Abstract
The highly conserved intraflagellar transport (IFT) proteins are essential for cilia formation in multiple organisms, but surprisingly, cilia form in multiple zebrafish ift mutants. Here, we detected maternal deposition of ift gene products in zebrafish and found that ciliary assembly occurs only during early developmental stages, supporting the idea that maternal contribution of ift gene products masks the function of IFT proteins during initial development. In addition, the basal bodies in multiciliated cells of the pronephric duct in ift mutants were disorganized, with a pattern suggestive of defective planar cell polarity (PCP). Depletion of pk1, a core PCP component, similarly led to kidney cyst formation and basal body disorganization. Furthermore, we found that multiple ift genes genetically interact with pk1. Taken together, these data suggest that IFT proteins play a conserved role in cilia formation and planar cell polarity in zebrafish.
The cilium is a cell surface organelle that is almost ubiquitously present on vertebrate cells. Protruding from the cell into its environment, the cilium is involved in multiple signaling pathways, including the Sonic hedgehog (Shh) pathway, the Wnt pathways, and the target of rapamycin (TOR) pathway.1–5 Not surprisingly, defects in the cilium have been linked to a growing list of human diseases, coined “ciliopathies,” ranging from laterality defects, retinal degeneration, polycystic kidney disease (PKD), and other hepatorenal fibrocystic disorders to obesity and diabetes (for a review, see reference 6).
Many studies have demonstrated that the formation and maintenance of the cilium depends on intraflagellar transport (IFT) particles, which are composed of at least 17 polypeptides.7,8 These IFT particles move along microtubules in cilia and are thought to act as vehicles for transporting cargos needed for the assembly, maintenance, and function of cilia. Homologs of IFT proteins have been found in a wide spectrum of organisms including Caenorhabditis elegans, Drosophila, and mammals and have also been shown to be required for cilia formation.3,9–12
In zebrafish, mutants of ift57, ift81, ift88, and ift172 have numerous defects commonly associated with ciliary abnormalities.13,14 However, cilia in these mutants are able to form initially but degenerate over time, giving rise to the hypothesis that IFT is essential for cilia maintenance rather than cilia assembly in zebrafish.14 Interestingly, products of many genes in zebrafish are deposited maternally. One hypothesis for the initial formation of cilia in zebrafish ift mutants is that maternal contribution of ift gene products masks the function of ift genes during early embryonic development. Accordingly, cilia formation is severely impaired in a maternal-zygotic mutant of ift88.15 In this study, we demonstrate that products of ift57 and ift172 are indeed maternally deposited. We further show that although cilia destined to form early in development show only maintenance defects in ift57hi3417 and ift172hi2211 mutants, cilia programmed to assemble later in development fail to form, providing further support for a conserved role of IFT in cilia formation in zebrafish.
One of the most extensively studied phenotypes associated with ciliary defects is the formation of kidney cysts. Both the canonical and the noncanonical Wnt pathway, or planar cell polarity (PCP) pathway, have been implicated in kidney cyst formation.4,16–21 However, in contrast to the well-established role of cilia in the hedgehog pathway,2,3,22 the role of cilia in the Wnt pathways is unclear.4,15,23,24 In this study, we demonstrate that, in the kidney duct of ift57hi3417 and ift172hi2211 mutants, the organization of basal bodies is impaired, a phenotype consistent with compromised PCP signaling. We further show that knockdown of prickle 1 (pk1), a core PCP player, leads to disorganization of basal bodies and kidney cyst formation. Finally, we provide evidence that ift57, ift88, and ift172 genetically interact with pk1 in convergence-extension (CE) movements during gastrulation, a process regulated by the PCP pathway. Together, these data support an intricate relationship between IFT and the PCP pathway.
Results
hi3417 and hi2211 are Zygotic Mutants of ift57 and ift172, Respectively
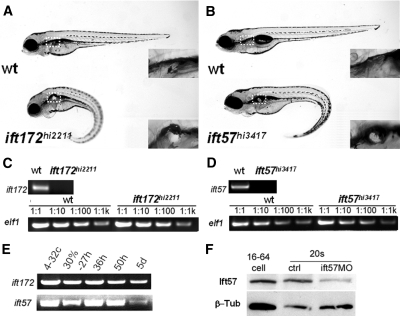
ift57hi3417 and ift172hi2211 were isolated from an insertional mutagenesis screen in zebrafish.13 Both mutants show similar morphologic phenotypes, including a ventrally curved body axis, kidney cyst formation in the glomerular-tubular region, and dilated kidney ducts (Figure 1, A and B; Supplemental Figure S1; and reference 13). In both mutants, the responsible proviral insertion is located in the 5′ side of the corresponding genes. Reverse transcription-polymerase chain reaction (RT-PCR) with primers spanning the proviral insertion site revealed that the wild-type transcripts are completely absent in each mutant (Figure 1, C and D).
Figure 1.
hi3417 and hi2211 are zygotic loss-of-function mutants of ift57 and ift172, respectively. (A and B) A wild-type sibling and a mutant at 4 dpf in side views. Box with dashed line: cyst (in mutant) and the lack of cyst (in wild-type sibling) enlarged in insets. (C and D) Loss of wild-type ift172 and ift57 transcripts in hi2211 and hi3417 mutants at 33 hpf, respectively. Upper panels: RT-PCR with a pair of primers spanning the proviral insertion site. Lower panels: loading controls with a pair of elf1-specific primers using cDNAs serially diluted 1:1, 1:10, 1:100, and 1:1000. (E) RT-PCR time course for ift172 and ift57 using wild-type samples. (F) Western blot against Ift57 (Ift57). β-Tub is used as loading control. β-Tub, β-Tubulin; ctrl, uninjected embryo control; ift57MO, ift57 morphants; wt, wild type; 4-32c, 4- to 32-cell stage; 30%–27h, mixture of samples from 30% epiboly to 27 hpf; 36h, 36 hpf; 50h, 50 hfp; 5d, 5dpf; 16–64 cell, sample from embryos at the 16- to 64-cell stage; 20s, samples from embryos at the 20-somite stage.
To further verify that inactivation of ift57 and ift172 are responsible for phenotypes seen in hi3417 and hi2211, respectively, we injected mRNA of each gene into embryos from heterozygous carriers of corresponding mutations. Results showed that ift172 mRNA can reduce the frequency of phenotypic embryo from 26 ± 0 to 6 ± 3% (P = 0.01, results from two independent experiments). Similarly, injection of ift57-GFP (GFP, green fluorescent protein) mRNA reduces the percentage of phenotypic embryos from 25 ± 3 to 2 ± 2% (P < 0.001, n = 3). These findings indicate that these two mutants can be rescued by overexpression of their corresponding gene.
In zebrafish, zygotic transcription commences at around the 512-cell stage.25 However, transcripts of a number of cilia-associated genes are maternally deposited and genes can be detected before this stage. To investigate for maternal contribution, we performed RT-PCR on samples from wild-type embryos at multiple developmental stages. The presence of ift57 and ift172 mRNA from embryos at the 4- to 32-cell stage verified that these mRNAs are maternally deposited (Figure 1E).
We further investigated whether IFT proteins can be detected before the maternal-zygotic transition by Western blot analysis. Results show that Ift57 can be readily detected at the 16- to 64-cell stage (Figure 1F). Any Ift57 protein present at this stage could be maternally deposited, translated from maternal mRNA, or both. Regardless of the source, the presence of an IFT protein at such an early stage suggests that it would be difficult to deplete IFT proteins completely with morpholino oligos, including translation-blocking morpholino oligos. To test this notion more directly, we used a morpholino oligo against the translation initiation site of ift57. Indeed, a significant quantity of Ift57 protein can still be detected in ift57 morphants, even when they are all phenotypic (Figure 1F).
Cilia Formation Is Affected in ift57 and ift172 Mutants in a Time-Dependent Manner
There are conflicting reports on the status of cilia formation in zebrafish ift mutants.14,15,26 Because cilia formation starts at different time points in different organs, the differences observed may be attributed to the gradual decay of maternally deposited gene products. Early in development, cilia are able to form using maternally contributed gene products, whereas later in development, with the decay of maternal contribution, cilia are unable to form. To test this hypothesis, we followed cilia formation in multiple organs in detail.
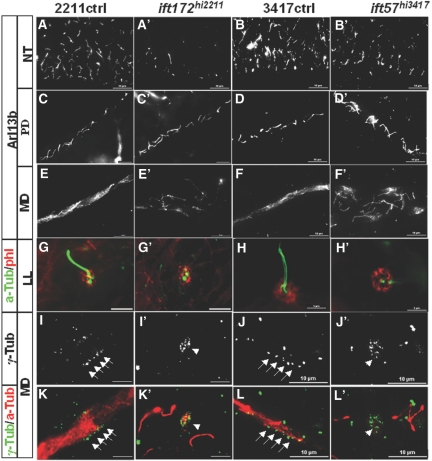
Cilia in the neural tube can be detected at 24 hpf (hours post-fertilization) and persist thereafter. We examined cilia formation at 24 hpf in at least 30 embryos from heterozygous carriers for each experiment. No significant difference was seen in any of the embryos, suggesting that cilia are able to form in the neural tube (data not shown). By contrast, at 34 hpf, a stage at which mutants can be reliably identified by their body curvature phenotype, cilia number in both mutants are drastically reduced (Figure 2, A through B'). Interestingly the reduction of cilia number in ift172hi2211 mutants is more pronounced than that in ift57hi3417 mutants (Figure 2, A through B'). Variations in mRNA or protein stability may account for this difference.
Figure 2.
Cilia formation is disrupted in ift172hi2211 and ift57hi3417 mutants in a time-dependent manner. (A through B') Cilia in the neural tube at 34 hpf as shown by anti-Arl13b labeling. (C through D') Cilia in the posterior pronephric duct at 2 dpf as shown by anti-Arl13b labeling. (E through F') Cilia in the MD at 2 dpf as shown by anti-Arl13b labeling. (G through H') Cilia in the lateral line organ as shown by anti–a-Tub in green at 2 dpf. phl labeling in red shows the presence of the LL organ. (I through L') Basal bodies in the MD at 3 pdf as shown by γ-Tub labeling. Anti–a-Tub in (K through L') labeled cilia. Arrows point to arrays of basal bodies, whereas arrowheads point to disorganized clusters of basal bodies. a-Tub, anti-acetylated Tubulin; γ-Tub, anti-γ-Tubulin; LL, lateral line; MD, mid region pronephric duct; NT, neural tube; PD, posterior pronephric duct; phl, Phalloidin; 2211ctrl, wild-type sibling of ift172hi2211; 3417ctrl, wild-type sibling of ift57hi347.
The zebrafish pronephric duct contains both single-ciliated cells (SCCs) and multiciliated cells (MCCs).27,28 Initially, at 24 hpf, most cells in the pronephric duct display a single cilium. By 2 dpf (days postfertilization), MCCs can be found in the mid region of the duct, whereas SCCs can be found in both the mid and posterior region of the duct. In both ift57hi3417 and ift172hi2211 mutants, there is no detectable difference in cilia in the pronephric duct at 24 hpf (data not shown). At 2 dpf, cilia in the posterior duct still appear relatively unaffected (Figure 2, C through D'). By contrast, severe ciliary defects can be readily observed in the mid region of the duct (Figure 2, E through F'). A simple explanation is that IFT is specifically involved in building cilia in MCCs, but not in SCCs. Alternatively, this difference may be attributed to the decay of maternally contributed ift gene products between the time of cilia formation in SCCs and MCCs.
To further clarify this picture, we investigated cilia formation in the lateral line organ, where cilia cannot be detected until 2 dpf. In both mutants, cilia formation in the lateral organ is severely affected (Figure 2, G through H').
We additionally observed disorganized basal bodies in MCCs of the pronephric duct. In wild-type embryos, basal bodies in a MCC are organized into a tight array at the apical side, as shown by γ-Tubulin staining (Figure 2, I, J, K, and L). In both ift57hi3417 and ift172hi2211mutants, basal bodies appeared as disorganized clusters, no longer tightly arrayed (Figure 2, I', J', K', and L'). We further investigated whether the apical localization of the basal body is affected in these mutants by labeling with the apical marker anti-atypical PKC. Results showed that basal bodies in MCCs are still localized to the apical side of the pronephric duct in both mutants, as evident by clusters in side views at the edge of the lumen (arrows in Supplemental Figure S2).
Knockdown of pk1 Leads to Disorganization of Basal Bodies in the Kidney Duct and Kidney Cyst Formation
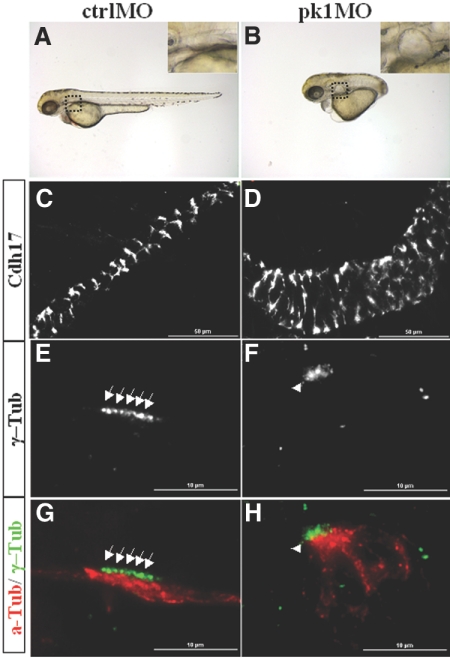
The PCP pathway has been implicated in kidney cyst formation.4,16,20,21 In addition, the disorganization of basal bodies in ift mutants is suggestive of a PCP defect. We therefore tested whether PCP defects can lead to cyst formation directly by knocking down the expression of a core PCP component, pk1, using a morpholino oligo.29 Injected at 2 ng, pk1 morphant showed a previously observed and typical PCP phenotype, namely, a severely shortened body axis (Figure 3, A and B).29,30 Interestingly, we additionally observed kidney cyst formation medial to the pectoral fins, a region where the glomerulus and the tubule are located (Figure 3, A and B). Further analysis with immunostaining using anti-Cadherin17 (Cdh17), an antibody that labels the basal-lateral membrane of the pronephric duct and the pronephric tubule, showed that the kidney duct is significantly enlarged in pk1 morphants as well (Figure 3, C and D). Moreover, similar to ift mutants, γ-Tubulin labeling revealed that basal bodies in MCCs of the pronephric duct of pk1 morphants are disorganized and more loosely arranged into an oval cluster (Figure 3, E through H; Supplemental Figure S2, E and F). It has recently been shown that pk1 is required for proper apical-basal polarity of the mouse epiblast.31 We therefore further investigated whether the basal bodies in MCCs of pk1 morphants are displaced from the apical side by colabeling with the apical marker anti-atypical PKC. Similar to ift mutants, the basal bodies of pk1 morphants are still localized to the apical side, albeit in a disorganized manner (Supplemental Figure S2, E and F).
Figure 3.
Inactivation of pk1 leads to kidney cyst formation and disorganization of basal bodies. (A and B) A control embryo and a pk1 morphant raised with 1-phenyl-2-thiourea at 3 dpf in side views. Insets: enlarged areas showing a cyst (B) and the lack of cyst (A). (C and D) Pronephric ducts labeled with anti-Cdh17 in side views. (E through H) Basal bodies as shown by anti–γ-Tub labeling and cilia by anti-a-Tub labeling. Arrows point to arrayed basal bodies, whereas arrowheads point to clusters of disorganized basal bodies. a-Tub, acetylated Tubulin; ctrlMO, embryos injected with a standard control morpholino; γ-Tub, γ-Tubulin; pk1MO, embryos injected with pk1 morpholino.
ift57 and ift172 Genetically Interact with pk1
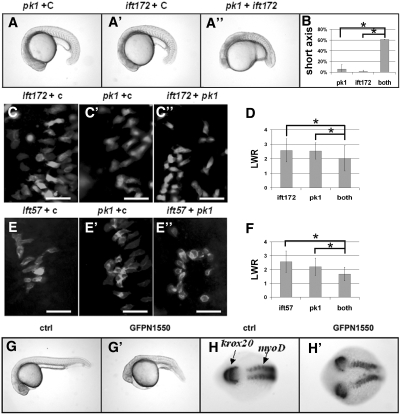
Multiple studies provide evidence to support a critical role of the PCP pathway in kidney cyst formation.16,20 However, reports on the role of cilia in the PCP pathway are conflicting.4,15,21 We therefore examined for potential genetic interactions between ift57, ift172, and pk1. We injected suboptimal doses of the ift172 morpholino (3.5 ng) and pk1 morpholino (0.6 ng), either together or with a control morpholino (0.6 and 3.5 ng, respectively). Results showed that the shortened body axis phenotype was found only in embryos that were co-injected with ift172 and pk1 morpholinos (Figure 4, A through B). Similarly, although a suboptimal dose of ift57 (0.35 ng) morpholino did not cause shortened body axis, this morpholino injected in combination with the pk1 morpholino did (Supplemental Figure S3). Interestingly, maternal-zygotic mutants of ift88 lack a PCP phenotype during gastrulation,15 raising the possibility that there are functional differences between ift88 and other ift genes. We therefore investigated whether ift88 genetically interacts with pk1. Results showed that a suboptimal dose (1.2 ng) of a previously published ift88 morpholino32 synergized with the pk1 morpholino in inducing the shortened body axis phenotype (Supplemental Figure S3), indicating that ift88 functions similarly to other ift genes in this assay.
Figure 4.
Interactions between ift57 or ift172 with the PCP pathway. (A through A”) Embryos injected with different combinations of morpholino oligos at the 20-somite stage in side views. (B) Tabulation of embryos with shortened body axis. *P < 0.05, from two independent experiments. (C through C”) and (E through E”) Paraxial cells labeled with a membrane-targeting GFP in dorsal views with the anterior to the top and the middle line to the right in embryos injected with different combinations of morpholinos. (D and F) Tabulation of LWR of paraxial cells along the lateral-medial axis. P < 0.0005. (G and G') A control embryo and an embryo injected with GFP-N1550 mRNA in side views at 1 dpf. (H and H') A control embryo and an embryo injected with GFP-N1550 mRNA labeled with krox20 and myoD in situ probes in dorsal views at the 10-somite stage. Scale bars: 50 μm. c, a standard control morpholino; GFPN1550, embryo injected with GFP-N1550 mRNA; pk1, pk1 morpholino; ift172, ift172 morpholino; ift57, ift57 morpholino.
To provide more direct support for our hypothesis that ift genes genetically interact with pk1 during CE movement in gastrulation, we analyzed the morphology of paraxial cells in late gastrulae by mosaically expressing a membrane-targeted enhanced GFP (eGFP). As reported previously for wild-type embryos, control embryos injected with a suboptimal dose of either ift172 morpholino or pk1 morpholino together with a control morpholino displayed paraxial cells that were elongated along the medial-lateral axis, with a length-to-width ratio (LWR) of 2.6 ± 0.8 and 2.5 ± 0.6, respectively. In contrast, embryos co-injected with suboptimal doses of ift172 and pk1 morpholinos exhibited a LWR that was significantly reduced to 2.0 ± 0.9 (Figure 4, C through D). Similarly, co-injection of suboptimal doses of ift57 and pk1 morpholino reduced the LWR to 1.7 ± 0.5 from 2.6 ± 0.8 and 2.2 ± 0.6 when compared with control embryos injected with ift57 or pk1 together with a control morpholino, respectively (Figure 4, E through F).
Further support for IFT's involvement in the PCP pathway comes from a dominant negative construct of ift172. Loss-of-function mutants of ift172 have been identified in Chlamydomonas and mouse.3,33 Both mutations are located in the C-terminal region of Ift172 (L1615P and L1564P, respectively), suggesting that the C-terminal region is critical for the function of Ift172. We therefore generated a C-terminal truncation of Ift172 (ift172N1550), which contains only the first 1550 amino acids from the N terminus of this protein. Surprisingly, overexpression of Ift172N1550 and GFP-N1550 (a GFP-tagged form of Ift172N1550) caused a shortened body axis at 1 dpf (Figure 4, G and G'). In situ hybridization with a krox20 and a myoD probe at the 10-somite stage of embryos injected with GFP-N1550 mRNA revealed that both the hindbrain and the somites failed to fuse at the midline (Figure 4, H and H'), consistent with defective CE movements. To test whether GFP-N1550 is a dominant negative or dominant active form of Ift172, we co-injected 150 pg of GFP-N1550 mRNA together with 2 ng of ift172 morpholino, which by itself does not cause an obvious body axis shortening defect. In a representative experiment, the inclusion of ift172 morpholino increased the percentage of embryos with shortened body axis from 18% to 36%, indicating that GFP-N1550 is a dominant negative form of Ift172.
Discussion
The Role of ift Genes in Cilia Assembly in Zebrafish
In both ift57hi3417 and ift172hi2211 mutants, at 1 dpf, cilia are able to form in the neural tube and the pronephric duct and are indistinguishable from cilia in wild-type embryos. A simple interpretation is that ift genes are not required for cilia assembly in zebrafish. As ift genes are highly conserved for ciliary assembly in a wide spectrum of organisms including Chlamydomonas and mice,3,9–12 we considered alternative explanations for this difference. In zebrafish, products of many genes are deposited maternally. Therefore, a homozygous null mutant can start its life with a significant quantity of functional products of the mutated gene provided from the mother, masking the function of that mutated gene during early development. Accordingly, we found transcripts of both ift57 and ift172 are supplied maternally. We also detected the presence of Ift57 protein as early as the 16- to 64-cell stage. Interestingly, although maternal ift57 transcripts decay by 33 hpf in mutants (Figure 1D), previous studies indicate that Ift57 protein can be detected at 48 hpf, but not at 4 dpf, in ift57hi3417 mutants.26,34 Together, these results suggest that maternally supplied Ift57 protein or transcript can persist for an extensive period of time. Consistent with the gradual degradation of maternal gene products, cilia assembly is initially present on the first day of development in ift57hi3417 and ift172hi2211 mutants, but is lost at later stages of development. Furthermore, although zygotic ift88oval mutants are initially able to form cilia, maternal-zygotic ift88oval mutants are not capable of cilia assembly.15 Together, these findings suggest that the role for ift genes in cilia assembly is conserved in zebrafish.
The PCP Pathway and Kidney Cyst Formation
PCP refers to the coordinated polarity within a sheet of epithelial cells. It is known that the proper establishment of PCP is regulated by the noncanonical Wnt pathway (for a review, see reference 35). Strikingly, recent studies indicate that the PCP pathway is critical for preventing cyst formation, which is the hallmark of polycystic kidney disease (PKD). Multiple PKD mouse mutants show misorientation of cell division axis during kidney development before visible kidney cyst formation.16 In addition, conditional inactivation of Four-jointed, a gene known to be involved in PCP, leads to kidney cyst formation in mouse.20 In this study, we demonstrate that inactivation of pk1 can directly lead to kidney cyst formation, providing further support for the involvement of the PCP pathway in preventing kidney cyst formation.
The Role of Cilia in the PCP Pathway
Multiple lines of evidence support an intricate relationship between the cilium and the PCP pathway. First, a number of known PCP pathway components, including Dishevelled, Inturned, and Fuzzy, are required for proper ciliogenesis during Xenopus development.36,37 Second, multiple genes have been shown to be involved in both cilia-mediated processes and the PCP pathway.4,21,38 Most directly, inactivation of ift88 in mice leads to CE movement defects during cochlear duct development, a phenotype associated with PCP abnormalities.39 Furthermore, conditional knockout of Ift20 in the mouse kidney leads to misorientation of cell division axis.40 The critical role of the cilium in PKD pathogenesis, and the close association between PKD and the PCP pathway, is also consistent with a role of cilia in the PCP pathway.
In this study, we show that both ift57 and ift172 mutants display disorganized basal bodies in the kidney duct, similar to that seen in pk1 morphants. We also demonstrate that ift57, ift88, and ift172 genetically interact with pk1 in body axis elongation and the alignment of paraxial cells in later gastrulae. Additionally, we show that overexpression of a dominant negative form of Ift172 disrupts the convergence of somites and the hindbrain during early somitogenesis. Together, these data support a functional involvement of IFT genes in the PCP pathway. However, a previous report has indicated that a maternal-zygotic zebrafish mutant of ift88oval showed no obvious defects in convergence extension during gastrulation.15 On the basis of these results, we propose that during zebrafish gastrulation IFT genes play a minor or redundant role that becomes apparent only when the PCP pathway is already comprised. Additionally, it remains possible that subtle PCP phenotypes exist in maternal-zygotic mutants of ift genes but are not easily detectable. Intriguingly, the PCP phenotypes observed in the mouse cochlear and kidney ducts of Ift88 and Ift20 mutants39,40 may suggest that there exists tissue and stage specificity in the role of IFT genes in this pathway. The genetic interaction between multiple ift genes and a core PCP component provides insight into a novel and intricate relationship between the cilium and planar cell polarity and furthers our understanding of the cilium as a key signaling center for vertebrate cells.
Concise Methods
Fish Husbandry
Zebrafish were maintained according to standard protocols.25 All lines were from the Hopkins laboratory and maintained in TAB background.13
Cloning
Full-length coding sequences of ift57 and ift172 were amplified from a zebrafish cDNA pool and cloned into pCS2 vector. GFP tags were added by PCR cloning. ift172N1550 was generated by PCR cloning.
Microinjection
mMESSAGE mMACHINE kit (Ambion) was used to synthesize capped mRNA. Morpholino oligos were purchased from Genetools. mRNA or morpholino were injected into embryos at the 1-cell stage at indicated concentrations. 5′-CATCCCTCTCTCTTTCTCTTTTCAC-3′ was used to block the translation of ift57 and 5′-GACTCAGGGCAGTTATAAGAACGTA-3′ was used to block the translation of ift172.13 Previously described morpholino oligos were used to knock down the expression of pk1 (5′-GCCCACCGTGATTCTCCAGCTCCAT-3′)29 and the expression of ift88 (5′-CTGGGACAAGATGCACATTCTCCAT-3′).32 A standard oligo (5′-CCTCTTACCTCAGTTACAATTTATA-3′) was injected as a control.
Histologic Analysis
Embryos were fixed in Bouin's fixative overnight at room temperature, washed three times with PBS, embedded in JB-4 resin from Polysciences, and cut at 4 μm. Slides were then stained with hematoxylin & eosin.
Protein Extraction and Western Blotting
Embryos were manually deyolked following a previously published protocol41 and then ground in 2× SDS sample buffer (4% SDS, 200 mM dithiothreitol, and 5% β-mercaptoethanol) with a disposable pestle, boiled for 5 minutes, cleared by microcentrifugation at top speed at room temperature for 2 minutes, and run on SDS-PAGE gels. Proteins were transferred to nitrocellulose membranes. Filters were then blotted in PBS buffer with 5% instant milk, incubated sequentially with anti-Ift57 (kindly provided by B. Perkins)34 at 1:2500 and horseradish peroxidase–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) at 1:5000 to 1:10,000. Signals were then detected with enhanced chemiluminescence detection using the Western Lightning kit from PerkinElmer Life Sciences.
Immunostaining
Immunostaining on fixed embryos was performed as described.42 Specifically, embryos were fixed in Dent's fixative. A monoclonal anti–acetylated Tubulin antibody (Sigma) was used at 1:5000. A monoclonal anti–γ-Tubulin antibody (Sigma) was used at 1:200. A rabbit anti-aPKC (Santa Cruz Biotechnology) was used at 1:200. A custom antibody made by Covance against the C-terminal region from amino acid 238 of Arl13b was used at 1:2000.43 Fluorescence-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) were used at 1:200. Stained embryos were manually deyolked and flat-mounted in Vectashield Hardset mounting medium (Vector Laboratories). Images were taken using a Nikon Eclipse epifluorescence microscope and a Leica stereoscope. Global contrast and intensity of fluorescence images were adjusted using the Elements software. Images were further cropped in Photoshop.
RT-PCR
RNA was extracted with trizol reagent (Invitrogen) following manufacturer's instructions. First strand cDNA was generated with the superscript II RT-PCR system (Invitrogen) and subsequently amplified with PCR. For ift57, primers 5′-CGGGATCCGGGATGGCGGAGGAGGAAGA-3′ and 5′-GGAATTCGTGTTTCAATAAGCCTGCGCA-3′ were used. For ift172, primers 5′-AAACGTGAAGTATGCAGCTTAAG-3′ and 5′-GGGTCAGCAGGCTTTGTTGTAAA-3′ were used. For elf1, primers 5′-CTTCTCAGGCTGACTGTGC-3′ and 5′-CCGCTAGCATTACCCTCC-3′ were used.
Analysis of Cell Polarity in Late Gastrulae
A previously published protocol, with minor modifications, was followed.44 mRNA encoding a membrane-anchored GFP was injected into one cell of embryos at the 16- to 32-cell stage, which leads to mosaic labeling of cells at later stages. Embryos at the 2- to 5-somite stage were imaged with a Nikon Eclipse epifluorescence microscope and analyzed using the Element software.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank members of the Sun Labs and members of Yale Center for PKD research for helpful discussions, Brian Perkins for anti-Ift57, Shiaulou Yuan for his critical reading of the manuscript and the cover image, the Reinke Lab and the Cooley Lab for use of their microscopes, and Nicole Semanchik for superb technical assistance. This project was supported by grants from NIDDK (RO1 DK069528 and P50 DK057328 (project #3)) and a research grant from PKD Foundation (to Z.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “More than Maintenance? A Role for IFT Genes in Planar Cell Polarity,” on pages 1240–1241.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.DiBella LM, Park A, Sun Z: Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Hum Mol Genet 18: 595–606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK: Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1: e53, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV: Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426: 83–87, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Kishimoto N, Cao Y, Park A, Sun Z: Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell 14: 954–961, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: From the Cover: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrandt F, Attanasio M, Otto E: Nephronophthisis: Disease mechanisms of a ciliopathy. J Am Soc Nephrol 20: 23–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL: A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A 90: 5519–5523, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum JL, Witman GB: Intraflagellar transport. Nat Rev Mol Cell Biol 3: 813–825, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Han YG, Kwok BH, Kernan MJ: Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol 13: 1679–1686, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC: IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J Biol Chem 278: 34211–34218, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL: Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141: 993–1008, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haycraft CJ, Schafer JC, Zhang Q, Taulman PD, Yoder BK: Identification of CHE-13, a novel intraflagellar transport protein required for cilia formation. Exp Cell Res 284: 251–263, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Tsujikawa M, Malicki J: Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 42: 703–716, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Huang P, Schier AF: Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136: 3089–3098, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P: Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romagnolo B, Berrebi D, Saadi-Keddoucci S, Porteu A, Pichard AL, Peuchmaur M, Vandewalle A, Kahn A, Perret C: Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated beta-catenin. Cancer Res 59: 3875–3879, 1999 [PubMed] [Google Scholar]
- 19.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C: Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20: 5972–5981, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H: Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G: Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37: 537–543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF: Vertebrate Smoothened functions at the primary cilium. Nature 437: 1018–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Reiter JF: Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol 10: 70–76, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Ocbina PJ, Tuson M, Anderson KV: Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One 4: e6839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westerfield M: The Zebrafish Book: a guide for the laboratory use of zebrafish (Danio rerio), University of Oregon Press, Eugene, OR, 2000 [Google Scholar]
- 26.Lunt SC, Haynes T, Perkins BD: Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect hedgehog signaling. Dev Dyn 238: 1744–1759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Pathak N, Kramer-Zucker A, Drummond IA: Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development 134: 1111–1122, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Ma M, Jiang YJ: Jagged2a-notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet 3: e18, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M: Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development 130: 4037–4046, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, Ueno N: The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol 13: 674–679, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Tao H, Suzuki M, Kiyonari H, Abe T, Sasaoka T, Ueno N: Mouse prickle1, the homolog of a PCP gene, is essential for epiblast apical-basal polarity. Proc Natl Acad Sci U S A 106: 14426–14431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA: Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development 132: 1907–1921, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Pedersen LB, Miller MS, Geimer S, Leitch JM, Rosenbaum JL, Cole DG: Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr Biol 15: 262–266, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Krock BL, Perkins BD: The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci 121: 1907–1915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solnica-Krezel L: Gastrulation in zebrafish—all just about adhesion? Curr Opin Genet Dev 16: 433–441, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB: Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40: 871–879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park TJ, Haigo SL, Wallingford JB: Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet 38: 303–311, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL: Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37: 1135–1140, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P: Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet 40: 69–77, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Jonassen JA, San Agustin J, Follit JA, Pazour GJ: Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 183: 377–384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Link V, Shevchenko A, Heisenberg CP: Proteomics of early zebrafish embryos. BMC Dev Biol 6: 1, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC: Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125: 4655–4667, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Duldulao NA, Lee S, Sun Z: Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development 136: 4033–4042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L: The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell 1: 251–264, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.