Abstract
Classical eyeblink conditioning is a well-characterized model paradigm that engages the septohippocampal cholinergic system. This form of associative learning is impaired in normal aging and severely disrupted in Alzheimer's disease (AD). Some nicotinic cholinergic receptor subtypes are lost in AD, making the use of nicotinic allosterically potentiating ligands a promising therapeutic strategy. The allosterically potentiating ligand galantamine (Gal) modulates nicotinic cholinergic receptors to increase acetylcholine release as well as acting as an acetylcholinesterase (AChE) inhibitor. Gal was tested in two preclinical experiments. In Experiment 1 with 16 young and 16 older rabbits, Gal (3.0 mg/kg) was administered for 15 days during conditioning, and the drug significantly improved learning, reduced AChE levels, and increased nicotinic receptor binding. In Experiment 2, 53 retired breeder rabbits were tested over a 15-wk period in four conditions. Groups of rabbits received 0.0 (vehicle), 1.0, or 3.0 mg/kg Gal for the entire 15-wk period or 3.0 mg/kg Gal for 15 days and vehicle for the remainder of the experiment. Fifteen daily conditioning sessions and subsequent retention and relearning assessments were spaced at 1-month intervals. The dose of 3.0 mg/kg Gal ameliorated learning deficits significantly during acquisition and retention in the group receiving 3.0 mg/kg Gal continuously. Nicotinic receptor binding was significantly increased in rabbits treated for 15 days with 3.0 mg/kg Gal, and all Gal-treated rabbits had lower levels of brain AChE. The efficacy of Gal in a learning paradigm severely impaired in AD is consistent with outcomes in clinical studies.
It has long been established that acetylcholine neurotransmission plays a crucial role in learning and memory, and more recently, the cholinergic system has been the focus of treatment for memory impairment in Alzheimer's disease (AD). The demonstrated role of acetylcholine in modulating the rate of learning in eyeblink classical conditioning in rabbits (1) makes this model system useful in preclinical investigations of cognition-enhancing drugs (2). More is known about the neural structures and systems that are involved in eyeblink classical conditioning than about any other learning and memory task. Although the neural circuitry essential for acquisition and retention of the conditioned eyeblink response resides in the cerebellum (3), the hippocampus is engaged during delay eyeblink classical conditioning (4). In the delay procedure, a neutral stimulus such as a tone conditioned stimulus (CS) is presented half a second before the onset of a corneal airpuff eyeblink-eliciting unconditioned stimulus (US). The organism learns to blink to the tone CS before the onset of the airpuff US, and the learned response is called the conditioned response (CR). It is our working hypothesis that selective loss of hippocampal pyramidal cells (5) and disruption of the septohippocampal cholinergic system in AD (6) impairs acquisition of delay eyeblink classical conditioning in AD beyond the impairment observed in normal aging. The hypothesis was supported (7, 8) and independently replicated (9).
Audioradiographic and histochemical studies of human brain tissue collected postmortem (10–13) and brain imaging studies in living AD patients (14) demonstrated specific loss of nicotinic cholinergic receptors and almost complete sparing of muscarinic cholinergic receptors in AD. Identification of nicotinic cholinergic receptors as the receptors impaired in AD led us to test a nicotinic cholinergic antagonist and nicotinic agonists in the animal model of eyeblink classical conditioning. By using a very low-dosage level of mecamylamine in young rabbits so that nicotinic cholinergic receptors would be selectively inhibited, we demonstrated a role for nicotinic cholinergic receptors in eyeblink conditioning because the acquisition of CRs was severely disrupted (15). A synthesized analog of the marine natural product anabasine (16) called GTS-21 [3-(2,4-dimethoxybenzylidene)anabaseine] has been found to preferentially interact with α7 neuronal nicotinic receptors. Several doses of GTS-21 were administered to older rabbits, and this drug enabled older animals to produce significantly more CRs than did vehicle-treated older rabbits (17).
Administration of nicotinic cholinergic agonists has promise in the treatment of cognition impairment in AD, but there are also some problems with this therapeutic strategy. It is difficult to establish the appropriate dose of a nicotinic cholinergic agonist, as higher-dose levels may cause desensitization rather than increased activation of nicotinic receptors (18). Additional problems include drug transport to the targeted nicotinic cholinergic receptors and the target selectivity of the receptor subtype. An alternative approach to drug treatment in AD is the application of allosteric modulators of nicotinic receptors (18, 19). Allosteric modulators are drugs that interact with the receptor through binding sites that are distinct from those for acetylcholine and nicotinic agonists and antagonists. Because these modulators are not directly involved in the neurotransmission process they affect, they typically do not induce compensatory processes that the agonists and antagonists induce. Thus, problems such as receptor desensitization and down-regulation of expression can be avoided with allosteric modulators.
AD has been associated with a deficit in nicotinic cholinergic neurotransmission. A means to up-modulate or potentiate the channel activity of nicotinic receptors in response to acetylcholine is to use allosterically potentiating ligands (APLs). Representative nicotinic APLs are the plant alkaloids physostigmine, galanthamine, and codeine and the neurotransmitter serotonin (20). Structural properties of APLs are different from the structural properties of inhibitors of the enzyme acetylcholinesterase (AChE), the type of drugs currently approved to treat cognition impairment in AD. Compared with conventional AChE inhibitors, galantamine (Gal) produces relatively less AChE inhibition. Codeine does not interact with AChE at all. In the covalent AChE inhibitor, physostigmine, removal of the carbamate function has no effect on potency as an APL, but this treatment reduces significantly the potency of physostigmine's AChE inhibition (20). The category of APLs has been limited to physostigmine, galanthamine, codeine, and serotonin on the basis of functional properties tested with nicotinic cholinergic agonists and antagonists (20). Functionally unique features of APLs include the ability to induce single-channel activity indistinguishable from single-channel activity induced by acetylcholine.
Having demonstrated that the nicotinic cholinergic drug GTS-21 ameliorated learning deficits in older rabbits, we wanted to determine whether the dual action of an APL would have even greater efficacy in the classical eyeblink-conditioning model paradigm. A nicotinic APL, Gal, was tested at doses of 0.0, 1.0, 2.0, 3.0, and 4.0 mg/kg (21). In 10 daily sessions, 40 older rabbits were tested in the 750-ms delay-conditioning paradigm. A dose of 3 mg/kg Gal was extremely effective in improving conditioning in older rabbits, enabling them to achieve learning criterion rapidly and to produce a very high percentage of CRs. Trials to learning criterion, a measure that is larger when learning is poorer, revealed a classical U-shaped response curve with doses of 1.0 and 2.0 mg/kg Gal producing nonsignificant effects over vehicle-treated rabbits, a dose of 3.0 mg/kg Gal reducing the number of trials to learning criterion to a mean significantly lower than vehicle-treated rabbits, and 4.0 mg/kg Gal producing a nonsignificant effect. Older rabbits treated with 3.0 mg/kg Gal achieved learning criterion 40% faster than older rabbits tested with the optimal dose of GTS-21.
Results with a dose of 3.0 mg/kg Gal were striking, but they were observed in a relatively small sample (21). We undertook the present experiments to explore further the effect of 3.0 mg/kg Gal on learning. There were three major aims: (i) to examine behavioral and pharmacological effects of the 3.0-mg/kg dose of Gal by testing the drug in young as well as older rabbits; (ii) to compare behavioral and pharmacological effects of Gal in larger groups of older rabbits at a dose that affected eyeblink conditioning in a 2-wk experiment (3.0 mg/kg) and a dose that was not different in its behavioral effect from vehicle (1.0 mg/kg); and (iii) to compare behavioral and pharmacological effects of short-term (3 wk of 5 daily injections per wk) versus longer-term (15 wk of 5 daily injections per wk) administration of 3.0 mg/kg Gal. We examined the effects of Gal in older rabbits over a time period (15 wk) that would simulate a human clinical trial, testing rabbits at monthly intervals for retention and relearning for 3 months after initial acquisition.
Methods
Subjects.
A total of 85 female specific pathogen free New Zealand White rabbits completed Experiments 1 and 2. Sixteen were young (4–6 months), and 69 were retired breeder rabbits. Birth dates of the rabbits were recorded by the breeder (Covance, Denver, PA). Older rabbits ranged in age from 15 to 43 months, with a mean of 29.1 months (SD = 5.7). Mean weight of the young rabbits was 2.7 kg (SD = 0.4), and the range was 2.0 to 3.7 kg. Mean weight of the older rabbits was 4.4 kg (SD = 0.5), and the range was 3.2 to 5.7 kg. Rabbits were individually housed in stainless steel cages in an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility. They had 24-h access to rabbit chow and tap water and a 12/12-h light/dark cycle.
Apparatus and Behavioral Conditioning Procedure.
At least 24 h after arrival at the animal facility, rabbits were adapted twice in Plexiglas restrainers for 1 h in sessions separated by 24 h. After the second adaptation session, rabbits were given a local ophthalmic anesthetic (proparacaine hydrochloride) in the left eye so that a 6–0 nylon suture loop could be placed in the temporal margin of the nictitating membrane (NM). A patch of fur (≈3 cm2) was shaved on the back to expose the skin for s.c. injections.
The classical conditioning equipment attached to the rabbit's head included elastic eyelid retractors and a platform holding a minitorque potentiometer (San Diego Instruments prototype model, San Diego, CA) for NM movement measurement that was secured under the animal's muzzle and behind the ears. The potentiometer was attached by a lever and a thread to the nylon suture loop in the NM. Analog output from the potentiometer was digitized and read into an IBM-PC-compatible system described by Chen and Steinmetz (22). This system also controlled the timing and presentation of conditioning stimuli.
For classical conditioning, the CS was an 850-ms, 85-dB, 1-kHz tone, followed 750 ms after its onset by a 100-ms, 3-psi (1 psi = 6.89 kPa) corneal airpuff US. The CS and US coterminated. The intertrial interval was random, ranging between 20 and 30 s at 1-s intervals. One training session lasted about 45 min. Rabbits were tested in separate conditioning chambers four at a time.
Each training session was controlled by a program written in C++ language (22) and run on an IBM-PC-compatible 386 computer. Data were collected about the position of the NM in 3-ms bins during the trials. A CR was scored if the NM was pulled back a minimum of 0.5 mm in the interval between 25 and 750 ms after CS onset. The dependent measure, learning criterion, was scored as the number of training trials it took the animal to produce eight CRs within nine consecutive trials. CR amplitude was scored as the mean NM amplitude in the interval between 25 and 750 ms after CS onset. Response latency was the latency of a response of 0.5 mm or greater in the CS or US period. Initially, response latency is over 750 ms (after US onset). As learning occurs, response latency shortens to less than 750 ms (becoming a CR). Data were collected in RAM and saved to a hard drive, and individual data summaries for each of the four rabbits run simultaneously were printed at the end of each session.
Rabbits in the explicitly unpaired condition were treated in a fashion identical to rabbits tested in the paired condition with the exception that the 850-ms, 85-dB, 1-kHz tone CS and 100-ms, 3-psi corneal air-puff US were never paired. In the unpaired condition, rabbits received a total of 90 stimuli, 45 tone CSs, and 45 corneal air-puff USs. Each stimulus was presented at an intertrial interval that was random and ranged between 20 and 30 s. Thus, the duration of the session and the intertrial interval were identical in the paired and unpaired sessions, and all rabbits were tested four at a time.
Pharmacological Analyses.
Plasma and brain AChE.
From each rabbit's ear vein, 3–5 ml of blood was removed 15 min after the 16th injection of drug or vehicle. Blood was processed in a Jouan CT4.22 centrifuge (Cedex, France) for 60 min at 3,000 rpm and frozen at −80°C. Young rabbits were killed after 15 days of conditioning and drug treatment with an overdose (70–100 mg/kg) of pentobarbital injected in the ear vein. Older drug-treated rabbits in Experiment 1 were killed 15 wk after training began, having received only 15 daily injections of 3.0 mg/kg Gal during eyeblink conditioning. Older rabbits in Experiment 2 received injections 5 days a wk for 15 wk of 0.0, 1.0, or 3.0 mg/kg Gal or 15 daily injections of 3.0 mg/kg Gal and injections 5 days a wk for the remaining 12 wk of vehicle. After sacrifice, animals were immediately decapitated. The brain of each animal was removed rapidly and frozen at −80°C. Plasma and frozen tissue were shipped on dry ice from Philadelphia to Tucson and immediately stored at −70°C until assayed. Sections from parietal/occipital cortex were prepared for neurochemical analysis of AChE activity according to the colorimetric method of Ellman et al. (23) by using a Beckman Coulter DU 640 spectrophotometer equipped with a Peltier temperature controller. The incubation solution contained the butyrylcholinesterase inhibitor tetraisopropyl pyrophosphoramide (iso-OMPA) at a final concentration of 100 μM to measure AChE activity specifically.
Brain nicotine receptor-binding studies.
Sections from sensorimotor cortex were homogenized and prepared for analysis according to the method of Flores et al. (24). Membrane suspensions were incubated (60 min at 4°C) with [3H]epibatidine (0.1–10 nM) in a 50-mM NaKHPO4 (pH 7.4) buffer, in a final volume of 1 ml, with or without unlabeled nicotine (0.1 mM) to define specific binding. [3H]Epibatidine was used to label the number of α4β2 nicotinic acetylcholine receptors. Separation of bound ligand from free was performed by filtering the samples through Whatman GF/C filters that had been presoaked with 0.3% polyethylenimine. The filters were washed three times with the buffer, and the radioactivity trapped on the filters was counted in a scintillation counter. All assays were performed in triplicate. The Bmax and the Kd were determined by saturation experiments with six different concentrations of labeled ligand. Data were analyzed by using the PHARM/PCS 4.2 program (MCS, Philadelphia, PA). Kd and Bmax values were determined after transformation of the data to fit the Rosenthal equation.
Drugs.
Galantamine was purchased from Tocris Cookson (Ballwin, MO). The vehicle solution was sterile saline. Drugs were freshly prepared in solution each wk and injected s.c. a minimum of 15 min before behavioral testing to ensure that peak blood levels of Gal were attained during the testing session. Rabbits were tested between 15 and 30 min after injections, and behavioral testing was completed a maximum of 1 h and 15 min after injection.
Research Design.
In Experiment 1, 3.0 mg/kg Gal or vehicle was injected s.c. daily for 15 days in young and older rabbits 15–30 min before sessions of eyeblink classical conditioning to examine behavioral and pharmacological effects. In Experiment 2, doses of 0.0, 1.0, and 3.0 mg/kg Gal were injected s.c. to older rabbits as indicated in Table 1. Equal volumes were injected into the animals, including vehicle-treated animals, based on weight.
Table 1.
Research design of Experiment 2: Acquisition 5 days/week for 3 weeks; 3 monthly retests of retention and relearning
| Group | n | Acquisition | Drug at acquisition | Retention | Drug at retention |
|---|---|---|---|---|---|
| Continuous drug | 24 (1.0 and 3.0 mg/kg Gal; 12 rabbits/dose) | 15 days; 750-ms delay procedure | Yes—1.0 or 3.0 mg/kg Gal | 3 retention and relearning tests; 4-week intervals* | Yes—1.0 or 3.0 mg/kg Gal |
| Acquisition only drug | 12† (3.0 mg/kg Gal) | 15 days; 750-ms delay procedure | Yes—3.0 mg/kg Gal | 3 retention and relearning tests; 4-week intervals | No |
| Vehicle control | 18 (sterile saline) | 15 days; 750-ms delay paradigm | No | 3 retention and relearning tests; 4-week intervals | No |
| Total | 54 | 3 weeks | 12 weeks |
Retention test: 20 CS-alone presentations on a Monday 4 weeks after previous testing; relearning test: paired CS-US trials for 3 days (Tuesday–Thursday) 4 weeks after previous testing.
† One rabbit in this group failed to complete the experiment and died of natural causes after the second monthly retest session.
Results
Experiment 1.
Behavioral analyses.
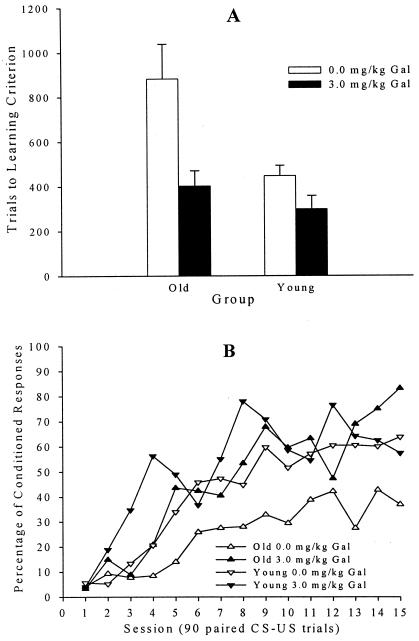
The dependent measure of trials to a learning criterion of eight CRs in nine consecutive trials was evaluated in a 2 (drug dose) by 2 (age) ANOVA. The effect of Gal was statistically significant, F (1, 28) = 11.44, P < 0.002. The effect of age was also statistically significant, F (1, 28) = 8.45, P < 0.007. The Gal by age-interaction effect approached statistical significance, F (1, 28) = 3.21, P = 0.084 (Fig. 1A). Post hoc analysis of the significant Gal effect indicated that a dose of 3.0 mg/kg Gal facilitated learning in young (P < 0.01) as well as older rabbits (P < 0.01). Post hoc analysis of the significant age effect indicated that vehicle-treated older rabbits took more trials to attain learning criterion than vehicle-treated younger rabbits (P < 0.05).
Figure 1.
(A) Trials to a learning criterion of eight CRs in nine consecutive trials for young and older rabbits treated with 3.0 mg/kg Gal or sterile saline vehicle. There were eight rabbits per group. (B) Percentage of CRs over 15 daily training sessions in the same 32 rabbits shown above.
Separate 2 (drug dose) by 2 (age) by 15 (training sessions) repeated-measures ANOVAs were carried out for three dependent measure assessments of learning (percentage of CRs, CR amplitude, and response latency). Gal had a significant effect on all three dependent measures: F (1, 28) = 9.25, P < 0.005 for percentage of CRs (Fig. 1B); F (1, 28) = 5.26, P < 0.03 for CR amplitude; and F (1, 28) = 12.70, P < 0.001 for response latency. The effect of age was significant for percentage of CRs, F (1, 28) = 4.91, P < 0.04, but there was not a significant age effect for CR amplitude or response latency. The drug dose by age-interaction effect did not reach statistical significance with any dependent measure of learning. A 2 (drug dose) by 2 (age) ANOVA was carried out on an assessment of the motor response, unconditioned response amplitude. The effect of age was significant, F (1, 28) = 6.23, P < 0.02, with older rabbits having greater unconditioned response amplitude (mean of 7.4 and 7.3 mm for Gal and vehicle, respectively) than young rabbits (mean of 6.2 and 6.2 mm for Gal and vehicle, respectively) because of the larger NM of older rabbits. No other effects were significant.
Behavior and pharmacological relationships.
Comparisons of plasma AChE, brain AChE, and nicotinic receptor binding were carried out with one-way ANOVAs. There were statistically significant group differences in plasma AChE, F (3,26) = 11.40, P < 0.0001. Post hoc comparisons indicated that the old Gal-treated rabbits had the lowest plasma AChE levels, which were significantly lower than the levels in the young vehicle-treated and young Gal-treated groups (P < 0.05). Young vehicle brains were not available for analyses, so comparisons for brain AChE and nicotinic receptor binding were analyzed for three groups. There was a significant difference in brain AChE levels, F (2, 12) = 4.91, P < 0.03. Young Gal-treated rabbits had the lowest brain AChE levels, whereas old vehicle-treated rabbits had the highest levels. There was a significant difference in brain nicotinic receptor binding (Bmax values), F (2, 15) = 6.81, P < 0.01. Old rabbits treated with Gal had the highest level of nicotinic receptor binding.
Correlations between the behavioral measures of trials to learning criterion and plasma AChE, brain AChE, and nicotinic receptor binding were carried out. There was a statistically significant correlation between brain AChE levels and trials to learning criterion, r = 0.621, P = 0.007. Neither the correlation between trials to learning criterion and plasma AChE nor the correlations between trials to learning criterion and Bmax or Kd attained statistical significance.
Experiment 2.
Behavioral analyses.
To compare the effects of various doses of Gal on the acquisition of CRs, a one-way ANOVA using the dependent measure of trials to learning criterion for the 4 treatment groups (0.0 Gal, 15 wk; 1.0 Gal, 15 wk; 3.0 Gal, 15 wk, 3.0 Gal, 3 wk) was carried out. There was a significant difference among the groups, F (3, 49) = 4.57, P = 0.007 (Fig. 2A). Post hoc comparisons using the Tukey honestly significant difference (HSD) test indicated that rabbits in the 3.0 Gal, 15-wk and 3.0 Gal, 3-wk groups took significantly fewer trials to attain learning criterion compared with rabbits in the 1.0 Gal, 15-wk and 0.0 Gal, 15-wk groups. The difference between 1.0 Gal and vehicle was not statistically significant.
Figure 2.
(A) Trials to a learning criterion of eight CRs in nine consecutive trials for four groups of older rabbits treated with 3.0 mg/kg Gal over 15 wk, 3.0 mg/kg Gal over 3 wk, 1.0 mg/kg Gal over 15 wk, or sterile saline vehicle. There was no difference during 15 days of acquisition in rabbits treated with 3.0 mg/kg Gal, so the groups are collapsed in B. (B) Response latency over 15 daily acquisition sessions in the older rabbits shown above treated with 3.0 mg/kg Gal, 1.0 mg/kg Gal, or 0.0 mg/kg Gal.
Because the two groups of rabbits treated with 3.0 mg/kg Gal during acquisition were similar in trials to learning criterion, the groups were collapsed into one group of 24 rabbits for additional analyses of behavioral acquisition data. Separate 3 (drug dose) by 15 (training sessions) repeated-measures ANOVAs were carried on for three dependent-measure assessments of learning (percentage of CRs, CR amplitude, and response latency) with a planned comparison between the vehicle and 3.0 mg/kg groups. The effect of 3.0 mg/kg Gal was significant for percentage of CRs and response latency, F (1, 39) = 4.88, P = 0.033 and F (1, 39) = 4.80, P = 0.034, respectively (Fig. 2B). Percentage of CRs was greater and response latency was shorter (more often preceding US onset) for rabbits in the 3.0 mg/kg Gal group. As expected, all three dependent measures changed significantly over training sessions, indicating learning in all groups. The drug dose by training session interaction effect was not significant for percentage of CRs and response latency, but for CR amplitude the interaction effect approached significance, F (14, 546) = 1.66, P = 0.061. A one-way ANOVA used to compare drug treatment on unconditioned response amplitude was not significant.
To examine retention, percentage of CRs in the 20 CS-alone trials was analyzed in a 4 (drug dose) by 3 (monthly retest) repeated-measures ANOVA. The effect of drug dose was statistically significant, F (3, 49) = 3.60, P = 0.020, as was the drug dose by monthly retest interaction, F (6, 98) = 2.38, P = 0.035 (Table 2). Post hoc analysis of the significant drug dose effect indicated that the group administered 3.0 mg/kg Gal over the 15-wk period had significantly greater retention than did the vehicle group in the 1-month retention session. As indicated in Table 2, the significant interaction resulted from better retention in the second and third retention tests in several groups.
Table 2.
Retention as measured by percentage of CRs to test trials and relearning as measured by trials to learning criterion in three 1-month retests for the four drug treatment groups
| Drug treatment | Retention (percentage of
CRs)
|
Relearning (trials to criterion)
|
||||
|---|---|---|---|---|---|---|
| Retest 1 | Retest 2 | Retest 3 | Retest 1 | Retest 2 | Retest 3 | |
| 3.0*Gal, 15 weeks | 52.3† (31.2)‡ | 33.3 (34.8) | 36.6 (35.2) | 97.5 (119.7) | 59.7 (97.0) | 66.7 (100.0) |
| 3.0 Gal, 3 weeks | 25.6 (30.0) | 26.8 (37.7) | 39.1 (30.6) | 103.6 (99.2) | 38.4 (43.0) | 24.6 (56.2) |
| 1.0 Gal, 15 weeks | 27.2 (32.7) | 26.8 (27.3) | 41.2 (29.0) | 95.5 (118.6) | 63.2 (98.1) | 43.1 (52.5) |
| Vehicle, 15 weeks | 17.2 (24.5) | 40.1 (32.7) | 40.4 (31.1) | 107.2 (127.4) | 120.2 (125.0) | 61.9 (106.0) |
mg/kg.
Mean.
SD.
To examine relearning, a 4 (drug dose) by 3 (monthly retest) repeated-measures ANOVA was carried out on the dependent measure of trials to learning criterion. The monthly retest effect was significant, F (2, 98) = 5.11, P = 0.008. At each retest, reacquisition occurred more rapidly than at the preceding retest (Table 2). The drug dose and interaction effects did not attain statistical significance.
Behavior and pharmacological relationships.
Comparisons of plasma AChE, brain AChE, and nicotinic receptor binding were carried out with one-way ANOVAs. There were statistically significant group differences in plasma AChE, F (3, 48) = 4.16, P = 0.011 (Fig. 3). Post hoc comparisons using the Tukey HSD test indicated that the 3.0 Gal, 3-wk group had significantly lower plasma AChE levels than the vehicle-treated rabbits. Differences among the three Gal-treated groups did not attain statistical significance in the post hoc comparisons. The correlation between trials to learning criterion and plasma AChE levels was low and did not approach statistical significance, (r = −0.066, P = 0.323).
Figure 3.
Plasma (Left) and brain (Right) AChE at the end of the 15-wk experiment in older rabbits treated with 3.0 mg/kg Gal over 15 wk, 3.0 mg/kg Gal over 3 wk, 1.0 mg/kg Gal over 15 wk, or sterile saline vehicle.
Six brains from rabbits in the 3.0 Gal, 15-wk group and six brains from vehicle-treated rabbits were analyzed for a different experiment, so comparisons for brain AChE and nicotinic receptor binding had six rabbits in the 3.0 mg/kg Gal group and 12 rabbits in the vehicle group. A one-way ANOVA comparing brain AChE levels indicated a significant difference in brain AChE levels, F (3, 38) = 6.34, P = 0.001 (Fig. 3). Post hoc comparisons using the Tukey HSD test indicated that all three groups treated with Gal had significantly lower brain AChE levels than vehicle-treated rabbits. Differences among the three Gal-treated groups did not attain statistical significance in the post hoc comparisons. The correlation between trials to learning criterion and brain AChE levels approached but did not attain statistical significance, r = 0.224, P = 0.082.
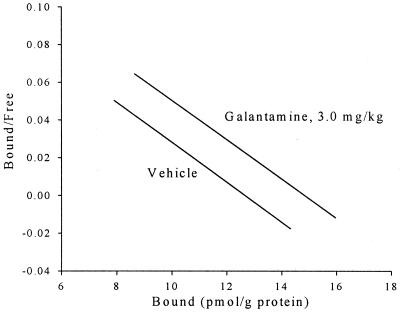
The saturation-binding experiments using [3H]epibatidine to label the α4β2 nicotinic acetylcholine receptors produced Bmax and Kd values. A one-way ANOVA comparing the brain nicotinic receptor-binding value, Bmax, indicated a significant effect, F (3, 38) = 6.95, P = 0.001. Post hoc comparisons using the Tukey HSD test indicated that the 3.0 Gal, 3-wk group differed significantly (P < 0.05) from the values for the other three groups, 3.0 Gal, 15 wk, 1.0 Gal, 15 wk, 0.0 Gal, 15 wk. Fig. 4 shows a Scatchard analysis of these results comparing the 3.0 Gal, 3-wk and 0.0 Gal, 15-wk groups. The correlation between trials to learning criterion and Bmax for the 24 rabbits in the 3.0 Gal, 3-wk and vehicle groups was −0.268 (P = 0.11), and the correlation between trials to learning criterion and Bmax for older rabbits in all four groups was −0.099 (P = 0.271).
Figure 4.
Scatchard analysis of nicotine receptor binding in older rabbit cortex comparing rabbits treated with 3.0 mg/kg Gal for 3 wk or sterile saline vehicle. For the 3.0 mg/kg Gal, 3 wk group, the Kd value was 0.075 (SD = 0.050) and the Bmax is 26.2 pmol/g. For the vehicle group, the Kd value was 0.054 (SD = 0.032), and the Bmax is 22.11 pmol/g (SD = 2.03).
Discussion
Galantamine at a dose of 3.0 mg/kg was effective in facilitating learning. In Experiment 1, the drug improved learning significantly in young as well as in older rabbits. Among the many cognition-enhancing drugs we have tested in 4-month-old rabbits (BMY-21502, donepezil, GTS-21, nefiracetam, nimodipine), Gal is the only drug that has facilitated learning in young rabbits. Young animals acquire CRs at close to ceiling levels (around 400 training trials), making it more difficult to demonstrate a significant effect. In the present study, the mean number of trials to criterion for young vehicle-treated rabbits was 445 trials (SD = 130). The 3.0-mg/kg dose of Gal enabled young rabbits to achieve learning criterion in a mean of 297 trials (SD = 166), and old rabbits treated with 3.0 mg/kg Gal achieved criterion in 401 trials (SD = 192). The 3.0-mg/kg dose of Gal caused older rabbits to learn at the same rate as young vehicle-treated rabbits.
In Experiment 2, 3.0 mg/kg Gal affected the rate of learning early in the acquisition process. On average, old rabbits treated with 3.0 mg/kg Gal learned on training day 4 or 5; old rabbits treated with 1.0 mg/kg Gal learned on training day 6 or 7; and old rabbits treated with vehicle learned on training day 9 or 10. Because all rabbits were trained for 15 sessions, the groups were relatively equal at the end of acquisition.
The significant effect of the 3.0-mg/kg dose of Gal on acquisition extended to retention in the case of the group continuously injected with 3.0 mg/kg Gal. When they were tested for retention at 1-, 2-, and 3-month intervals after acquisition, the continuously injected group treated with 3.0 mg/kg Gal showed significantly greater retention at the 1-month retest (52% CRs versus 17% CRs for vehicle-treated rabbits). The significant retention effect did not occur in the group treated with 3.0 mg/kg Gal only for the 15 days of acquisition training. Indeed, the group treated continuously with 1.0 mg/kg Gal had a numerically higher retention score in the 1-month retest than did the group treated with 3.0 mg/kg Gal for 15 days. There was no significant drug dose effect on relearning.
Associated with the facilitated learning in Experiments 1 and 2 were statistically significant correlations between learning and brain (but not plasma) AChE levels. Greater inhibition of brain AChE correlated significantly with faster acquisition. It should be noted that this correlation was between learning that was tested more than 12 wk before the blood was sampled and the brain was removed. The drug that had been administered to the animal was injected 15 min before blood was sampled; the animal was killed, and the brain was removed. Thus, the AChE levels probably reflect the characteristic response of the individual animals, and this is why the correlation between brain AChE level and learning is significant. Similarly, nicotinic receptor binding was assessed in the brains removed after 15 wk of treatment, whereas learning was affected by Gal dose in the first 3 wk of the experiment. We assume that nicotinic receptor-binding increases occurred during the first 3 wk of the experiment when learning was improved, but we did not examine rabbit brains at that time. This assumption is based on the reported findings that treatment with nicotinic receptor agonists could increase cortical and hippocampal nicotinic receptor number after only 10 days of treatment in rats (25) and between 2 to 4 wk in mice (26). Furthermore, in the experiment with rats, the elevation in nicotinic receptor number correlated with the rate of acquisition in the Morris water-maze task (25).
Plasma levels of AChE in Experiment 1 were lowest in older rabbits treated with Gal, and these levels were significantly lower than plasma AChE in both groups of young rabbits, suggesting an age effect. The lower levels of AChE observed in Gal-treated old rabbits in the present study might be due to the fact that aged animals are more vulnerable to the effects of AChE inhibitors. In a recent study, the inhibitors donepezil and tacrine produced greater decline in AChE activity in the brains of aged rats, as compared with young rats, and this decline might have been related to the higher concentrations of these drugs achieved within the brain of older animals (27). In addition, the degree of AChE inhibition achieved in the different aged animals might also be related to the endogenous level of AChE enzyme. The age-related decline in AChE activity varies across different brain regions and rodent strains (28–30), and specific molecular forms of the enzyme may be more vulnerable than others (31).
Using [3H]epibatidine to label the α4β2 nicotinic acetylcholine receptors produced Bmax values indicating that nicotinic binding was elevated significantly in older rabbits treated with 3.0 mg/kg Gal in both Experiments 1 and 2. The higher-dose Gal therapy initially induced a significant up-regulation of nicotinic sites. However, after long-term therapy for 15 wk, the response to Gal showed that tolerance and up-regulation of nicotinic sites had attenuated by the time of sacrifice. A similar tolerance to the effects of chronic nicotinic agonist treatment was observed for locomotor depression in mice after 7 wk of therapy (26).
These findings are consistent with a report (32) that also demonstrated a similar effect of chronic Gal therapy on nicotinic receptor density. Although Barnes et al. (32) did not find a significant improvement in spatial memory in their aged rats, they did find a significant positive correlation between the durability of long-term potentiation and the Bmax of nicotinic receptors within the hippocampus that was induced by chronic Gal therapy. Taken together with the results of the current study, these data suggest that chronic Gal therapy can effectively and consistently increase the density of nicotinic receptors in selected brain regions that are involved in learning and memory. It is our conclusion that this increase in nicotinic receptor number, and the resultant changes in electrophysiological indicators of neural plasticity (32), may underlie aspects of the cognitive benefits produced by long-term therapy with Gal in humans with AD.
Acknowledgments
We thank Michael Ewers, Tara Orlando, Michelle Pak, and Isagani Santos for assistance with animal data collection. This research was supported by grants from Janssen Pharmaceutica (to D.S.W.-P. and G.L.W.) and by U.S. Public Health Service Contract Grant AG10546 (to G.L.W.).
Abbreviations
- APL
allosterically potentiating ligand
- AChE
acetylcholinesterase
- AD
Alzheimer's disease, CR, conditioned response
- CS
conditioned stimulus
- Gal
galantamine
- NM
nictitating membrane
- US
unconditioned stimulus
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031584398.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031584398
References
- 1.Berger T W, Berry S D, Thompson R F. In: The Hippocampus. Isaacson R L, Pribram K H, editors. New York: Plenum; 1986. pp. 203–239. [Google Scholar]
- 2.Woodruff-Pak D S. CNS Drug Rev. 1995;1:107–128. [Google Scholar]
- 3.Thompson R F. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- 4.Berger T W, Thompson R F. Brain Res. 1978;145:323–346. doi: 10.1016/0006-8993(78)90866-1. [DOI] [PubMed] [Google Scholar]
- 5.West M J, Coleman P D, Flood D G, Troncoso J C. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 6.Coyle J T, Price D L, DeLong M R. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 7.Woodruff-Pak D S, Finkbiner R G, Sasse D K. NeuroReport. 1990;1:45–48. doi: 10.1097/00001756-199009000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff-Pak D S, Papka M, Romano S, Li Y-T. Neurobiol Aging. 1996;17:505–512. [PubMed] [Google Scholar]
- 9.Solomon P R, Levine E, Bein T, Pendlebury W W. Neurobiol Aging. 1991;12:283–287. doi: 10.1016/0197-4580(91)90004-4. [DOI] [PubMed] [Google Scholar]
- 10.Nordberg A, Winblad B. Neurosci Lett. 1986;72:115–119. doi: 10.1016/0304-3940(86)90629-4. [DOI] [PubMed] [Google Scholar]
- 11.Perry E, Morris C, Court J, Cheng A, Fairbairn A, McKeith I, Irving D, Brown A, Perry R. Neuroscience. 1995;64:385–395. doi: 10.1016/0306-4522(94)00410-7. [DOI] [PubMed] [Google Scholar]
- 12.Schroder B, Reinhardt S, Schrattenholz A, McLane K E, Kretschmer A, Conti-Tronconi B M, Maelicke A. J Biol Chem. 1994;269:10407–10416. [PubMed] [Google Scholar]
- 13.Whitehouse P J, Martino A M, Antuono P G, Lowenstein P R, Coyle J T, Price D L, Kellar K J. Brain Res. 1986;371:146–151. doi: 10.1016/0006-8993(86)90819-x. [DOI] [PubMed] [Google Scholar]
- 14.Nordberg A, Lundqvist A, Hartvig P, Lilja A, Langstrom B. Alzheimer Dis Assoc Disord. 1995;9:21–27. doi: 10.1097/00002093-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Woodruff-Pak D S, Li Y-T, Kazmi A, Kem W R. Behav Neurosci. 1994;108:486–493. doi: 10.1037//0735-7044.108.3.486. [DOI] [PubMed] [Google Scholar]
- 16.Kem W R, Abbott B C, Coates R M. Toxicon. 1971;9:15–22. doi: 10.1016/0041-0101(71)90039-0. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff-Pak D S, Li Y-T, Kem W R. Brain Res. 1994;645:309–317. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- 18.Maelicke A, Albuquerque E X. Drug Discov Today. 1996;1:53–59. [Google Scholar]
- 19.Maelicke A, Schrattenholz A, Schroder H. Semin Neurosci. 1995;7:103–114. [Google Scholar]
- 20.Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albuquerque E X. Behav Brain Res. 2000;113:199–206. doi: 10.1016/s0166-4328(00)00214-x. [DOI] [PubMed] [Google Scholar]
- 21.Woodruff-Pak D S, Santos I. Behav Brain Res. 2000;113:11–19. doi: 10.1016/s0166-4328(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Steinmetz J E. Behav Res Methods Instrum Comput. 1998;30:384–391. [Google Scholar]
- 23.Ellman G L, Courtney K D, Andres V, Jr, Featherstone R M. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 24.Flores C M, Davila-Garcia M I, Ulrich Y M, Kellar K J. J Neurochem. 1997;69:2216–2219. doi: 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- 25.Abdulla F A, Bradbury E, Calaminici M R, Lippiello P M, Wonnacott S, Gray J A, Sinden J D. Psychopharmacology. 1996;124:323–331. doi: 10.1007/BF02247437. [DOI] [PubMed] [Google Scholar]
- 26.Pietila K, Lahde T, Attila M, Ahtee L, Nordberg A. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;357:176–182. doi: 10.1007/pl00005152. [DOI] [PubMed] [Google Scholar]
- 27.Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. Eur J Pharmacol. 1999;386:7–13. doi: 10.1016/s0014-2999(99)00741-4. [DOI] [PubMed] [Google Scholar]
- 28.Michalek H, Fortuna S, Pintor A. Neurobiol Aging. 1989;10:143–148. doi: 10.1016/0197-4580(89)90023-7. [DOI] [PubMed] [Google Scholar]
- 29.Springer J E, Rayrien M W, Loy R. Brain Res. 1987;407:180–184. doi: 10.1016/0006-8993(87)91235-2. [DOI] [PubMed] [Google Scholar]
- 30.Decker M W. Brain Res Bull. 1987;12:423–438. doi: 10.1016/0165-0173(87)90007-5. [DOI] [PubMed] [Google Scholar]
- 31.Skau K A, Triplett C G. Mol Chem Neuropathol. 1998;35:13–21. doi: 10.1007/BF02815113. [DOI] [PubMed] [Google Scholar]
- 32.Barnes C A, Meltzer J, Houston F, Orr G, McGann K, Wenk G L. Neuroscience. 2000;99:17–23. doi: 10.1016/s0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]