Abstract
Aims
To assess the effect of rosiglitazone on cardiovascular performance and cardiac function.
Methods and results
One hundred and fifty type 2 diabetes patients with cardiovascular disease (CVD) or ≥1 other CVD risk factor were randomized to receive rosiglitazone vs. placebo for 6 months. The primary outcome was peak oxygen uptake indexed to fat-free mass (VO2peak–FFM) during maximum exercise. A subset of 102 subjects underwent cardiac magnetic resonance imaging (cMRI). On hundred and eight subjects completed the study, including 75 completing the cMRI substudy. No significant differences were observed in mean VO2peak–FFM between rosiglitazone and placebo (26.1 ± 7.0 vs. 27.6 ± 6.6 mL/kg-FFM/min; P = 0.26). Compared with placebo, the rosiglitazone group had lower hematocrit (38 vs. 41%; P < 0.001) and more peripheral oedema (53.7 vs. 33.3%; P = 0.03). In the cMRI substudy, compared with placebo, the rosiglitazone group had larger end-diastolic volume (128.1 vs. 112.0 mL; P = 0.01) and stroke volume (83.7 vs. 72.9 mL; P = 0.01), and a trend toward increased peak ventricular filling rate (79.4 vs. 60.5; P = 0.07).
Conclusion
Rosiglitazone increased peripheral oedema but had no pernicious effects on cardiovascular performance or cardiac function, with modest improvement in selected cMRI measures. Changes in indirect markers of plasma volume suggest expansion with rosiglitazone.
Trial registration: clinicaltrials.gov identifier: NCT00424762.
Keywords: Diabetes mellitus, Drugs, Heart failure, Exercise
Introduction
The thiazolidinedione (TZD) drugs rosiglitazone and pioglitazone for type 2 diabetes mellitus (T2DM) cause peripheral oedema, and less commonly, incident or worsening heart failure (HF),1–3 leading to product cautions against their use in the setting of HF.4–5 The mechanistic underpinning and clinical consequences of TZD-associated peripheral oedema and HF remain poorly understood.
Few studies have evaluated the cardiac effects of the TZDs, and these are limited by several factors including: (i) echocardiographic assessment,6–8 a method relatively insensitive to small cardiac changes;9 (ii) open-label designs;6,8 (iii) high attrition;6–8 (iv) the study of patients with low cardiovascular disease (CVD) risk;6,8,10 and (v) cardiac assessments only at rest.6–8,10 Given these limitations, we conducted a randomized, double-blind, placebo-controlled trial to assess the effect of rosiglitazone treatment on peak integrated cardiovascular performance during maximal exercise, and on magnetic resonance imaging (MRI) measures of cardiac structure and function in patients at high CVD risk.
Methods
Study design
The trial was a randomized, single-centre, double-blind, placebo-controlled trial, with the design previously published.11 In brief, participants had T2DM and either CVD (coronary artery disease, stroke or transient ischaemic attack, or carotid or peripheral atherosclerosis) or ≥1 other CVD risk factor, including smoking, hypertension, hypercholesterolaemia, albuminuria, family history of coronary artery disease, or high-sensitivity C-reactive protein >3 mg/L. Exclusions included TZD treatment within the prior 6 months; prior TZD intolerance; prior HF; AST or ALT>3X upper limit of normal; or inability to perform treadmill exercise.
Participants were recruited from outpatient clinics, prior research participants, and public advertisement, with planned recruitment of approximately one-third each of White, Black, and Hispanic patients. The study complies with the Declaration of Helsinki, was approved by the University of Texas Southwestern Medical Center at Dallas Institutional Review Board, and all participants provided informed consent.
Study treatments
Participants were randomized 1:1 stratified by race (White, Black, Hispanic, or Other) to receive rosiglitazone 4 mg daily or placebo added to existing T2DM treatment, increased after 1 month to 8 mg daily for an additional 5 months. Investigators and subjects were blinded to assignment, and both groups were treated with open-label non-TZD drugs throughout the study with target A1C <7.0%.
Outcome measures
The primary outcome measure was peak oxygen uptake indexed to fat-free mass (VO2peak–FFM) during maximal treadmill exercise.11 Scaling of VO2 to FFM was used as the primary outcome parameter given the (i) prevalent adiposity of the T2DM population; (ii) broad age range studied; (iii) inclusion of men and women; and (iv) increased adiposity expected with TZD treatment, all of which confound the more standard indexation of VO2 to total body mass.12–13
Key secondary measures included new peripheral oedema; new HF defined as symptoms coupled with exam or imaging evidence of volume overload; changes in circulating brain natriuretic peptide (BNP); changes in weight, and MRI parameters of cardiac structure and function.
Study assessments
Clinical history, exam, and blood testing including glycosylated haemoglobin (HbA1c) were performed at baseline and at Months 1, 2, 3, and 6 and with compliance assessed by pill count. At baseline and at end-of-study, plasma samples were collected and frozen at −80°C for BNP batch assay at the end of the study (Biosite, Inc., San Diego, CA, USA).
Cardiopulmonary exercise testing
At baseline and after 6 months of study treatment, each subject performed a graded exercise treadmill test to exhaustion with integrated cardiovascular performance assessed by measuring oxygen consumption (VO2peak) using a MedGraphics™ Cardiopulmonary Exercise System (CPX/D; Medical Graphics Corporation, St. Paul, MN, USA) for breath-by-breath measurement.11,13
Skin-fold thickness was measured with calipers prior to each exercise test from four locations in duplicate and averaged, and percent body fat was calculated by the method of Durnin and Womersley.14
Cardiac magnetic resonance imaging
The first 102 subjects enrolled who volunteered to participate in the imaging substudy underwent cardiac MRI (cMRI) at baseline and after 6 months of study treatment using methods previously described,11 with all images analysed by a single-investigator (J.M.) blinded to study treatment. Dynamic cine images were used to manually quantify left ventricular (LV) diastolic and systolic volumes using short-axis slices. Left ventricular mass was computed as the product of end-diastolic LV volume and myocardial density (1.05 g/mL), indexed to body surface area. Systolic function was estimated by ejection fraction and diastolic function estimated by early diastolic peak filling rate.15
Statistical methods
Between group differences of the observed values at each visit were compared using Student's t-test or Wilcoxon rank-sum for continuous and χ2 or Fisher's Exact for categorical variables. Within-group comparisons were performed using paired t-tests. The original analysis plan comprised an intention-to-treat strategy;11 however, due to high attrition and incompleteness of ascertainment of the primary outcome parameter precluding intention-to-treat methods, the primary analysis strategy was modified to analyse data from those completing the study. For sensitivity analyses, we also performed mixed-effects model analyses as well as last-observation-carried-forward analyses of primary and key secondary measures, which yielded qualitatively similar results for all analyses except where noted (data not shown). All testing was two-tailed at a significance level of 0.05, with analyses performed using SAS Version 9.1.3 (Cary, NC, USA). The study was powered according to the primary endpoint of VO2peak–FFM, with 150 patients providing ≥80% power to detect a ≥10% difference in VO2peak–FFM.11
Results
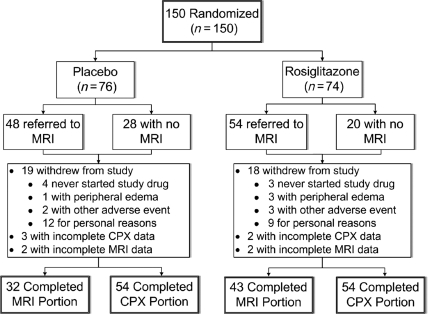
The study cohort comprised 150 subjects, including 74 randomized to rosiglitazone and 76 to placebo, recruited between February 2005 and October 2006. The disposition of patients in the trial is shown in Figure 1. Evaluable baseline and end-of-study assessments of the primary outcome, VO2peak–FFM, were available for 108 subjects (72%), 54 in each group. Of those completing the final study assessment, 103 completed all 6 months of therapy, with five participants undergoing early final assessments due to oedema and/or HF (four rosiglitazone; one placebo) at a mean treatment duration of 61 days. Baseline characteristics are presented in Table 1. Individuals not completing the study were younger (51.1 vs. 56.3 years; P = 0.001); more commonly Hispanic (33.3 vs. 16.7%; P = 0.03); and were less commonly treated with beta-blockers (19.1 vs. 38.9%; P = 0.02) and ACE inhibitors/angiotensin II receptor blockers (47.6 vs. 64.8%; P = 0.05). Among the subjects completing the trial comprising the primary analysis population, baseline characteristics were well-matched between the groups. Those completing the study had a mean age of 56 years, and included 41% women, 44% black, and 17% Hispanic subjects. On average, body mass index (BMI) was 34.1 kg/m2, with a mean duration of T2DM >9 years including >40% treated with insulin. The prevalence of hypertension and hyperlipidaemia were each about 75%, 35% had prior CVD, and 17% were smokers.
Figure 1.
Flow of patients through the trial (MRI, magnetic resonance imaging; CPX, cardiopulmonary exercise test).
Table 1.
Baseline demographic and clinical characteristics at the time of randomization for those not completing the study, and among participants completing the trial, by treatment group
| Non-Completers | Non-completers vs. Completers P-value | Completers |
|||
|---|---|---|---|---|---|
| Placebo (n = 54) | Rosiglitazone (n = 54) | Rosiglitazone vs. Placebo P-value | |||
| Age, mean (SD), yr | 51.1 (9.4) | 0.001 | 55.7 (8.3) | 57.0 (8.7) | 0.44 |
| Women, No (%) | 18 (42.9) | 0.81 | 21 (38.9) | 23 (42.6) | 0.70 |
| Race/ethnicity, No (%) | 1.00 | ||||
| White | 12 (28.6) | 0.51 | 18 (33.3) | 19 (35.2) | |
| Black | 13 (31.0) | 0.16 | 24 (44.4) | 23 (42.6) | |
| Hispanic | 14 (33.3) | 0.03 | 9 (16.7) | 9 (16.7) | |
| Other | 3 (7.1) | 0.71 | 3 (5.6) | 3 (5.6) | |
| Weight, mean (SD), kg | 104.6 (25.6) | 0.24 | 97.2 (19.4) | 97.9 (20.1) | 0.87 |
| BMI, mean (SD), kg/m2 | 35.6 (8) | 0.43 | 34.1 (7.3) | 34.1 (6.4) | 0.88 |
| % Body fat, mean (SD) | 31.8 (7.4) | 0.80 | 31.1 (8.1) | 31.8 (7) | 0.67 |
| Current smoking, No (%) | 7 (16.7) | 1.0 | 7 (13.0) | 11 (20.4) | 0.30 |
| Duration of diabetes, mean (SD), yr | 7.4 (5.4) | 0.29 | 8.7 (8.4) | 9.5 (6.5) | 0.17 |
| Hypertension, No (%) | 26 (61.9) | 0.09 | 40 (74.1) | 42 (77.8) | 0.65 |
| Hyperlipidaemia, No (%) | 30 (71.4) | 0.74 | 41 (75.9) | 39 (72.2) | 0.66 |
| Prior CVD, No (%) | 13 (31.0) | 0.55 | 20 (37.0) | 19 (35.2) | 0.84 |
| Medication use, No (%) | |||||
| Aspirin | 14 (33.3) | 0.15 | 25 (46.3) | 25 (46.3) | 1.00 |
| Beta-blocker | 8 (19.1) | 0.02 | 20 (37.0) | 22 (40.7) | 0.69 |
| ACE inhibitor or ARB | 20 (47.6) | 0.05 | 33 (61.1) | 37 (68.5) | 0.42 |
| Statin | 20 (47.6) | 0.24 | 30 (55.6) | 33 (61.1) | 0.56 |
| Insulin | 17 (40.5) | 0.89 | 21 (38.9) | 24 (44.4) | 0.56 |
| Metformin | 24 (57.1) | 0.15 | 42 (77.8) | 33 (61.1) | 0.06 |
| Sulfonylurea | 13 (31.0) | 0.87 | 14 (25.9) | 18 (33.3) | 0.40 |
CVD, cardiovascular disease; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; SD, standard deviation; yr, year; No, number.
Clinical and laboratory results
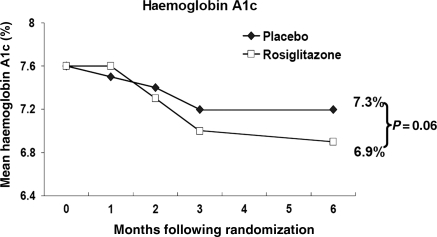
Table 2 summarizes clinical and laboratory values at baseline and study end for the 108 subjects who completed the study, with no statistically significant differences at baseline between the groups. At study entry, HbA1c was 7.6% in both treatment groups, declining during the study in both groups to 7.2% in the placebo vs. 6.9% in the rosiglitazone-treated group (P = 0.06; Figure 2).
Table 2.
Baseline and end-of-study clinical parameters and laboratory values for those participants completing the trial
| Placebo (n = 54) |
Rosiglitazone (n = 54) |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | End-of-study | P-value within group | Baseline | End-of-study | P-value within group | P-value between groups at end-of-study | |
| Blood pressure, mean (SD), mmHg | |||||||
| Systolic | 148.4 (22.5) | 144.6 (23.1) | 0.25 | 151.3 (25.8) | 143 (20.0) | 0.07 | 0.87 |
| Diastolic | 82.9 (11.7) | 79.8 (11.6) | 0.08 | 83.6 (13.1) | 78.2 (9.7) | 0.004 | 0.49 |
| Weight, mean (SD), kg | 97.2 (19.4) | 97.3 (19.0) | 0.89 | 97.9 (20.1) | 100.8 (20.5) | <0.0001 | 0.36 |
| BMI, mean (SD), kg/m2 | 34.1 (7.3) | 34.2 (7.4) | 0.75 | 34.1 (6.4) | 35.1 (6.6) | <0.0001 | 0.48 |
| % Body fat, mean (SD) | 31.1 (8.1) | 31.4 (7.7) | 0.58 | 31.8 (7.0) | 33.6 (7.1) | <0.0001 | 0.12 |
| New/worse peripheral oedema, No (%) | — | 18 (33.3%) | — | — | 29 (53.7%) | — | 0.03 |
| Laboratory values | |||||||
| HbA1c, mean (SD), % | 7.6 (1.8) | 7.2 (1.3) | 0.03 | 7.6 (1.8) | 6.9 (1.6) | 0.001 | 0.06 |
| LDL, mean (SD), mg/dl | 104.2 (36.6) | 93.2 (45.8) | 0.01 | 108.3 (52.9) | 117.5 (56.7) | 0.10 | 0.02 |
| HDL, mean (SD), mg/dL | 46.4 (11.5) | 46.3 (10.2) | 0.64 | 44.0 (8.6) | 46.6 (10.1) | 0.01 | 0.86 |
| Triglycerides, mean (SD), mg/dl | 149.8 (85.2) | 154.7 (142.0) | 0.30 | 189.2 (141.3) | 198.3 (147.6) | 0.78 | 0.05 |
| Hematocrit, mean (SD), % | 42.1 (3.9) | 41.1 (3.5) | 0.01 | 40.7 (3.8) | 38.0 (4.9) | <0.0001 | 0.0003 |
| BNP, median (IQR), pg/mL | 6.4 (0, 28.4) | 6.2 (3.1, 23.5) | 0.57 | 6.1 (3, 34.1) | 13.7 (3.2, 73) | 0.04 | 0.14 |
kg, kilogram; kg/m2, kilogram per square meter; HbA1c, haemoglobin A1c; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BNP, brain natriuretic peptide.
Figure 2.
Changes in mean haemoglobin A1c during the trial.
Cardiopulmonary testing results
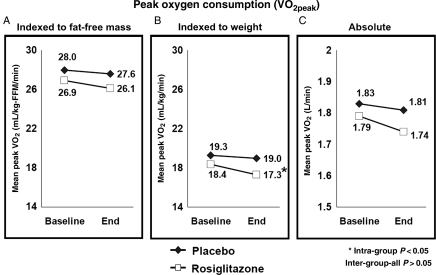
The exercise test results are presented in Table 3 and Figure 3. After 6 months of study therapy, no significant differences were observed in the primary endpoint of mean VO2peak–FFM between rosiglitazone and placebo (26.1 vs. 27.6 mL/kg-FFM/min; P = 0.26); similarly, absolute VO2 (mL/min) or VO2 indexed to total body weight (mL/kg/min) were not statistically different between the groups.
Table 3.
Results of cardiopulmonary exercise testing among participants who completed the trial
| Placebo (n = 54) |
Rosiglitazone (n = 54) |
P-value between groups at end-of-study | |||||
|---|---|---|---|---|---|---|---|
| Baseline | End-of-study | P-value within group | Baseline | End-of-study | P-value within group | ||
| Primary outcome | |||||||
| VO2 (mL/kg-FFM/min) | 28.0 (7) | 27.6 (6.6) | 0.49 | 26.9 (6.3) | 26.1 (7) | 0.06 | 0.26 |
| At rest | |||||||
| Heart rate | 75 (11.1) | 75 (13.8) | 0.84 | 73 (12.6) | 76 (14.2) | 0.08 | 0.92 |
| Blood pressure | |||||||
| Systolic, mmHg | 148 (22.5) | 145 (23.1) | 0.25 | 151 (25.8) | 143 (20.0) | 0.07 | 0.87 |
| Diastolic, mmHg | 83 (11.7) | 80 (11.3) | 0.08 | 84 (13.1) | 78 (9.6) | 0.004 | 0.49 |
| Peak exercise | |||||||
| Heart rate | 152 (21.2) | 150 (23.3) | 0.36 | 144 (24.5) | 142 (23.9) | 0.29 | 0.09 |
| Blood pressure | |||||||
| Systolic, mmHg | 191 (28.7) | 190 (24.8) | 0.92 | 195 (29.2) | 194 (25.6) | 0.75 | 0.41 |
| Diastolic, mmHg | 91 (19.8) | 95 (25.1) | 0.76 | 93 (17.9) | 91 (19.4) | 0.41 | 0.52 |
| VO2 (mL/min) | 1835 (490) | 1815 (503) | 0.55 | 1788 (567) | 1736 (592) | 0.07 | 0.46 |
| VO2 (mL/kg/min) | 19.3 (5.5) | 19 (5.2) | 0.29 | 18.4 (4.6) | 17.3 (5.0) | 0.0003 | 0.06 |
All values reported as mean (standard deviation); VO2, oxygen consumption; mL, millilitre; kg, kilogram; FFM, fat-free mass.
Figure 3.
Mean peak oxygen consumption during maximal treadmill exercise (VO2peak) by treatment group at baseline and at study end (A) indexed to fat-free mass (primary endpoint); (B) indexed to total body weight; and (C) absolute measure without indexation.
Within groups, VO2 indexed to total body weight (mL/kg/min) significantly decreased in the rosiglitazone group, with the magnitude of decline of 1.1 mL/kg/min, or 6% relative decline (P = 0.003); there were no significant changes in any of the other VO2 parameters within either group. In sensitivity analyses using baseline-observations-carried-forward for those not completing the study (n = 20; 27%), similar to analyses of those completing the study, there was no significant difference between rosiglitazone and placebo groups at study end in the primary outcome measure of VO2peak scaled to fat-free mass (26.35 vs. 27.49 mL/kg-ffm/min; P = 0.26). However, within the rosiglitazone group, the decline from baseline to study end was statistically significant (26.95 vs. 26.35 mL/kg-ffm/min; P = 0.026), though representing a relative change of only 2%.
Cardiac magnetic resonance imaging results
Of the 102 participants volunteering to undergo cMRI, 75 (74%) had complete baseline and end-of-study data, with results presented in Table 4. No significant differences were observed between the groups in LV mass index (83.2 vs. 75.2 g/m2; P = 0.06), end-systolic volume (44.4 vs. 39.1 mL; P = 0.28), or ejection fraction (66.1 vs. 65.9%; P = 0.9). Rosiglitazone vs. placebo was associated with significantly higher end-diastolic volume (128.1 vs. 112.0 mL; P = 0.01), stroke volume (83.7 vs. 72.9 mL; P = 0.01), and a trend toward improved peak filling rate (79.4 vs. 60.5; P = 0.07). Within groups, no significant changes were observed from baseline to end-of-study in cMRI parameters in the placebo group, whereas rosiglitazone was associated with statistically significant increases in end-diastolic volume (117.9 vs. 128.1 mL; P = 0.001); stroke volume (74.9 vs. 83.7 mL; P = 0004); and ejection fraction (63.8 vs. 66.1%; P = 0.03).
Table 4.
Results from cardiac magnetic resonance imaging
| Placebo (n = 32) |
Rosiglitazone (n = 43) |
P-value between groups at end-of-study | |||||
|---|---|---|---|---|---|---|---|
| Baseline | End-of-study | P-value within group | Baseline | End-of-study | P-value within group | ||
| End systolic volume, mL | 39.9 (12) | 39.1 (13.5) | 0.63 | 43.0 (18.3) | 44.4 (18.7) | 0.47 | 0.28 |
| End diastolic volume, mL | 114.8 (18.9) | 112 (24.4) | 0.34 | 117.9 (26.9) | 128.1 (25.6) | 0.001 | 0.01 |
| Ejection fraction, % | 65.4 (7.6) | 65.9 (6.8) | 0.70 | 63.8 (10.7) | 66.1 (9.8) | 0.03 | 0.90 |
| LV mass index, g/m2 | 76.9 (18.0) | 75.2 (14.3) | 0.28 | 85.9 (21.5) | 83.2 (22.7) | 0.14 | 0.06 |
| Stroke volume, mL | 74.8 (13.8) | 72.9 (15.4) | 0.36 | 74.9 (21.2) | 83.7 (20) | 0.0004 | 0.01 |
| Peak filling rate, mL/s | 68.1 (42.5) | 60.5 (24.9) | 0.37 | 70.8 (32.6) | 79.4 (41.9) | 0.38 | 0.07 |
All values reported as means (SD).
Evidence of volume expansion in subjects treated with rosiglitazone
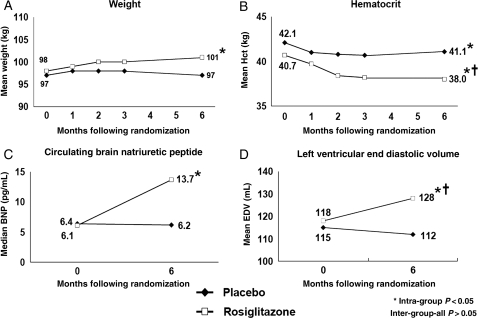
Within the rosiglitazone group, significant increases in weight, BNP, and LV end-diastolic volume, and a significant decrease in hematocrit occurred over time, with no significant changes in these parameters in the placebo group (Figure 4A–D). Likewise, new or worse peripheral oedema was more common with rosiglitazone vs. placebo (53.7 vs. 33.3%; P = 0.03; Table 2).
Figure 4.
Changes in selected parameters during the trial suggesting plasma volume expansion, including (A) weight; (B) hematocrit; (C) circulating brain natriuretic peptide; and (D) left ventricular end-diastolic volume.
Safety and tolerability
Premature study drug discontinuation occurred in 20 subjects in the rosiglitazone group and 22 in the placebo group, most commonly for reasons unrelated to study therapy, excepting three patients in the rosiglitazone group and one in the placebo group stopping due to new or worsening peripheral oedema, and one in the rosiglitazone group for incident HF. Among those completing the study, compliance with study drug prescription was high and similar between the groups (89.9%). Three patients treated with rosiglitazone developed incident HF, two of whom had complete end-of-study assessments; no patients treated with placebo developed HF. Two patients had acute coronary syndrome events, both in the placebo group.
Discussion
The principal observations from the present study include (i) no discernable adverse effects on cardiac structure, function, or peak cardiovascular performance associated with rosiglitazone use; and (ii) changes in a number of markers (i.e. hematocrit; BNP; end-diastolic volume; weight) that in aggregate indirectly suggest plasma volume expansion among rosiglitazone-treated patients.
The concern for HF persists for the TZD medications,3,16 with product label warnings against use in patients with HF.4–5 Despite these warnings, the use of TZDs remains high, even in HF populations.17 Moreover, the mechanistic underpinning for increased HF remains poorly understood. Whereas consistently observed with both available drugs, the net increase in HF risk is modest in absolute terms, with an estimated incidence over placebo of ∼0.25–0.45%/year,18–19 contrasted with up to 15% increased peripheral oedema risk with TZDs, suggesting possible non-cardiac aetiologies for these observations.2,20
Cardiac structure and function
Several animal studies using ex vivo preparations21–22 and intact models22–25 suggest favourable cardiac effects of the TZDs, and human echocardiographic studies have demonstrated no adverse cardiac effects with TZD therapy,6–8,26 with noted limitations of echocardiography to discriminate small but potentially important cardiac changes.9,27 In addition, with assessments only at rest, no information is available on cardiovascular performance during activity. Therefore, the present study evaluated the effect of rosiglitazone on integrated cardiovascular performance during peak exercise to assess TZD effects on performance, complemented by cMRI as a measure more sensitive than echocardiography to assess effects on cardiac structure and function.9,27
Peak VO2 is the gold standard for assessment of cardiovascular performance, an integrated measure of cardiac and peripheral components.28 A decline of integrated cardiovascular performance resulting in symptomatic HF occurs from compromise of one or more of these parameters and therefore should be reflected as a decline in peak VO2, as used clinically in estimating severity of HF and candidacy for heart transplantation.29 In contrast, improvements in any of these parameters should be reflected in improved peak VO2.
Only one prior study has evaluated the effects of a TZD on exercise performance, demonstrating improved peak VO2 with rosiglitazone.30 However, that study was limited by a small sample size (n = 20), a relatively low-risk cohort, only 4 weeks of study treatment, and the use of correlated data analyses with each subject serving as their own control. The present study was designed to perform independent sample comparisons with a longer duration of therapy, and although we did not observe improved peak VO2, we were able to demonstrate no statistical differences between subjects treated with rosiglitazone vs. placebo.
These observations are complemented by the cMRI data. Compared with placebo, rosiglitazone was not associated with adverse effects on cardiac structure or function, with increased end-diastolic volume reflecting volume expansion, and matched by a commensurate increase in stroke volume. In addition, among patients treated with rosiglitazone, compared with baseline measures, ejection fraction improved modestly but significantly. The validity of the observed absence of adverse effects on cardiac performance is strengthened by the fact these observations were made in the context of high rates of new or worsening peripheral oedema observed in the rosiglitazone-treated group.
Plasma volume expansion
Both animal and human data suggest that the TZDs may cause plasma volume expansion via effects on renal sodium handling.31–33 The PPAR-γ is expressed in the renal collecting duct epithelium, where its activation up-regulates epithelial sodium channel (ENaC)-γ expression and increases distal sodium reclamation.31 In mouse models, knockout of renal PPAR-γ eliminates plasma volume expansion with TZDs.31–32 and amiloride (an ENaC-γ antagonist) prevents plasma volume expansion with pioglitazone in wild-type mice.31 In a randomized clinical trial, aldactone, an indirect antagonist of ENaC-γ, reversed plasma volume expansion in patients treated with rosiglitazone.33
In the present study, changes in a number of markers related to plasma volume (increased weight, BNP, and end-diastolic volume with decreased hematocrit) with rosiglitazone treatment, in the absence of negative cardiac effects, provide additional indirect support for plasma volume expansion as the primary mechanism of TZD-associated peripheral oedema and HF.
Limitations
The trial has a number of limitations. First, only 72% of enrolled subjects completed the study. However, this rate of attrition is comparable to or lower than the reported echocardiographic studies of the TZDs,6–8 and similar to other studies employing similarly intensive evaluations that have no direct benefit to the study participants.34–35 Furthermore, drop-out was relatively balanced between the groups and was uncommonly related to study drug, with those completing vs. not completing the study on average at higher risk based on comparison of baseline characteristics. Owing to large numbers without primary outcome assessment at study end, we were unable to perform the planned intention to treat analyses, and are therefore unable to account for biases introduced by the study drop-out. However, the stability of the results of sensitivity analyses using both mixed-effects modelling and last-observation-carried-forward imputation increases the probable validity of our primary observations. Second, only about two-third of subjects underwent the cMRI portion of the study, with complete MRI data on 74% of those imaged, limiting both the statistical power to detect differences in the measures of interest and the generalizability of the observations. Third, the interpretation of the results is limited to rosiglitazone, with inability to determine drug vs. class effects. Fourth, the 6-month study duration precludes the ability to ascertain the longer term effects weight gain and volume expansion on integrated cardiopulmonary performance and cardiac structure. Finally, although there is ongoing uncertainty with regard to the association between rosiglitazone and atherosclerotic vascular disease risk,2 the present study was not designed or powered to provide incremental information in that regard.
Conclusions
In conclusion, using extensive cardiovascular interrogation in a high-risk cardiovascular cohort of patients with T2DM and despite the high incidence of new or worsening peripheral oedema observed with rosiglitazone, we were unable to demonstrate any adverse cardiac effects of rosiglitazone, with a number of indirect measures in aggregate suggesting significant plasma volume expansion in this setting.
Funding
This work was supported by research grants from Glaxo Smith Kline; Biosite, Inc.; the Donald W. Reynolds Foundation; and the United States Public Health Services General Clinical Research Center (grant #M01-RR00633) from National Institutes of Health/National Center Research Resources-Clinical Research. The funding organizations did not participate in study design, data collection, analysis, or interpretation, writing of the report, or the decision to submit the paper for publication. Representatives of GSK reviewed the manuscript prior to submission, but did not contribute to its content. The corresponding author had full access to all of the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, and had final responsibility for the decision to submit for publication.
Conflict of interest: D.K.M. reports receiving honoraria from Pfizer and Takeda and consultancy fees from Novo Nordisk, Tethys Bioscience, and AstraZeneca. M.H.D. reports receiving honoraria from Glaxo Smith Kline. L.S.S. reports receiving grant support from Glaxo Smith Kline and Takeda. S.M.A., A.K., J.M., J.A.L., and P.G.S. and C.R.A. report no financial relationships.
References
- 1.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. doi:10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 2.McGuire DK, Inzucchi SE. New drugs for the treatment of diabetes mellitus: part I: thiazolidinediones and their evolving cardiovascular implications. Circulation. 2008;117:440–449. doi: 10.1161/CIRCULATIONAHA.107.704080. doi:10.1161/CIRCULATIONAHA.107.704080. [DOI] [PubMed] [Google Scholar]
- 3.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. doi:10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 4.GlaxoSmithKline. Avandia (rosiglitazone): [prescribing information] Research Triangle Park. NC: USA: GlaxoSmithKline; 2007. [Google Scholar]
- 5.Takeda. Actos [prescribing information] Lincolnshire, IL: Takeda Pharmaceuticals; 2007. [Google Scholar]
- 6.St John Sutton M, Rendell M, Dandona P, Dole JF, Murphy K, Patwardhan R, Patel J, Freed M. A comparison of the effects of rosiglitazone and glyburide on cardiovascular function and glycemic control in patients with type 2 diabetes. Diabetes Care. 2002;25:2058–2064. doi: 10.2337/diacare.25.11.2058. doi:10.2337/diacare.25.11.2058. [DOI] [PubMed] [Google Scholar]
- 7.Dargie HJ, Hildebrandt PR, Riegger GA, McMurray JJ, McMorn SO, Roberts JN, Zambanini A, Wilding JP. A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association Functional Class I or II Heart Failure. J Am Coll Cardiol. 2007;49:1696–1704. doi: 10.1016/j.jacc.2006.10.077. doi:10.1016/j.jacc.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 8.Ghazzi MN, Perez JE, Antonucci TK, Driscoll JH, Huang SM, Faja BW, Whitcomb RW. Cardiac and glycemic benefits of troglitazone treatment in NIDDM. The Troglitazone Study Group. Diabetes. 1997;46:433–439. doi: 10.2337/diab.46.3.433. doi:10.2337/diabetes.46.3.433. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann R, von Bardeleben S, ten Cate F, Borges AC, Kasprzak J, Firschke C, Lafitte S, Al-Saadi N, Kuntz-Hehner S, Engelhardt M, Becher H, Vanoverschelde JL. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J. 2005;26:607–616. doi: 10.1093/eurheartj/ehi083. doi:10.1093/eurheartj/ehi083. [DOI] [PubMed] [Google Scholar]
- 10.van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. doi: 10.1161/CIRCULATIONAHA.108.803916. doi:10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- 11.McGuire DK, See R, Abdullah SM, Snell PG, McGavock JM, Ayers CR, Szczepaniak LS. The effect of rosiglitazone on integrated cardiovascular performance, cardiac structure, function and myocardial triglyceride: trial design and rationale. Diab Vasc Dis Res. 2009;6:43–50. doi: 10.3132/dvdr.2009.009. doi:10.3132/dvdr.2009.009. [DOI] [PubMed] [Google Scholar]
- 12.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1–92. [PubMed] [Google Scholar]
- 13.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation. 2001;104:1350–1357. [PubMed] [Google Scholar]
- 14.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. doi:10.1079/BJN19740060. [DOI] [PubMed] [Google Scholar]
- 15.Kudelka AM, Turner DA, Liebson PR, Macioch JE, Wang JZ, Barron JT. Comparison of cine magnetic resonance imaging and Doppler echocardiography for evaluation of left ventricular diastolic function. Am J Cardiol. 1997;80:384–386. doi: 10.1016/s0002-9149(97)00375-5. [DOI] [PubMed] [Google Scholar]
- 16.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2008;31:173–175. doi: 10.2337/dc08-9016. doi:10.2337/dc08-9016. [DOI] [PubMed] [Google Scholar]
- 17.Masoudi FA, Wang Y, Inzucchi SE, Setaro JF, Havranek EP, Foody JM, Krumholz HM. Metformin and thiazolidinedione use in medicare patients with heart failure. J Am Med Assoc. 2003;290:81–85. doi: 10.1001/jama.290.1.81. doi:10.1001/jama.290.1.81. [DOI] [PubMed] [Google Scholar]
- 18.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. doi:10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 19.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes–an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. doi:10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 20.Patel C, Wyne KL, McGuire DK. Thiazolidinediones, peripheral oedema and congestive heart failure: what is the evidence? Diab Vasc Dis Res. 2005;2:61–66. doi: 10.3132/dvdr.2005.010. doi:10.3132/dvdr.2005.010. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto K, Ohki R, Lee RT, Ikeda U, Shimada K. Peroxisome proliferator-activated receptor gamma activators inhibit cardiac hypertrophy in cardiac myocytes. Circulation. 2001;104:1670–1675. doi: 10.1161/hc4001.097186. doi:10.1161/hc4001.097186. [DOI] [PubMed] [Google Scholar]
- 22.Asakawa M, Takano H, Nagai T, Uozumi H, Hasegawa H, Kubota N, Saito T, Masuda Y, Kadowaki T, Komuro I. Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation. 2002;105:1240–1246. doi: 10.1161/hc1002.105225. doi:10.1161/hc1002.105225. [DOI] [PubMed] [Google Scholar]
- 23.Sakai S, Miyauchi T, Irukayama-Tomobe Y, Ogata T, Goto K, Yamaguchi I. Peroxisome proliferator-activated receptor-gamma activators inhibit endothelin-1-related cardiac hypertrophy in rats. Clin Sci (Lond) 2002;103(Suppl. 48):16S–20S. doi: 10.1042/CS103S016S. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji T, Mizushige K, Noma T, Murakami K, Ohmori K, Miyatake A, Kohno M. Pioglitazone improves left ventricular diastolic function and decreases collagen accumulation in prediabetic stage of a type II diabetic rat. J Cardiovasc Pharmacol. 2001;38:868–874. doi: 10.1097/00005344-200112000-00008. doi:10.1097/00005344-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Furuse Y, Ogino K, Shimoyama M, Sasaki N, Hisatome I. Ca(2+)-sensitizing effect is involved in the positive inotropic effect of troglitazone. Br J Pharmacol. 2001;133:1307–1313. doi: 10.1038/sj.bjp.0704212. doi:10.1038/sj.bjp.0704212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirayama H, Sugano M, Abe N, Yonemoch H, Makino N. Troglitazone, an antidiabetic drug, improves left ventricular mass and diastolic function in normotensive diabetic patients. Int J Cardiol. 2001;77:75–79. doi: 10.1016/s0167-5273(00)00411-3. doi:10.1016/S0167-5273(00)00411-3. [DOI] [PubMed] [Google Scholar]
- 27.Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–228. doi: 10.1016/0895-7061(94)00178-E. doi:10.1016/0895-7061(94)00178-E. [DOI] [PubMed] [Google Scholar]
- 28.Levine BD. VO2max: what do we know, and what do we still need to know? J Physiol. 2008;586:25–34. doi: 10.1113/jphysiol.2007.147629. doi:10.1113/jphysiol.2007.147629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber KT, Janicki JS, McElroy PA. Determination of aerobic capacity and the severity of chronic cardiac and circulatory failure. Circulation. 1987;76:VI40–VI45. [PubMed] [Google Scholar]
- 30.Regensteiner JG, Bauer TA, Reusch JE. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care. 2005;28:2877–2883. doi: 10.2337/diacare.28.12.2877. doi:10.2337/diacare.28.12.2877. [DOI] [PubMed] [Google Scholar]
- 31.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. doi:10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA. 2005;102:9406–9411. doi: 10.1073/pnas.0501744102. doi:10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karalliedde J, Buckingham R, Starkie M, Lorand D, Stewart M, Viberti G. Effect of various diuretic treatments on rosiglitazone-induced fluid retention. J Am Soc Nephrol. 2006;17:3482–3490. doi: 10.1681/ASN.2006060606. doi:10.1681/ASN.2006060606. [DOI] [PubMed] [Google Scholar]
- 34.Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D'Agostino RB, Sr, Perez A, Provost JC, Haffner SM. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. J Am Med Assoc. 2006;296:2572–2581. doi: 10.1001/jama.296.21.joc60158. doi:10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- 35.Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochelliere R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. J Am Med Assoc. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. doi:10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]