Abstract
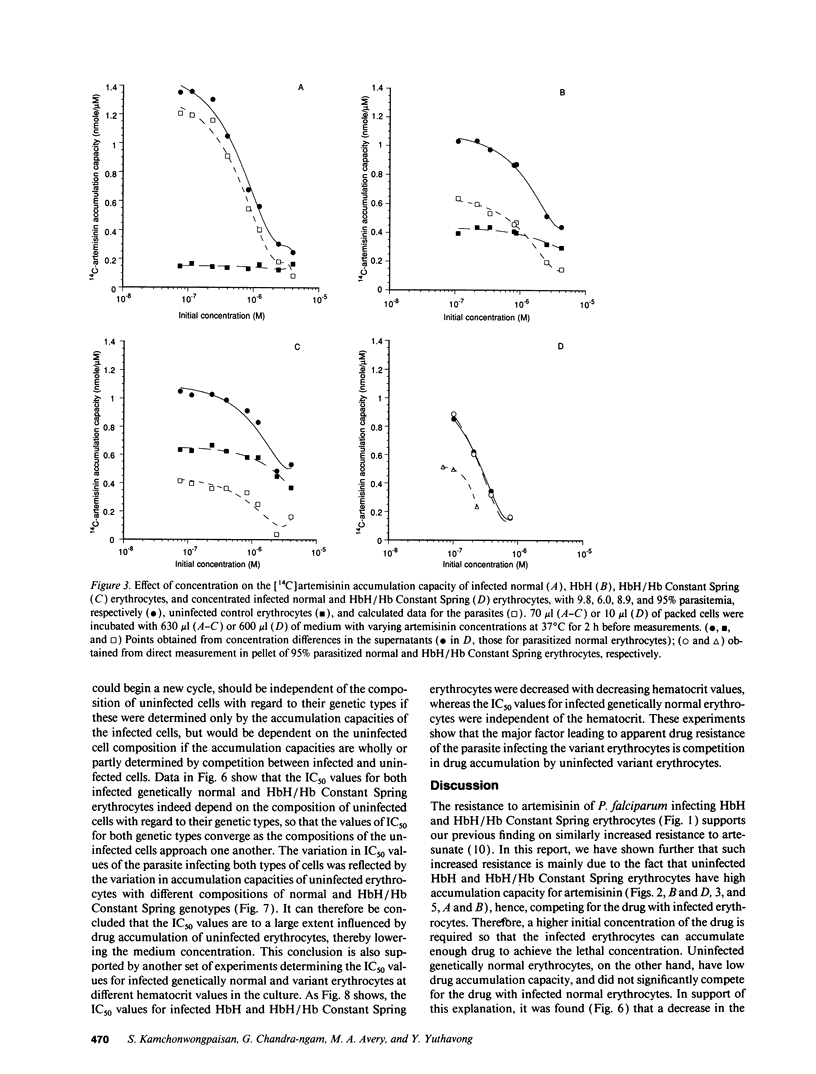
Plasmodium falciparum infecting hemoglobin (Hb)H and/or Hb Constant Spring erythrocytes has higher resistance to artemisinin in vitro than when infecting normal erythrocytes. This is due to low drug accumulation of infected erythrocytes resulting from competition with uninfected variant erythrocytes, which have a higher accumulation capacity than genetically normal cells. Drug accumulation of the parasite was shown to be saturable and dependent on metabolic energy. The 50% inhibitory concentrations (IC50's) for the parasite in HbH/Hb Constant Spring erythrocytes were decreased when normal erythrocytes were added to the infected cells, and correspondingly, the IC50's in normal erythrocytes were increased when HbH/Hb Constant Spring erythrocytes were added to the infected cells. The changes of IC50 corresponded to the variation in drug accumulation of mixtures of normal and variant erythrocytes of different compositions. The IC50's for the parasite in variant erythrocytes were also greatly decreased when the hematocrit of the culture was lowered, while the IC50's in normal erythrocytes were independent of the hematocrit. The increase in IC50 values for the parasites infecting variant erythrocytes was also related to the decrease in parasite accumulation, indicating that drug accumulation capacity of the parasite also has a role in determining drug sensitivity. Artemisinin sensitivity therefore is determined by its accessibility to the parasite, which is decreased in infected variant erythrocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejkriengkraikhul P., Santiyanont R., Wilairat P. A rapid method for concentrating schizont-infected red cells of Plasmodium falciparum. Southeast Asian J Trop Med Public Health. 1982 Dec;13(4):663–665. [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. J. Oxidant damage mediates variant red cell resistance to malaria. Nature. 1979 Jul 19;280(5719):245–247. doi: 10.1038/280245a0. [DOI] [PubMed] [Google Scholar]
- Gu H. M., Warhurst D. C., Peters W. Uptake of [3H] dihydroartemisinine by erythrocytes infected with Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1984;78(2):265–270. doi: 10.1016/0035-9203(84)90296-7. [DOI] [PubMed] [Google Scholar]
- Ifediba T. C., Stern A., Ibrahim A., Rieder R. F. Plasmodium falciparum in vitro: diminished growth in hemoglobin H disease erythrocytes. Blood. 1985 Feb;65(2):452–455. [PubMed] [Google Scholar]
- Klayman D. L. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985 May 31;228(4703):1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Krungkrai S. R., Yuthavong Y. The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress. Trans R Soc Trop Med Hyg. 1987;81(5):710–714. doi: 10.1016/0035-9203(87)90003-4. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Levander O. A., Ager A. L., Jr, Morris V. C., May R. G. Qinghaosu, dietary vitamin E, selenium, and cod-liver oil: effect on the susceptibility of mice to the malarial parasite Plasmodium yoelii. Am J Clin Nutr. 1989 Aug;50(2):346–352. doi: 10.1093/ajcn/50.2.346. [DOI] [PubMed] [Google Scholar]
- Luzzi G. A., Merry A. H., Newbold C. I., Marsh K., Pasvol G., Weatherall D. J. Surface antigen expression on Plasmodium falciparum-infected erythrocytes is modified in alpha- and beta-thalassemia. J Exp Med. 1991 Apr 1;173(4):785–791. doi: 10.1084/jem.173.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshnick S. R., Thomas A., Ranz A., Xu C. M., Pan H. Z. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol Biochem Parasitol. 1991 Dec;49(2):181–189. doi: 10.1016/0166-6851(91)90062-b. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Roth E. F., Jr Malaria and red cell genetic defects. Blood. 1989 Sep;74(4):1213–1221. [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Bradley T. B., Blumberg W. E. Role of haemichromes in the formation of inclusion bodies in haemoglobin H disease. Nature. 1969 Apr 19;222(5190):248–250. doi: 10.1038/222248a0. [DOI] [PubMed] [Google Scholar]
- Scott M. D., Meshnick S. R., Williams R. A., Chiu D. T., Pan H. C., Lubin B. H., Kuypers F. A. Qinghaosu-mediated oxidation in normal and abnormal erythrocytes. J Lab Clin Med. 1989 Oct;114(4):401–406. [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wasi P., Pootrakul P., Fucharoen S., Winichagoon P., Wilairat P., Promboon A. Thalassemia in southeast Asia: determination of different degrees of severity of anemia in thalassemia. Ann N Y Acad Sci. 1985;445:119–126. doi: 10.1111/j.1749-6632.1985.tb17181.x. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J. Common genetic disorders of the red cell and the 'malaria hypothesis'. Ann Trop Med Parasitol. 1987 Oct;81(5):539–548. doi: 10.1080/00034983.1987.11812155. [DOI] [PubMed] [Google Scholar]
- Wernsdorfer W. H. The development and spread of drug-resistant malaria. Parasitol Today. 1991 Nov;7(11):297–303. doi: 10.1016/0169-4758(91)90262-m. [DOI] [PubMed] [Google Scholar]
- Wozencraft A. O., Dockrell H. M., Taverne J., Targett G. A., Playfair J. H. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984 Feb;43(2):664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuthavong Y., Butthep P., Bunyaratvej A., Fucharoen S. Decreased sensitivity of artesunate and chloroquine of Plasmodium falciparum infecting hemoglobin H and/or hemoglobin constant spring erythrocytes. J Clin Invest. 1989 Feb;83(2):502–505. doi: 10.1172/JCI113910. [DOI] [PMC free article] [PubMed] [Google Scholar]