Abstract
Membranous nephropathy is one of the most common causes of nephrotic syndrome in adults. Recent reports suggest that treatment with adrenocorticotropic hormone (ACTH) reduces proteinuria, but the mechanism of action is unknown. Here, we identified gene expression of the melanocortin receptor MC1R in podocytes, glomerular endothelial cells, mesangial cells, and tubular epithelial cells. Podocytes expressed most MC1R protein, which colocalized with synaptopodin but not with an endothelial-specific lectin. We treated rats with passive Heymann nephritis (PHN) with MS05, a specific MC1R agonist, which significantly reduced proteinuria compared with untreated PHN rats (P < 0.01). Furthermore, treatment with MC1R agonists improved podocyte morphology and reduced oxidative stress. In summary, podocytes express MC1R, and MC1R agonism reduces proteinuria, improves glomerular morphology, and reduces oxidative stress in nephrotic rats with PHN. These data may explain the proteinuria-reducing effects of ACTH observed in patients with membranous nephropathy, and MC1R agonists may provide a new therapeutic option for these patients.
Membranous nephropathy (MN) is one of the most common causes of nephrotic syndrome in adults.1 One-third of the patients have a good prognosis with spontaneous remission.2 Even so, approximately 50% of the remainder manifest an unchanged disease state, whereas 50% progress into ESRD and dialysis.2 The nephrotic syndrome is a glomerular disease characterized by proteinuria, edema, hypoalbuminemia, and hyperlipidemia. Most MN cases are idiopathic and the mechanisms underlying the disease are still largely unknown.1 However, it has recently been shown that there is an association with antibodies against the phospholipase A2 receptor (PLA2R); these antibodies are present in 70% of patients with MN.3 Treatments are nonspecific and often include steroids with anti-inflammatory actions, sometimes in combination with cytotoxic agents.4 These drugs affect multiple tissues, causing cytotoxic effects, osteoporosis, adrenal insufficiency, hypertension, peptic ulcers, and increased risk of glucose intolerance and infections.5 Therefore, more effective and specific treatment options are needed.
In 1999, Berg et al.6 by accidental observation in a lipid study found that adrenocorticotropic hormone (ACTH) lowered the urinary albumin excretion by 90% and increased the GFR by 25% in MN-nephrotic patients (n = 14). A randomized controlled study4 and case reports7–9 confirmed this observation. The observation has also been repeated in another controlled study, presented in a preliminary form.10 ACTH is an endogenous peptide hormone and agonist for all melanocortin receptors 1 to 5 (MC1–5R), of which MC2R specifically binds ACTH.11 α-melanocyte stimulating hormone (α-MSH) is also a small endogenous peptide hormone, structurally related to ACTH, which binds all of the MCRs except MC2R. MS05 is a peptide of 13 amino acids specific for human MC1R,12 which has been used in in vitro and in vivo studies but not in renal research.13 The mechanism by which ACTH mediates its effect on proteinuria is still unknown and no one seems to have addressed the question. This led us to explore potential mechanisms behind the beneficial effect of ACTH in glomerular disease.
The aim of this study was to confirm ACTH-induced clinical parameters in MN-nephrotic patients and to test the hypothesis that ACTH exerts its antiproteinuric effect via one or several of its natural receptors: MC1–5R. We first explored the expression of all MCRs in human kidney tissue and cells. To study the effects in vivo, we used the passive Heymann nephritis (PHN) model in the rat, which resembles human MN. The disease is characterized by immune deposits formed in situ in the glomerulus, triggered by the antigens expressed on the surface of podocytes.14 Proteinuria reaches a peak level 14 days after disease induction. New immune deposit formation eventually ceases; however, proteinuria persists throughout life.14 Thus, PHN nephrotic rats were treated with different MCR agonists and proteinuria was followed over time.
Results
ACTH Improved Clinical Parameters in MN-Nephrotic Patients
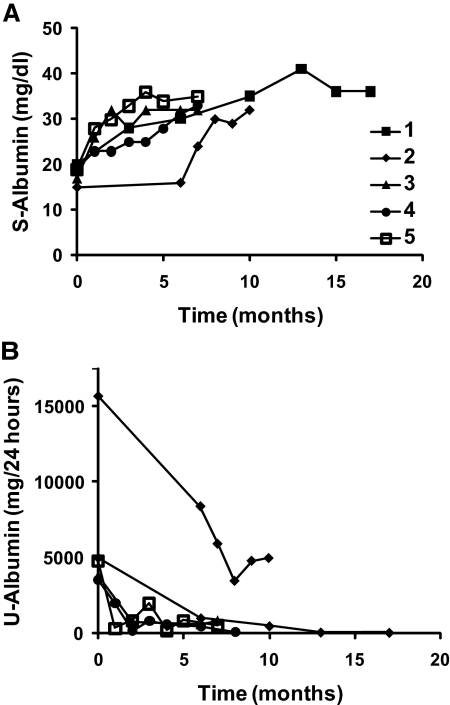
Before ACTH treatment, the patients were relentlessly nephrotic for at least 15 months. After the introduction of ACTH, the nephrotic condition improved markedly in all five patients (Table 1 and Figure 1). The effects were significant, rapid, and pronounced: serum albumin increased with 88 ± 7% from 18 ± 0.9 to 34 ± 0.8 mg/dl (P < 0.001, Figure 1A), while proteinuria fell with 86 ± 6% from 6550 ± 2300 to 1310 ± 930 mg/24 h (P < 0.001, Figure 1B). The effect was sustained in all of the patients in an observation period of at least 1 month after ACTH treatment ended. In addition, three patients were followed up for another 15 months and still showed no signs of remission into proteinuria.
Table 1.
Clinical parameters in idiopathic MN patients treated with ACTH
| Patient | Age (years) | Gender | Observation Period Before ACTH Treatment (months) | Time with ACTH (months) | S-Albumin (mg/dl) Start; Enda | tU-Albumin (mg/24 h) Start; Enda |
|---|---|---|---|---|---|---|
| 1 | 50 | Male | 24 | 15 | 20; 36 | 4982; 59 |
| 2 | 26 | Male | 15 | 10 | 15; 32 | 15,667; 4984 |
| 3 | 53 | Male | 48 | 7 | 17; 32 | 3800; 840 |
| 4 | 69 | Female | 204 | 7 | 19; 33 | 3500; 120 |
| 5 | 50 | Female | 30 | 7 | 19; 35 | 4800; 531 |
aSerum albumin (S-albumin) and total urinary albumin (tU-albumin) at the start point for ACTH treatment and within 5 months after treatment withdrawal.
Figure 1.
ACTH improves clinical parameters in MN-nephrotic patients. (A) Serum albumin (S-Albumin) and (B) urinary albumin (U-Albumin) were followed in five patients (patients 1 to 5) at least 6 months after the start of ACTH treatment. The last time point of each patient represents values measured within 5 months after end of treatment. S-Albumin increased and U-Albumin decreased rapidly within a few months and the effect was sustained after treatment withdrawal.
MC1R Is Expressed in the Kidney and in Most Renal Cells
To explore potential mechanisms behind the beneficial effect of ACTH in patients, we analyzed gene expression of all MCRs in relevant tissues and cell types. As can be seen in Table 2, only MC1R was clearly detected in human kidney cortex and in the specific renal cell types: podocytes, glomerular endothelial, mesangial, and tubular epithelial cells (CT ≤ 30). MC1R mRNA was also detected in rat glomeruli (CT ≤ 30). RNA from testis, brain, and adrenal gland were used as controls. Minute and biologically insignificant amounts of MC4R were detected in podocytes, endothelial cells, and tubular epithelial cells (30 ≤ CT ≤ 35).
Table 2.
Expression of MCRs in human and rat tissue and kidney cells
| Tissue/Cell Type | MC1R | MC2R | MC3R | MC4R | MC5R |
|---|---|---|---|---|---|
| Kidney | + | – | – | – | – |
| Podocyte | + | – | – | (+) | – |
| Glomerular endothelial cell | + | – | – | (+) | – |
| Mesangial cell | + | – | – | – | – |
| Tubular epithelial cell | + | – | – | (+) | – |
| Rat glomeruli | + | * | * | * | * |
| Testis | + | (+) | (+) | (+) | (+) |
| Adrenal gland | (+) | + | + | – | – |
| Brain | + | – | (+) | + | – |
+, mRNA expression CT ≤ 30; (+) weak mRNA expression, 30 ≤ CT < 35; –, no mRNA expression, CT value ≥ 35;
*, not done.
MC1R Colocalizes with Synaptopodin in Podocytes
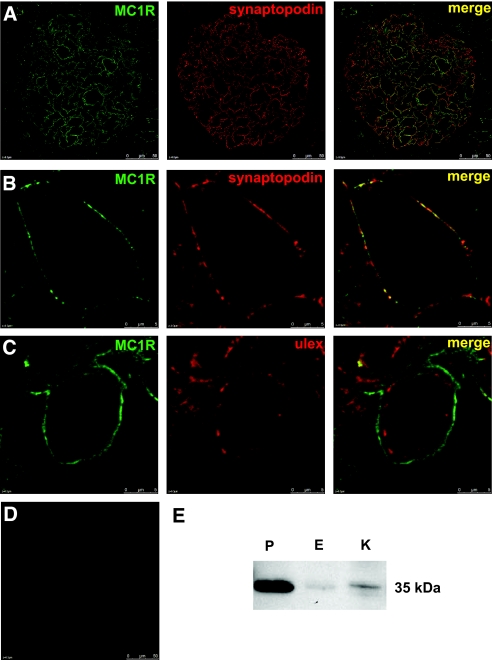
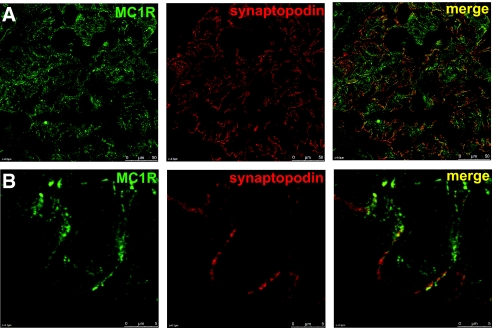
To further explore the expression of MC1R, we performed immunohistochemical analysis in human kidney tissue. MC1R protein expression was confirmed in glomeruli (Figure 2). For additional information about the localization, we performed colocalization studies with the podocyte-specific protein synaptopodin (Figure 2, A and B) and the endothelial-specific lectin Ulex europaeus agglutinin I (UEA I; Figure 2C). There was a clear colocalization of MC1R with synaptopodin in glomerular podocytes. On the other hand, MC1R did not colocalize with the endothelial cell marker UEA I. Protein expression of MC1R was further confirmed in human kidney tissue with western blotting (Figure 2E). MC1R was clearly detected in podocytes and to a small extent in endothelial cells and kidney tissue. As shown in Figure 3, MC1R is also present in patients with MN. Colocalization studies with synaptopodin revealed a similar pattern as compared with healthy kidney with MC1R expression in podocytes.
Figure 2.
MC1R is expressed in normal human kidney tissue. MC1R colocalized with synaptopodin in a podocyte-specific manner. (A) Confocal microscopic analysis of cryosections from human kidney tissue confirmed expression of MC1R (green) and synaptopodin (red). Colocalization of these proteins was shown in merge (yellow). (B) Enlarged images of one glomerular capillary loop. (C) MC1R (green) and the endothelial-specific lectin UEA I (red) did not colocalize (merge). (D) Omission of primary antibody yielded no staining. Scale bar = 50 μm. (E) Western blotting was performed with an anti-MC1R antibody. MC1R was detected in high amounts primarily in podocytes. P, podocytes; E, endothelial cells; K, kidney tissue.
Figure 3.
MC1R is expressed in kidney tissue from a patient with MN. (A) Staining with MC1R (green), synaptopodin (red), and merge (yellow) also confirm MC1R expression in MN patients. (B) Enlarged images of one glomerular capillary loop. Scale bar = 50 μm.
PHN—A Stable Disease Model for Human MN
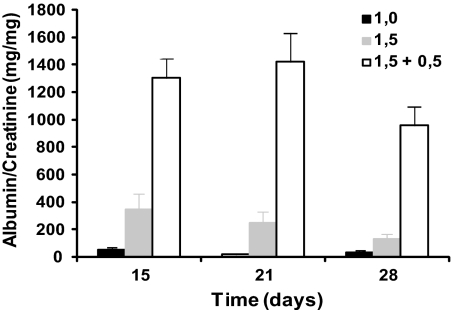
To mimic the situation in human patients, we treated nephrotic rats over several weeks. To ensure that we had a stable model with a high level of proteinuria throughout several weeks, we first conducted a dose-response study. To induce PHN, rats were injected with the commercially available anti-Fx1A IgG antibody (30 mg/ml, Probetex Inc., San Antonio, TX). A dose of 1.0 ml (n = 8) resulted in very low levels of proteinuria 15 and 28 days after injection: 49 ± 21 mg/mg and 35 ± 12 mg/mg, respectively (Figure 4). A dose of 1.5 ml (n = 8) resulted in higher levels of proteinuria 15 days after injection: 343 ± 118 mg/mg. However, contrary to what we had expected, the level of proteinuria was steadily reduced and 2 weeks later reached 131 ± 40 mg/mg. A dose of 1.5 ml (n = 8) at day 0 followed by a booster dose of 0.5 ml at day 7 resulted in a high and steady level of proteinuria at all time points: 1304 ± 141 mg/mg after 15 days, 1423 ± 211 mg/mg after 21 days, and 959 ± 140 mg/mg after 28 days. On the basis of these results, the last mentioned regimen was chosen for further studies.
Figure 4.
A high dose combined with a booster dose of the anti-Fx1A antibody induces a stable PHN disease model with a long-lasting and high level of proteinuria. The antibody was intravenously injected at a volume of 1.0 (black bars) or 1.5 ml (gray bars) at day 0 or a volume of 1.5 and 0.5 ml at day 0 and 7 (transparent bars), respectively. Spot urine was collected on day 15, 21, and 28 and analyzed for urinary albumin and creatinine content. Data are presented as mean ± SEM (n = 8).
MC1R Agonists Reduced Proteinuria in Nephrotic Rats
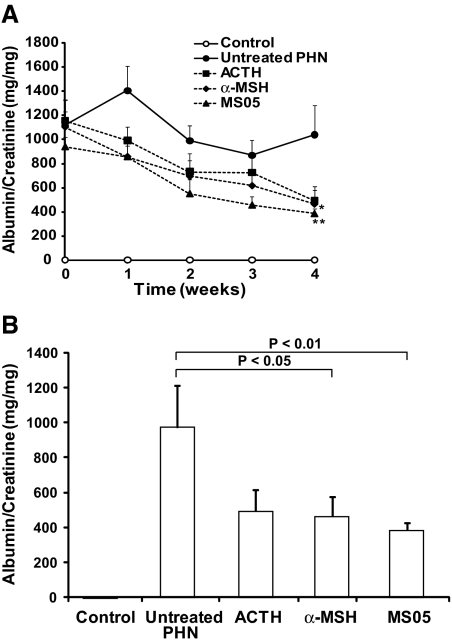
As can be seen in Figure 5A, the level of proteinuria in MCR-agonist-treated PHN rats was gradually reduced during the treatment period, from a mean range of 940 to 1157 mg/mg to 387 to 496 mg/mg. Spot urine analyses collected twice a week made it possible to follow proteinuria in all rats during the whole treatment period. Controls remained at a low and stable level at <3.0 mg/mg. The level of proteinuria in untreated PHN was high, with a mean range of 872 to 1406 mg/mg throughout the study. Interestingly, already after 1 week there was a tendency for a reduced level of proteinuria in MCR-agonist-treated rats.
Figure 5.
MCR agonists reduce proteinuria in nephrotic rats. Proteinuria was followed over time in PHN nephrotic rats treated for 4 weeks with MCR agonists (A). After 4 weeks, treatment with α-MSH and MS05 significantly reduced the level of proteinuria compared with untreated PHN rats (B). Proteinuria was analyzed in each animal from spot urine samples. Urinary albumin concentration, measured by rat albumin ELISA, was related to urinary creatinine concentration measured by the Jaffé creatinine assay. The samples were collected twice a week and a mean from these two samples was calculated, except for the last time point at week 4 for all groups, and at week 0 for controls, which is based on a single measurement. Note that controls coincide with the x-axis because of the low level of albumin in the urine. Data are presented as mean ± SEM. Untreated PHN: n = 7 to 8; controls: n = 4; MS05: n = 7 to 8; α-MSH: n = 7 to 8; ACTH: n = 7 to 8. *P < 0.05, **P < 0.01.
Figure 5B shows the main findings at the end point of the study. The specific MC1R agonist MS05 (n = 8) reduced proteinuria by 60% compared with untreated PHN (n = 8, P < 0.01). Within the MS05 group, this corresponds to a 65 ± 5.3% decrease in the level of proteinuria when comparing end of treatment with the proteinuria peak level at treatment start (n = 7, P < 0.001). The unspecific MCR agonist α-MSH (n = 8) also significantly reduced proteinuria by 52% compared with untreated PHN (n = 8; P < 0.05). Within the α-MSH group, this corresponds to a 55 ± 11% decrease in proteinuria after 4 weeks of treatment compared with treatment start (n = 7, P < 0.05). ACTH (n = 8) had a tendency to reduce the level of proteinuria compared with untreated PHN, but it did not reach statistical significance.
The weight of all animals was followed throughout the study (Table 3). There was no difference in the weight between controls, α-MSH, or MS05 compared with untreated PHN rats. However, ACTH-treated rats did not increase in weight to the same extent as untreated PHN rats (P < 0.05).
Table 3.
Weight, systolic BP, and proteinuria after 4 weeks of MCR agonist treatment
| n | Weight (g) | Systolic BP (mmHg) | Albumin/Creatinine (mg/mg) | |
|---|---|---|---|---|
| Control | 4 | 399 ± 13 | 134 ± 11b | 1.58 ± 0.43b |
| Untreated PHN | 8 | 397 ± 8.3 | 173 ± 5.6 | 1040 ± 240 |
| ACTH | 8 | 363 ± 8.5a | 126 ± 17b | 496 ± 120 |
| α-MSH | 8 | 416 ± 10 | 138 ± 9.2b | 466 ± 110a |
| MS05 | 8 | 418 ± 9.3 | 140 ± 6.6b | 387 ± 41b |
Data are presented as mean ± SEM.
aP < 0.05 compared with untreated PHN rats;
bP < 0.01 compared with untreated PHN rats.
Systolic blood pressure (BP) data (Table 3) corresponded to the degree of proteinuria. At the end of the study, the average systolic BP of untreated PHN rats (n = 8) was 173 ± 5.6 mmHg and significantly higher than controls rats: 134 ± 11 mmHg (n = 4; P < 0.01). Treatment with MCR agonists significantly lowered BP compared with untreated PHN rats (P < 0.01) and did not differ from controls—MS05: 140 ± 6.6 mmHg (n = 8); α-MSH: 138 ± 9.2 mmHg (n = 8), and ACTH: 126 ± 17 mmHg (n = 3).
Improved Morphology in MCR-Agonist-Treated PHN Rats
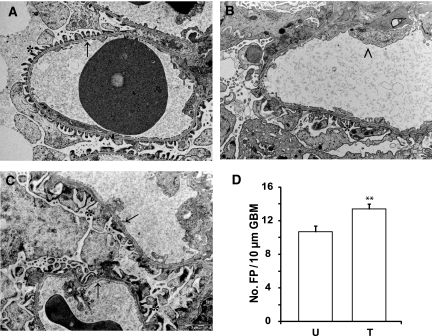
To confirm that the PHN rat experimental model resembled human MN, kidney morphology was examined in an unbiased fashion by a blinded pathologist using transmission electron microscopy (TEM). Morphologically, the controls displayed normal structure and thickness of the glomerular basement membrane (GBM), and the foot processes showed normal spacing and width (Figure 6A). In contrast, distinct pathologic changes resembling those seen in human MN were seen in untreated PHN rats, in which the normal ultrastructure of the glomerulus was severely altered by subepithelial deposits, spike formation, and foot process effacement (Figure 6B). MCR agonist treatment was shown to improve glomerular morphology (Figure 6C). Compared with nephrotic PHN rats, the degree of foot process effacement was not as pronounced in the treated group, and the architecture of the GBM was normalized with absence of spike formation. To quantify the degree of glomerular damage, the average number of foot processes/10 μm of GBM was determined (Figure 6D). Controls had 27.1 ± 0.68 foot processes/10 μm of GBM (n = 32). MCR-agonist-treated PHN rats had an increased number of foot processes (13.4 ± 0.57 [n = 49]) compared with untreated PHN rats (10.7 ± 0.71 [n = 27; P < 0.01]).
Figure 6.
MCR agonist treatment improves glomerular morphology in PHN rats. Kidneys were collected for morphologic analysis after 4 weeks of MCR agonist treatment. Slides were taken in a Leo 912AB Omega electron microscope and examined in a blinded fashion by a pathologist. Representative images of kidneys from (A) control (n = 32), (B) untreated PHN (n = 27), and (C) MCR-agonist-treated PHN rats (n = 49). Controls display a normal structure of the foot processes and GBM. Untreated PHN rats demonstrate foot process effacement and spike formation, whereas in treated PHN rats, foot process effacement is less pronounced with normalized GBM. *Normal foot process; → normal GBM; > disrupted glomerular barrier structure: loss of foot processes and thick GBM. Scale bar = 2 μm. (D) The number of foot processes per 10 μm of GBM was quantified. MCR-agonist-treated PHN rats had a significantly higher number of foot processes compared with untreated PHN. Data are presented as mean ± SEM. FP, foot processes; U, untreated PHN rats; T, treated PHN rats. **P < 0.01.
Glomerular Deposition of C5b-9 in PHN Nephrotic Rats
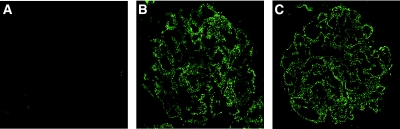
Glomerular deposition of the membrane attack complex C5b-9 is characteristic for MN. As expected, no deposition of C5b-9 was found in controls (Figure 7A). On the contrary, glomerular deposition was verified in untreated and MS05-treated PHN nephrotic rats (Figure 7, B and C) with no clear difference between the groups.
Figure 7.
Glomerular deposition of C5b-9 in PHN nephrotic rats. Cryosections from (A) control, (B) untreated PHN, and (C) MS05-treated PHN nephrotic rats were stained with a C5b-9 antibody. Glomerular deposition was found in untreated PHN and MS05-treated PHN rats but was absent in controls. No visible difference was seen between the untreated PHN and the MS05-treated PHN rats. Omission of primary antibody yielded no staining (data not shown). Magnification: ×63.
MC1R Agonists Reduced Oxidative Stress
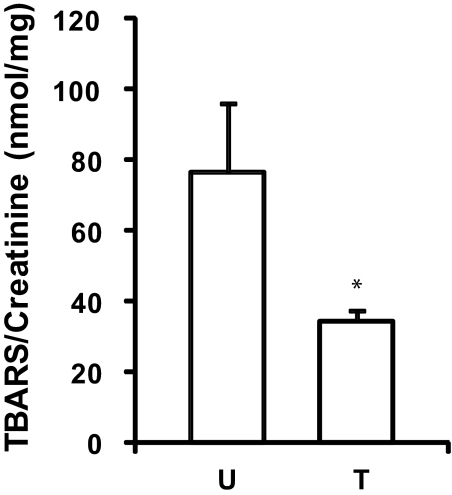
Lipid peroxidation is an estimation of oxidative stress, which can be measured through thiobarbituric acid-reactive substances (TBARS). As seen in Figure 8, TBARS were significantly reduced in rats treated with MS05 (34.3 ± 3.2 nmol TBARS/mg creatinine [n = 8]) compared with untreated PHN (76.8 ± 19.4 nmol TBARS/mg creatinine [n = 6, P < 0.05]), whereas control TBARS was 36.1 ± 7.2 nmol TBARS/mg creatinine (n = 4).
Figure 8.
MC1R agonist treatment reduces oxidative stress in PHN rats. Urine samples were collected after 4 weeks of treatment. TBARS were measured and related to the creatinine concentration. TBARS were significantly decreased in MS05-treated PHN rats (T, n = 8) compared with untreated PHN (U, n = 6). Data are presented as mean ± SEM, *P < 0.05.
Discussion
In this study, we reproduce the beneficial clinical effect of ACTH on patients with membranous nephropathy. With the hypothesis that ACTH exerts its antiproteinuric effect on renal cells, we studied the gene and protein expression of melanocortin receptors (MCRs). Only MC1R was found in the kidney. Colocalization using immunofluorescence with synaptopodin showed that MC1R indeed was mainly expressed in podocytes (Table 2 and Figures 2 and 3). Also, specific MC1R agonists reduced the level of proteinuria, improved morphology, and reduced oxidative stress in nephrotic rats with PHN. Our findings suggest a new role for specific MC1R agonists, which could be used as therapy for nephrotic patients, hopefully without the pronounced side effects seen in the treatment options currently available.
MC1R expression was confirmed at the RNA and protein level in human kidney tissue. Staining of MC1R in patients with MN showed the same pattern as in healthy tissue with synaptopodin colocalization in podocytes. We further confirmed expression of MC1R protein in podocytes and other renal cells with western blotting. Expression of MC1R in podocytes and synaptopodin colocalization data suggest a specific role for MC1R in these cells. Thus, it is widely accepted that podocyte morphology is crucial for maintaining an intact glomerular barrier. Altered morphology leads to proteinuria, a hallmark of MN and glomerular disease. However, further studies are required to explore the signaling cascade induced by MC1R activation during nephrotic conditions.
We detected MC1R, but no other melanocortin receptor subtype, in glomerular cells. On the basis of this observation and the knowledge that ACTH binds MCRs, we hypothesized that ACTH exerts its antiproteinuric effect through MC1R. To test the hypothesis, we studied rats with PHN, an experimental form of MN. Indeed, a specific MC1R agonist, MS05, significantly reduced the level of proteinuria in PHN nephrotic rats (Figure 5). Morphologic examination confirmed an improved glomerular structure in rats treated with MCR agonists, with an increased number of foot processes compared with untreated PHN rats (Figure 6). It has previously been shown that high amounts of reactive oxygen species (ROS) are produced by podocytes in PHN.15 ROS are associated with lipid peroxidation and the production of TBARS, which in turn can lead to modification of podocyte membrane proteins and components of the GBM.16 In our study, MC1R agonist treatment significantly reduced TBARS compared with untreated PHN rats (Figure 8). Because we also demonstrated MC1R expression in podocytes, there might be a connection between that receptor and ROS inactivation. However, the expression of C5b-9 in MS05-treated PHN rats was not visibly reduced compared with untreated PHN rats (Figure 7) in this experimental setup. Taken together, these findings support the hypothesis that the effect of ACTH is mediated by one MCR, most likely through MC1R, in the podocytes of the kidney. However, further studies are required to understand the beneficial effect behind MC1R agonist treatment.
The first study demonstrating that ACTH reduced proteinuria in nephrotic patients was published in 1999.6 Although others have repeated the findings,4,7–9 the mechanisms behind the effect have not been studied. We report five additional patients who experienced beneficial effects of ACTH treatment. All patients had an observation period of at least 15 months before treatment was started, and the improvement came soon after the introduction of ACTH. It is therefore unlikely that the beneficial effects on serum and urinary albumin were due to spontaneous remission. The specific ACTH receptor (MC2R) is mainly located in the adrenal gland, where stimulation regulates steroid output.17 Meta-analyses have shown that corticosteroids alone do not ameliorate MN.18,19 Furthermore, the amount of cortisol released by ACTH is very small in comparison to the corticosteroid doses conventionally administered to patients with MN. Thus, the antiproteinuric effect of ACTH is probably not mediated via increased release of cortisol from the adrenal glands. However, long-term treatment with ACTH is associated with MC2R-mediated side effects.
The MCRs are widely distributed in the body, including melanocytes (MC1R),20 immune cells (MC1- and 3R),21 exocrine glands (MC5R),22 the central nervous system (MC3–4R),23 and testis (MC5R).24 MC1R is best known for its expression in melanocytes, where it affects skin pigmentation.25 Before this study, MCR expression had been detected in human kidney tissue,26 but there was no information about specific location (i.e., which cell types express the receptors). Our hypothesis included a MCR-specific response in the kidney; therefore, we first analyzed the expression in relevant tissue and cells. In contrary to Chhajlani and co-workers, who only found a weak expression of MC5R,26 we did find a robust RNA and protein expression of MC1R in kidney cortex and specifically in podocytes. However, none of the other MCRs were expressed significantly.
Although treatment with MC1R agonists ameliorated symptoms in nephrotic rats, it did not completely cure the PHN. One explanation for the incomplete remission could be the difference in human and rat protein structures. The amino acid sequence is only 76% identical between human and murine MC1R.27 Also, the specific MC1R agonist, MS05, was developed for the human MC1R, but it has been used in animal studies. In addition, the dose of MS05 is probably low because we used a constant infusion and such a small peptide is rapidly filtered in the kidneys. Patients have been treated with ACTH as intermittent bolus injections intramuscularly or subcutaneously twice a week for up to 12 months.6 In the rats, the level of proteinuria gradually fell over several weeks (Figure 5A) and it is possible that a longer study period or a different administration would result in an even better outcome and possibly cure the disease.
Systolic BP was decreased in all treated compared with nontreated nephrotic rats (Table 3), but it is unlikely that the decrease in BP itself was responsible for the improvement of the glomerular morphology. Previous studies have shown that decreasing BP with angiotensin converting enzyme inhibitors in PHN nephrotic rats does not reduce proteinuria at all28 or only by 25%.29 In comparison, MCR agonists in this study reduced proteinuria by more than 50%. The antiproteinuric effect is therefore probably not secondary to reduced BP.
In conclusion, we have found that MC1R, but no other MCR subtype, is expressed in glomerular cells, and in particular in the podocytes. Furthermore, treating rats with PHN with a selective MC1R agonist markedly reduces the level of proteinuria, reduces oxidative stress, and improves glomerular morphology. On the basis of these findings, we propose that ACTH exerts its beneficial effects in nephrotic patients with MN through MC1R on the podocytes. Therefore, specific MC1R agonists could be a new treatment option for nephrotic patients. Such a therapy is likely to be more effective and less toxic than the currently used treatment options.
Concise Methods
Clinical Data
Five patients, 26 to 69 years old, were subcutaneously treated with synthetic ACTH (Synachten Depot, Novartis, Switzerland) according to the protocols by Berg et al.6,7 published in 1999 and 2004 at the University Hospitals of Lund and Sahlgrenska in Sweden. All patients had severe nephrotic syndrome due to idiopathic MN proven by kidney biopsy. After an observation period of at least 15 months, ACTH treatment was started and the treatment period continued for 7 to 15 months. Patients were treated symptomatically with angiotensin II antagonists and/or angiotensin converting enzyme inhibitors, statins, and diuretics. Warfarin or low-molecular-weight heparin was used if serum albumin was <20 mg/dl. Clinical parameters such as serum albumin and proteinuria were followed during the treatment period, at least 1 month after treatment withdrawal, and almost 2 years in three of the patients.
Real-Time PCR
The following tissues and cells were used for preparation of RNA according to the RNeasy Mini Protocol with DNAse digestion (Qiagen Nordic, Solna, Sweden): healthy parts of kidney tissue removed by nephrectomy (approved by the local ethical committee at the University of Gothenburg); human podocytes, described and characterized elsewhere;30 human glomerular endothelial cells, described and characterized elsewhere;31 human mesangial cells (Cambrex Bio Science Walkersville, Inc., Walkersville, MD); human tubular epithelial cells prepared from healthy parts of kidney tissue removed by nephrectomy; and rat glomeruli collected on a 75-μm sieve. Total RNA from testis (AM7972), adrenal gland (AM7994), and brain (AM6050) used in real-time PCR was ordered from Applied Biosystems Incorporated (ABI). RNA quality was confirmed using RNA6000 nanochips on an Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). RNA was quantified using a NanoDrop spectrophotometer (Nanodrop Technologies Inc., Wilmington, DE).
Reverse transcription of RNA was performed using a standard protocol at a total volume of 20 μl and final cDNA concentration of 50 ng/μl. Fifty nanograms of sample cDNA was used to quantify the mRNA level of each target gene by real-time PCR on the ABI Prism 7900 sequence detection system (TaqMan, ABI, Foster City, CA) as described previously.31 The following primers and probes (all tested by ABI) were used to detect MCR mRNA: MC1R (Hs00267167_s1), MC2R (Hs00265039_s1), MC3R (Hs00252036_s1), MC4R (Hs00271877_s1), MC5R (Hs00271882_s1), and rat MC1R (custom made, GenBankID AB306978.1). All samples were run in duplicates. RNA samples without performing reverse transcription were used as negative controls. The threshold cycle (CT) is defined as the cycle number at which the reporter fluorescence reaches a certain level (i.e., usually 10 times the SD of the baseline). A CT level <30 was considered as significant mRNA expression, whereas a CT level >35 was considered as no expression.
Western Blotting
Sources of protein were the same as for RNA synthesis (see Real-Time PCR section), and concentrations were determined with a BCA Protein Assay Kit (Thermoscientific, Rockford, IL). Samples were analyzed by electrophoresis under reducing conditions and transferred to polyvinylidene fluoride membranes according to standard protocols. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline-Tween at 4°C overnight, followed by incubation with an anti-MC1R antibody (Alomone Labs, Ltd., Israel) at a dilution of 1:500 at 4°C overnight. After washing, membranes were incubated with an anti-rabbit horseradish-peroxidase-conjugated secondary antibody (GE Healthcare, United Kingdom) at a dilution of 1:5000 for 1 hour. Immunoreactive bands were visualized using the Immun-Star WesternC chemiluminescent kit and a CCD camera (Molecular Imager Chemidoc XRS+ Systems, Bio-Rad Laboratories Inc., Hercules, CA).
Immunohistologic Analyses
Cryosections (4 μm thick) of healthy kidney tissue removed by nephrectomy or biopsies from patients with MN were blocked with 2% FCS, 2% BSA, and goat IgG at a dilution of 1:1000 in PBS. To detect MC1R, we used an anti-MC1R antibody at a 1:100 dilution (Alomone Laboratories). For podocyte colocalization studies, we used a mouse monoclonal antibody to synaptopodin at a dilution of 1:100 (Abcam, Ltd., United Kingdom); for endothelial colocalization studies, we used the endothelial-specific lectin rhodamine UAE I (Vector Laboratories, CA) at a dilution of 1:200. Cryosections from controls, untreated PHN, and MS05-treated PHN rats were blocked with 2% FCS, 2% BSA, and donkey serum in PBS. Staining was performed with a C5b-9 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a dilution of 1:50. Secondary antibodies were goat or donkey Alexa Fluor 488 diluted 1:2000 (Invitrogen, Carlsbad, CA) or a goat Texas Red antibody diluted 1:500 (Abcam, Ltd., United Kingdom). The sections were mounted using ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA) and analyzed with a confocal microscope (Leica TCS SP5).
Induction of PHN
All experiments were performed on male Sprague–Dawley rats (Charles River, Germany) of initial body weight of 125 to 165 g. The rats had free access to standard food and water and were housed in a room with a 12-hour dark-light cycle. The local ethical committee approved all experiments. Anesthesia was induced and maintained by inhalation of isoflurane (2 to 3% vol/vol, Schering-Plough, Stockholm, Sweden) mixed with air (approximately 1 L/min) in an isoflurane vaporizer (Ohmeda Isotec 5, Simtec engineering, Askim, Sweden). To induce PHN, anti-Fx1A IgG antibody (Probetex Inc., San Antonio, TX) at 30 mg/ml was slowly injected into the jugular vein. In the dose-response study, rats were divided into three groups (n = 8 in all groups). One group received 1.0 ml of antibody at day 0, the second group received 1.5 ml at day 0, and the third group received 1.5 ml at day 0 followed by 0.5 ml at day 7. All rats were followed at least 28 days after the first injection. In the treatment study, the last administration volume was used (1.5 ml at day 0 and 0.5 ml at day 7).
Experimental Protocol
PHN was induced (see Induction of PHN). Instead of the PHN-inducing antibody, controls (n = 4) received sterile saline. At day 15 treatments were started: rats were again anesthetized and an osmotic pump (Alzet Osmotic Pumps, Cupertino, CA) was placed subcutaneously in the neck. The pump contained one of the following substances diluted in sterile saline: ACTH1-24 (Novartis, Switzerland), α-MSH (Sigma, St. Louis, MO), or MS05 (custom-made peptide from Sigma, St. Louis, MO). Untreated PHN received only sterile saline. All treatment groups included eight rats. Substances were administered at a dose of 10 μg/d for 4 weeks. The dose was based on a dose-response study with ACTH1-24 (data not shown). Controls did not receive an osmotic pump and were left untreated. Temgesic (0.1 ml/100 g body weight, Schering-Plough) was given as a postoperative pain reliever in all small operative procedures. Weight was followed twice a week. Systolic BP was measured after 4 weeks of treatment with tail-cuff (Harvard Apparatus, Holliston, MA) monitored by a computer using AcqKnowledge version 3.7.3 (Biopac Systems, Inc., Goleta, CA). BP values are means of at least three values.
Urine Protein Analyses
During the 4 weeks of treatment, spot urine samples were collected twice a week. Albumin was analyzed using the Rat Albumin Elisa Quantitation Kit (Bethyl Laboratories Inc., Montgomery, TX). All urine samples were corrected for the urine-creatinine concentration measured with the Jaffé reaction using a creatinine standard solution (Sigma, St. Louis, MO). In both reactions, optical density was measured on a Spectra max plus reader (Molecular Devices, Sunnyvale, CA).
Measurement of Oxidation Products
Urine samples collected from PHN rats after 4 weeks of treatment were analyzed for lipid peroxidation. TBARS were determined by the method of Yagi et al.32 Fluorescence was measured at 553 nm with 515-nm excitation. All urine samples were corrected for the urine-creatinine concentration as described above. The level of TBARS was expressed as nanomoles of malondialdehyde per milligrams of creatinine.
Electron Microscopy
Kidneys from controls, untreated PHN rats, and MCR-agonist-treated PHN rats were collected for morphologic analysis after 4 weeks of treatment. The renal artery and vein were clamped and the kidney was fixed by subcapsular injection of Karnovsky's fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.05 M Na-cacodylate buffer, pH 7.2). The kidneys were cut into millimeter slices and processed by standard procedure as described previously.33 Slides were taken with a Leo 912AB Omega electron microscope (Leo Electron Microscopy, Ltd., Cambridge, United Kingdom) and examined in a blinded fashion by a pathologist. The number of foot processes per 10 μm of GBM was determined (control: n = 32; untreated PHN: n = 27; MCR-agonist-treated PHN: n = 49).
Statistical Analysis
All results are presented as mean ± SEM. Differences in clinical parameters, systolic BP, weight, number of foot processes, and measurement of oxidation products were determined using the t test. Because of non-normal distribution, differences in proteinuria were determined with the exact Mann–Whitney nonparametric test. P ≤ 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
Technical assistance by K. Askerlund is gratefully acknowledged. The Swedish Medical Research Council grants 9898 and 14764, Vinnova, the National Association for Kidney Diseases, the John and Brit Wennerströms Research Foundation, the Ingbritt and Arne Lundberg Research Foundation, Gambro AB, and Sahlgrenska University Hospital grant LUA75450 supported this study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Ponticelli C: Membranous nephropathy. J Nephrol 20: 268–287, 2007 [PubMed] [Google Scholar]
- 2.Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85–89, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, Mandolfo S, Messa P: A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 47: 233–240, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Schacke H, Docke WD, Asadullah K: Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96: 23–43, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Berg AL, Nilsson-Ehle P, Arnadottir M: Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int 56: 1534–1543, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Berg AL, Arnadottir M: ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant 19: 1305–1307, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Picardi L, Villa G, Galli F, Piazza V, Bovio G, Efficace E, Montagna G, Semeraro L, Segagni S, Salvadeo A: ACTH therapy in nephrotic syndrome induced by idiopathic membranous nephropathy. Clin Nephrol 62: 403–404, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Rauen T, Michaelis A, Floege J, Mertens PR: Case series of idiopathic membranous nephropathy with long-term beneficial effects of ACTH peptide 1–24. Clin Nephrol 71: 637–642, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Berg AL, Stefánsson B, Arnadottir M: A randomized, controlled study on treatment with adrenocorticotropic hormone in idiopathic membranous nephropathy. Presented at the 2006 American Society of Nephrology Renal Week (abstract number F-PO1112), San Diego, CA, 2006 [Google Scholar]
- 11.Eberle A: The Melanotropins, Basel, Switzerland, Karger, 1988 [Google Scholar]
- 12.Szardenings M, Muceniece R, Mutule I, Mutulis F, Wikberg JE: New highly specific agonistic peptides for human melanocortin MC(1) receptor. Peptides 21: 239–243, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Getting SJ, Christian HC, Lam CW, Gavins FN, Flower RJ, Schioth HB, Perretti M: Redundancy of a functional melanocortin 1 receptor in the anti-inflammatory actions of melanocortin peptides: Studies in the recessive yellow (e/e) mouse suggest an important role for melanocortin 3 receptor. J Immunol 170: 3323–3330, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Salant DJ, Cybulsky AV: Experimental glomerulonephritis. Methods Enzymol 162: 421–461, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Neale TJ, Ullrich R, Ojha P, Poczewski H, Verhoeven AJ, Kerjaschki D: Reactive oxygen species and neutrophil respiratory burst cytochrome b558 are produced by kidney glomerular cells in passive Heymann nephritis. Proc Natl Acad Sci U S A 90: 3645–3649, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neale TJ, Ojha PP, Exner M, Poczewski H, Ruger B, Witztum JL, Davis P, Kerjaschki D: Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest 94: 1577–1584, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefkowitz RJ, Roth J, Pricer W, Pastan I: ACTH receptors in the adrenal: Specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A 65: 745–752, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogan SL, Muller KE, Jennette JC, Falk RJ: A review of therapeutic studies of idiopathic membranous glomerulopathy. Am J Kidney Dis 25: 862–875, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Perna A, Schieppati A, Zamora J, Giuliano GA, Braun N, Remuzzi G: Immunosuppressive treatment for idiopathic membranous nephropathy: A systematic review. Am J Kidney Dis 44: 385–401, 2004 [PubMed] [Google Scholar]
- 20.Loir B, Perez Sanchez C, Ghanem G, Lozano JA, Garcia-Borron JC, Jimenez-Cervantes C: Expression of the MC1 receptor gene in normal and malignant human melanocytes. A semiquantitative RT-PCR study. Cell Mol Biol (Noisy-le-grand) 45: 1083–1092, 1999 [PubMed] [Google Scholar]
- 21.Catania A: The melanocortin system in leukocyte biology. J Leukoc Biol 81: 383–392, 2007 [DOI] [PubMed] [Google Scholar]
- 22.van der Kraan M, Adan RA, Entwistle ML, Gispen WH, Burbach JP, Tatro JB: Expression of melanocortin-5 receptor in secretory epithelia supports a functional role in exocrine and endocrine glands. Endocrinology 139: 2348–2355, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Catania A, Gatti S, Colombo G, Lipton JM: Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev 56: 1–29, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Labbe O, Desarnaud F, Eggerickx D, Vassart G, Parmentier M: Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry 33: 4543–4549, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA: Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology 137: 1627–1633, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Chhajlani V: Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int 38: 73–80, 1996 [PubMed] [Google Scholar]
- 27.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD: The cloning of a family of genes that encode the melanocortin receptors. Science 257: 1248–1251, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Zoja C, Corna D, Rottoli D, Cattaneo D, Zanchi C, Tomasoni S, Abbate M, Remuzzi G: Effect of combining ACE inhibitor and statin in severe experimental nephropathy. Kidney Int 61: 1635–1645, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Benigni A, Corna D, Maffi R, Benedetti G, Zoja C, Remuzzi G: Renoprotective effect of contemporary blocking of angiotensin II and endothelin-1 in rats with membranous nephropathy. Kidney Int 54: 353–359, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Bjornson A, Moses J, Ingemansson A, Haraldsson B, Sorensson J: Primary human glomerular endothelial cells produce proteoglycans, and puromycin affects their posttranslational modification. Am J Physiol Renal Physiol 288: F748–F756, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Yagi K: A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med 15: 212–216, 1976 [DOI] [PubMed] [Google Scholar]
- 33.Jeansson M, Haraldsson B: Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J Am Soc Nephrol 14: 1756–1765, 2003 [DOI] [PubMed] [Google Scholar]