Abstract
Toll-like receptors (TLRs) can orchestrate an inflammatory response upon activation by pathogen-associated motifs and release of endogenous stress ligands during tissue injury. The kidney constitutively expresses most TLRs, including TLR4. The function of TLR4 during the inflammation, tubular atrophy, and fibrosis that accompany progressive renal injury is unknown. Here, we subjected wild-type (WT) and TLR4-deficient mice to unilateral ureteral obstruction and observed elevated levels of TLR4 mRNA in the kidney after obstruction. One day after unilateral ureteral obstruction, TLR4-deficient mice had fewer proliferating tubular epithelial cells and more tubular damage than WT mice; however, TLR4-deficient mice developed considerably less renal fibrosis despite decreased matrix metalloproteinase activity and without significant differences in myofibroblast accumulation. In vitro, TLR4-deficient primary tubular epithelial cells and myofibroblasts produced significantly less type I collagen mRNA after TGF-β stimulation than WT cells. The reduced fibrosis in TLR4-deficient mice associated with an upregulation of Bambi, a negative regulator of TGF-β signaling. In conclusion, TLR4 attenuates tubular damage but promotes renal fibrosis by modulating the susceptibility of renal cells to TGF-β. These data suggest that TLR4 signaling may be a therapeutic target for the prevention of renal fibrosis.
Fibroproliferative diseases, including progressive renal disease, are a leading cause of morbidity and mortality worldwide.1 Renal tubular damage, inflammation, and interstitial fibrosis are main predictors for the risk for progression toward end-stage renal failure.2 Progression of renal fibrosis involves a cascade of pathophysiologic processes, including disruption of tubular integrity, a robust inflammatory response, accumulation of (myo)fibroblasts, tubular atrophy, and an increased deposition of extracellular matrix (ECM) components, resulting in fibrogenesis.3–5
The group of Toll-like receptors (TLRs) may be one of the receptor families that orchestrate this cascade of inflammation, myofibroblast accumulation, and fibrosis in the kidney. TLRs can initiate an inflammatory response upon recognition of specific pathogen-associated molecular patterns. It is widely accepted that not only pathogen-associated molecular patterns can trigger TLR-mediated immune responses but endogenous danger molecules that are released upon tissue or cell injury as well.6–11 We already found that several of these endogenous ligands that can potentially activate both TLR2 and TLR49,11–13 are strongly upregulated in murine kidneys after unilateral ureteral obstruction (UUO).14,15 We demonstrated that TLR2 does not play a role in the development of fibrosis or injury after UUO.14 Until now, the role of TLR4 in progressive renal injury and fibrosis has remained unknown. In a model of hepatic fibrogenesis, it was demonstrated that TLR4 can enhance TGF-β signaling and myofibroblast activation, suggesting that TLR4 can function as a molecular link between proinflammatory and profibrogenic signals in liver tissue.16 Interestingly, TLR4 is widely and constitutively expressed in the kidney (e.g., on tubular epithelial cells [TECs]).17,18 We and others have shown that renal-associated TLR2 and TLR4 can induce an exaggerated inflammatory response in the kidney upon acute ischemic renal injury with subsequent detrimental effects on renal histology and function.19–21 To study the role of TLR4 in progressive renal injury and renal fibrosis, we subjected wild-type (WT) and TLR4−/− mice to UUO.
Results
Increased TLR4 mRNA Expression upon UUO
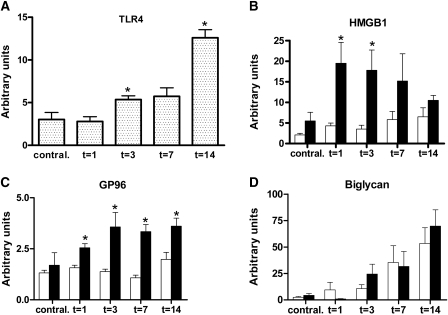
To evaluate the expression of TLR4 and some potential endogenous danger ligands upon progressive renal injury, we quantified TLR4, HMGB1, GP96, and biglycan mRNA levels at several time points after UUO. TLR4 mRNA levels were significantly enhanced 3 and 14 days after UUO compared with contralateral kidneys (Figure 1A).
Figure 1.
Enhanced TLR4 mRNA expression after UUO and higher expression of danger ligands in TLR4−/− kidneys. (A through D) Expression levels of TLR4 mRNA (A) in WT obstructed kidneys and its endogenous danger ligands HMGB1 (B), GP96 (C), and biglycan (D) in WT (□) and TLR4−/− (■) mice after UUO. TLR4 is markedly upregulated upon UUO when compared with contralateral kidneys. HMGB1 and GP96 mRNAs are enhanced in TLR4−/− mice compared with WT mice, whereas biglycan levels are similar. Data are means ± SEM of six mice per group. *P < 0.05.
Interestingly, HMGB1 and GP96 mRNA was higher in obstructed kidneys of TLR4−/− mice after UUO when compared with WT mice, whereas biglycan mRNA expression was similar (Figure 1, B through D). These data suggest a potential role for TLR4 activation and signaling after UUO.
TLR4 Attenuates Tubular Injury in Obstructed Kidneys
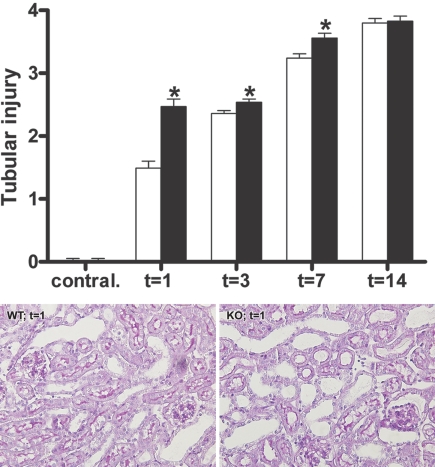
After UUO, WT mice demonstrated progressive tubular injury, such as widespread dilation and epithelial flattening. Interestingly, TLR4−/− mice developed more severe tubular damage in the obstructed kidneys than WT mice at 1, 3, and 7 days after UUO (Figure 2). After 14 days, ESRD was reached in both groups of mice, as demonstrated by maximal score of injury.
Figure 2.
TLR4 attenuates tubular injury after UUO. Semiquantitative score of tubular injury in WT (□) and TLR4−/− (■) mice after UUO. (Top) TLR4−/− mice develop more severe tubular injury when compared with WT mice 1, 3, and 7 days after UUO. Data are means ± SEM of seven to eight mice per group. Contral., contralateral kidneys of mice subjected to 14 days of UUO. *P < 0.05. (Bottom) Representative microphotographs of renal tissue of WT and TLR4−/− mice 1 day after UUO. Magnification, ×200.
Impaired Proliferation Early upon UUO in TLR4−/− Mice
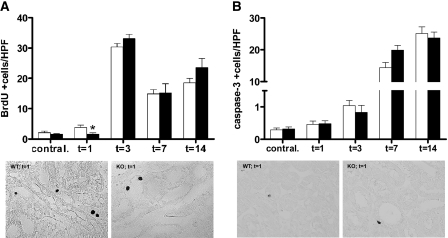
To assess whether the increased tubular injury in TLR4−/− obstructed kidneys was associated with a disturbed balance between proliferation and apoptosis, we quantitatively determined the number of proliferating and apoptotic TECs by immunohistochemistry; 14 days after UUO, we determined the total number of positive cells. The number of proliferating TECs was significantly lower in the obstructed kidneys of TLR4−/− mice 1 day after UUO but similar at later time points (Figure 3A). The number of apoptotic cells was comparable between WT and TLR4−/− mice at all time points (Figure 3B).
Figure 3.
TLR4 deficiency affects early proliferation but not apoptosis of tubular epithelial cells upon UUO. (A and B) Proliferating (A) and apoptotic (B) cells in the obstructed and contralateral kidneys from WT (□) or TLR4−/− (■) mice after UUO. The number of proliferating (BrdU+) TECs is lower in the obstructed kidneys of TLR4−/− mice compared with WT mice 1 day after UUO, whereas there are no differences at later time points. The number of apoptotic (active caspase-3+) cells is similar in obstructed kidneys of WT and TLR4−/− mice at all time points. The number of positive stained TECs is counted in 10 nonoverlapping high-power fields. Because tubular atrophy is very severe 14 days after UUO in both groups, identification of epithelial cells is difficult; therefore, the total amount of positive cells is quantified at this time point. Representative pictures of WT and TLR4−/− mice are of 1 day after UUO. Data are means ± SEM of seven to eight mice per group. Contral., contralateral kidneys of mice subjected to 14 days of UUO. *P < 0.05. Magnification, ×400.
Comparable Inflammatory Response and Influx of Macrophages in Obstructed Kidneys of WT and TLR4−/− Mice
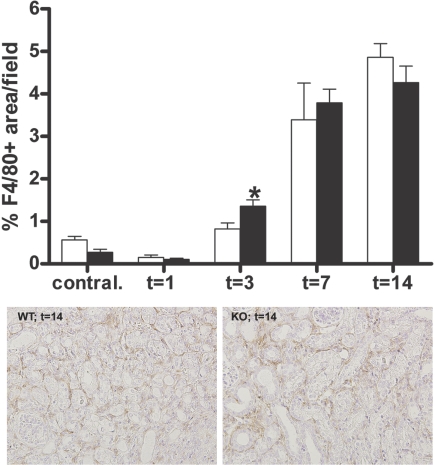
To obtain insight into the role of TLR4 in renal inflammation after UUO, we determined the concentration of the proinflammatory chemokines monocyte chemoattractant protein 1 (MCP-1) and keratinocyte chemoattractant (KC) in renal homogenates and the accumulation of macrophages. No differences were observed in the levels of KC and MCP-1 in renal homogenates of both groups at all time points (data not shown). Macrophage infiltrate progressively increased in the obstructed kidneys after UUO. In line with the chemokine data, no differences were found in the accumulation of macrophages between obstructed kidneys of WT and TLR4−/− mice 1, 7, and 14 days after UUO (Figure 4). Surprisingly, 3 days after UUO, TLR4−/− mice displayed enhanced macrophage influx compared with WT mice.
Figure 4.
TLR4 deficiency does not reduce macrophage accumulation after UUO. (Top) Quantitative analysis of interstitial macrophages in obstructed and contralateral kidneys of WT (□) and TLR4−/− (■) mice after UUO. In WT and TLR4−/− mice, an increase of interstitial macrophages is observed in the obstructed kidneys, peaking at 14 days after UUO. The amount of macrophages is higher in the TLR4−/− mice 3 days after UUO when compared with WT mice. (Bottom) Representative pictures of WT and TLR4−/− mice are of 14 days after UUO. Data are means ± SEM of seven to eight mice per group. Contral., contralateral kidneys of mice subjected to 14 days of UUO. *P < 0.05. Magnification, ×200.
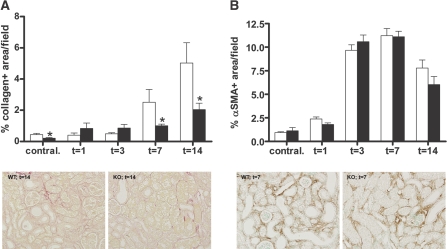
TLR4 Enhances Collagen Deposition but Does not Affect Myofibroblast Accumulation
Total collagen deposition was determined by quantitative analysis of picrosirius red staining. This revealed that WT mice had a progressive increase in the amount of total collagen deposition. Interestingly, total collagen deposition was less in the obstructed kidneys of TLR4−/− mice 7 and 14 days after UUO when compared with WT mice (Figure 5A). Because myofibroblasts are major matrix producing cells in the kidney,22,23 we quantified the presence of myofibroblasts. A strong accumulation of α-smooth muscle actin (α-SMA)-positive myofibroblasts was observed in obstructed kidneys after UUO; however, no differences were observed between WT and TLR4−/− mice at all time points (Figure 5B).
Figure 5.
TLR4 deficiency reduces renal fibrosis but does not affect myofibroblast accumulation after UUO. (A and B) Quantitative analysis of total collagen deposition (A) and myofibroblast accumulation (B) in obstructed and contralateral kidneys of WT (□) and TLR4−/− (■) mice after UUO. (A) The amount of total collagen deposition progressively increases after UUO and is significantly lower in the TLR4−/− mice 7 and 14 days after UUO. (B) No difference is found in the accumulation of α-SMA–positive myofibroblasts between WT and TLR4−/− mice. The percentage of picrosirius red– and α-SMA–positive staining is quantified by digital analysis. Representative pictures of WT and TLR4−/− mice are of 14 days after UUO (A) or 7 days after UUO (B). Data are means ± SEM of seven to eight mice per group. Contral., contralateral kidneys of mice subjected to 14 days of UUO. *P < 0.05.
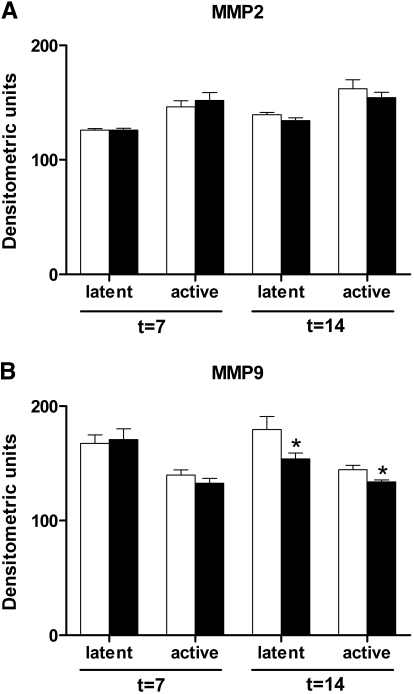
TLR4 Deficiency Lowers MMP9 Activity after UUO
By zymography, we evaluated whether TLR4 deficiency influenced the activity of MMP2 or MMP9. The levels of both latent and active MMP2 were equal in the obstructed kidneys of WT and TLR4−/− mice 7 and 14 days after UUO (Figure 6A). In contrast, a slight but significant decrease in the levels of latent and active MMP9 was found in the obstructed kidneys of TLR4−/− mice when compared with WT mice 14 days after UUO (Figure 6B).
Figure 6.
TLR4 deficiency reduces MMP9 but not MMP2 activity after UUO. (A and B) Quantification of latent and active MMP2 (A) and MMP9 (B) in obstructed kidneys of WT (□) and TLR4−/− (■) mice after UUO. No differences are observed in the renal levels of latent or active MMP2 between WT and TLR4−/− mice. The levels of latent and active MMP9 are significantly lower in the kidneys of TLR4−/− mice 14 days after UUO. Data are means ± SEM of seven mice per group. *P < 0.05.
TLR4 Deficiency Alters TGF-β Susceptibility after UUO
To investigate whether TLR4 deficiency affected the balance between the profibrotic protein TGF-β and the antifibrotic cytokine hepatocyte growth factor (HGF), we determined concentrations of both proteins in kidney homogenates. The concentration of total and active TGF-β protein was similar in homogenates of WT and TLR4−/− mice at all time points (data not shown). In addition, concentrations of HGF were comparable between renal homogenates of WT and TLR4−/− mice, except for 3 days after UUO (1422.1 ± 71.0 versus 891.6 ± 53.3 pg/mg protein of renal homogenate; WT versus TLR4−/−; P = 0.003).
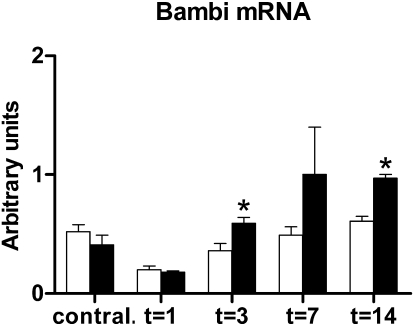
To asses whether TLR4 deficiency altered the susceptibility toward TGF-β signaling, we determined the renal expression of bone morphogenic protein and activin membrane-bound inhibitor (Bambi). Bambi mRNA was significantly elevated 3 and 14 days after UUO in the obstructed kidneys of TLR4−/− mice when compared with kidneys of WT mice (Figure 7).
Figure 7.
TLR4 deficiency enhances renal Bambi mRNA expression after UUO. Bambi mRNA levels in obstructed kidneys of WT (□) and TLR4−/− (■) mice after UUO. Bambi expression is significantly enhanced in obstructed kidneys of TLR4−/− mice compared with WT mice 3 and 14 days after UUO. Data are means ± SEM of six to seven mice per group. Contral., contralateral kidneys of mice subjected to 14 days of UUO. *P < 0.05.
Renal TECs and Myofibroblasts Promote Fibrosis via TLR4
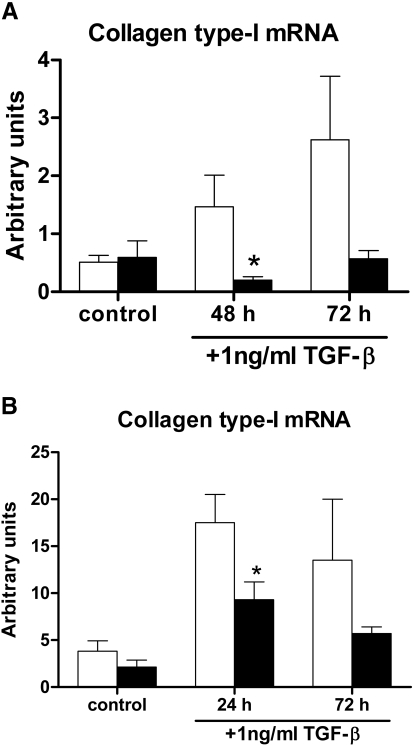
To explore the mechanism by which TLR4 contributes to renal fibrogenesis, we stimulated primary TECs and myofibroblasts of WT and TLR4−/− mice with TGF-β, after which we determined the relative levels of collagen type I mRNA. The level of collagen type I mRNA expression was enhanced in both WT TECs and myofibroblasts after TGF-β stimulation when compared with unstimulated WT TECs and myofibroblasts, respectively. Interestingly, TLR4−/− TECs and myofibroblasts produced significantly less collagen type I mRNA after TGF-β stimulation compared with WT TECs and myofibroblasts, respectively. A similar trend was still observed after 72 hours of TGF-β stimulation (Figure 8).
Figure 8.
Primary renal TECs and myofibroblasts produce type-I collagen in a TLR4-dependent manner after TGF-β stimulation. (A and B) Relative collagen type I mRNA expression by primary TECs (A) and primary myofibroblasts (B) from WT (□) and TLR4−/− (■) mice, when subjected to stimulation with 1 ng/ml TGF-β. Control cells remain for 72 hours in culture medium. TLR4−/− primary TECs and TLR4−/− primary myofibroblasts produce significantly less collagen type I mRNA compared with WT primary TECs and with WT primary myofibroblasts after TGF-β stimulation. *P < 0.05.
Bambi mRNA expression was significantly elevated in both unstimulated TLR4−/− TECs and TLR4−/− TECs that were stimulated for 72 hours with TGF-β when compared with their specific WT control TECs (unstimulated WT versus TLR4−/− TECs 0.47 ± 0.13 versus 1.42 ± 0.51 arbitrary units [AU; P < 0.05]; stimulated WT versus TLR4−/− TECs 0.22 ± 0.06 versus 1.11 ± 0.34 AU [P < 0.05]). Moreover, unstimulated TLR4−/− myofibroblasts showed significant enhanced Bambi mRNA expression when compared with unstimulated WT myofibroblasts (0.17 ± 0.04 versus 0.44 ± 0.16 AU; P < 0.05). After 24 and 72 hours of TGF-β stimulation, TLR4−/− myofibroblasts showed a tendency toward enhanced Bambi expression when compared with WT myofibroblasts (24-hour stimulated WT versus TLR4−/− myofibroblasts 0.21 ± 0.03 versus 0.32 ± 0.08 AU [P = 0.083]; 72-hour stimulated WT versus TLR4−/− myofibroblasts 0.19 ± 0.03 versus 0.28 ± 0.03 AU [P = 0.083]).
Discussion
Irrespective of the primary insult, the final common pathway of many chronic kidney diseases is the development of renal fibrosis. More insights into the primary mechanisms that cause renal fibrosis may contribute to the development of specific therapeutic strategies aimed at blocking or slowing progression of renal diseases.
Most TLRs, including TLR4, are expressed in the kidney and have been shown to play a pivotal role in various experimental models of renal injury and in renal transplantation.24,25 We have reported that the endogenous danger ligands hyaluronan,15 HMGB1, biglycan, and GP96,14 which have the potential to activate TLR2 and TLR4,9,11–13 are significantly upregulated during UUO. We also demonstrated that TLR2 deficiency does not affect fibrogenesis and renal injury during chronic obstructive nephropathy.14 Because these ligands can also activate TLR4, we aimed to elucidate the role of TLR4 in chronic obstructive nephropathy. In this study, we found that TLR4 mRNA is progressively enhanced after UUO. This may reflect an increased expression of TLR4 by TECs and/or a local accumulation of TLR4-positive macrophages and myofibroblasts. In addition, we showed that TLR4 attenuates tubular injury after UUO, which may be a consequence of an early increase in tubular proliferation in WT kidneys compared with TLR4−/− kidneys. This finding is consistent with previous studies demonstrating that TLR4 is involved in cell proliferation.26,27 Another explanation for the protective role of TLR4 on tubular injury could be that TLR4 activation after UUO induces the expression of several adhesion molecules that may be essential for the preservation of tubular architecture and integrity. Indeed, it has already been demonstrated that TLR4 activation can trigger fibroblasts,28 epithelial cells,29 and endothelial cells30 to express several adhesion molecules. In addition, an effect of TLR4 deficiency on epithelial-to-mesenchymal transition processes can also be of importance, because epithelial-to-mesenchymal transition processes contribute to disturbance of the epithelium.31,32 This should be investigated in more detail in the future.
One of the characteristics of tubulointerstitial injury is an excessive inflammatory response, mainly characterized by infiltrating macrophages that contribute to renal fibrosis.33 Because TLRs induce inflammation upon activation, we hypothesized that TLR4 deficiency diminishes inflammation with a subsequent dampening effect on progressive injury in the obstructed kidneys. Surprisingly, inflammatory parameters were comparable in obstructed kidneys of WT and TLR4−/− mice, as reflected by similar levels of renal chemokines KC and MCP-1 and comparable numbers of infiltrating macrophages after UUO, whereas 3 days after UUO, macrophage accumulation was significantly higher in TLR4−/− mice. This result is in sharp contrast with previous findings that showed a severely reduced inflammatory response in kidneys of TLR4−/− mice compared with WT mice after acute renal ischemic injury.20,21 Apparently, TLR4 activation leads to a profoundly different outcome of injury and inflammation during acute (ischemic) or chronic (UUO) renal injury. One possible explanation for the similar degree of inflammation in both groups of mice after UUO is that the increased renal injury in TLR4−/− mice leads to an increased amount of multiple endogenous stress ligands that can activate several receptors, including members of the TLR/IL-1 superfamily. As a consequence, the net effect may be a profound inflammation, thereby neutralizing the negative impact of TLR4 deficiency on inflammation. In support of this hypothesis, we observed that TLR4−/− mice displayed enhanced levels of endogenous danger ligands in obstructed kidneys after UUO, including HMGB1 and GP96, which can also signal via TLR4-independent cascades.9,12 The significance of the higher macrophage accumulation 3 days after UUO in TLR4−/− mice is not clear, however, and further studies will be necessary to elucidate this. Even though TLR4−/− mice showed a similar degree of inflammation compared with WT mice, they displayed dramatically lower amounts of fibrosis. Apparently, the magnitude of inflammation as a result of TLR4 activation does not correlate with the extent of fibrosis.
Our data reveal that despite the extensive tubular injury observed in the TLR4−/− mice after UUO, renal fibrosis is clearly attenuated, as reflected by reduced collagen deposition. This result is in agreement with the report of Seki et al.16 that demonstrated the importance of TLR4 in hepatic fibrosis after bile duct ligation. Moreover, another study demonstrated that fibroblasts produced profibrotic chemokines (e.g., MCP-1) upon activation in a TLR4-dependent manner in a model of systemic sclerosis.34 The reduced fibrosis in our study can be the consequence of a disturbed balance between ECM component synthesis and degradation. We first investigated whether TLR4 deficiency affects the accumulation of myofibroblasts after UUO, because activated fibroblasts can become matrix-producing myofibroblasts by several stimuli that are associated with tissue injury.22 Despite a progressive accumulation of myofibroblasts upon UUO, no differences were observed between WT and TLR4−/− mice. Although these data show that TLR4 does not affect the accumulation of myofibroblasts in obstructed kidneys, it does not rule out a role for TLR4 in the activation of (myo)fibroblasts that subsequently can enhance their matrix-synthesis capacity. Indeed, it has been described that (myo)fibroblasts can express TLR435,36 and that TLR4 drives myofibroblast activation in the liver.16 To determine whether the reduced renal fibrosis observed in the TLR4−/− mice could be ascribed to elevated ECM-degradation, we assessed the activity of MMP2 and MMP9. The role of MMPs and their regulators in tubulointerstitial injury has already been investigated extensively.37–39 Our results showed no difference in the activity of MMP2 between both groups. Surprisingly, we observed that MMP9 activity was slightly lower in renal homogenates of TLR4−/− mice 14 days after UUO. These data suggest that MMP-mediated ECM breakdown is not responsible for the markedly decreased fibrogenesis in TLR4−/− mice.
In progression of renal diseases, TGF-β and HGF exert reciprocal and essential functions. TGF-β is known to promote fibrosis, whereas HGF has been described to act in a renoprotective manner.40,41 To investigate whether TLR4 deficiency alters the balance between TGF-β and HGF, we determined protein levels in the renal homogenates. Despite major differences in renal fibrosis between WT and TLR4−/− mice, similar concentrations were observed for total and active TGF-β protein. In addition, TLR4 deficiency did not alter concentrations of HGF, except for 3 days after UUO. To determine whether TLR4 could affect the susceptibility toward TGF-β signaling, we analyzed the renal levels of Bambi. Previous studies demonstrated that Bambi lacks an intracellular kinase domain and as a consequence inhibited TGF-β signaling by forming nonproductive complexes with TGF-β receptors.42 We observed that TLR4−/− mice had elevated levels of Bambi mRNA when compared with WT mice after UUO. This result is in line with the study of Seki et al.16 revealing that TLR4 can sensitize hepatic stellate cells to TGF-β–mediated signals by downregulation of Bambi. Thus, the lower amount of renal fibrosis that we observed in TLR4−/− mice might be explained by a decreased susceptibility toward TGF-β–mediated fibrosis as a result of higher expression of the negative regulator Bambi.
Finally, we explored the mechanism by which TLR4 contributes to renal fibrogenesis. We found that TECs and myofibroblasts induced a profibrogenic response upon TGF-β stimulation in a TLR4-dependent manner, as reflected by a reduced collagen type I mRNA synthesis by TLR4−/− TECs and TLR4−/− myofibroblasts. Interestingly, TGF-β–stimulated primary TLR4−/− TECs demonstrated elevated Bambi mRNA expression when compared with stimulated WT TECs. We even found elevated Bambi mRNA expression in unstimulated primary TLR4−/− TECs and myofibroblasts compared with nonstimulated WT TECs and myofibroblasts, respectively. Together, these data suggest that Bambi may sensitize TECs and myofibroblasts to TGF-β(–induced signals) and as a consequence to reparative or reactive processes. An alternative explanation is that TECs and myofibroblasts are directly stimulated to produce collagens via TLR4 and stress ligand interaction. Taken together, these data show that TLR4 can increase renal fibrosis most probably by altering the susceptibility of renal cells toward TGF-β signaling, which is associated with a downregulation of Bambi mRNA.
In a previous study, we did not find a role for TLR2 in the development of renal fibrosis and injury after UUO, although TLR2 deficiency reduced the accumulation of myofibroblasts.14 Likely, the contrasting function of TLR2 and TLR4 in this experimental setting reflects the individual functions these molecules may have during progressive renal injury, among which the exclusive link between TLR4 signaling and Bambi-mediated TGF-β susceptibility. Hence, these data highlight the importance of unraveling individual TLR signaling pathways. Despite similarity in potential endogenous ligands (biglycan, HMGB1, and GP96 can activate both TLR2 and TLR4), individual TLRs exert different functions. Indeed, Seki et al.16 reported that TLR4 and not TLR2 were required for hepatic fibrosis.
In conclusion, our results demonstrate that TLR4 exerts important functions during chronic obstructive nephropathy. This study is the first to show that TLR4 attenuates early tubular damage and exerts profibrogenic properties after UUO via increased TGF-β susceptibility. Thus, TLR4 inhibition may be a new therapeutic target to prevent the progression of chronic renal diseases.
Concise Methods
Mice
Pathogen-free 8-week-old female WT C57Bl/6 mice were purchased from Charles River Laboratories. TLR4−/− mice were a gift of Dr. S. Akira and generated as described previously,43 backcrossed six times to a C57Bl/6 background, and bred into the animal facility of the Academic Medical Center in Amsterdam, Netherlands. Only age- and gender-matched mice were used in experiments. The Animal Care and Use Committee of the University of Amsterdam approved all experiments.
Unilateral Ureteral Obstruction
UUO was induced as described previously.15 Briefly, all mice received preoperative analgesia (subcutaneous injection of 50 μg/kg buprenorphin [Temgesic; Shering-Plough]); subsequently, the right ureter was ligated with 6-0 silk through a small abdominal incision under 2.0% isoflurane-induced anesthesia. The abdomen was closed in two layers, and mice were allowed to recover from surgery for 12 hours at 28°C in a ventilated stove. The contralateral nonobstructed kidney served as control. Mice (n = 7 to 8 per group) were killed 1, 3, 7, and 14 days after UUO. For detection of proliferating cells, 5-bromo-2′-deoxyuridine (BrdU; Sigma Chemical Co., St. Louis, MO) was injected intraperitoneally 1 hour before mice were killed (40 mg/kg body wt).
Quantitative Real-Time Reverse Transcription–PCR
Total RNA was extracted from 30 frozen renal tissue sections (20 μm) of WT and TLR4−/− mice with Trizol reagent (Invitrogen) according to the manufacturer's protocol. All RNA samples were quantified by spectrophotometry and stored at −80°C until processed for reverse transcription. RNA was converted to cDNA by using oligo-dT as primer. Mouse TLR4, HMGB1, GP96, biglycan, Bambi, α-SMA, E-cadherin, and collagen type I mRNA expression was analyzed by real-time quantitative reverse transcription–PCR (RT-PCR) performed on a Roche lightcycler with SYBR green PCR master mix. Specific gene expression was normalized to household genes (mouse HPRT, TBP, and CycloG gene expression). SYBR green dye intensity was analyzed with linear regression analysis.
Scoring of Histopathology
Renal tissues were fixed for 24 hours in 10% formalin and subsequently embedded in paraffin. All histopathologic scorings were made in the cortex using Periodic acid-Schiff Diastase–stained renal sections and performed on coded slides. The percentage of tubular injury was estimated by a pathologist in a blinded manner using a four-point scale according to the following criteria: Tubular dilation, cast deposition, brush border loss, and necrosis in 10 randomly chosen, nonoverlapping high-power fields (magnification, ×200). Degree of injury was graded on a scale from 0 to 4: 0 = normal; 1 = mild, involvement of <25% of the cortex; 2 = moderate, involvement of 25 to 50% of the cortex; 3 = severe, involvement of 50 to 75% of the cortex; 4 = extensive damage involving >75% of the cortex.
Detection of Apoptosis and Proliferation
For detection of apoptosis, antigen retrieval was performed by boiling tissue sections of 5 μm for 10 minutes in 10 mM Tris/1 mM EDTA, followed by blocking endogenous peroxidase activity and incubation with rabbit anti-human active caspase-3 polyclonal antibody (Cell Signaling Technology). For staining for BrdU, DNA was denatured in 2 M HCl, and antigen retrieval was performed by 0.4% pepsin in 0.01 M HCl. Sections were subsequently exposed to mouse IgG1 anti-BrdU antibodies (Sigma-Aldrich). The slides were developed using 1% H2O2 and DAB (Sigma-Aldrich) in 0.05 M Tris-HCl (pH 7.9) and counterstained with methyl green (Sigma-Aldrich). Caspase-3 and BrdU immunostainings were quantified by counting the numbers of positive TECs in at least 10 nonoverlapping high-power fields (magnification, ×400). Because tubular atrophy was very severe 14 days after UUO in both groups, identification of epithelial cells was difficult; therefore, the total amount of positive cells was quantified at this time point.
Detection of Macrophage Infiltrates
Renal sections were deparaffinized, and antigen retrieval was performed by 0.1% trypsin digestion (BDH). Slides were subsequently exposed to rat anti-mouse F4/80 IgG2b mAb (Serotec), followed by incubation with rabbit anti-rat biotin (DakoCytomation) and subsequently incubated with streptavidin-ABC solution (DakoCytomation). The slides were developed as described already and counterstained with methyl green. The immunostaining for macrophages was quantified digitally using Image Pro Plus 5.0.
Detection of Fibrosis
For staining of total collagen, slides were incubated with 0.2% picrosirius red solution (pH 2.0) for 1 hour followed by incubation within 0.01 M HCL. For (myo)fibroblast staining, antigen retrieval was performed by boiling for 10 minutes in 10 mM sodium citrate buffer (pH 6.0). Subsequently, slides were exposed to mouse anti-human α-SMA–IgG2a (DAKO), followed by incubation with goat anti-mouse IgG2a–horseradish peroxidase (Southern Biotech). Slides were developed as described already and counterstained with methyl green. The percentage of positive α-SMA and picrosirius red staining was quantified digitally using Image Pro Plus 5.0.
Preparing Kidney Homogenate
For cytokine measurements, snap-frozen kidneys were homogenized in Greenberger Lysis buffer (150 mM NaCl, 15 mM Tris, 1 mM MgCl-H2O, 1 mM CaCl2, and 1% Triton-X) and incubated for 30 minutes at 4°C. Homogenates were subsequently centrifuged at 14,000 rpm for 10 minutes, after which the supernatants were stored at −80°C until ELISAs were performed. For correcting for protein content, Bradford Protein Assay (BioRad) was used with IgG as standard to determine protein levels.
ELISA
Cytokines and chemokines (KC, MCP-1, HGF, and active and latent TGF-β) were measured in kidney homogenates using specific ELISA (R&D Systems) according the manufacturer's protocol. Activation of latent TGF-β was done according to the protocol supplied by the manufacturer. The detection limits were 12 pg/ml (KC), 6 pg/ml (MCP-1), 156 pg/ml (HGF), and 30 pg/ml (TGF-β).
Zymography
Total protein was isolated from frozen renal tissue sections of each animal using RIPA buffer (without protease inhibitors). Equal amounts of protein (12 μg) were loaded onto a 10% SDS-PAGE gel containing 2 mg/ml gelatin-B (Bloom 225; Sigma). Gels were washed twice with 2.5% Triton-X (BioRad Laboratories) and subsequently incubated overnight at 37°C in a buffer containing 50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 5 mM CaCl2 and 0.02% Brij-35 (Sigma). For visualization of MMP activity, gels were stained with Coommassie Brilliant Blue, subsequently destained, and photographed. Levels of latent and active MMP2 and MMP9 were determined by densitometric analysis.
Isolation of Primary Renal TECs and Myofibroblasts and In Vitro Stimulation Assays
Primary renal TECs from WT (n = 4) and TLR4−/− (n = 3) mice were harvested and cultured as described previously21 and grown to confluence in HK2 culture medium, supplemented with 10% FCS, 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine (all Invitrogen), 1% ITSe, and 1% S1 hormone mixture (Sigma). For obtaining primary renal myofibroblasts, WT and TLR4−/− mice (n = 4 per group) were subjected for 7 days to UUO to enhance interstitial fibrosis. Cells of obstructed kidneys were subsequently isolated as described previously21 and cultured for 2 weeks in standard culture medium (RPMI 1640; Life Technologies), supplemented with 10% FCS, 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine (all Invitrogen) to improve selective outgrowth of myofibroblasts. Cultured cells disclosed a clear fibroblast-like morphology by phase-contrast microscopy (elongated spindle-shaped cells). For verification of myofibroblast-like origin of cells, quantitative RT-PCR analysis was performed for E-cadherin and α-SMA as described already. E-cadherin PCR was negative, whereas both genotypes demonstrated equal levels of α-SMA mRNA expression, indicating presence of comparable levels of myofibroblasts between WT and TLR4−/− samples (data not shown). After washing with PBS, cells were serum-starved for 2 hours and subsequently stimulated with 1 ng/ml recombinant human TGF-β (R&D Systems). Unstimulated control cells remained in normal medium for 3 days. After stimulation, total RNA was isolated using Trizol reagent (Invitrogen) and converted to cDNA as described already. As marker for fibrogenesis, collagen type I mRNA levels were measured by RT-PCR in the obtained samples and corrected for housekeeping gene expression.
Statistical Analysis
Differences between groups were analyzed using Mann-Whitney U test. Values are expressed as mean ± SEM. P < 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
We thank Dr. Joris Roelofs for carefully reading the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Wynn TA: Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker GJ, Hewitson TD: The role of tubulointerstitial injury in chronic renal failure. Curr Opin Nephrol Hypertens 9: 133–138, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Eddy AA: Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Klahr S, Morrissey J: Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bascands JL, Schanstra JP: Obstructive nephropathy: Insights from genetically engineered animals. Kidney Int 68: 925–937, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson GB, Brunn GJ, Platt JL: Activation of mammalian Toll-like receptors by endogenous agonists. Crit Rev Immunol 23: 15–44, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Johnson GB, Brunn GJ, Kodaira Y, Platt JL: Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168: 5233–5239, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL: Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem 282: 18265–18275, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E: Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370–7377, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H: HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277: 15107–15112, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ: The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115: 2223–2233, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, Schild H: The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem 277: 20847–20853, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW: Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Leemans JC, Butter LM, Pulskens WP, Teske GJ, Claessen N, van der Poll T, Florquin S: The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS ONE 4: e5704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouschop KM, Sewnath ME, Claessen N, Roelofs JJ, Hoedemaeker I, van der Neut R, Aten J, Pals ST, Weening JJ, Florquin S: CD44 deficiency increases tubular damage but reduces renal fibrosis in obstructive nephropathy. J Am Soc Nephrol 15: 674–686, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF: TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13: 1324–1332, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van't Veer C: In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T: Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 169: 2026–2033, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S: Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ: TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC: Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS ONE 3: e3596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strutz F, Zeisberg M: Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17: 2992–2998, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Qi W, Chen X, Poronnik P, Pollock CA: The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int J Biochem Cell Biol 38: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Anders HJ, Banas B, Schlondorff D: Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867, 2004 [DOI] [PubMed] [Google Scholar]
- 25.El-Achkar TM, Dagher PC: Renal Toll-like receptors: Recent advances and implications for disease. Nat Clin Pract Nephrol 2: 568–581, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT: Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology 131: 862–877, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Graaf R, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F: Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect 8: 1859–1865, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kumagai N, Fukuda K, Fujitsu Y, Lu Y, Chikamoto N, Nishida T: Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Invest Ophthalmol Vis Sci 46: 114–120, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Cetin S, Ford HR, Sysko LR, Agarwal C, Wang J, Neal MD, Baty C, Apodaca G, Hackam DJ: Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem 279: 24592–24600, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Sawa Y, Ueki T, Hata M, Iwasawa K, Tsuruga E, Kojima H, Ishikawa H, Yoshida S: LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J Histochem Cytochem 56: 97–109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wynn TA: Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns WC, Kantharidis P, Thomas MC: The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs 185: 222–231, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Schreiner GF, Harris KP, Purkerson ML, Klahr S: Immunological aspects of acute ureteral obstruction: Immune cell infiltrate in the kidney. Kidney Int 34: 487–493, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Fineschi S, Goffin L, Rezzonico R, Cozzi F, Dayer JM, Meroni PL, Chizzolini C: Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of toll-like receptor 4. Arthritis Rheum 58: 3913–3923, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Otte JM, Rosenberg IM, Podolsky DK: Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology 124: 1866–1878, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, Gay RE, Gay S, Kyburz D: Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum 58: 3684–3692, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Oda T, Lopez-Guisa J, Wing D, Edwards DR, Soloway PD, Eddy AA: TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 12: 736–748, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Nishida M, Okumura Y, Ozawa S, Shiraishi I, Itoi T, Hamaoka K: MMP-2 inhibition reduces renal macrophage infiltration with increased fibrosis in UUO. Biochem Biophys Res Commun 354: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Legallicier B, Trugnan G, Murphy G, Lelongt B, Ronco P: Expression of the type IV collagenase system during mouse kidney development and tubule segmentation. J Am Soc Nephrol 12: 2358–2369, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Leask A, Abraham DJ: TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Klahr S, Morrissey J: Obstructive nephropathy and renal fibrosis: The role of bone morphogenic protein-7 and hepatocyte growth factor. Kidney Int Suppl S105–S112, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C: Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 401: 480–485, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S: Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol 162: 3749–3752, 1999 [PubMed] [Google Scholar]