Abstract
The clinical importance of preexisting HLA antibodies at the time of transplantation, identified by contemporary techniques, is not well understood. We conducted an observational study analyzing the association between preexisting donor-specific HLA antibodies (HLA-DSA) and incidence of acute antibody-mediated rejection (AMR) and survival of patients and grafts among 402 consecutive deceased-donor kidney transplant recipients. We detected HLA-DSA using Luminex single-antigen assays on the peak reactive and current sera. All patients had a negative lymphocytotoxic cross-match test on the day of transplantation. We found that 8-year graft survival was significantly worse (61%) among patients with preexisting HLA-DSA compared with both sensitized patients without HLA-DSA (93%) and nonsensitized patients (84%). Peak HLA-DSA Luminex mean fluorescence intensity (MFI) predicted AMR better than current HLA-DSA MFI (P = 0.028). As MFI of the highest ranked HLA-DSA detected on peak serum increased, graft survival decreased and the relative risk for AMR increased: Patients with MFI >6000 had >100-fold higher risk for AMR than patients with MFI <465 (relative risk 113; 95% confidence interval 31 to 414). The presence of HLA-DSA did not associate with patient survival. In conclusion, the risk for both AMR and graft loss directly correlates with peak HLA-DSA strength. Quantification of HLA antibodies allows stratification of immunologic risk, which should help guide selection of acceptable grafts for sensitized patients.
Anti-HLA immunization constitutes an immunogenetic hurdle to transplantation, leading to increasingly protracted waiting times for sensitized kidney transplant recipients.1–3 In France, 25% of patients on the waiting list have a panel-reactive antibody (PRA) level of >5%,4 and in the United States, 32% of patients awaiting transplantation are sensitized.1 Despite efforts to diminish the risk for sensitization by use of recombinant erythropoietin, leukocyte-depleted transfusions, and the cessation of pregraft transfusion protocols, the number of sensitized patients on transplant lists remains substantial. Moreover, loss of a previous graft has become the primary cause of anti-HLA sensitization.
Patel and Terasaki5 in 1969 demonstrated the efficacy of complement-dependent lymphocytotoxic cross-match (CXM) in defining immunologic risk in renal transplantation. This became the standard method, still used today, for graft allocation. It became clear with time that it did not identify all preexisting donor-specific HLA antibodies (HLA-DSA). In recent years, techniques for detection of HLA antibodies have become more sensitive with the introduction of solid-phase assays, including ELISA, and multiple bead–based technology, of which the Luminex-based assays are the most frequently used. The clinical impact of the antibodies detected by these more sensitive techniques has yet to be fully evaluated in terms of graft survival and definition of acceptable grafts.3 Studies of the clinical relevance of HLA-DSA in patients who receive a transplant with a negative CXM have been contradictory.6–9
The ability to quantify these antibodies10 has added a dimension of complexity to the equation. The semiquantitative ELISA technique was used to advantage by our group in a cohort of 237 patients with renal transplants, showing an increase in the occurrence of acute antibody-mediated rejection (AMR) with increasing HLA-DSA levels detected in historic sera.11 The Luminex technique has been used in recent studies to choose the type of desensitization according to HLA-DSA strength12 and to determine acceptable HLA-DSA levels, allowing for successful kidney transplantation after desensitization.13 Forty years after the initial definition of immunologic risk by Terasaki and Patel, the introduction of these more sensitive techniques revives and carries to a new level the basic question of the clinical relevance of donor-specific anti-HLA antibodies and their integration into current strategies of transplantation. Indeed, no single study has compared the sensitivity, specificity, and positive predictive value (PPV) of classic or flow CXM, ELISA, and Luminex techniques in the prediction of AMR and graft survival.
The objective of this study was to appraise the full clinical potential of HLA-DSAs detected before transplantation, with reference to the previously described ELISA single-antigen technique. We used the capacity of the Luminex technique to identify with precision and to quantify HLA-specific antibodies to grade increasing immunologic risk. This observational, single-center study of 402 consecutive deceased-donor kidney transplant patients examined the impact of the strength of HLA-DSA detected on historic and current sera on the risk for AMR occurrence and graft survival in deceased-donor kidney graft patients. Our graft strategy was the current worldwide strategy based on a negative National Institutes of Health lymphocytotoxic CXM test.
Results
Pretransplantation HLA Antibodies in Kidney Transplant Recipients
Historic (Peak) Sera
Among the 402 renal graft patients, 61 (15.2%) had a PRA level of ≥1%. A total of 118 (29.4%) patients had antibodies against class I or class II HLA on any pretransplantation serum and were considered as sensitized. Of these, 46 (39%) had HLA-DSA identified by ELISA and 83 (70.3%) had HLA-DSA identified by single-antigen flow-beads Luminex testing (peak SAFB HLA-DSA).
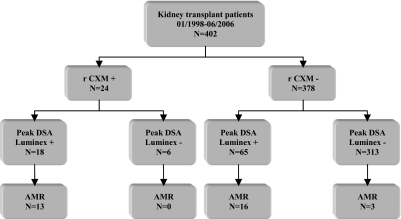
Twenty-four (6%) patients presented a remote positive CXM: Nine patients with IgG T cell complement-dependent cytotoxicity CXM (CDCXM), two patients with IgG T cell antiglobulin enhanced complement-dependent cytotoxicity (AHG-CDCXM), two patients with B cell CXM, eight patients with IgG T and B cell CXM, and three patients with IgG B and T cell AHG-CDCXM. Figure 1 shows the distribution of peak SAFB HLA-DSAs according to the positivity/negativity of remote CXM (rCXM).
Figure 1.
Distribution of donor-specific anti-HLA antibodies in kidney transplant recipients. rCXM+, patients with positive remote CDCXM; rCXM−, patients with negative remote CDCXM; peak HLA-DSA Luminex+, patients with donor-specific HLA antibodies detected by SAFB Luminex technique on the peak sera; peak HLA-DSA Luminex−, patients without donor-specific HLA antibodies detected by SAFB Luminex technique on the peak sera.
Current Sera
Seventy-six (18.9%) patients showed HLA-DSA at the time of transplantation (current SAFB HLA-DSA). The mean of the highest ranked SAFB HLA-DSA mean fluorescence intensity (MFI) detected on the current sera was 1293 ± 200, not significantly different from that of HLA-DSAs detected on peak sera (1137 ± 178; NS). There was no statistically significant intraindividual variation between the maximum SAFB HLA-DSAs of peak and current sera (P = 0.67).
Survival Rates of Patients and Grafts
Patients
The mean follow-up time was 51.4 ± 30.6 months (range 1.0 to 132.0 months). Patient 8-year survival was similar in nonsensitized patients, sensitized patients without peak SAFB HLA-DSA, and patients with peak SAFB HLA-DSA (90.2 versus 91.2 and 90.9%, respectively; P = 0.98).
Grafts
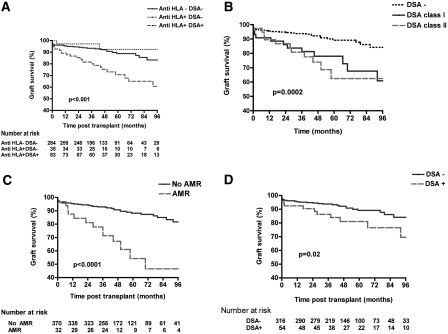
Five- and 8-year death-censored graft survivals were 89.2 and 83.6% in nonsensitized patients, 92.5 and 92.5% in sensitized patients with no HLA-DSA recognized on peak, and 71.2 and 60.8% in patients with HLA-DSA, detected by Luminex technique on the peak sera. Kaplan-Meier analysis revealed that patients with peak SAFB HLA-DSA had a significantly lower graft survival as compared with sensitized patients with no HLA-DSA recognized and nonsensitized patients (P < 0.001, respectively; Figure 2A). There was no difference in graft survival analyzed according to the class of the maximum HLA-DSA identified on the peak sera (P = 0.8). Patients with HLA-DSA had poorer graft survival regardless of whether the maximum HLA-DSA was class I or II (P < 0.0002; Figure 2B).
Figure 2.
The presence of HLA-DSAs on the highest rank pregraft serum associates with a significantly decreased graft survival (A), regardless of whether HLA-DSAs were class I or II (B). (C) The occurrence of acute AMR associates with a significantly decreased graft outcome. (D) Even in the absence of acute AMR, patients with preexisting HLA-DSAs have poorer graft survival as compared to patients without preexisting HLA-DSAs. Donor-specific HLA antibodies are detected by SAFB Luminex technique. P values are calculated with the use of the log-rank test.
Acute AMR Episodes
Acute AMR occurred in 8% of kidney transplant patients. The 5- and 8-year graft survivals of patients who had an episode of AMR were 54.3 and 45.5%, respectively, significantly worse than that of the remaining transplant population (88.5 and 81.9%, respectively; P < 0.0001; Figure 2C). The relative risk (RR) for graft loss for patients who had an episode of AMR was 4.1 (95% confidence interval [CI] 2.2 to 7.7) as compared with patients without AMR. Even in patients without any episode of AMR, the presence of SAFB HLA-DSA on the peak serum was still associated with a significantly lower graft survival as compared with patients without HLA-DSA (69.5 versus 84.4% at 96 months respectively; P = 0.02; Figure 2D).
Peak Sera
Analysis of the PPV, sensitivity, and specificity for AMR of the various methods of identifying preexisting HLA-DSA is shown in Table 1. A positive rCXM has a high predictive performance of 54.2% with a high specificity at 97% but the lowest sensitivity at 40.6%. The Luminex technique has the highest sensitivity of these techniques (90.6%) but a low PPV of 34.9%, whereas the combination of Luminex and rCXM has the highest PPV of 72.2%.
Table 1.
Relationship of pretransplantation anti-HLA antibody status to acute AMR occurrence
| Parameter | Positive Values (n) | Negative Values (n) | AMR |
||
|---|---|---|---|---|---|
| PPV | Sensitivity (%) | Specificity (%) | |||
| PRA ≥1% | 61 | 341 | 36.1 | 68.8 | 89.5 |
| rCXM | 24 | 378 | 54.2 | 40.6 | 97.0 |
| Peak ELISA HLA-DSA | 46 | 356 | 41.3 | 59.4 | 92.7 |
| Peak Luminex HLA-DSA | 83 | 319 | 34.9 | 90.6 | 85.4 |
| Current Luminex HLA-DSA | 76 | 326 | 31.6 | 75.0 | 86.2 |
| rCXM/peak Luminex HLA-DSA | 18 | 384 | 72.2 | 40.6 | 98.7 |
| rCXM/current Luminex HLA-DSA | 20 | 382 | 60.0 | 37.5 | 97.8 |
Current Sera
The presence of SAFB HLA-DSA on the current serum has a PPV for AMR of 31.6% (sensitivity 75%, specificity 86.2%). The incidence of AMR was 40% (24 of 60 patients) in patients with HLA-DSA detectable on both the peak serum and the current serum, versus 21.7% (five of 23 patients) in those with HLA-DSAs detectable on the peak serum but not on the current serum.
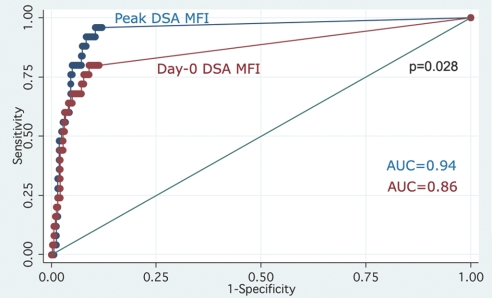
The receiver operating characteristic (ROC) curve analyses of HLA-DSA strength in the prediction of AMR showed that maximum peak HLA-DSA MFI area under the curve (AUC) was significantly higher than that of maximum current HLA-DSA MFI (0.94 ± 0.02 versus 0.86 ± 0.04, respectively; P = 0.028; Figure 3).
Figure 3.
Peak HLA-DSA MFIs are better predictors of acute AMR than current HLA-DSA MFIs. Peak versus current ROC AUC was compared using χ2 test with Bonferroni correction.
Graft Survival and Risk for AMR According to Quantification of Donor-Specific Anti-HLA Antibodies by SAFB Assays
ROC curve analysis determined that an MFI of 465 for the highest single or an MFI of 820 for total HLA-DSA MFI on the peak serum is associated with maximal specificity and sensitivity regarding the occurrence of AMR (AUC of 0.94 ± 0.02 and 0.91 ± 0.02, respectively; each P < 0.0001). The following analysis uses the highest single MFI values.
The prevalence of AMR rises significantly with increasing MFI of highest pregraft HLA-DSA detected by Luminex technique on peak pregraft serum: 0.9% in patients with MFI <465, 18.7% in those with MFI between 466 and 3000, 36.4% for MFI between 3001 and 6000, and 51.3% for patients with MFI >6000 (χ2 = 138.1, P < 0.0001). The RR for AMR according to MFI is shown in Table 2.
Table 2.
RR for acute AMR according to the MFI of highest pregraft ranked DSA detected by Luminex (logistic regression)
| DSA MFImax class | RR (95% CI) | P |
|---|---|---|
| ≤465 | 1.0 | |
| 465 to 1500 | 24.8 (4.6 to 134.8) | <0.001 |
| 1500 to 3000 | 23.9 (3.5 to 160.8) | 0.001 |
| 3000 to 6000 | 61.3 (11.5 to 327) | <0.001 |
| >6000 | 113.0 (30.8 to 414) | <0.001 |
The 1-, 3-, and 8-year graft survivals decrease progressively with rising peak HLA-DSA MFI: 95.0, 93.8, and 82.5% in patients with MFI <465; 100.0, 92.1, and 78.4% for patients with MFIs between 466 and 3000; and 85.0, 75.0, and 60.6% for patients with MFIs >3000 (P < 0.001; Figure 4A). The graft survival in patients with MFIs >3000 was significantly lower than that of patients with MFIs ≤3000 (P < 0.0001).
Figure 4.
The graft survival in patients with preexisting HLA-DSA MFIs >3000 is significantly lower than in patients with HLA-DSA MFIs ≤3000. Kaplan-Meier estimates of graft survival according to the MFImax of preexisting HLA-DSAs in the entire cohort of kidney transplant patients (A) and in the subgroup of patients without acute AMR (B).
The RR for graft loss for patients who underwent transplantation with peak HLA-DSAs >3000 was 3.8 (95% CI 3.5 to 18.4; P < 0.0001) as compared with those with MFI HLA-DSA <3000. These results were also valid in patients without AMR (RR 2.8; 95% CI 1.5 to 16.9; P = 0.009; Figure 4B). In patients with MFIs >3000, 57.1% of the graft losses at 1 year were due to AMR.
Significance of the Association of SAFB HLA-DSA/Remote CDCXM
A total of 18 (21.7%) patients with peak SAFB HLA-DSA had a remote positive IgG T or B cell CXM. Their mean of the maximum HLA-DSA MFI was 7700.6 ± 1139.0, not significantly different from the mean of the maximum HLA-DSA MFI of patients with negative rCXM (5782.6 ± 672.3; NS). In patients with preformed HLA-DSA, the presence of peak complement-fixing HLA-DSA on rCXM increased significantly the risk for AMR (peak HLA-DSA+/rCXM+ versus peak HLA-DSA+/rCXM−; P = 0.0005) and significantly reduced graft survivals (P < 0.01). One- and 8-year graft survivals were 95.6 and 74.5% in patients with peak HLA-DSA+/rCXM− and, respectively, 76.4 and 29.1% in patients with peak HLA-DSA+/rCXM+.
Discussion
This study shows the clinical relevance of precise immunologic characterization of patients before transplantation, using single-antigen flow-beads technology. Long-term outcomes of kidney grafts in patients with preexisting HLA-DSA detected by SAFB Luminex technique are significantly worse as compared with patients who undergo transplantation without HLA-DSA, confirming the recent results reported by Amico et al.9 Our study furthers those observations by showing that there is a gradation of the risk for AMR and of kidney graft survival according to the levels of HLA-DSA detected before transplantation. It also underlines the pertinence and importance of analysis of peak sera by SAFB techniques in defining the immunologic risk for patients on the waiting list. The high sensitivity of Luminex permits definition of the cutoff points above which antibody levels detected before the graft are clinically relevant. We have shown a dramatic increase in the risk for AMR with increasing levels of preexisting HLA-DSA above 465 as detected by Luminex. We have also shown that patients who undergo transplantation with HLA-DSA MFIs >3000 have a 3.8 increased risk for graft loss as compared with patients who undergo transplantation with HLA-DSA MFIs <3000.
Our study also refines the current definition of immunologic risk3,9,14 in specifying the importance of the temporal element (peak and current serum) and of quantification of HLA-DSAs. The combination of different techniques for detecting HLA-DSA contributes to increasing their predictive performance. We have shown here that a precise estimate of immunologic risk before transplantation is possible, much earlier than any CXM assay and in advance of the point where grafts are accepted from deceased donors. This permits transfer of the focus from the simple identification of contraindication to transplantation (i.e., the CXM veto) to a personalized appraisal of immunologic risk and helps define the transplant strategy for any patient on the waiting list. This strategy should help in weighing the risk/benefit ratio on the basis of defined immunologic risk before transplantation. More generally, this early definition of immunologic risk should allow the transplant community to establish (1) an active policy for the transplantation of immunized patients, with priority programs for highly sensitized patients and the reduction of unnecessary shipping of organs, and (2) protocols for immunosuppression therapy and monitoring adapted to the immunologic risk of the recipients.
Avoiding important confounding factors in graft survival, the design of this observational study has permitted an accurate analysis of the clinical relevance of HLA-DSAs. The Luminex analysis was performed retrospectively at the end of the study, avoiding possible bias in the decision to accept the graft and in modifications of immunosuppressive treatment. We have also excluded the patients for whom a pregraft conditioning was performed and patients for whom decisions were made taking into account sensitive techniques for detecting HLA-DSA (living donors, kidney-pancreas transplants). These data are important in the current context of widespread use of sensitive techniques for HLA-DSA detection but with practices varying considerably from one transplantation center to another.
In recent years, a major change in renal transplantation has come from the recognition of the importance of AMR, recognized initially only as the cause of hyperacute rejection but now known to be responsible for acute and chronic lesions.15 Our study underlines that AMR is a major factor in the evolution of HLA-incompatible kidney transplants and is associated with higher rates of graft loss, even though interpretation of the long-term graft survival of patients with AMR suffers from variation of the approach to treatment of AMR over time. For treatment of AMR, we used a specific treatment based on intravenous Ig (IVIg) products known to have powerful immunomodulatory effects.16 Treatment of AMR has evolved from IVIg-based regimens to combination therapies using plasmapheresis, IVIg, and rituximab, leading to an amelioration of short-term graft survival of patients with AMR.17–20 The immunologic and histologic profiles of patients with AMR and poor prognosis have largely been defined.17,21 The concept of quantification of HLA-DSA posttreatment and the optimization of treatment according to the DSA levels17,22 should allow the histologic and immunologic evolution of AMR to be better defined over the long term as well.
Importantly, our study shows that even in the absence of clinical AMR, the long-term graft course is worse in patients with preexisting HLA-DSA. The recently described entity of subclinical AMR23,24 in which progressive morphologic lesions are found on biopsy in the absence of overt clinical rejection may account for this different course. A recent study demonstrated that subclinical AMR is a frequent finding in patients with preformed HLA-DSA (31.1% at 3 months) and is associated with worse GFR at 1 year.25 These progressive lesions lead to chronic humoral rejection, first described in 200126 and now recognized to be a distinct cause of late graft dysfunction and loss.25,27,28
Our study shows the major impact that pregraft Luminex-based HLA-specific antibody screening is having on the field of transplantation. The Luminex technique is more sensitive in detection of HLA-DSAs than the other two techniques analyzed, ELISA and CDCXM. Luminex analysis permits pregraft characterization of the antibody profiles in sensitized patients and gives improved definition of safe (antibody-negative) and at-risk (antibody-positive) HLA specificities. The first step in the transplant strategy for sensitized patients is to define whether a graft with minimal immunologic risk is possible. Whenever possible, kidney transplantation should be performed in the absence of DSAs. Virtual cross-matching, recently promoted by the United Network for Organ Sharing,29 consists of selecting potential donors without HLA specificities against a recipient's antibodies, predicting a negative CXM. The use of the virtual cross-matching permits expanding the geographic regions from which kidneys can be drawn and reduces waiting time and deaths on the waiting list.30–32 It is, furthermore, a good indicator for reducing the risk of AMR.33
In practice, we must evaluate, for each sensitized patient on the waiting list, whether the listing of forbidden antigens permits a donor pool sufficient to ensure transplantation. For some highly sensitized patients, use of virtual cross-matching leads to an insufficient number of donors. Thus, alternatively, the access to transplantation for these patients can be augmented in three ways: Priority programs, desensitization regimens, or increased immunologic risk of transplantation with preexisting HLA-DSA.
Priority programs such as the Eurotransplant “Acceptable Mismatch” program34 raise the probability for a hypersensitized patient to receive a kidney with a negative CXM,35 with excellent graft survivals.34 Desensitization protocols based on high-dosage IVIgs36–38 have been used with success. Other therapies39,40 can be efficacious in desensitizing patients who are awaiting transplantation. Transplantation in the presence of HLA-DSA requires a careful estimation of the immunologic risk, as assessed by rCXMs and levels of antibodies detected by Luminex technique. For such patients at high immunologic risk, specific posttransplantation protocols41–44 and close monitoring are indispensable.
Access to and results of transplantation can therefore be improved in sensitized patients. The data presented in this article emphasize the importance of precisely characterizing the status of the anti-HLA pretransplantation immunization using sensitive techniques. This should allow optimization of donor immunologic selection, immunosuppressive regimen, and posttransplantation monitoring.
Concise Methods
Patients
The study included 402 consecutive deceased-donor single-organ kidney transplants recipients who underwent transplantation in Saint-Louis Hospital (Paris, France) between January 1998 and June 2006. Patients with multiple transplants (20 patients with combined liver-kidney transplants and 14 with pancreas-kidney transplants), living-donor transplants (36 patients), or pregraft conditioning (17 patients) and those for whom historic sera were not available for further research (24 patients) were excluded.
All patients were followed up through January 2009. Data on the HLA typing of transplant donors and recipients and rCXM results were recorded on day 0 (day of transplantation). Data on survival of patients and grafts, AMR episodes, serum creatinine values, and posttransplantation immunosuppressive therapy were obtained at months 3 and 6; at years 1, 3, 5, and 8; and at the end of follow-up.
Criteria for Accepting a Donor
CXMs were performed by complement-dependent cytotoxicity (CDCXM) and T cell antiglobulin enhanced complement-dependent cytotoxicity (AHG-CDCXM) on lymph nodes and by complement-dependent cytotoxicity on separated B lymphocytes or spleen cells, according to National Institutes of Health recommendations. Peak and current sera were tested for all patients according to European Federation for Immunogenetics standards. Sera were tested both diluted and undiluted, with and without dithiothreitol. For all kidney transplant recipients, negative current IgG T cell and B cell CDCXMs were required. CXMs positive only for IgM were not considered as a contraindication to transplantation.
Detection of HLA Antibodies
HLA typing of transplant recipients was performed by molecular biology (Innolipa HLA typing kit; Innogenetics, Gent, Belgium). For all kidney transplant donors, HLA-A/B/DR/DQ tissue typing was performed using the microlymphocytotoxicity technique with One Lambda Inc. tissue-typing trays and was controlled by molecular biology. HLA-CW and HLA-DP typing of the donor was performed when an isolated HLA-CW or HLA-DP HLA-DSA was potentially present.
All pretransplantation (historic) sera were screened by the most sensitive routine screening test available at the beginning of the study, ELISA assays (LAT-M; One Lambda, Canoga Park, CA), to determine the presence or absence of HLA class I or class II antibodies of the IgG isotype. HLA class I antibodies were then identified by complement-dependent cytotoxicity on a frozen cell tray of 30 selected HLA-typed lymphocytes (Serascreen FCT30 Frozen Cell Trays; Gen Trak, Liberty, NC). PRAs of the IgG class directed against HLA class I molecules were calculated from this complement-dependent cytotoxicity assay.
All patients in whom HLA antibodies were detected were screened for the presence of HLA-DSA in pretransplantation peak sera by two techniques: ELISA and SAFB Luminex assays. Peak serum was determined on the basis of the serum's having the highest % PRA. The interval between the peak serum values and the actual transplantation was 29.4 ± 11.8 months.
Detection of HLA-DSA Using ELISA
Identification of ELISA HLA-DSA class I was done using a high-definition single-antigen ELISA (LAT-1HD; One Lambda). For ELISA HLA-DSA class II identification, we performed an ELISA (LAT 2-40; One Lambda) test, which identified DR and DQ subtypes on a panel of purified HLA antigens. Both ELISA tests were performed as recommended by the manufacturer and described previously.11 Detection of HLA-DSA by ELISA on the peak sera was performed retrospectively between June and November 2006.
Detection of HLA-DSA Using SAFB on Luminex Platform
Identification of class I and class II HLA-DSA by Luminex analysis (One Lambda) uses sets of 96 beads (class I) and 76 beads (class II), respectively; each bead is coated with a single HLA glycoprotein, permitting precise identification of antibody specificity. Presence of antibodies was detected using a goat anti-human IgG coupled with phycoerythrin. The fluorescence of each bead was detected by a reader (LABscan) and recorded as the MFI. All beads showing a normalized MFI >300 were considered positive. For each patient, we recorded the number of HLA-DSA and the maximum HLA-DSA MFI defined as the highest ranked donor-specific bead.
All current (day 0) sera were screened for the presence of HLA-DSA by class I and class II SAFB Luminex assays. Luminex analyses were performed retrospectively between January and March 2009 for the peak sera and in November 2009 for the current sera.
Posttransplantation Induction Protocols and Maintenance Immunosuppressive Therapy
Immunosuppression protocols were defined according to the immunologic risk, determined by the current system using lymphocytotoxic PRAs and T and B cell–based assays. Patients received induction therapy consisting of rabbit antithymocyte globulin (1.5 mg/kg per d for 10 days; Thymoglobulin; Genzyme) with maintenance immunosuppression consisting of tacrolimus (Prograf; Astellas) or cyclosporine (Neoral; Novartis), mycophenolate mofetil (CellCept; Roche), and steroids. Patients with remote positive IgG T and B cell CXM received IVIg at the time of transplantation (2 g/kg body wt on days 0 to 1, 20 to 21, and 40 to 41).
Diagnosis and Treatment of Acute AMR Episodes
Among the patients with clinical acute graft dysfunction, three had episodes of borderline changes, 18 had episodes of acute T cell–mediated rejection (IA for six patients, IB for three patients, IIA for four patients, IIB for two patients, and III for three patients), and 32 patients had episodes of AMR. All rejection episodes were biopsy-proven. Biopsy specimens were evaluated by light microscopy and immunofluorescence (C4d and Igs). Findings were graded according to the Banff '07 classification.45 C4d was detected by a two-step indirect immunofluorescence method using a mAb specific for complement fragment C4d on frozen tissue (Quidel, Santa Clara, CA). All patients with AMR had characteristic histologic lesions delineated by the Banff classification of allograft rejection,46 positive C4d staining, and HLA-DSAs detected by SAFB assays at the time of diagnosis.
All patients with AMR were treated with methylprednisolone pulses (500 mg/d for 3 days), with switch to tacrolimus for patients who were previously on cyclosporine and a protocol of high-dosage IVIg (2 g/kg, repeated every 3 weeks for four administrations). Between January 1998 and December 2003, eight patients received an additional treatment with plasma exchange (five patients) or muromonab-CD3 (OKT3; three patients). After January 2004, all patients received as additional treatment four plasmaphereses and two weekly doses of rituximab (375 mg/m2 body surface area; MabThera; Roche, Meylan, France).17
Statistical Analysis
Results are expressed as mean ± SD for continuous variables, with the exception of MFIs, or which mean ± SEM is used. Comparisons were based on the χ2 test for categorical data and the t test for normally distributed continuous data. For parameters without Gaussian distribution (MFI), the Mann-Whitney U test was used. For individual HLA-DSA MFI evolution between peak and current sera, the Wilcoxon matched-pairs test was used. Death-censored graft survivals were calculated by Kaplan-Meier analysis. Differences between survivals were calculated by log-rank analysis. P < 0.05 was regarded as statistically significant.
For studying the usefulness of HLA-DSA MFIs as a predictor of AMR, ROC curves were plotted to estimate the cutoff of MFImax HLA-DSA and total HLA-DSA MFIs in terms of yielding the highest sensitivity + specificity/2. We determined the AUC to evaluate its significance. For peak versus current ROC curves, AUC were compared using χ2 test with Bonferroni correction.
The association of AMR occurrence and MFI strength classes was determined by univariate logistic regression. The risk for graft failure according to the occurrence of AMR and HLA-DSA MFIs was determined using univariate Cox analyses.
All tests were two-sided. All statistical analyses were performed using STATA 10.0 software (Stata Corp., College Station, TX).
Disclosures
None.
Acknowledgments
We thank Astellas France for the contribution to the statistical analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Organ Procurement and Transplantation Network: Scientific registry of transplant recipients. Available at: http://optn.transplant.hrsa.gov/data/ Accessed May 21, 2010 [DOI] [PubMed]
- 2.Cho YW, Cecka J: Crossmatch tests: An analysis of UNOS data from 1991 to 2000. In: Clinical Transplants, edited by Terasaki P, Los Angeles: UCLA Immunogenetics Center, 2001, pp 237–246 [PubMed] [Google Scholar]
- 3.Gebel HM, Bray RA, Nickerson P: Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant 3: 1488–1500, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Rapport d'activité de prélèvement et de greffe Agence de la Biomédecine. Available at: http://www.agence-biomedecine.fr/uploads/document/RA_1_2_entier.pdf Accessed May 26, 2010
- 5.Patel R, Terasaki PI: Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280: 735–739, 1969 [DOI] [PubMed] [Google Scholar]
- 6.Vlad G, Ho EK, Vasilescu ER, Colovai AI, Stokes MB, Markowitz GS, D'Agati VD, Cohen DJ, Ratner LE, Suciu-Foca N: Relevance of different antibody detection methods for the prediction of antibody-mediated rejection and deceased-donor kidney allograft survival. Hum Immunol 70: 589–594, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Patel AM, Pancoska C, Mulgaonkar S, Weng FL: Renal transplantation in patients with pre-transplant donor-specific antibodies and negative flow cytometry crossmatches. Am J Transplant 7: 2371–2377, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Iveson V, Varagunam M, Bodger S, Sinnott P, Thuraisingham RC: Pretransplant donor-specific antibodies in cytotoxic negative crossmatch kidney transplants: Are they relevant? Transplantation 85: 1200–1204, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Amico P, Honger G, Mayr M, Steiger J, Hopfer H, Schaub S: Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation 87: 1681–1688, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Mizutani K, Terasaki P, Hamdani E, Esquenazi V, Rosen A, Miller J, Ozawa M: The importance of anti-HLA-specific antibody strength in monitoring kidney transplant patients. Am J Transplant 7: 1027–1031, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, Gautreau C, Charron D, Glotz D: Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant 8: 324–331, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Akalin E, Dinavahi R, Friedlander R, Ames S, de Boccardo G, Sehgal V, Schroppel B, Bhaskaran M, Lerner S, Fotino M, Murphy B, Bromberg JS: Addition of plasmapheresis decreases the incidence of acute antibody-mediated rejection in sensitized patients with strong donor-specific antibodies. Clin J Am Soc Nephrol 3: 1160–1167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinsmoen NL, Lai CH, Vo A, Cao K, Ong G, Naim M, Wang Q, Jordan SC: Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation 86: 820–825, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Bray RA, Nolen JD, Larsen C, Pearson T, Newell KA, Kokko K, Guasch A, Tso P, Mendel JB, Gebel HM: Transplanting the highly sensitized patient: The Emory algorithm. Am J Transplant 6: 2307–2315, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gloor J, Cosio F, Lager DJ, Stegall MD: The spectrum of antibody-mediated renal allograft injury: Implications for treatment. Am J Transplant 8: 1367–1373, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kazatchkine MD, Kaveri SV: Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 345: 747–755, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C: Comparison of combination plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 9: 1099–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Becker YT, Becker BN, Pirsch JD, Sollinger HW: Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant 4: 996–1001, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Faguer S, Kamar N, Guilbeaud-Frugier C, Fort M, Modesto A, Mari A, Ribes D, Cointault O, Lavayssiere L, Guitard J, Durand D, Rostaing L: Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation 83: 1277–1280, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Mulley WR, Hudson FJ, Tait BD, Skene AM, Dowling JP, Kerr PG, Kanellis J: A single low-fixed dose of rituximab to salvage renal transplants from refractory antibody-mediated rejection. Transplantation 87: 286–289, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, Glotz D: Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 7: 832–841, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES: Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 9: 1063–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Gloor JM, Cosio FG, Rea DJ, Wadei HM, Winters JL, Moore SB, DeGoey SR, Lager DJ, Grande JP, Stegall MD: Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant 6: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Haas M, Montgomery RA, Segev DL, Rahman MH, Racusen LC, Bagnasco SM, Simpkins CE, Warren DS, Lepley D, Zachary AA, Kraus ES: Subclinical acute antibody-mediated rejection in positive crossmatch renal allografts. Am J Transplant 7: 576–585, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, Martinez F, Thervet E, Mejean A, Charron D, van Huyen JP, Bruneval P, Legendre C, Nochy D: Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant 9: 2561–2570, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, Williams WW, Cosimi AA, Schneeberger EE, Colvin RB: Chronic humoral rejection: Identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol 12: 574–582, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Regele H, Bohmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, Watschinger B, Kerjaschki D, Exner M: Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol 13: 2371–2380, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, Griffin MD, Larson TS, Cosio FG: Transplant glomerulopathy: Subclinical incidence and association with alloantibody. Am J Transplant 7: 2124–2132, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Nikaein A, Cherikh W, Nelson K, Baker T, Leffell S, Bow L, Crowe D, Connick K, Head MA, Kamoun M, Kimball P, Klohe E, Noreen H, Rebellato L, Sell T, Sullivan K, Land G: Organ Procurement and Transplantation Network/United Network for Organ Sharing Histocompatibility Committee collaborative study to evaluate prediction of crossmatch results in highly sensitized patients. Transplantation 87: 557–562, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Zangwill SD, Ellis TM, Zlotocha J, Jaquiss RD, Tweddell JS, Mussatto KA, Berger S: The virtual crossmatch: A screening tool for sensitized pediatric heart transplant recipients. Pediatr Transplant 10: 38–41, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Zangwill S, Ellis T, Stendahl G, Zahn A, Berger S, Tweddell J: Practical application of the virtual crossmatch. Pediatr Transplant 11: 650–654, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Zachary AA, Sholander JT, Houp JA, Leffell MS: Using real data for a virtual crossmatch. Hum Immunol 70: 574–579, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Tambur AR, Ramon DS, Kaufman DB, Friedewald J, Luo X, Ho B, Skaro A, Caicedo J, Ladner D, Baker T, Fryer J, Gallon L, Miller J, Abecassis MM, Leventhal J: Perception versus reality? Virtual crossmatch—How to overcome some of the technical and logistic limitations. Am J Transplant 9: 1886–1893, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claas FH, Witvliet MD, Duquesnoy RJ, Persijn GG, Doxiadis II: The acceptable mismatch program as a fast tool for highly sensitized patients awaiting a cadaveric kidney transplantation: Short waiting time and excellent graft outcome. Transplantation 78: 190–193, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Doxiadis II, Duquesnoy RJ, Claas FH: Extending options for highly sensitized patients to receive a suitable kidney graft. Curr Opin Immunol 17: 536–540, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Glotz D, Antoine C, Julia P, Suberbielle-Boissel C, Boudjeltia S, Fraoui R, Hacen C, Duboust A, Bariety J: Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg). Am J Transplant 2: 758–760, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Jordan SC: Management of the highly HLA-sensitized patient: A novel role for intravenous gammaglobulin. Am J Transplant 2: 691–692, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Jordan SC, Tyan D, Stablein D, McIntosh M, Rose S, Vo A, Toyoda M, Davis C, Shapiro R, Adey D, Milliner D, Graff R, Steiner R, Ciancio G, Sahney S, Light J: Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: Report of the NIH IG02 trial. J Am Soc Nephrol 15: 3256–3262, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S: A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant 6: 346–351, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC: Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 359: 242–251, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Akalin E, Bromberg JS: Intravenous immunoglobulin induction treatment in flow cytometry cross-match-positive kidney transplant recipients. Hum Immunol 66: 359–363, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Akalin E, Ames S, Sehgal V, Murphy B, Bromberg JS, Fotino M, Friedlander R: Intravenous immunoglobulin and thymoglobulin induction treatment in immunologically high-risk kidney transplant recipients. Transplantation 79: 742, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Anglicheau D, Loupy A, Suberbielle C, Zuber J, Patey N, Noel LH, Cavalcanti R, Le Quintrec M, Audat F, Mejean A, Martinez F, Mamzer-Bruneel MF, Thervet E, Legendre C: Posttransplant prophylactic intravenous immunoglobulin in kidney transplant patients at high immunological risk: A pilot study. Am J Transplant 7: 1185–1192, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Burns JM, Cornell LD, Perry DK, Pollinger HS, Gloor JM, Kremers WK, Gandhi MJ, Dean PG, Stegall MD: Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant 8: 2684–2694, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria: An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]