Abstract
Aims
The aim of this study was to evaluate the safety and efficacy of transcatheter closure for perimembranous ventricular septal defect (pmVSD) and its long-term results. The most common congenital heart condition is pmVSD. Transcatheter closure of pmVSD is a recently described technique with limited results for mid- to long-term follow-up.
Methods and results
Between June 2002 and June 2008, 848 patients with pmVSD were enrolled in our study and treated percutaneously with pmVSD occluders. All patients were followed up until December 2008, an average of 37 months. According to colour Doppler transthoracic echocardiography before the intervention and ventriculography, the average end-diastolic pmVSD size was 5.1 and 5.4 mm, respectively. Placement of the device was successful in 832 patients (98.1%) and the median device size was 8.6 mm. During follow-up, 103 adverse events (12.4%) were reported. Most adverse events were categorized as minor and there were nine major adverse events (8.7%), including two complete atrioventricular block requiring pacemaker implantation. Kaplan–Meier estimates showed >85% freedom from major or minor adverse events during a maximal follow-up of 79 months.
Conclusions
In experienced hands, transcatheter pmVSD closure can be performed safely and successfully with low morbidity and mortality. Long-term prognostic results are favourable, and the transcatheter approach provides a less-invasive alternative that may become the first choice in selected pmVSD patients.
This trial is registered with ClinicalTrials.gov, number NCT00890799.
Keywords: Catheterization, Heart defects, Congenital, Echocardiography, Follow-up studies
Ventricular septal defect (VSD) is a common congenital heart defect in both children and adults.1 Perimembranous VSD (pmVSD), which involves the membranous septum and the adjacent portion of the muscular septum, accounts for about 70% of cases. Standard treatment for pmVSD is open surgery, which is widely performed with minimal operative mortality but still carries risks, such as complete atrioventricular block (cAVB), residual shunt, postpericardiotomy syndrome, and wound infection, etc.1 Since the first report of transcatheter VSD closure in 1988,2 this catheter-based approach for VSD has been shown to be an alternative to surgical closure with acceptable mortality and morbidity as well as encouraging results.3–10 However, until now, reports on this treatment for pmVSD have been limited to small series with insufficient follow-up.11–15 According to a review by Butera et al.14, the total number of percutaneous closures of pmVSD worldwide was 366, and the average follow-up period did not exceed 1 year. Both large-scale and long-term follow-up results for this technique are rare. Thus, this study was designed to evaluate the safety and efficacy of transcatheter closure of pmVSD and to characterize patients’ long-term clinical outcomes.
Methods
Patients
From June 2002 to June 2008, 848 patients with pmVSD were enrolled for attempted transcatheter closure using the pmVSD occluders available at our centre. The study was registered with ClinicalTrials.gov, number NCT00890799, was approved by the ethics committee of Xijing Hospital, and was carried out in accordance with the Declaration of Helsinki (1996) and all relevant Chinese laws.
The occluders
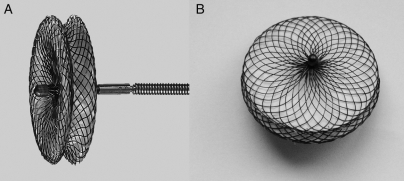
Two types of pmVSD occluders (pmVSD-O) were used in the study: the Amplatzer pmVSD-O (AGA Medical, Golden Valley, MN, USA) and the domestic Shanghai pmVSD-O (Shape Memory Alloy Ltd, Shanghai, China). The Amplatzer pmVSD-O and delivery system have been previously described in detail.16,17 The Shanghai pmVSD-O is a symmetrical double-disk device (Figure 1)10,18 made of 0.005 in. nitinol wire mesh and fabric inside. The diameter of both discs is 4 mm larger than that of the waist. The thickness of the waist of Shanghai pmVSD-O is 3 mm while that of Amplatzer pmVSD-O is 2 mm. The Shanghai pmVSD-O was approved in 2003 by the State Food and Drug Administration, P.R. China (SFDA), and received CE mark in 2008. Device sizes are available from 4 to 20 mm in 1 mm increments.
Figure 1.
(A) Lateral view of the Shanghai symmetrical perimembranous ventricular septal defect occluder; (B) front view of the Shanghai symmetrical perimembranous ventricular septal defect occluder.
Peri-operative protocol
In all pmVSD patients, transcatheter closure was indicated for haemodynamic, symptomatic, or other medical reasons (Table 1). All patients were routinely screened by conventional two-dimensional and colour Doppler transthoracic echocardiography (TTE) with a Sonos 5500 ultrasound system (Hewlett Packard, USA). The following inclusion criteria were used in this study: (i) congenital pmVSD as shown by echocardiography; (ii) body weight >10 kg and age >2 years; (iii) maximum VSD diameter <20 mm by TTE; (iv) defect located at the 9 to 11 o’clock positions of an analog clock in the short-axis parasternal view by TTE; (v) a distance of >1 mm from the pmVSD to the aortic valve; (vi) left-to-right shunt; and (vii) calculated pulmonary vascular resistance <8 Wood units. The exclusion criteria were as follows: (i) defects associated with other cardiac lesions requiring a surgical approach; (ii) body weight <10 kg and age <2 years; (iii) irreversible pulmonary vascular disease with calculated pulmonary vascular resistance >8 Wood units; (iv) severe aortic regurgitation; (v) severe aortic valve prolapse; and (vi) right-to-left shunt. Before intervention, an informed written consent was obtained from all patients or their parents. Physical examination, a chest X-ray, standard 12-lead electrocardiogram (ECG), and TTE were routinely performed in all patients.
Table 1.
Baseline characteristics of the patients underwent transcatheter procedure (median with range)
| Patients (n) | 832 |
| Sex (F/M) [n(%)] | 406 (48.8%)/426 (51.2%) |
| Age (years) | 9.0 (2–73) |
| Age groups [n (%)] | |
| <10 years | 439 (52.8%) |
| 10–20 years | 283 (34.0%) |
| >20 years | 110 (13.2%) |
| Weight (kg) | 30.5 (10–88) |
| Indications | |
| Symptoms (frequent respiratory infections, oedema, NYHA functional class II or greater.) | 337 (40.5%) |
| Haemodynamic changes (cardiomegaly on chest X-ray, left atrial, or left ventricular enlargement verified by echocardiography) | 462 (55.5%) |
| Asymptomatic with heart murmur | 23 (2.8%) |
| Previous SBE | 10 (1.2%) |
| Echocardiography | |
| Subaortic rim (mm) | 2.8 (1.0–17.0) |
| Subtricuspid rim (mm) | 5.3 (2.0–21.0) |
| VSD size (mm) | 5.1 (1.3–15.5) |
| Heart function (NYHA grade) | |
| I | 786 (94.5%) |
| II | 34 (4.1%) |
| III | 12 (1.4%) |
| IV | 0 |
| LVEDD (mean ± SD, Z score) | 1.9 ± 1.6 |
LVEDD, left ventricular end-diastolic dimension; NYHA, New York Heart Association; SBE, subacute bacterial endocarditis.
Device implantation
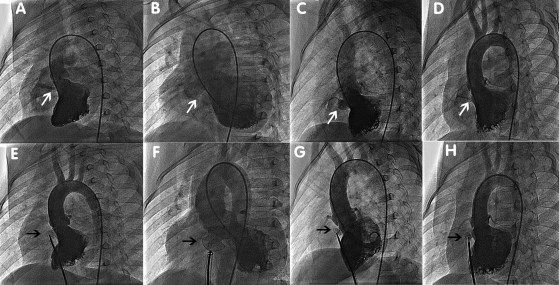
The catheterization procedure was performed under basal anaesthesia without tracheal intubation for patients <10 years of age and under local anaesthesia and conscious sedation for patients >10 years of age. Heparin (100 IU/kg) and antibiotics were administered intravenously during the procedure. Access was through the right femoral vein and right femoral artery. Angiography in the left ventricle at a 55°/20° left anterior oblique projection/cranial was used to profile the pmVSD. Location, size of the pmVSD, and its relationship with the aortic valve were assessed. Each pmVSD was categorized by shape as tubular, window-like, aneurysmal, or conical (Figure 2A–D). The diameter of the pmVSD was measured at the largest diastolic phase, and an occluder was selected based on this measurement. The defect was then passed from the left ventricle by a 5 Fr partly cut pigtail catheter or a right Judkins catheter. An arteriovenous circuit was set up through a femoral vein approach on the same side. A long sheath (6–12 Fr) was advanced to the left ventricle through the arteriovenous circuit and positioned beneath the aortic valve. Through the long sheath, the pmVSD occluder was deployed under fluoroscopic control and echocardiographic guidance. Angiography in the left ventricle and ascending aorta was performed again to verify complete occlusion and to identify any new-onset aortic valve regurgitation (Figure 2E–H). After the intervention, patients were transferred to the general wards. Continuous ECG monitoring was used during the first 24 h after the procedure. Aspirin (5 mg/kg daily) was administered for 6 months in all patients.
Figure 2.
Classification of perimembranous ventricular septal defect and ventriculography before the release of the occluders. (A) In the tubular type, the shunt jet is long, and the diameter in the left and right sides of the septum is the same. (B) In the window-like type, the shunt jet is scattered immediately after crossing the septum. (C) In the aneurysmal type, the shunt jet has an aneurysmal shape. (D) In the conical type, the shunt jet is wide on the left side of the septum and narrow on the right side. (E) Ventriculography before the release of occluders in tubular perimembranous ventricular septal defect, same patient as in (A). (F) Ventriculography before the release of occluders in window-like perimembranous ventricular septal defect, same patient as in (B). (G) Ventriculography before the release of occluders in aneurysmal perimembranous ventricular septal defect, same patient as in (C). (H) Ventriculography before the release of occluders in conical perimembranous ventricular septal defect, same patient as in (D).
Follow-up protocol
All patients were followed up until December 2008, and the median follow-up period was 37 months. Clinical examination, Holter or electrocardiographic monitoring, chest roentgenogram, and TTE were performed at 1, 6, and 12 months after the procedure and yearly thereafter. Adverse events were ascertained at each assessment on the basis of clinical evaluation. Thrombi, valve regurgitation, and residual shunts were looked for using TTE. Complications were prospectively recorded and divided into major and minor adverse events.
Adverse events
Events were graded as major or minor adverse events. Major adverse events included but were not limited to death during or after the procedure because of complications of the procedure, cAVB requiring pacemaker implantation, thrombo-embolism, and new-onset valvular regurgitation requiring surgical repair. Minor adverse events included but were not limited to groin haematoma, blood loss requiring transfusion, device embolization with transcatheter removal, any cardiac arrhythmia that required medication, new or increased valvular regurgitation less than two grades, haemolysis requiring medication, fever >38.5°C, rash, and loss of peripheral pulse. These minor adverse events required medical intervention but were not life threatening; they had no long-term sequelae and did not require long-term therapy.
Data analysis
All continuous variables are expressed as mean ± standard deviation (SD) or median with range as appropriate, and discrete variables are presented as frequencies and/or percentages. We used SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA) for the statistical analysis. Analysis of categorical variables was performed with the χ2 test or Fisher's exact test. Freedom from major or minor adverse events was assessed by the Kaplan–Meier product-limit method.
Results
Patients
Among all 848 patients in whom we attempted transcatheter closure of pmVSD, 832 patients (98.1%) were successfully treated by transcatheter intervention. There were 406 male and 426 female patients. According to colour Doppler TTE before intervention, the average end-diastolic pmVSD size was 5.1 mm. Demographic and diagnostic data are summarized in Table 1.
Procedural details
According to ventriculography, the pmVSDs were classified into four categories as described by Qin:10 tubular, window-like, aneurysmal, and conical. Altogether, 729 Shanghai pmVSD-Os and 103 Amplatzer pmVSD-Os were implanted. The mean measured pmVSD size on ventriculography was 5.4 mm (1.2–17.2 mm), and the mean pmVSD-Os size was 8.6 mm. A total of 62 combined procedures were performed simultaneously, including 24 atrial septal defect (ASD) closures, 18 patent foramen ovale (PFO) closures, 15 patent ductus arteriosus (PDA) closures, 2 muscular VSD closures, and 3 pulmonary valvuloplasties. Immediate closure was seen in 447 (53.7%) patients and all residual shunts diminished 3 months after the intervention. In our study, five pmVSD patients who failed with the symmetric domestic Shanghai pmVSD-O due to aortic regurgitation were successfully closed using the Amplatzer device. The median hospital stay was 3.3 days (1–25 days) and median time to return to normal activities was 13.6 days (5–98 days) (Table 2).
Table 2.
Procedural data and devices used (median with range)
| Fluoroscopic time (min) | 12.5 (4–149) |
| Procedure time (min) | 41.2 (22–342) |
| Catheterization | |
| VSD size in ventriculography (mm) | 5.4 (1.2–17.2) |
| PA systolic pressure (mmHg) | 28.2 (18–75) |
| PA mean pressure (mmHg) | 17.6 (14–56) |
| PA diastolic pressure (mmHg) | 15.3 (11–42) |
| Qp/Qs | 2.1 (1.5–8.5) |
| Defect shape, as determined by ventriculography [n (%)] | |
| Tubular | 239 (28.7%) |
| Window-like | 77 (9.3%) |
| Aneurysmal | 155 (18.6%) |
| Infundibular | 361 (43.4%) |
| Type of device used [n (%)] | |
| Amplatzer pmVSD-O | 103 (12.4%) |
| Shanghai pmVSD-O | 729 (87.6%) |
| Mean size of the device used (mm) | 8.6 (4–20) |
| Combined procedures (n) | |
| ASD closure | 24 |
| PFO closure | 18 |
| PDA closure | 15 |
| Muscular VSD closure | 2 |
| Pulmonary valvuloplasty | 3 |
| Median hospital stay (days) | 3.3 (1–25) |
| Time to return to normal activities (days) | 13.6 (5–98) |
VSD, ventricular septal defect; PA, pulmonary artery; Qp/Qs, pulmonary to systemic flow ratio; pmVSD-O, perimembranous ventricular septal defect occluder; ASD, atrial septal defect; PFO, patent foramen ovale; PDA, patent ductus arteriosus.
Adverse events and follow-up evaluation
The median follow-up was 37 months (6–78.7 months). A total of 103 adverse events (12.4%) were reported in patients who underwent attempted VSD closure. In our group, a residual shunt was considered to be present when colour Doppler flow mapping showed a left-to-right shunt across the interventricular septum. It was experienced in 352 of all 729 patients using Shanghai pmVSD-Os and 53 of 103 patients implanting Amplatzer pmVSD-Os. Comparison of the residual shunt rate between the two groups showed no statistical significance (χ2 = 0.363; P = 0.547).
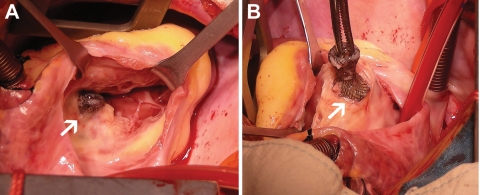
Altogether, nine major adverse events were observed in this study. One 19-year-old girl died 7 days after transcatheter closure, using an 8 mm Amplatzer pmVSD-O, in hospital because of concomitant cerebral haemorrhage demonstrated by computer tomography. The speculated reason was congenital cerebral vascular malformation. Other major adverse events included two cAVBs requiring pacemaker implantation, two cases of new-onset valvular regurgitation requiring surgical repair or replacement, two device embolizations requiring surgical removal, and one case of bacterial endocarditis. Complete atrioventricular block occurred in a 2-year-old boy 4h after placement of a 6 mm Amplatzer pmVSD-O and also in a 2.5-year-old girl 7 days after placement of a 7 mm Shanghai symmetric pmVSD-O device. Both patients received permanent pacemaker implantation and showed no signs of rhythm recovery. Two patients suffered moderate-to-severe tricuspid valvular regurgitation (180 and 746 days after the procedure using the Amplatzer and Shanghai pmVSD-O, respectively) and underwent open repair. In one patient, the tricuspid valve was encroached upon by the occluder, leading to severe tricuspid valve insufficiency (Figure 3). The device was fully endothelialized and removed. Aortic valvular regurgitation was found in a 13-year-old girl who received a Shanghai pmVSD-O, which was followed up closely. The regurgitation gradually increased, and the girl underwent aortic valve replacement 2.5 years later. Device embolizations were seen in two patients: one occurred instantly, with a 6 mm Amplatzer pmVSD-O embolizing into the pulmonary artery after release, while the other was noted at the 3 month follow-up, with the Shanghai pmVSD-O migrating just to the bifurcation of the iliac artery. Both patients underwent open surgery and recovered well. Another severe adverse event, thrombo-embolization of the ophthalmic artery, was seen in a 6-year-old girl with insufficient aspirin therapy at 95 days after transcatheter VSD closure. Her right eyesight remained poor even after active medication. Altogether, there were five major adverse events in the Shanghai pmVSD-O group (0.69%) and four major adverse events in the Amplatzer pmVSD-O group (3.9%). Fisher's exact test showed significant difference between the two devices (P = 0.017).
Figure 3.
Removal of the perimembranous ventricular septal defect occluder in a 13-year-old boy 746 days post-intervention due to moderate tricuspid insufficiency. (A) The tricuspid valve was encroached upon by the occluder, leading to moderate tricuspid insufficiency; (B) The Shanghai perimembranous ventricular septal defect occluder was explanted, and the perimembranous ventricular septal defect was repaired.
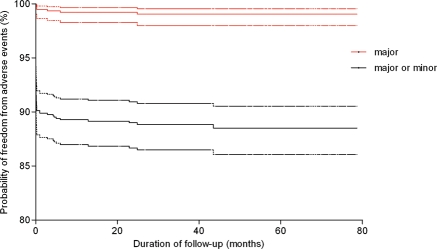
Most adverse events (91.3%) were categorized as minor, including 13 groin hematomas, 2 blood transfusions because of blood loss, 2 device embolizations with successful transcatheter removal, 56 different types of cardiac arrhythmia that required medication, 7 new or increased valvular regurgitation less than two grades, 5 instances of haemolysis requiring medication, and 9 other minor adverse events (Table 3). Kaplan–Meier estimates for freedom from major or minor adverse events are described in Figure 4.
Table 3.
Adverse events
| Events | No. |
|---|---|
| Major adverse events | 9 |
| Death | 1 |
| cAVB that required pacemaker implantation | 2 |
| New-onset valvular regurgitation that required surgical repair | 3 |
| Device embolization that required surgical removal | 2 |
| Thromb-oembolism | 1 |
| Minor adverse events | 94 |
| Haematoma of the groin | 13 |
| Blood transfusion because of blood loss | 2 |
| Device embolization with transcatheter removal | 2 |
| Junctional rhythm | 32 |
| cAVB recovered after medication | 3 |
| LBBB | 6 |
| RBBB | 15 |
| New or increased valvular regurgitation less than two grades | 7 |
| Hemolysis required medication | 5 |
| Others (fever >38.5°C, rash, loss of peripheral pulse.) | 9 |
cAVB, complete atrioventricular block; LBBB, left bundle-branch block; RBBB, right bundle-branch block.
Figure 4.
Kaplan–Meier estimates of outcomes (freedom from major adverse events and mixed major and minor adverse events). Dotted lines are the 95% confidence interval levels.
Except for the adverse events mentioned above, there were no deaths, cardiac perforations, incidents of infective endocarditis, device embolizations or malpositions, thrombus formations, significant arrhythmias, late cAVBs, or other morbidities during a median 37 month follow-up period. All patients were in normal sinus rhythm and had a normal P–R interval, and there was no evidence of conduction disturbance on ECG.
Discussion
Perimembranous ventricular septal defect represents the most common congenital cardiac malformation, accounting for almost one-fifth of all defects, and it is more frequent in Asian countries.14 Our previous study and literature from other major centres showed that both open surgery and the transcatheter technique can achieve extraordinarily good results in closing a pmVSD.19–21 Meanwhile, transcatheter closure has the advantages of reduced psychological impact, less pain, and discomfort due to the procedure, shorter hospital stay, no need for admission to an intensive care unit, faster time to normal activities.1,8,14 Different devices have been used to close pmVSD,2,22–24 among which the Amplatzer pmVSD occluder and similar devices have been shown to cause few complications and yield good results.5,8,11,13,16,25 However, long-term follow-up results with these devices in a sufficient number of patients have been lacking.8,15 This study was designed to evaluate the safety and efficacy of transcatheter closure of pmVSD and its long-term clinical outcomes.
Safety and effectiveness of transcatheter device closure of perimembranous ventricular septal defect
We have presented data from 848 patients who underwent attempted transcatheter device closure of pmVSDs with a median of 37 months of follow-up. In this study, we considered effectiveness in terms of procedural success, complications, hospital stay, and time to return to normal activities. The present study demonstrates an excellent immediate outcome and follow-up results of transcatheter pmVSD device closure using pmVSD occluders. Most pmVSDs were effectively closed, as evidenced by stable device position and significantly decreased interventricular flow by echocardiography at all post-implantation time points.
Adverse events
Complications of transcatheter pmVSD closure in our study were rare and occurred early in the vast majority of patients. Among all 103 adverse events (12.1% of the study population), only 9 major adverse events occurred, with 1 non-procedure-related death. Most adverse events (92 of 103) took place in the early stage of the procedure, and only 11 events were observed during the follow-up period after discharge. This outcome is quite different from previous reports.8,12,15,26 In our cohort, the most common complications associated with transcatheter pmVSD closure were heart rhythm disturbances (58 of 103). Other common adverse events included valvular regurgitation, device embolization, haemolysis, haematoma, and fever. These adverse events were generally manageable and did not outweigh the benefits.
Many types of cardiac arrhythmia may take place during the transcatheter procedure. In our 832 cases, the most frequent arrhythmia was a junctional rhythm, which occurred in 32 patients (3.9%). Bundle branch block and transient AVB occurred in only 2.9% of patients (24 of 832) who underwent device closure. Five patients experienced cAVB, of whom two patients were implanted with permanent pacemakers; the other three patients recovered to normal rhythm after effective treatment with dexamethasone and isoproterenol. cAVB has been reported to be the most significant complication in both the early phase and follow-up period, with an incidence varying from 3.5 to 8.6%.12,27,28 In our study, the cAVB rate was 0.23%, and no permanent cAVBs were experienced by Qin et al.10 using domestic symmetric devices. One large series, reported by Andersen et al.,19 of 2079 open VSD repair patients showed a comparable cAVB rate of <1%. The underlying mechanism may be related to the modification of the domestic symmetric devices by enlarging the thickness of the waist from 2 to 3 mm and using softer nitinol wire mesh to braid the occluder. Nonetheless, careful monitoring of the heart rhythm remains mandatory throughout follow-up due to the severe impact of and the characteristically late onset of cAVB.
Valvular regurgitation was another major consideration in transcatheter closure of pmVSD. Impingement of the occluder on the valve leaflets may cause instant aortic or tricuspid regurgitation by interfering with the chordae tendineae.27,29 Thus, echocardiography (TTE) and angiography are crucial for confirming correct device deployment. Among the 16 failed cases in our study, 12 were attributed to new-onset moderate-to-severe aortic regurgitation (2 due to the inability to pass the VSD, 1 cAVB during the procedure, and another 1 failed with the largest occluder available). We also identified one case of delayed moderate aortic regurgitation (occurring 90 days post-intervention) that required surgical repair, as well as two instances of late moderate tricuspid regurgitation (occurring at 180 and 746 days post-intervention) during follow-up. The mechanism may be associated with improper placement of the occluder on the tricuspid septal leaflets, migration of the occluder, shape memory of nithinol wires, or rupture of the chordae tendineae.27,30
Technical consideration of transcatheter perimembranous ventricular septal defect closure
Perimembranous ventricular septal defect involves the entire membranous septum and adjacent structures and may have many variations. The aortic valve, tricuspid chordae tendineae, atrioventricular node, conduction bundle are all closely related to the pmVSD. Therefore, technical consideration of transcatheter pmVSD closure may be more important than in other types of congenital heart diseases.
First, the inclusion/exclusion criteria for transcatheter pmVSD closure should be strict. Severe aortic valve prolapse, a large VSD with pulmonary hypertension, abnormal tricuspid chordae tendineae origination, small infants with low body weight should be referred for open surgery and excluded from transcatheter intervention. In our centre, more pmVSD patients (n = 1798) were referred directly for surgery after TTE screening during the same study period. It is likely due to this strict screening that the success rate of transcatheter pmVSD closure in our group reached 98.1%.
Second, passing the guide-wire and catheter across the defect is a crucial step in transcatheter pmVSD closure.10 Different types of guide catheters can be useful in passing through the pmVSD, such as the AR-1, 3DRC, right Judkins, and partly cut pigtail catheter. Once the guide-wire passes the defect and is captured in the pulmonary artery or vena cava, caution should be exercised in examining the shape of the arteriovenous circuit. Only when the creation of the arteriovenous loop is not met with any resistance and this loop is straight, without kinks on frontal image fluoroscopy can we proceed with the procedure; otherwise, the tricuspid chordae tendineae may be twisted, and repeating the passing procedure should be mandatory. Before releasing the device, angiography of the ascending aorta is recommended to determine whether aortic valve insufficiency is present. If new-onset aortic valvular regurgitation is identified, either asymmetric devices or abandonment of the procedure should be considered to prevent severe complications.
Third, device selection is another important technical consideration. The Amplatzer pmVSD occluder is an asymmetric device, whereas the Shanghai pmVSD-O is symmetrical. In our study, most pmVSDs could be successfully closed with the domestic symmetrical double-disk device. In pmVSDs with a short subaortic rim or when aortic valve insufficiency was seen on the aortogram before the release of the device, an asymmetrical Amplatzer pmVSD occluder was selected. In addition, over-sized devices should be avoided to prevent the occurrence of cAVB.
Lastly, transoesophageal echocardiography (TEE) is the imaging modality most frequently used to guide transcatheter pmVSD closure according to previous studies.6,8,13,14 In our group, TEE was used in the early stages of the study period and was gradually replaced by TTE. Although TEE provides clearer images and is more accurate, it extends the procedural time and is more complicated. We used TTE combined with angiography to guide transcatheter pmVSD closure in our study, which remarkably reduced the whole procedural time.
Limitations
Although our study demonstrated significant benefits of transcatheter pmVSD closure, it nevertheless had some limitations. First, this was a single-centre, non-randomized study, and the experiences of one centre may not be universally representative. Second, all operators in this study were very experienced, having each performed >200 cases. We admit that only in very experienced hands can these techniques be carried out safely and can the complications be managed properly.26 Third, more pmVSD patients (1798) were referred for surgery directly after TTE screening in our institute and were not considered due to the strict inclusion/exclusion criteria; this may demonstrate the importance of patient selection in assuring the safety and effectiveness of this transcatheter technique.
Conclusions
This study has proved that transcatheter device closure is an effective method in treating pmVSD patients with excellent results in experienced hands. The success rate was high, and the long-term follow-up result was favourable. Adverse events were rare and were generally manageable. The transcatheter approach provides a less-invasive alternative to open surgery and may become the treatment of choice for selected patients with pmVSD.
Funding
Funding to pay the Open Access publication charges for this article was provided by the authors of this article.
Conflict of interest: none declared.
References
- 1.Minette MS, Sahn DJ. Ventricular septal defects. Circulation. 2006;114:2190–2197. doi: 10.1161/CIRCULATIONAHA.106.618124. doi:10.1161/CIRCULATIONAHA.106.618124. [DOI] [PubMed] [Google Scholar]
- 2.Lock JE, Block PC, McKay RG, Baim DS, Keane JF. Transcatheter closure of ventricular septal defects. Circulation. 1988;78:361–368. doi: 10.1161/01.cir.78.2.361. [DOI] [PubMed] [Google Scholar]
- 3.Bridges ND, Perry SB, Keane JF, Goldstein SA, Mandell V, Mayer JE, Jr, Jonas RA, Casteneda AR, Lock JE. Preoperative transcatheter closure of congenital muscular ventricular septal defects. N Engl J Med. 1991;324:1312–1317. doi: 10.1056/NEJM199105093241903. [DOI] [PubMed] [Google Scholar]
- 4.Kalra GS, Verma PK, Dhall A, Singh S, Arora R. Transcatheter device closure of ventricular septal defects: immediate results and intermediate-term follow-up. Am Heart J. 1999;138:339–344. doi: 10.1016/s0002-8703(99)70122-5. doi:10.1016/S0002-8703(99)70122-5. [DOI] [PubMed] [Google Scholar]
- 5.Pedra CA, Pedra SR, Esteves CA, Pontes SC, Jr, Braga SL, Arrieta SR, Santana MV, Fontes VF, Masura J. Percutaneous closure of perimembranous ventricular septal defects with the Amplatzer device: technical and morphological considerations. Catheter Cardiovasc Interv. 2004;61:403–410. doi: 10.1002/ccd.10797. doi:10.1002/ccd.10797. [DOI] [PubMed] [Google Scholar]
- 6.Butera G, Carminati M, Chessa M, Piazza L, Abella R, Negura DG, Giamberti A, Claudio B, Micheletti A, Tammam Y, Frigiola A. Percutaneous closure of ventricular septal defects in children aged <12: early and mid-term results. Eur Heart J. 2006;27:2889–2895. doi: 10.1093/eurheartj/ehl340. doi:10.1093/eurheartj/ehl340. [DOI] [PubMed] [Google Scholar]
- 7.Holzer R, de Giovanni J, Walsh KP, Tometzki A, Goh T, Hakim F, Zabal C, de Lezo JS, Cao QL, Hijazi ZM. Transcatheter closure of perimembranous ventricular septal defects using the amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv. 2006;68:620–628. doi: 10.1002/ccd.20659. doi:10.1002/ccd.20659. [DOI] [PubMed] [Google Scholar]
- 8.Thanopoulos BD, Rigby ML, Karanasios E, Stefanadis C, Blom N, Ottenkamp J, Zarayelyan A. Transcatheter closure of perimembranous ventricular septal defects in infants and children using the Amplatzer perimembranous ventricular septal defect occluder. Am J Cardiol. 2007;99:984–989. doi: 10.1016/j.amjcard.2006.10.062. doi:10.1016/j.amjcard.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 9.Xunmin C, Shisen J, Jianbin G, Haidong W, Lijun W. Comparison of results and complications of surgical and Amplatzer device closure of perimembranous ventricular septal defects. Int J Cardiol. 2007;120:28–31. doi: 10.1016/j.ijcard.2006.03.092. doi:10.1016/j.ijcard.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 10.Qin Y, Chen J, Zhao X, Liao D, Mu R, Wang S, Wu H, Guo H. Transcatheter closure of perimembranous ventricular septal defect using a modified double-disk occluder. Am J Cardiol. 2008;101:1781–1786. doi: 10.1016/j.amjcard.2008.02.069. doi:10.1016/j.amjcard.2008.02.069. [DOI] [PubMed] [Google Scholar]
- 11.Hijazi ZM, Hakim F, Haweleh AA, Madani A, Tarawna W, Hiari A, Cao QL. Catheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: initial clinical experience. Catheter Cardiovasc Interv. 2002;56:508–515. doi: 10.1002/ccd.10292. doi:10.1002/ccd.10292. [DOI] [PubMed] [Google Scholar]
- 12.Knauth AL, Lock JE, Perry SB, McElhinney DB, Gauvreau K, Landzberg MJ, Rome JJ, Hellenbrand WE, Ruiz CE, Jenkins KJ. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation. 2004;110:501–507. doi: 10.1161/01.CIR.0000137116.12176.A6. doi:10.1161/01.CIR.0000137116.12176.A6. [DOI] [PubMed] [Google Scholar]
- 13.Fu YC, Bass J, Amin Z, Radtke W, Cheatham JP, Hellenbrand WE, Balzer D, Cao QL, Hijazi ZM. Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the U.S. phase I trial. J Am Coll Cardiol. 2006;47:319–325. doi: 10.1016/j.jacc.2005.09.028. doi:10.1016/j.jacc.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Butera G, Chessa M, Carminati M. Percutaneous closure of ventricular septal defects. Cardiol Young. 2007;17:243–253. doi: 10.1017/S1047951107000431. [DOI] [PubMed] [Google Scholar]
- 15.Chessa M, Butera G, Negura D, Bussadori C, Giamberti A, Fesslova V, Carminati M. Transcatheter closure of congenital ventricular septal defects in adult: mid-term results and complications. Int J Cardiol. 2009;133:70–73. doi: 10.1016/j.ijcard.2007.11.098. doi:10.1016/j.ijcard.2007.11.098. [DOI] [PubMed] [Google Scholar]
- 16.Thanopoulos BD, Tsaousis GS, Konstadopoulou GN, Zarayelyan AG. Transcatheter closure of muscular ventricular septal defects with the amplatzer ventricular septal defect occluder: initial clinical applications in children. J Am Coll Cardiol. 1999;33:1395–1399. doi: 10.1016/s0735-1097(99)00011-x. doi:10.1016/S0735-1097(99)00011-X. [DOI] [PubMed] [Google Scholar]
- 17.Carminati M, Butera G, Chessa M, Drago M, Negura D, Piazza L. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardio. 2005;96:52–58. doi: 10.1016/j.amjcard.2005.09.068. doi:10.1016/j.amjcard.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Yi D, Zuo J, Yang L, Li J, Zhang J, Sun L, Cui H. Clinical follow-up of transcatheter closure of perimembranous ventricular septal defects using domestic symmetric double-disk occluders. Chinese J Interv Cardiol. 2008;16:124–128. [Google Scholar]
- 19.Andersen H, de Leval MR, Tsang VT, Elliott MJ, Anderson RH, Cook AC. Is complete heart block after surgical closure of ventricular septum defects still an Issue? Ann Thorc Surg. 2006;82:948–956. doi: 10.1016/j.athoracsur.2006.04.030. doi:10.1016/j.athoracsur.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Q, Zuo J, Yang J, Wang H, Yu S, Yi D. A comparative study: early results and complications of percutaneous and surgical closure of ventricular septal defect. Cardiology. 2009:238–243. doi: 10.1159/000232405. doi:10.1159/000232405. [DOI] [PubMed] [Google Scholar]
- 21.Backer CL. Ventricular septal defect closure: what is the role for transcatheter closure? Cardiology. 2009;114:235–237. doi: 10.1159/000232404. [DOI] [PubMed] [Google Scholar]
- 22.Janorkar S, Goh T, Wilkinson J. Transcatheter closure of ventricular septal defects using the Rashkind device: initial experience. Catheter Cardiovasc Interv. 1999;46:43–48. doi: 10.1002/(SICI)1522-726X(199901)46:1<43::AID-CCD12>3.0.CO;2-T. doi:10.1002/(SICI)1522-726X(199901)46:1<43::AID-CCD12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Vogel M, Rigby ML, Shore D. Perforation of the right aortic valve cusp: complication of ventricular septal defect closure with a modified Rashkind umbrella. Pediatr Cardiol. 1996;17:416–418. doi: 10.1007/s002469900093. doi:10.1007/s002469900093. [DOI] [PubMed] [Google Scholar]
- 24.Gu X, Han YM, Titus JL, Amin Z, Berry JM, Kong H, Rickers C, Urness M, Bass JL. Transcatheter closure of membranous ventricular septal defects with a new nitinol prosthesis in a natural swine model. Catheter Cardiovasc Interv. 2000;50:502–509. doi: 10.1002/1522-726x(200008)50:4<502::aid-ccd29>3.0.co;2-8. doi:10.1002/1522-726X(200008)50:4<502::AID-CCD29>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Hijazi ZM, Hakim F, Al-Fadley F, Abdelhamid J, Cao QL. Transcatheter closure of single muscular ventricular septal defects using the amplatzer muscular VSD occluder: initial results and technical considerations. Catheter Cardiovasc Interv. 2000;49:167–172. doi: 10.1002/(sici)1522-726x(200002)49:2<167::aid-ccd11>3.0.co;2-s. doi:10.1002/(SICI)1522-726X(200002)49:2<167::AID-CCD11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, Abella R, Giamberti A, Frigiola A. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol. 2007;50:1189–1195. doi: 10.1016/j.jacc.2007.03.068. doi:10.1016/j.jacc.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan ID. Transcatheter closure of perimembranous ventricular septal defect: is the risk of heart block too high a price? Heart. 2007;93:284–286. doi: 10.1136/hrt.2006.103671. doi:10.1136/hrt.2006.103671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carminati M, Butera G, Chessa M, De Giovanni J, Fisher G, Gewillig M, Peuster M, Piechaud JF, Santoro G, Sievert H, Spadoni I, Walsh K. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. Eur Heart J. 2007;28:2361–2368. doi: 10.1093/eurheartj/ehm314. doi:10.1093/eurheartj/ehm314. [DOI] [PubMed] [Google Scholar]
- 29.Michel-Behnke I, Le TP, Waldecker B, Akintuerk H, Valeske K, Schranz D. Percutaneous closure of congenital and acquired ventricular septal defects–considerations on selection of the occlusion device. J Interv Cardiol. 2005;18:89–99. doi: 10.1111/j.1540-8183.2005.04051.x. doi:10.1111/j.1540-8183.2005.04051.x. [DOI] [PubMed] [Google Scholar]
- 30.Szkutnik M, Qureshi SA, Kusa J, Rosenthal E, Bialkowski J. Use of the Amplatzer muscular ventricular septal defect occluder for closure of perimembranous ventricular septal defects. Heart. 2007;93:355–358. doi: 10.1136/hrt.2006.096321. doi:10.1136/hrt.2006.096321. [DOI] [PMC free article] [PubMed] [Google Scholar]