Abstract
The activation of heterotrimeric G protein signaling is a key feature in the pathophysiology of polycystic kidney diseases (PKD). In this study, we report abnormal overexpression of activator of G protein signaling 3 (AGS3), a receptor-independent regulator of heterotrimeric G proteins, in rodents and humans with both autosomal recessive and autosomal dominant PKD. Increased AGS3 expression correlated with kidney size, which is an index of severity of cystic kidney disease. AGS3 expression localized exclusively to distal tubular segments in both normal and cystic kidneys. Short hairpin RNA–induced knockdown of endogenous AGS3 protein significantly reduced proliferation of cystic renal epithelial cells by 26 ± 2% (P < 0.001) compared with vehicle-treated and control short hairpin RNA–expressing epithelial cells. In summary, this study suggests a relationship between aberrantly increased AGS3 expression in renal tubular epithelia affected by PKD and epithelial cell proliferation. AGS3 may play a receptor-independent role to regulate Gα subunit function and control epithelial cell function in PKD.
Polycystic kidney disease (PKD) is one of the most common genetic diseases found in humans.1 The genetic mutation(s) associated with PKD results in fundamental changes in the signal processing of multiple extrinsic cues, leading to abnormal proliferation, fluid secretion, cell polarity and differentiation, and proliferation of the epithelial cells within the kidney and other organs.1 Heterotrimeric G proteins are key components in the control and integration of the signaling pathways activated in the pathogenesis of fluid-filled cyst or ectatic duct formation in PKD. The traditional activation of intracellular signal transduction pathways after hormonal or mechanical stimulation of target cells was believed to be exclusively through cell surface G protein–coupled receptors (GPCR). In fact, polycystin 1, the major causative cystic protein in autosomal dominant PKD (ADPKD), is considered to behave as a GPCR and regulate heterotrimeric G protein signaling.2–4 In this report, however, we identified a novel GPCR-independent mechanism to regulate heterotrimeric G protein function in renal epithelial cells through the actions of activator of G protein signaling 3 (AGS3). AGS3 was originally discovered using a yeast-based screening system5,6 and classified as a group II guanine dissociation inhibitor because of its ability to bind preferentially to inactive Gαi/o subunits complexed with guanine dinucleotide phosphate (GDP) at multiple G protein regulatory or GoLoco motif repeats.7 The biologic role of AGS3 has been studied in lower invertebrates and nonrenal mammalian organs; AGS3 regulates the orientation of the mitotic spindle, cAMP production, membrane protein transport, and asymmetric cell division.7 These functions have relevance to the pathogenesis of PKD, so we initiated this study to investigate whether AGS3 could be involved in mediating increased proliferation in cystic renal tubular epithelia.
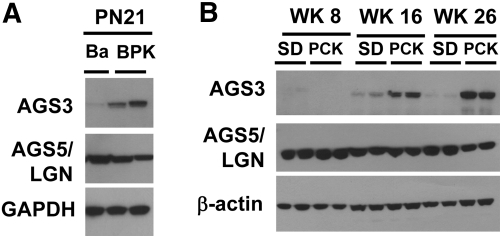
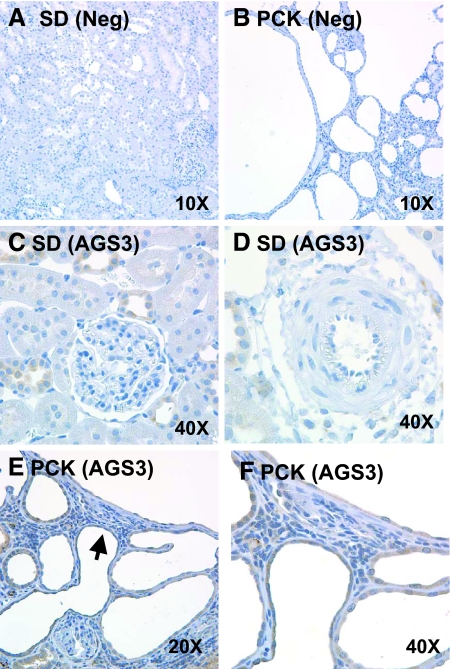
Immunoblot analysis demonstrated a marked increase in renal AGS3 protein expression in two distinct murine models of autosomal recessive PKD (ARPKD) compared with their genetic control strains (Figure 1). Increased renal AGS3 protein was detected in the Balb/c polycystic kidney (BPK) mouse at postnatal day 21, which exhibits an advanced stage of cystogenesis, compared with Balb/c mice (Ba; Figure 1A). Similarly, renal AGS3 mRNA (Supplemental Figure 1) and protein (Figure 1B) were observed to increase temporally from weeks 8 to 26 in the polycystic kidney (PCK) rats versus Sprague-Dawley (SD) rats. The low to absent expression of AGS3 in normal rat kidneys is consistent with previously published findings.8–10 This may be attributed to the exclusive localization to the distal tubular epithelial cells (Figure 2), which compose only a small percentage of the total renal cell population in the kidney. No visible expression was detected in the proximal tubules (Figure 2C), glomeruli (Figure 2C), and blood vessels (Figure 2D). It is interesting to note that the liver, which is the most prevalent extrarenal organ affected by ARPKD, showed increased AGS3 expression with exclusive localization to the biliary epithelial cells (Supplemental Figure 2).
Figure 1.
Increased expression of AGS3 protein in the kidneys from murine models of ARPKD. (A and B) Representative immunoblot analysis for AGS3 and AGS5/LGN expression in mouse (A) and rat kidneys (B). (A) Ba and BPK rat kidney lysates (n = 3 to 4 kidneys per group) at postnatal day 21 (PN21) are examined. (B) SD and PCK rat kidney lysates (n = 4 to 6 rat samples per time point and group) at postnatal weeks 8 (WK8), 16 (WK16), and 26 (WK26) are examined. From these findings, the PCK rat kidneys exhibit a temporal increase in the AGS3 protein between weeks 8 and 26. No change in the expression of AGS5/LGN is determined. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; A) or β-actin (B) is used to ensure equal loading of the protein samples.
Figure 2.
Immunolocalization of AGS3 in ARPKD rat kidneys. AGS3 localization is performed in the kidneys of SD (A, C, and D) and PCK (B, E, and F) rats. Affinity-purified AGS3 antibody (pep32) is incubated at a 1:250 dilution on kidney sections from SD (C and D) and PCK (E and F) rats. As a negative control, SD (A) and PCK (B) rat kidneys are incubated with the primary AGS3 antibody in the presence of the competing AGS3 peptide conjugate or normal rodent serum (data not shown). The brown diaminobenzidine staining demonstrates the specific localization of the AGS3 protein within distinct cell types in the kidney. Sections are counterstained with hematoxylin. Arrowhead in E signifies the magnified view of the area in F. n = 4 kidneys per group.
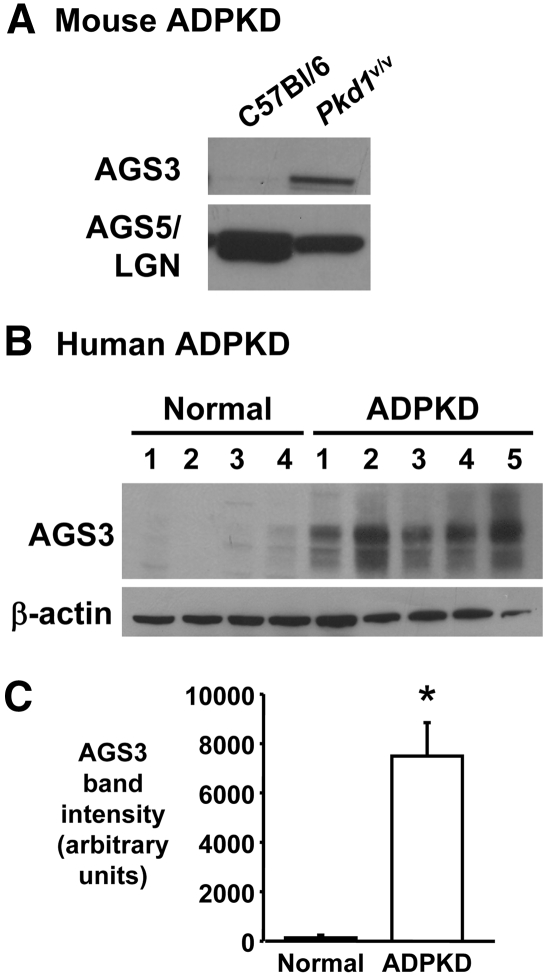
Similar increases in AGS3 protein expression were noted in ADPKD models (Figure 3). The Pkd1v/v mouse kidney exhibited higher expression of AGS3 compared with the normal C57Bl/6 mouse kidney at postnatal day 14 (Figure 3A). Moreover, robust expression of AGS3 was observed in human ADPKD kidneys (n = 5 kidneys) with minimal, if any, expression detected in normal human kidneys (n = 4 kidneys; Figure 3, B and C). The overexpression of AGS3 in the kidney seemed to be specific to PKD, because other hypertensive rat models with (Dahl S) or without (SHR) renal damage expressed little to no expression of AGS3 protein (Supplemental Figure 3). The expression of AGS5/LGN (Figure 1 and Supplemental Figure 3), a closely related AGS3 homolog (approximately 60% amino acid sequence identity), was found to be unchanged among the various rat kidney groups.
Figure 3.
Increased renal AGS3 expression in a mouse model and humans with ADPKD. (A and B) Representative immunoblot analysis is performed in kidneys obtained from Pkd1v/v hypomorphic mouse (A) and humans with ADPKD kidneys (B). (A) Normal C57Bl/6 and cystic Pkd1v/v hypomorph kidneys are harvested at postnatal day 14 and analyzed for AGS3 and AGS5/LGN protein expression. The Pkd1v/v mouse model of ADPKD exhibits a point mutation in the Pkd1 gene, resulting in inefficient cleavage of polycystin 1 (PC1), which is necessary for optimal activity of PC1.28 (B) Normal human kidneys (n = 4) and human ADPKD kidneys (n = 5) are analyzed for the expression of AGS3. (C) Densitometry of the human AGS3 bands are analyzed (P < 0.001). β-actin is used to determine equal loading of the lanes.
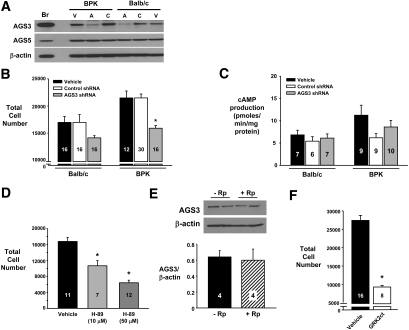
To investigate the relationship between AGS3 with epithelial cell proliferation, we generated lentiviral vectors expressing short hairpin RNA (shRNA) molecules targeted to AGS3 and evaluated their efficacy using an AGS3 overexpression system derived from a porcine renal epithelial LLC-PK1 cell line (Supplemental Figure 4). Using the two most efficient AGS3 shRNA-expressing lentiviral vectors (3 and 4), as determined in Supplemental Figure 4, endogenous AGS3 protein was specifically decreased in only the renal epithelial Ba and BPK cells expressing the combined AGS3 shRNA (denoted as “A” in Figure 4A). The decreased AGS3 protein was associated with a significant reduction (P < 0.001) by 26 ± 2% in the proliferation of the BPK epithelial cells overexpressing the AGS3 shRNA versus the control shRNA. No significant change in either AGS3 protein expression (Figure 4A) or cell proliferation (P > 0.05; Figure 4B) was detected in the control shRNA-expressing cells (denoted as “C”) compared with the vehicle-treated cells (denoted as “V” in Figure 4A).
Figure 4.
AGS3 promotes increased epithelial cell number in a Gβγ-dependent pathway. Ba and BPK epithelial cells are transduced at a multiplicity of infection of 40 with lentiviral vectors expressing shRNA (control or specifically targeted to AGS3) or GRK2ct cDNA. (A through C) For the AGS3 knockdown experiments, the two most effective AGS3 shRNAs (see Supplemental Figure 4) are used for the Western blot (A), cell proliferation assay (B), and cAMP assay (C). (A) Western blot analysis demonstrates a specific reduction in AGS3 expression after combined AGS3 shRNA knockdown (A) compared with control shRNA (C) or vehicle-treated cells (V). No off-target knockdown of AGS5/LGN or β-actin is detected using the AGS3 shRNA. Br, brain (positive control); V, vehicle; A, AGS3 shRNA; C, control (scrambled) shRNA. (B) The genetically modified Ba and BPK cells are aliquotted into 96-well plates and examined for cell number 24 hours later by CyQuant fluorescence assay. *P < 0.001, significant difference between vehicle-treated cells. (C) cAMP levels are examined by ELISA using cell lysates obtained from the genetically modified Ba and BPK cells treated with vehicle or transduced with lentiviral vectors expressing control or specific AGS3 shRNA (n = 6 to 10 samples per group). (D) BPK epithelial cells are incubated with H-89 (10 and 50 μM) for 24 hours before determination of cell numbers by CyQuant fluorescence assay. (E) BPK epithelial cells are incubated with or without Rp-adenosine-3′,5′-monophosphorothioate (20 μM), a specific PKA inhibitor, for 5 hours. Cells are collected for protein isolation to determine the expression of AGS3 by Western blot analysis. Densitometry is performed to determine the band intensities. (F) BPK epithelial cells genetically modified to overexpress GRK2ct are aliquotted into a 96-well plate; 24 hours later, the cell proliferation is measured using the CyQuant fluorescence assay and compared with vehicle-treated BPK cells. *P < 0.001, significant difference between vehicle-treated cells. Numbers of samples in each group are shown in each graph.
The mechanism(s) by which AGS3 regulates heterotrimeric G protein activity in the renal epithelia has yet to be elucidated. AGS3 competes with free Gβγ subunits for binding to Gαi/o-GDP subunits, and this would provide the opportunity for AGS3 to regulate downstream signal transduction pathways by either inhibiting Gαi- or activating Gβγ-dependent pathways. Gαi subunits directly inhibit adenylyl cyclase (AC) activity.11 Elevated levels of cAMP are a common observance in many animal models of PKD,12 and several currently active clinical trials are based on reducing cyst formation through the targeting of AC-dependent pathways.12 Thus, we initially postulated that AGS3 in renal epithelial cells may play an important role in regulating the activation of cAMP/PKA pathways. In our study, however, we did not observe a relationship between endogenous AGS3 expression with cAMP production (Figure 4C). Reductions in the levels of AGS3 did not result in any difference in cAMP levels in either the presence (data not shown) or the absence (Figure 4C) of a phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine. The inability of AGS3 to regulate cAMP production is consistent with a previous study by Sato et al.13 that used transfected cell lines overexpressing AGS3. Conversely, there is evidence of the activation of cAMP/PKA pathways in neuronal cells as a result of AGS3-mediated effects.14,15 After drug withdrawal, AGS3 was found to potentiate the activity of AC and cAMP production as a result of a PKA-dependent increase of AGS3 expression.14 In our study, we measured a dosage-dependent reduction in cell number after inhibition with a specific PKA inhibitor (H-89; Figure 4D), which suggests that basal epithelial cell activity of cAMP/PKA pathways can mediate proliferation. Unlike the findings in the neuronal cells,14 we did not observe an increase in AGS3 protein expression after inhibition with another specific PKA antagonist, Rp-adenosine-3′,5′-monophosphorothioate (Figure 4E). The lack of a regulatory effect by AGS3 on the cAMP/PKA pathway may be attributed to low basal AC activity in the renal epithelia using serum-free cell culture conditions. It is likely that additional stimuli, which are normally available in the mammalian circulation, may be required to prime the renal epithelial cells to uncover the AGS3-dependent role on cAMP/PKA similar to the previous neuronal cell studies.14,15
Instead of altering cAMP production, we examined whether the sequestration of Gαi subunits by AGS3 prevented the re-association with free Gβγ dimers to activate Gβγ-mediated downstream signaling cascades, as observed in previous behavior modification15,16 and mammalian brain development studies.17 To block the activity of Gβγ dimers in renal epithelia, the C-terminal region of the bovine GPCR kinase 2 (GRK2ct) was overexpressed in the BPK renal epithelia using lentiviral vectors. The GRK2ct contains a pleckstrin homology domain that binds free Gβγ subunits to regulate its signaling activity.18 The cell proliferation in the GRK2ct-expressing cells was significantly reduced (P < 0.001) by 68 ± 1% compared with the vehicle-treated BPK cells (Figure 4F). The AGS3 mediated Gβγ-dependent mechanism to promote cell proliferation has not been well defined, but there may be direct interactions with AC to promote cAMP production,14 mitogen-activated protein kinase pathways19 or possibly through the regulation of mitotic spindle orientation.17 Sanada et al.17 showed that either reductions in endogenous AGS3 or overexpression of GRK2ct in neural progenitor cells shifted the axis of cell cleavage from a horizontal to vertical plane. From this study, the orientation of the progenitor cell divisions was correlated with cell fate (i.e., there was increased neuronal fate with a concomitant loss of the progenitor cell pool).17 At the present time, there is an emerging albeit controversial role for the regulation of the mitotic spindle in PKD. Dysregulation of the mitotic spindle in renal epithelial cells is believed to be associated with promoting aberrant epithelial cell proliferation, tubular dilation, and cyst formation.20–23 On a subcellular level, AGS3 was found to co-localize with Gαi3 at the plasma membrane and mitotic spindle poles (Supplemental Figure 5), which could suggest a potential role for AGS3 in mitotic spindle pole orientation. Our findings in conjunction with previously published studies in other organs and invertebrate systems suggest that there is a unique role for AGS3 to regulate proliferative signaling cascades through a Gβγ-dependent manner.
In summary, our study provides the first evidence demonstrating aberrant expression of AGS3, a novel receptor-independent regulator of heterotrimeric G protein, in the kidneys from multiple mammalian models of ARPKD and ADPKD. Our data suggest a novel mechanism by which AGS3-Gα signaling cassettes may be involved in activating noncanonical G protein pathways to promote renal epithelial cell proliferation. AGS3 may play a fundamentally important role in the integration of multiple converging signaling pathways to transition normal renal tubular epithelia toward a pathologically cystic disease phenotype. Our study may open the door to new investigations into the development of anticystic drugs to target AGS3-Gα complexes to treat PKD and possibly other proliferative diseases.
Concise Methods
Animal Models and Humans with PKD
We obtained kidneys from three distinct animal models and humans with PKD for our study. To study models of ARPKD, we used the BPK mice and PCK rats. To examine animal and humans with ADPKD, we studied the Pkd1V/V knock-in mouse and humans. The details regarding each of the models of PKD are described next.
BPK Mouse
Kidneys were harvested from the BPK mouse × ImmortoMouse at postnatal day 21 as described previously by our laboratory.24 Detailed description of the BPK mouse has been reviewed elsewhere.25 For control mice, Ba × ImmortoMouse mouse kidneys were harvested at postnatal day 21. The BPK and Ba mouse kidneys were used to isolate the conditionally immortalized renal epithelial cells as described by Sweeney et al.26
Polycystic Kidney (PCK) Rats
Rats were purchased from Charles River Laboratories (Benton Harbor, MA) at postnatal week 4 and allowed ad libitum access to food and water. Kidneys and livers were harvested at weeks 8, 16 and 26. The PCK rat is the only orthologous animal model that resembles human ARPKD, and is caused by a splicing mutation (IVS35-2A3T) in Pkhd1 leading to the skipping of exon 36.27 The PCK rat is a more indolent model of ARPKD compared with the BPK mouse.
Pkd1V/V Knock-in Mice
Normal C57Bl/6 and Pkd1V/V knock-in C57Bl/6 mouse kidneys were harvested at postnatal day 14, and kidney lysates were isolated as described previously by Yu et al.28
Human ADPKD Kidneys
Discarded de-identified kidney biospecimens from normal humans and humans with ADPKD phenotype were obtained from Dr. Patricia D. Wilson under institutional review board–approved and Health Insurance Portability and Accountability Act–compliant protocols.
Renal Protein Isolation and AGS3 Immunoblot Analysis
Protein homogenates were isolated from normal and cystic kidneys from multiple animal models and humans with PKD. To examine the effect of PKA inhibition on endogenous AGS3 expression in vitro, we added Rp-adenosine-3′,5′-monophosphorothioate to the renal epithelial cells at a concentration of 20 μM for 5 hours before cell harvesting. AGS3 expression was determined using standard Western blot techniques. The primary antibodies for AGS3 and AGS5/LGN were provided by Dr. S.M. Lanier (Medical University of South Carolina, Charleston, SC), which have been extensively used and characterized.8,10,13,29 All other primary antibodies were obtained from Sigma (St. Louis, MO): Mouse anti–glyceraldehyde-phosphate dehydrogenase (1:4000 dilution; cat. no. G8795); mouse anti–β-actin (1:4000 dilution; cat. no. A5441), and mouse β-tubulin (clone tub2.1). Densitometry was performed to quantify band intensity using ImageJ software (National Institutes of Health, Bethesda, MD).
Reverse Transcription Quantitative PCR for AGS3
Total RNA was extracted from SD and PCK rat kidneys using TRIzol reagent, and reverse transcription quantitative PCR was performed using a slight modification in the PCR amplification conditions with SYBR Green reagent.24 Specific primers used for the PCR assay were as follows: sense 5′-TCCAGGATTGATGACCAGCG-3′ and antisense 5′-CACTGGCCTCTTGGTCTGGA-3′ (Integrated DNA Technologies, Coralville, IA). The AGS3 amplicon was normalized to 18S RNA using the ΔΔCT method. PCR products were sequenced to verify their authenticity (SeqWright, Houston, TX).
Immunohistochemical Localization of AGS3 in the Kidney and Liver
SD and PCK rat kidneys and livers were fixed in zinc formalin and paraffin-embedded. Tissues were sectioned (4 μm) and deparaffinized for performance of antigen retrieval. Slides were stained for AGS3 protein using a DAKO Autostainer Plus (Carpenteria, CA) using the affinity-purified polyclonal AGS3 antibody (pep32) at a dilution of 1:250 for 60 minutes at room temperature. Diaminobenzidine was applied to the slides after the secondary antibody conjugated with a horseradish peroxidase polymer, counterstained in Mayer's hematoxylin solution, and coverslipped.
Subcellular Distribution of AGS3 in Nonrenal and Renal Epithelial Cells
Immunocytochemistry was performed essentially as described previously on Chinese Hamster Ovary cells.8 For renal epithelial cells isolated from the Ba and BPK mouse, we used the following imaging methods. In brief, the cells were visualized using an inverted laser-scanning confocal microscope (Eclipse TE2000-U; Nikon, Tokyo, Japan) using Plan Apo ×60 oil NA 1.40 objective lens. Each 512 × 512-pixel image was acquired using EZ-C1 software (EZ-C1 2.10; Nikon). Simultaneous detection of TO-PRO-3–stained nuclei and immunolabeled AGS3 protein with AGS3-specific (pep32) antisera was obtained using red He/Ne laser (λex = 633 nm, λem = 650 nm long pass) and argon laser (λex = 488 nm, λem = 515/30 nm), respectively.
Lentiviral Vector Transfer Plasmid and Vector Production
Lentiviral vector transfer plasmids were cloned using standard molecular techniques with the following cDNA inserts: (1) Full-length rat AGS3 cDNA from pEGFPN1-AGS3-WT and (2) the C-terminal region from bovine GRK2ct cDNA encoding residues 495 through 689 from pcDNA3-GRK2-CT (provided by Dr. J. Benovic; Thomas Jefferson University, Philadelphia, PA). Lentiviral vector transfer plasmids containing specific AGS3 shRNA were obtained from Open Biosystems (Huntsville, AL). Lentiviral vector transfer plasmids containing control shRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The lentiviral vectors were produced by triple plasmid transfection as previously described.30
Epithelial Cell Proliferation Assay
Normal Ba and cystic BPK renal collecting duct epithelial cells were treated with vehicle or transduced with VSV-G pseudotyped lentiviral vectors expressing (1) control shRNA, (2) AGS3-specific shRNA, or (3) GRK2ct. The cells were transduced at a multiplicity of infection of approximately 40 and expanded until they were aliquotted into a 96-well plate format. In some experiments, BPK epithelial cells were treated overnight with H-89 (10 and 50 μM) to determine the role of PKA inhibition on cell proliferation. All cell numbers were subsequently determined 24 hours later using the CyQuant Direct Cell Proliferation Assay as per the manufacturer's protocol (Life Technologies). Cell numbers were calculated using a standard curve generated on each plate.
Measurement of cAMP Levels
Normal Ba and cystic BPK renal collecting duct epithelial cells were treated with vehicle or transduced at a multiplicity of infection of 40 with VSV-G pseudotyped lentiviral vectors expressing either control or AGS3-specific shRNA. The epithelial cells were expanded in 60-mm plates for 48 to 72 hours. The cells were harvested in 0.1 N HCl, and the cAMP levels were determined by ELISA (Assay Designs).
Statistical Analysis
The significance of differences between groups was tested by a one-way ANOVA with the use of Prism 3.0 statistical software. When a probability value of P < 0.05 was obtained, the Tukey test was used for comparison of each individual group with the appropriate control groups.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was funded by the Advancing a Healthier Wisconsin grant (F.P.), PKD Foundation (F.P.), institutional Medical College of Wisconsin laboratory start-up funds (F.P.), NS24821 (S.M.L.), DA025896 (S.M.L.), F32MH65092 (J.B.B.), GM086510 (J.B.B.), and P01 DK62345 (P.D.W.).
We thank Dr. Ellis D. Avner for access to reagents related to the completion of these studies; Dr. J.L. Benovic (Department of Biochemistry and Molecular Biology, Thomas Jefferson University, Philadelphia, PA) for the pcDNA3-GRK2 CT plasmid; Christine Naughton for help with the immunohistochemistry of the AGS proteins; and Maureen Fallon, Heather Bainbridge, and Sandra Klatt for technical assistance. We also thank Nathalie Pizzinat (Institut National de la Santé et de la Recherche Médicale U388) for generating the AGS3-EGFP construct.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Grantham JJ: Polycystic kidney disease: From the bedside to the gene and back. Curr Opin Nephrol Hypertens 10: 533–542, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Delmas P, Nomura H, Li X, Lakkis M, Luo Y, Segal Y, Fernandez-Fernandez JM, Harris P, Frischauf AM, Brown DA, Zhou J: Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J Biol Chem 277: 11276–11283, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP: The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun 251: 625–631, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Parnell SC, Magenheimer BS, Maser RL, Zien CA, Frischauf AM, Calvet JP: Polycystin-1 activation of c-Jun N-terminal kinase and AP-1 is mediated by heterotrimeric G proteins. J Biol Chem 277: 19566–19572, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, Lanier SM, Duzic E: Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol 17: 878–883, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S, 3rd, Duzic E, Lanier SM: Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem 274: 33202–33205, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Blumer JB, Cismowski MJ, Sato M, Lanier SM: AGS proteins: Receptor-independent activators of G-protein signaling. Trends Pharmacol Sci 26: 470–476, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Blumer JB, Chandler LJ, Lanier SM: Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality: Localization of LGN to the midbody during cytokinesis. J Biol Chem 277: 15897–15903, 2002 [DOI] [PubMed] [Google Scholar]
- 9.De Vries L, Fischer T, Tronchere H, Brothers GM, Strockbine B, Siderovski DP, Farquhar MG: Activator of G protein signaling 3 is a guanine dissociation inhibitor for Galpha i subunits. Proc Natl Acad Sci U S A 97: 14364–14369, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizzinat N, Takesono A, Lanier SM: Identification of a truncated form of the G-protein regulator AGS3 in heart that lacks the tetratricopeptide repeat domains. J Biol Chem 276: 16601–16610, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Chabardes D, Imbert-Teboul M, Elalouf JM: Functional properties of Ca2+-inhibitable type 5 and type 6 adenylyl cyclases and role of Ca2+ increase in the inhibition of intracellular cAMP content. Cell Signal 11: 651–663, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato M, Gettys TW, Lanier SM: AGS3 and signal integration by Galpha(s)- and Galpha(i)-coupled receptors: AGS3 blocks the sensitization of adenylyl cyclase following prolonged stimulation of a Galpha(i)-coupled receptor by influencing processing of Galpha(i). J Biol Chem 279: 13375–13382, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Fan P, Jiang Z, Diamond I, Yao L: Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol Pharmacol 76: 526–533, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao L, McFarland K, Fan P, Jiang Z, Inoue Y, Diamond I: Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci U S A 102: 8746–8751, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang SJ, Janak PH, Bonci A, Diamond I: Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci U S A 105: 12533–12538, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanada K, Tsai LH: G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell 122: 119–131, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ: Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem 269: 6193–6197, 1994 [PubMed] [Google Scholar]
- 19.Schwindinger WF, Robishaw JD: Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene 20: 1653–1660, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Fischer E, Pontoglio M: HNF1beta and defective nephrogenesis: A role for interacting partners? Kidney Int 74: 145–147, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ: Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41: 793–799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H: Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Park F, Sweeney WE, Jia G, Roman RJ, Avner ED: 20-HETE mediates proliferation of renal epithelial cells in polycystic kidney disease. J Am Soc Nephrol 19: 1929–1939, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guay-Woodford LM: Murine models of polycystic kidney disease: Molecular and therapeutic insights. Am J Physiol Renal Physiol 285: F1034–F1049, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Sweeney WE, Jr, Kusner L, Carlin CR, Chang S, Futey L, Cotton CU, Dell KM, Avner ED: Phenotypic analysis of conditionally immortalized cells isolated from the BPK model of ARPKD. Am J Physiol Cell Physiol 281: C1695–C1705, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC: The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Hackmann K, Gao J, He X, Piontek K, Garcia-Gonzalez MA, Menezes LF, Xu H, Germino GG, Zuo J, Qian F: Essential role of cleavage of polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc Natl Acad Sci U S A 104: 18688–18693, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard ML, Peterson YK, Chung P, Jourdan J, Lanier SM: Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J Biol Chem 276: 1585–1593, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Park F, Ohashi K, Chiu W, Naldini L, Kay MA: Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat Genet 24: 49–52, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.