status, lead characteristics, arrhythmias detected, therapies delivered, mean heart rate, per cent ventricular pacing, and daily patient activity.20,22 In case of technical flaw or arrhythmia, a central data processing facility may alert the physician via e-mail or mobile phone text messaging, and simultaneously post a detailed report on a secure website.22,25,26 The event-triggered reports may include intracardiac EGM with marker channels.27,28
Following the AFSSAPS warning regarding the Sprint Fidelis lead, the ECOST Safety Committee recommended modifications of the standard ventricular impedance and absence of HM transmission values that trigger events, in order to increase the sensitivity of ventricular lead failure detection (Table 2).
Table 2.
Recommended settings for the trigger of Home Monitoring (HM) events for Sprint Fidelis and other leads
| Settings |
||
|---|---|---|
| Triggers | Other leads | Sprint Fidelis Lead |
| Right ventricular impedance | <250 and >1500 Ω | <250 and >1000 if meana ≤70 Ω |
| <250 and >1500 if meana >700 Ω | ||
| Right atrial impedance | <250 and >1500 Ω | |
| Shock impedance | <30 and >100 Ω | |
| SVT, VT1, VT2, or VF | ON | |
| 30 J shock ineffective | ON | |
| Duration of mode switching | >75% (18 h) | |
| Mean ventricular heart rate | >100 bpm | |
| Elective replacement indicator | ON | |
| Absence of HM transmission | >14 days | >5 days |
amean, mean chronic impedance; SVT, supraventricular tachycardia; VT, ventricular tachycardia; VF, ventricular fibrillation.
Follow-up and data analysis
Besides the systematic scheduled follow-ups at 3-month intervals, the recipients of Sprint Fidelis leads were seen at unscheduled visits following HM-triggered events or upon their request. We examined the contributions and reliability of HM in the early detection of lead failure, and measured the number of contributory follow-ups, defined as an ambulatory visit prompting a change in patient management or ICD reprogramming, or yielding important information communicated to the patient. The contributions of scheduled and non-scheduled follow-ups were compared. Absolute values, per cents, means ± standard deviation, median, and lower/upper quartile were computed as appropriate. Continuous variables were evaluated with Kolmogorov–Smirnov test for normal distribution. Data analysis was performed with SPSS V18.0 software.
Results
Patient population
Between January 2007 and October 2007, when AFSSAPS issued the warning, 40 patients enrolled in ECOST received the Sprint Fidelis lead, of whom 22 were assigned to the control and 18 to the test group. Their mean age was 64 ± 12 years, and 35 (87.5%) were men. The indications for ICD implantation and the underlying heart disease are related in Table 3. The mean left ventricular ejection fraction was 35 ± 14%. New York Heart Association functional class was II or III in 85% of patients; no patients were in functional class IV.
Table 3.
Indication for implantable cardioverter–defibrillator implantation and underlying heart disease in the 40 recipients of Sprint Fidelis leads
| Study group (n = 40) | |
|---|---|
| Indications for ICD implantation | |
| Primary prevention | 26 (65%) |
| Secondary prevention | 14 (35%) |
| Underlying heart disease | |
| Ischaemic heart disease | 32 (80%) |
| Dilated non-ischaemic cardiomyopathy | 3 (7.5%) |
| Genetic disorders | 2 (5%) |
| Others | 3 (7.5%) |
Values are number and (%) of patients.
Follow-up
The mean follow-up after ICD implantation was 22 ± 4 months. The mean number of follow-up visits after the warning issued by AFSSAPS was 5.1 ± 1.5, over 17 ± 3 months (median 18; range 8–18), representing 3.8 ± 0.9 visits per patient per year.
In four patients, HM triggered urgent, unscheduled visits, which, in three patients, confirmed the presence of lead fractures, prompting their extractions and replacements. No signs of lead failure were detected at the time of scheduled follow-up visits or in HM transmissions in the remaining 36 patients.
Case no. 1: lead fracture with sensing of artifact and no abnormal change in impedance
Lead fracture was detected 20 months after implantation. The event was a single episode of ‘non-sustained VF’ reported by HM. The online recording of EGM (Figure 2) revealed the presence of noise, without change in ventricular lead impedance (Figure 3). Lead fracture was confirmed by embedded Holter EGM recorded 3 days later (Figure 4). The lead was replaced 1 week later. This lead fracture occurred 10 days after the last scheduled ambulatory follow-up visit, when no lead dysfunction was observed.
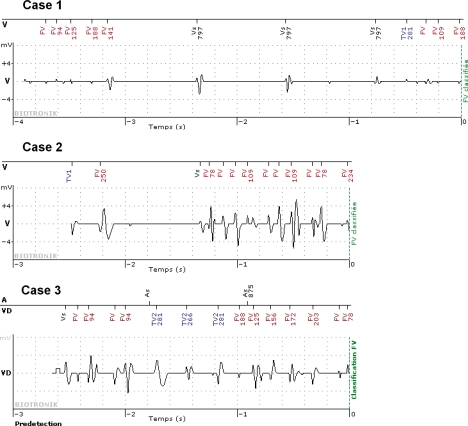
Figure 2.
Home Monitoring (HM) detected VF in the three cases of confirmed lead fractures. The EGM of cases 1–3 were automatically recorded before the diagnosis of VF (0 s point) and were transmitted with the other HM data. Patients nos 1 and 2 did not receive ICD shocks since the ‘arrhythmia’ ended before the confirmation phase. Patient no. 3 received three inappropriate shocks. With the devices used in this study, EGM are recorded for 3 s before VF is detected. FV, ventricular fibrillation; TV, ventricular tachycardia; Vs, sensed ventricular beats.
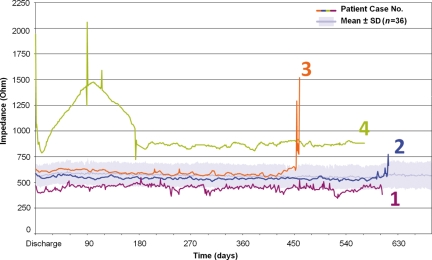
Figure 3.
Variations in ventricular lead impedance in the four cases described in the text, compared with the mean impedance (± SD) in the remaining 36 patients (light blue area in background). Among the three patients with confirmed lead fractures, an abnormal rise in impedance was observed in two (cases 2 and 3). In case no. 4, an abnormal impedance was observed during the first 6 months after ICD implantation, without other signs of lead dysfunction. The impedance values were integrated in the Home Monitoring data and triggered an event when they exceeded pre-selected values.
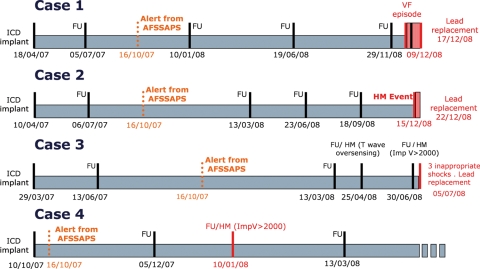
Figure 4.
Summaries of clinical cases. Dates are shown in dd/mm/yy format. Red print indicates abnormalities detected by HM, confirmed at subsequent ambulatory visits, and lead replacement times. Light red areas indicate time intervals between lead fracture diagnosis and replacement procedures (days at risk). AFSSAPS, the legal authority of the French Agency for the Sanitary Safety of Health Products; HM, Home Monitoring; ICD, implantable cardioverter–defibrillator; ImpV, ventricular lead impedance.
Case no. 2: lead fracture with sensing of artifact and impedance rise
An episode of ‘non-sustained VF’ was reported by HM, 20 months after ICD implantation (Figure 2). An increase in ventricular lead impedance from 540 to 773Ω was observed on the preceding day (Figures 3 and 4). The patient underwent urgent ICD interrogation, when noise artifacts were observed on the EGM channel upon motion of the left upper extremity. The lead was replaced 4 days later. This lead fracture occurred 3 months after the last scheduled ambulatory follow-up visit, when no lead dysfunction had been observed.
Case no. 3: lead fracture with sensing of artifacts, impedance rise, and shocks delivery
A HM event was triggered 15 months after ICD implantation by a sudden rise in ventricular lead impedance from 600 to 1294 Ω (Figures 3 and 4). No abnormality was found during an unscheduled ambulatory visit on the same day, when the lead impedance was 734 Ω. However, 5 days later, the patient was admitted to the hospital for immediate lead replacement, after having received three inappropriate shocks caused by noise artifacts (Figure 2). This lead fracture occurred 2.5 months after the last scheduled ICD follow-up, which had showed no sign of lead dysfunction.
Case no. 4: no lead fracture and rise in impedance >2000 Ω
A HM event was triggered by a high ventricular lead impedance, 3 months after ICD implantation. A subsequent review of HM data recorded since device implantation revealed a 1950 Ω impedance at the time of discharge of the patient from the hospital, decreasing to 800 Ω over the next few days, and increasing up to 1500 Ω and briefly spiking over 2000 Ω in the 3 months after device implantation (Figure 3). Repeated measurements in the ambulatory department revealed a ventricular capture threshold at 0.6 V/0.5 ms and a lead impedance at 1344 and 1521 Ω, without sensing of artifacts. Subsequent daily observations by HM transmissions up to 6 months after implantation showed a gradual decrease in lead impedance, which stabilized at 850 Ω. The lead has remained functional at a follow-up of 18 months after ICD implantation.
Other follow-ups triggered by Home Monitoring
Home Monitoring triggered seven other unscheduled ambulatory visits, of which four contributed clinical information, including T-wave oversensing in one, inappropriate shock caused by a supraventricular tachyarrhythmia in one, and non-sustained ventricular tachycardia and VF in one patient each. In three other patients, HM was triggered by (i) three episodes of ventricular arrhythmia, (ii) a rise in atrial impedance, and (iii) a mean daily heart rate >110 bpm, respectively. Including case no. 4, 4 out of 11 unscheduled ambulatory follow-up visits (36%) prompted by HM were classified as non-contributory.
Contributions of scheduled follow-ups
Out of 198 scheduled follow-ups, 178 (89.8%) were classified as non-contributory. A change in ICD programming, the disclosure to the patient of the Sprint Fidelis advisory information, as recommended by the health authorities, or both, occurred during 16 ambulatory follow-up visits. Changes in medical management, patient reassurance, or both occurred for atrial fibrillation, pulse generator pocket infection, near syncope, and phantom shocks in one patient each. Table 4 summarizes the contributions of scheduled and unscheduled follow-up visits. The contributions of unscheduled follow-ups triggered by HM in 7 out of 11 cases (63%) were considerably greater than in 20 out of 198 scheduled follow-up visits (10%).
Table 4.
Contributions of follow-ups in the 40 recipients of Sprint Fidelis leads
| Follow-ups |
|||
|---|---|---|---|
| Contributory | Non-contributory | Total | |
| Scheduled | 20 | 178 | 198 (94.8) |
| Triggered by HM | 7 | 4 | 11 (5.2) |
| Total | 27 (12.9) | 182 (87.1) | 209 (100) |
Values are numbers (%) of observations.
HM, Home Monitoring.
Discussion
By accurately detecting three lead fractures during this study, HM made important contributions to the follow-up of ICD leads under advisory, in contrast to the absence of useful information contributed by the systematically scheduled ambulatory surveillance of ICDs. In a study by Hauser et al.,29 no lead dysfunction was detected among six recipients of Sprint Fidelis leads, followed at 4-month intervals, during ambulatory visits, which took place between 2 weeks and 3 months before the diagnosis of lead fractures. These six patients, who were not under HM, received a total of 57 inappropriate shocks. While Theuns et al.25 detected four lead failures in 146 patients (2.7%) under HM over a mean follow-up of 22 months, we detected, during a similar observation period, three lead fractures among 40 patients (7.5%), highlighting the particularly important contributions made by HM in recipients of leads under advisory.
Lead dysfunction is a major concern in ICD recipients, whether it is due to a systematic manufacturing defect, or to random failure or dislodgement. A recent study in nearly 1000 recipients of various ICD lead models between 1992 and 2005 reported failure rates of 15% at 5, and 40% at 8 years, accelerating over time.1,24 Lead dysfunction must be detected early in order to prevent major adverse clinical events, including potentially fatal failure to defibrillate or pace, or delivery of inappropriate shocks.2–4 This early detection has relied on ambulatory visits, the frequency of which cannot be optimal. Moreover, frequent visits lower the patients' quality of life, and are costly and time-consuming. An ideal early detection strategy needs no patient intervention, particularly since some patients cannot hear the alarms that should warn them of possible ICD dysfunctions.12,18 In a retrospective study, Spencker et al.16 found that fewer ICD shocks were delivered to patients monitored remotely than to patients followed in ambulatory departments, before undergoing replacements of dysfunctional leads.
Home Monitoring allows the detection of initially intermittent dysfunctions, as shown by the three cases in this study. Spencker et al.16 found that in up to 90% of patients presenting with lead failure, the first inappropriate shock was preceded by non-sustained noise artifacts detected as VF. Home Monitoring also enables to focus on patients who require particularly close attention, and plan provocative manoeuvres to be performed during ambulatory visits when a lead fracture is suspected. The diagnosis of lead fracture may be challenging and cannot solely be based on an impedance increase, as illustrated by our case no. 4. On the other hand, daily measurements of lead impedance do not identify all lead failures.12,18 New lead-integrity algorithms can alert the patients, physicians, or both, of noise oversensing or abnormal impedance.12,13 These algorithms, which may increase the number of intervals triggering the detection of VF, are the first kind of ICDs monitoring function that prompts real-time changes in VF detection criteria to reduce the risk of inappropriate shocks.13 Because the rate of false positive detections of lead fracture ranges between 21 and 35%,13 EGM need to be verified, which can easily be accomplished with HM.
This study illustrates different presentations of lead fracture, including non-sustained episodes of ventricular tachycardia or VF, and impedance increase. However, the diagnosis might also be suspected from other signals, such as an increase percent pacing, which suggests undersensing, or the presence of PVC in the counters, suggesting oversensing. The suddenness of these signals and their lack of clinical explanation are consistent with lead failure. In contrast to ambulatory visits, HM allows the immediate detection of these signals.
All links in the data transmission chain, including triage, interpretation, and clinical management of the data must be in place for the HM system to function properly. The assistance from the service centre in ensuring the continuity of HM transmissions was of great importance. The physician was automatically alerted when breaks in HM transmissions exceeded 5 days.
Limitation of the study
This study is limited by its observational design. The broader contributions of HM need to be confirmed by the randomized ECOST trial.
Conclusions
Over a mean follow-up of 22 ± 4 months after implantation, the failure rate of the Sprint Fidelis lead in our registry was 7.5%. Home Monitoring allowed the early and reliable detection of three lead fractures, manifest by sensing of noise artefact, abrupt rise in pacing impedance, or both, without requiring the intervention of patients in the diagnosis or decision-making process.
Funding
The ECOST trial is sponsored by BIOTRONIK SE & Co. KG. Funding to pay the Open Access publication charges for this article was provided by Biotronik SE & Co. KG.
Conflict of interest: none declared.
Acknowledgements
The authors thank Nicolas Canot and Sophie Fauquembergue for their expert technical assistance, and Rodolphe Ruffy, MD, and Dejan Danilovic, PhD, for their help in the composition of the manuscript.
Appendix
The following investigators participated in this study: Didier Klug, MD, Centre Hospitalier Régional Universitaire, Lille; Claude Kouakam, MD, Centre Hospitalier Régional Universitaire, Lille; Christelle Marquié, MD, Centre Hospitalier Régional Universitaire, Lille; Antoine Da Costa, MD, Centre Hospitalier Universitaire, Saint Etienne; Nathalie Elbaz, MD, Hôpital Henri Mondor, Créteil; Philippe Chevalier, MD, Centre Hospitalier Universitaire, Lyon; Jean-Sylvain Hermida, MD, Centre Hospitalier Universitaire, Amiens; Pierre Graux, MD, Hôpital Saint Philibert, Lomme; Jean-Luc Pasquie, MD, Centre Hospitalier Universitaire, Montpellier; Jean-Claude Deharo, Hôpital de la Timone, Marseille; Gilles Lascault, MD, Centre Cardiologique du Nord, Saint Denis.
Serious Adverse Events Committee: Jacques Clementy, MD, Hôpital Haut-Lévêque, Pessac; Dominique Lacroix, MD, Centre Hospitalier Régional Universitaire, Lille; Nicolas Sadoul, MD, CHU de Nancy, Vandoeuvre Les Nancy.
Electrogram Analysis Board: Laurence Guédon-Moreau, MD, Centre Hospitalier Régional Universitaire, Lille; Xavier Laroche, Ing, Biotronik.
References
- 1.Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider S, Saggau W, Weisse U, Seidl K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115:2474–2480. doi: 10.1161/CIRCULATIONAHA.106.663807. doi:10.1161/CIRCULATIONAHA.106.663807. [DOI] [PubMed] [Google Scholar]
- 2.Undavia M, Fischer A, Mehta D. Fatal outcome in a pacemaker-dependent patient. Pacing Clin Electrophysiol. 2009;32:550–553. doi: 10.1111/j.1540-8159.2009.02320.x. doi:10.1111/j.1540-8159.2009.02320.x. [DOI] [PubMed] [Google Scholar]
- 3.Chung EH, Casavant D, John RM. Analysis of pacing/defibrillator lead failure using device diagnostics and pacing maneuvers. Pacing Clin Electrophysiol. 2009;32:547–549. doi: 10.1111/j.1540-8159.2009.02319.x. doi:10.1111/j.1540-8159.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson DM, Singh JP, Heist EK, Mela T, Ruskin JN, Das S. Multiple ICD discharges associated with lead fracture without triggering of high impedance alert. Pacing Clin Electrophysiol. 2009;32:543–546. doi: 10.1111/j.1540-8159.2009.02318.x. doi:10.1111/j.1540-8159.2009.02318.x. [DOI] [PubMed] [Google Scholar]
- 5.Amin MS, Ellenbogen KA. Focus on management of pacemaker and ICD advisories, recalls, and alerts. Curr Treat Options Cardiovasc Med. 2006;8:347–352. doi: 10.1007/s11936-006-0038-2. doi:10.1007/s11936-006-0038-2. [DOI] [PubMed] [Google Scholar]
- 6.Maisel WH, Sweeney MO, Stevenson WG, Ellison KE, Epstein LM. Recalls and safety alerts involving pacemakers and implantable cardioverter-defibrillator generators. JAMA. 2001;286:793–799. doi: 10.1001/jama.286.7.793. doi:10.1001/jama.286.7.793. [DOI] [PubMed] [Google Scholar]
- 7.Hauser RG, Hayes DL, Epstein AE, Cannom DS, Vlay SC, Song SL, Tyers GF. Multicenter experience with failed and recalled implantable cardioverter-defibrillator pulse generators. Heart Rhythm. 2006;3:640–644. doi: 10.1016/j.hrthm.2006.02.011. doi:10.1016/j.hrthm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Groves R. Urgent medical device information: Sprint Fidelis lead patient management recommendations. 15 October 2007, Letter to physicians. http://www.medtronic.com/fidelis/physician-letter.html. (27 December 2009)
- 9.AFSSAPS, MEDTRONIC. Information concernant les sondes de défibrillation Sprint Fidelis—MEDTRONIC - Modèles 6930, 6931, 6948 et 6949. http://www.afssaps.fr/content/download/14270/170934/version/1/file/dm071009.pdf ; http://www.afssaps.fr/content/download/14271/170942/version/1/file/dm071009_correctif.pdf. (27 December 2009)
- 10.Gould PA, Krahn AD. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;295:1907–1911. doi: 10.1001/jama.295.16.1907. doi:10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]
- 11.Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Reiser C. Clinical study of the laser sheath for lead extraction: the total experience in the United States. Pacing Clin Electrophysiol. 2002;25:804–808. doi: 10.1046/j.1460-9592.2002.t01-1-00804.x. doi:10.1046/j.1460-9592.2002.t01-1-00804.x. [DOI] [PubMed] [Google Scholar]
- 12.Vollmann D, Erdogan A, Himmrich E, Neuzner J, Becker D, Unterberg-Buchwald C, Sperzel J. Patient alert to detect ICD lead failure: efficacy, limitations, and implications for future algorithms. Europace. 2006;8:371–376. doi: 10.1093/europace/eul023. doi:10.1093/europace/eul023. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow CD, Gunderson BD, Ousdigian KT, Abeyratne A, Stadler RW, Gillberg JM, Patel AS, Ellenbogen KA. Downloadable algorithm to reduce inappropriate shocks caused by fractures of implantable cardioverter-defibrillator leads. Circulation. 2008;118:2122–2129. doi: 10.1161/CIRCULATIONAHA.108.796136. doi:10.1161/CIRCULATIONAHA.108.796136. [DOI] [PubMed] [Google Scholar]
- 14.Hauck M, Bauer A, Voss F, Weretka S, Katus HA, Becker R. ‘Home monitoring’ for early detection of implantable cardioverter-defibrillator failure: a single-center prospective observational study. Clin Res Cardiol. 2009;98:19–24. doi: 10.1007/s00392-008-0712-3. doi:10.1007/s00392-008-0712-3. [DOI] [PubMed] [Google Scholar]
- 15.Varma N. Remote monitoring for advisories: automatic early detection of silent lead failure. Pacing Clin Electrophysiol. 2009;32:525–527. doi: 10.1111/j.1540-8159.2009.02314.x. doi:10.1111/j.1540-8159.2009.02314.x. [DOI] [PubMed] [Google Scholar]
- 16.Spencker S, Coban N, Koch L, Schirdewan A, Muller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace. 2009;11:483–488. doi: 10.1093/europace/eun350. doi:10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 17.FDA statement on Medtronic's voluntary market suspension of their Sprint Fidelis defibrillator leads. 15 October 2007. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109007.htm. (27 December 2009)
- 18.Kallinen LM, Hauser RG, Lee KW, Almquist AK, Katsiyiannis WT, Tang CY, Melby DP, Gornick CC. Failure of impedance monitoring to prevent adverse clinical events caused by fracture of a recalled high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2008;5:775–779. doi: 10.1016/j.hrthm.2008.02.039. doi:10.1016/j.hrthm.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 19.Vlay SC. Limitation of programmed alerts to predict ICD lead failures. Pacing Clin Electrophysiol. 2009;32:554–555. doi: 10.1111/j.1540-8159.2009.02321.x. doi:10.1111/j.1540-8159.2009.02321.x. [DOI] [PubMed] [Google Scholar]
- 20.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30(Suppl. 1):S2–S12. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 21.Jung W, Rillig A, Birkemeyer R, Miljak T, Meyerfeldt U. Advances in remote monitoring of implantable pacemakers, cardioverter defibrillators and cardiac resynchronization therapy systems. J Interv Card Electrophysiol. 2008;23:73–85. doi: 10.1007/s10840-008-9311-5. doi:10.1007/s10840-008-9311-5. [DOI] [PubMed] [Google Scholar]
- 22.Burri H, Senouf D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace. 2009;11:701–709. doi: 10.1093/europace/eup110. doi:10.1093/europace/eup110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruffy R. The device recalls nightmare: help might be on the way. Pacing Clin Electrophysiol. 2007;30(Suppl. 1):S1. doi: 10.1111/j.1540-8159.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 24.Marine JE. Remote monitoring for prevention of inappropriate implantable cardioverter defibrillator shocks: is there no place like home? Europace. 2009;11:409–411. doi: 10.1093/europace/eup009. doi:10.1093/europace/eup009. [DOI] [PubMed] [Google Scholar]
- 25.Theuns DA, Rivero-Ayerza M, Knops P, Res JC, Jordaens L. Analysis of 57,148 transmissions by remote monitoring of implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2009;32(Suppl. 1):S63–S65. doi: 10.1111/j.1540-8159.2008.02230.x. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen JC, Kottkamp H, Zabel M, Aliot E, Kreutzer U, Bauer A, Schuchert A, Neuser H, Schumacher B, Schmidinger H, Stix G, Clementy J, Danilovic D, Hindricks G. Automatic home monitoring of implantable cardioverter defibrillators. Europace. 2008;10:729–735. doi: 10.1093/europace/eun099. doi:10.1093/europace/eun099. [DOI] [PubMed] [Google Scholar]
- 27.Perings C, Klein G, Toft E, Moro C, Klug D, Bocker D, Trappe HJ, Korte T. The RIONI study rationale and design: validation of the first stored electrograms transmitted via Home Monitoring in patients with implantable defibrillators. Europace. 2006;8:288–292. doi: 10.1093/europace/eul009. doi:10.1093/europace/eul009. [DOI] [PubMed] [Google Scholar]
- 28.Ritter O, Bauer WR. Use of ‘IEGM Online’ in ICD patients—early detection of inappropriate classified ventricular tachycardia via Home Monitoring. Clin Res Cardiol. 2006;95:368–372. doi: 10.1007/s00392-006-0390-y. [DOI] [PubMed] [Google Scholar]
- 29.Hauser RG, Kallinen LM, Almquist AK, Gornick CC, Katsiyiannis WT. Early failure of a small-diameter high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2007;4:892–896. doi: 10.1016/j.hrthm.2007.03.041. [DOI] [PubMed] [Google Scholar]