Abstract
Barttin is an accessory subunit that modifies protein stability, subcellular distribution, and voltage-dependent gating of ClC-K chloride channels expressed in renal and inner ear epithelia. ClC-K channels are double-barreled channels with two identical protopores that may be opened by individual or common gating processes. Using heterologous expression in mammalian cells and patch-clamp recordings, we studied the effects of barttin on gating of rat ClC-K1 and human ClC-Ka. In the absence of barttin, rClC-K1 channels displayed two gating processes with distinct kinetics and voltage dependence. A fast gating process, activated by membrane hyperpolarization, opens and closes individual rClC-K1 protopores. In addition, slow common gating steps, stimulated by membrane depolarization, act on both protopores together. Coexpression of barttin results in voltage-independent open probabilities of the common gate, causing increased channel activity at physiologic potentials. In contrast to rClC-K1, human ClC-Ka is functional only when coexpressed with barttin. Single-channel recordings of hClC-Ka/barttin show double-barreled channels with fast protopore gating without apparent cooperative gating steps. These findings demonstrate that barttin stimulates chloride flux through ClC-K channels by modifying cooperative gating of the double-barreled channels and highlight a physiologic role for gating of epithelial ClC chloride channels.
ClC-K channels form a subgroup of ClC channels expressed in the kidney and the inner ear.1–3 ClC-K isoforms were cloned from rats (rClC-K1 and rClC-K24,5) and humans (hClC-Ka and hClC-Kb6) and shown to fulfill distinct cellular functions. ClC-K1/ClC-Ka mediates apical Cl− influx and basolateral Cl− efflux in the thin ascending limb of Henle, and ClC-K2/ClC-Kb represents the anion efflux pathway necessary for NaCl resorption in the thick ascending limb of Henle. In the stria vascularis, both isoforms cooperate in mediating basolateral Cl− efflux necessary for active K+ secretion by marginal cells.1,7,8 For the majority of ClC-K channels, heterologous expression results only in measurable anion currents when expressed together with the accessory subunit barttin.1,3,9–11 Rat ClC-K1 is the only ClC-K isoform that is functional also in the absence of barttin.4,11
Barttin regulates the number and the activity of ClC-K channels by modifying stability,12,13 intracellular trafficking,9–12 and also the function of these channels.11,13 Naturally occurring mutations in the gene encoding barttin (BSND) result in Bartter syndrome type IV characterized by sensorineural deafness and salt-losing nephropathy. The modification of channel function by barttin appears to determine the severity of renal symptoms. We studied various disease-associated barttin mutations found in patients with Bartter syndrome type IV and demonstrated that mutations associated with severe renal phenotype abolish channel activity.13 In contrast, a BSND mutation that affects only the chaperone function of barttin and leaves the function of ClC-K/barttin channels unaffected caused nonsyndromic deafness.14
To further understand how barttin modifies the function of ClC-K channels, we studied voltage-dependent gating of two ClC-K isoforms, rat ClC-K1 and human ClC-Ka, expressed either alone or together with barttin. ClC channels exhibit a unique double-barreled architecture with two independent ion conduction pathways, the so-called protopores. This architecture results in two structurally distinct gating processes, the opening and closing of individual protopores, and also the cooperative gating of both protopores. Our experiments demonstrate that barttin activates ClC-K channels by constitutively opening common gates.
Results
Fast and Slow Gating of rClC-K1
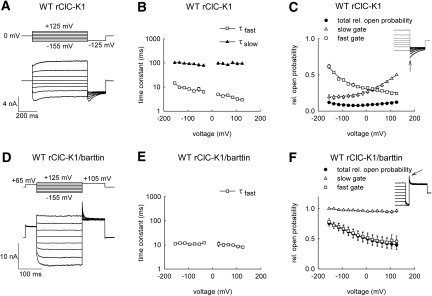
Figure 1 shows whole-cell recordings from mammalian cells heterologously expressing rat ClC-K1, either alone (Figure 1, A through C) or together with the accessory subunit barttin (Figure 1, D through F). In the absence of barttin, rClC-K1 currents increased instantaneously upon voltage steps in the positive as well as in the negative direction (Figure 1A). At positive voltages, instantaneous increases were followed by time-dependent current activation, whereas—upon membrane hyperpolarization—currents first activated and then subsequently inactivated. The biphasic time dependence at negative potentials in the absence of barttin (Figure 1A) suggests that rClC-K1 exhibits two gating processes with distinct kinetics and voltage dependence, resembling fast and slow gates of other ClC channels, such as ClC-0, ClC-1, and ClC-2.3
Figure 1.
Barttin locks the slow gate of WT rCIC-K1 in a voltage-independent open state. (A and D) Pulse protocols and representative current responses of tsA201 cells expressing WT rClC-K1, either expressed alone (A) or together with barttin (D). (B and E) Voltage dependencies of fast and slow activation and deactivation time constants for WT rClC-K1, either expressed alone (B, n = 6) or together with barttin (E, n = 5 to 9). (C and F) Voltage dependencies of relative probabilities of the channel to be conductive and of relative open probabilities of the fast and slow gates for rClC-K1 without (C, n = 5) or with barttin (F, n = 6). Solid lines in (C) and (F) represent fits with Boltzmann functions. Insets show representative experiments used to determine voltage dependence of slow gate openings.
Fits to the time course of current relaxations at various voltages provided fast and slow activation and deactivation time constants that are only moderately voltage-dependent (Figure 1B). To determine the voltage dependencies of the two different gating processes, we first plotted normalized isochronal current amplitudes immediately after a voltage step to −125 mV versus the preceding voltage. The so-obtained open probabilities did not change significantly with voltage (Figure 1C, filled circles). However, after depolarizing prepulses, rClC-K1 tail currents at −125 mV activated to maximum current amplitudes followed by a slower deactivation, indicating that the open probabilities of fast and slow gates are voltage-dependent. We took advantage of the different time courses of the two gates to separate voltage dependencies of fast and slow open probabilities. Depending on the slow gate open probability at the end of the prepulse, currents activated on a fast time course to certain maximum amplitudes and then slowly deactivated. Extrapolating the slow current decay to the beginning of the test steps using a monoexponential function (Figure 1C, inset) allowed determination of the voltage dependence of the relative open probabilities of the slow gate at the end of the variable prepulse (Figure 1C, open triangles). Assuming that fast and slow gating are independent of each other, relative open probabilities of the fast gate were then calculated by dividing the channel activation curve by the relative open probabilities of the slow gate (Figure 1C, open squares).
Coexpression of the accessory subunit barttin modifies the time and voltage dependence of rClC-K1 currents11 (Figure 1D). When coexpressed with barttin, rClC-K1 currents activated upon hyperpolarization and deactivated upon depolarization. There were no indications for depolarization-induced activation, such as those observed in rClC-K1 without barttin. Kinetics and voltage dependence of rClC-K1/barttin gating resembled fast gating of channels without barttin. Activation and deactivation could be fit with a single exponential function with time constants around 10 milliseconds (Figure 1E). Separation of fast and slow gates by inserting a 20-millisecond step to −180 mV revealed that the slow gate is open throughout the entire tested voltage range without appreciable voltage dependence (Figure 1F, inset).
Insertion of a Gating Glutamate into rClC-K1 Inverts the Voltage Dependence of Fast and Slow Gating
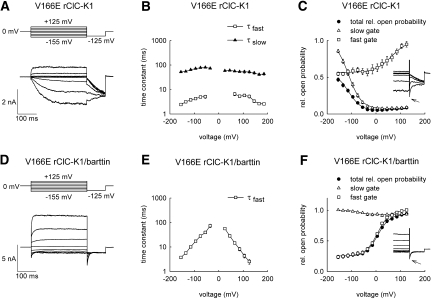
A glutamate belonging to a conserved motif (GKE/VLGP) at the amino terminus of the F helix was proposed to serve as the fast gate in various ClC channels.15–17 ClC-K channels are the only mammalian ClC isoforms that carry a neutral side chain at this position. A point mutation inserting a negative charge—V166E—inverts the voltage dependence of both the fast and the slow gates of rClC-K1. Hyperpolarization caused slow activation of V166E rClC-K1, whereas depolarization elicited faster activation (Figure 2A). Time constants of the fast and the slow gates lay within a similar range between wild-type (WT) and V166E rClC-K1, albeit with altered voltage dependence (Figure 2B). In the absence of barttin, the relative open probability of V166E rClC-K1 channels increased with more negative voltages (Figure 1C, filled circles). To separate both the fast and slow gates of V166E rClC-K1, we inserted a 2- to 5-millisecond voltage step to +180 mV (Figure 2C, inset). This short pulse is long enough to fully activate the fast gate without significantly affecting the slow gate. The dependence of the isochronal tail current amplitudes on the preceding voltages yielded the relative open probability of the slow gate (Figure 2C, open triangles). The fast gate activation curve was calculated by dividing the probability of channel opening by the slow gate open probability (Figure 2C, open squares).
Figure 2.
V166E inverts the voltage dependence of fast and slow gating of rCIC-K1 but leaves modification by barttin unaffected. (A and D) Pulse protocols and representative current responses of tsA201 cells expressing V166E rClC-K1, either expressed alone (A) or together with barttin (D). (B and E) Voltage dependencies of fast and slow activation and deactivation time constants for V166E rClC-K1, either expressed alone (B, n = 4 to 18) or together with barttin (E, n = 5). (C and F) Voltage dependencies of relative probabilities of the channel to be conductive and of relative open probabilities of the fast and the slow gates for mutant rClC-K1 without (C, n = 9) or with barttin (F, n = 5). Solid lines in (C) and (F) represent fits with Boltzmann functions. Insets show current responses to pulse protocols used to determine voltage dependence of slow gate openings.
Coexpression with barttin resulted in V166E rClC-K1 channels that activate upon membrane depolarization and deactivate upon hyperpolarization (Figure 2D).11 Activation/deactivation could be fit with a single exponential function, resulting in time constants with pronounced voltage dependence (Figure 2E). Separation of the fast and slow gates demonstrated that—in the presence of barttin—the slow gate is voltage-independent and open throughout the entire voltage range (Figure 2F). We recently determined a maximum absolute open probability of V166E rClC-K1/barttin channels of 1,11 indicating that the slow gate of mutant rClC-K1 is always open in the presence of barttin. Barttin also modified fast gating of V166E rClC-K1. In the presence of barttin, the minimum relative open probability was reduced, suggesting that either the maximum absolute open probability was increased and/or the absolute minimum open probability decreased (Figure 2, C and F).
The Fast Gate Opens and Closes Individual Protopores and the Slow Gate Mediates Common Gating Steps of rClC-K1
As a member of the ClC family of channels and transporters, individual ClC-K channels are expected to exhibit two ion conduction pathways (protopores).15 In double-barreled channels, two distinct types of gating transitions are possible, i.e., individual gating processes that open or close protopores separately, or common gating processes affecting both ion conduction pathways together. To separate individual and common gating processes, we performed single-channel analyses in rCIC-K1 without barttin (Supplemental Figure 1). For WT channels, open times and open probabilities were extremely small, and a single conductance state only was observed in most patches (Supplemental Figure 1, A through E). In patches containing V166E rClC-K1, multiple conductance states were regularly observed (Supplemental Figure 1, F through J). Such multiple states could correspond to openings of either multiple protopores or multiple channels. We were not able to distinguish between these two possibilities because the low open probability prevented accurate determination of the number of channels per patch and thus identification of deviation from binomial behavior.
We therefore used noise analysis to distinguish individual and common gating processes. Ion channel noise arises from the random opening and closing of individual channels.18 In double-barreled ion channels, noise may arise from common openings and closings of both protopores together, from gating of individual protopores or from a combination of both. In one extreme case, characterized by all protopores with their individual gates continuously open, unitary events correspond to simultaneous openings of both protopores by the common gate. In the other extreme, common gates that are completely open and individual protopores switching between open and closed states, currents and variances will be identical to values obtained with twice the number of channels displaying the same unitary conductance as a protopore. We reasoned that if one process gates individual protopores and the other one both protopores together, a voltage protocol that results in fast activation and consecutive slow deactivation will reveal different current variances at the same current amplitude, depending on whether it is measured during the activating or the deactivating phase (Supplemental Text).
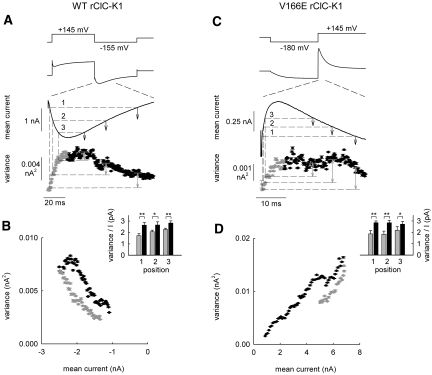
Figure 3A gives the time courses of mean current amplitudes and variances at voltage protocols designed to observe activation of the fast gate and subsequent deactivation of the slow gate during the test step to −155 mV for WT rClC-K1 without barttin. Current variances calculated during this period were then plotted versus the corresponding mean current amplitude (Figure 3B). These plots reveal a looplike dependence, deviating from the parabolic relationship usually observed in this kind of analysis.19,20 Current amplitudes during the rising phase immediately after the voltage step are associated with lower variances than the variances associated with the same current amplitude during the decaying phase (Figure 3B). This behavior closely resembles the predictions for noise analysis of ClC channels in the case that fast gating mediates individual and slow gating common opening and closing transitions (Supplemental Text and Supplemental Figure 2). Similar results could be obtained for V166E rClC-K1 (Figure 3, C and D). Current amplitudes during the rising phase immediately after the voltage step are associated with lower variances, in contrast to the same current amplitude during the decaying phase (Figure 3D), indicating that—also for V166E rClC-K1—individual gating processes are fast and common ones slow.
Figure 3.
Nonstationary noise analysis of rClC-K1 reveals an open channel substructure. (A and C) Time dependencies of the mean current amplitudes and variances upon pulse protocols that elicit biphasic current responses for WT rClC-K1 (A) and V166E rClC-K1 (C). (B and D) Plot of variances versus mean current amplitudes from the experiments shown in panels (A) and (C). Insets show ratios of mean current variances by mean currents for three points in time referred to as positions 1 through 3 in (A) and (C) (n = 5, 6).
Single-Channel Recordings Reveal the Occurrence of Double-Barreled Channels with Fast Protopore Gating in ClC-K/Barttin Channels
We next used single-channel recordings to describe the structural basis of gating in ClC-K/barttin channels. In patches containing WT rClC-K1/barttin, more than one open state was always observed (Supplemental Figure 3). The probability of being in these different open states was binomially distributed, as is to be expected if common gates are always completely open. However, we could not exclude the fact that multiple conductance states correspond to two or more channels that exclusively display common opening and closing.
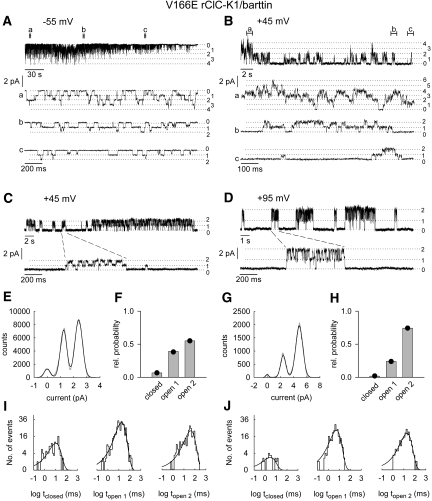
For V166E rClC-K1/barttin, distinction between individual and common gating processes was made possible by a rundown process that caused time-dependent changes in channel activity after patch excision (Figure 4, A and B). Under these conditions, long nonconducting periods were followed by channel activity with two conductance states at positive potentials (Figure 4, C and D). Within these bursts the open probabilities of the two conductance states were binomially distributed (Figure 4, E through H), indicating that the protopores gate independently of each other. Dwell times in the closed state and in the states with one or both protopores being open followed a monoexponential distribution (Figure 4, I and J). After partial rundown V166E rClC-K1/barttin channels resemble double-barreled channels with fast individual and slow common gating.21,22
Figure 4.
Single channel recordings show fast protopore gating in V166E rCIC-K1/barttin channels. (A and B) Representative single-channel recordings in excised patches at −55 mV (A) and +45 mV (B). Portions of the recordings shown on an expanded time scale are indicated as (a, b, and c). (C and D) Single-channel recordings from an excised inside-out patch at a holding potential of +45 mV (C) or +95 mV (D). (E and G) Amplitude histograms from bursts of the registrations shown in C and D and fits with the sum of three equidistant Gaussian distributions (E, single burst of 23-second duration with maximum dwell time <5 milliseconds in zero conductance state; G, sum of seven bursts with maximum dwell time <2 milliseconds in zero conductance state). (F and H) Probabilities of the three current levels, closed, open 1, and open 2, at +45 mV (F) and +95 mV (H). The measured state probabilities are represented by bars, whereas the symbols denote the predicted binomial distribution of state probabilities calculated from P0 = 0.74 in (F) and P0 = 0.86 in (H). (I and J) Dwell-time distribution of current levels, closed, open 1, and open 2, from bursts of the registrations shown in (C) and (D). Curves represent monoexponential fits to the log-binned data (I: +45 mV, 760 events, τclosed = 10 milliseconds, τopen 1 = 18 milliseconds, τopen 2 = 42 milliseconds; J: +95 mV, 491 events, τclosed = 3 milliseconds, τopen 1 = 6 milliseconds, τopen 2 = 19 milliseconds).
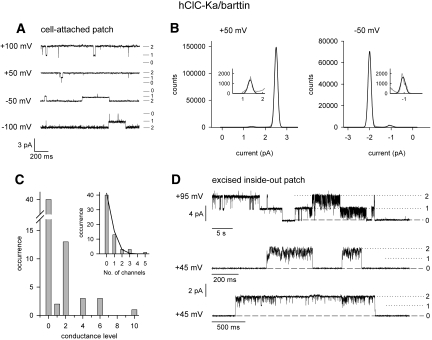
Figure 5 gives single-channel recordings from a human ClC-K isoform, hClC-Ka/barttin. Representative recordings from a cell-attached patch containing hClC-Ka/barttin showed two equally spaced conductance levels (Figure 5A). The distribution of the time spent in the distinct states followed a binomial distribution with open probabilities of at least 97% (Figure 5B). In cell-attached patches, the behavior of hClC-Ka/barttin thus resembled a channel with two independently gated conduction pathways without a cooperative gating process. The high absolute open probabilities of hClC-Ka/barttin14 made possible the accurate determination of the number of ion pores per patch and thus allowed another approach to distinguish individual or common gating. If the observed fast gating affects individual protopores, an even number of conductance states must be observed in each patch. In contrast, even and uneven conductance state numbers are possible if protopores are always open and only common gating processes occur. Figure 5C shows numbers of current levels. In 20 out of 22 patches containing channels, an even number of conductance levels was observed, and the number of conductance states divided by 2 followed a Poisson distribution (Figure 5C, inset), the distribution expected if double-barreled channels are randomly inserted in membrane patches of comparable size. Moreover, in excised patches containing hClC-Ka/barttin, channel rundown caused long-lasting common closures and binomial burst behavior between these episodes (Figure 5D). We conclude that individual protopores are opened and closed by the fast gate also in the presence of barttin.
Figure 5.
Single channel recordings show fast protopore gating in hCIC-Ka/barttin channels. (A) Single-channel recordings from a cell-attached patch at holding potentials between −100 and +100 mV. (B) Amplitude histograms of the registrations shown in (A) and fits with the sum of three equidistant Gaussian distributions. (C) Frequency distribution of patches containing a certain number of current levels. (D) Single-channel recordings from excised inside-out patches at holding potentials of +95 or +45 mV.
Modification of Unitary Current Amplitudes by Barttin
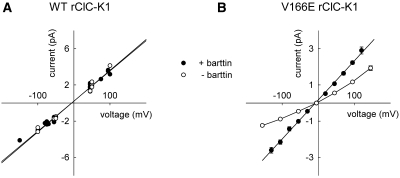
Single-channel analysis allows direct determination of unitary current amplitudes in the absence and in the presence of barttin. The unitary conductance of rClC-K1 was 33 pS with barttin and 34 pS without barttin (Figure 6A). V166E reduced the unitary conductance to 10 pS in the absence of barttin and 22 pS in the presence of barttin (Figure 6B), in agreement with earlier data.11 Barttin modifies single-channel amplitudes only in mutant but not in WT rClC-K1.
Figure 6.
Barttin increases the single-channel amplitude of V166E, but not of WT rClC-K1. (A) Voltage dependencies of single protopore current amplitudes from WT rClC-K1 channels alone (open symbols, 7 patches) and with barttin (closed symbols, 12 patches). (B) Voltage dependencies of single protopore current amplitudes from V166E rClC-K1 channels alone (open symbols, n = 5 to 16) and with barttin (closed symbols, n = 3 to 15).
Discussion
This study demonstrates how barttin modifies gating of two ClC-K channels, rat ClC-K1 and human ClC-Ka. Rat ClC-K1 is active in the absence of barttin and displays two gating processes with different kinetics and voltage dependence (Figures 1 and 2). Using noise analysis, we could demonstrate that—in the absence of barttin—fast gating mediates individual opening and closing and slow gating cooperative processes. The variance of rClC-K1 currents assumes a smaller value when measured during fast activation than for the same current amplitude determined during slow deactivation (Figure 3). Because a double-barreled structure is firmly established for all ClC proteins15 and because single-channel analyses reveal equally spaced conductance levels for ClC-K channels (Figures 4 and 5, Supplemental Figure 1), these results indicate that the slow gates mediate common openings/closings of both protopores, whereas the fast gate mediates individual gating steps.
When cotransfected with barttin, channels display only one gating process that resembles macroscopic fast gating of the same channel in the absence of barttin (Figures 1 and 2). Single V166E rClC-K1/barttin and human ClC-Ka/barttin channels exhibit double-barreled channels with fast protopore gating (Figures 4 and 5). In the presence of barttin, the open probability of the slow gate assumed a voltage-independent value (Figures 1 and 2). Absolute open probabilities could not be accurately determined for WT rClC-K1/barttin. However, for V166E rClC-K1/barttin and hClC-Ka/barttin, we could demonstrate that the absolute open probability of the slow gate is 1 in whole-cell11 and cell-attached recordings14 (Figure 5), respectively. Taken together, these results demonstrate that protopore gating is fast both in the presence and in the absence of barttin and that barttin constitutively opens the common slow gate of ClC-K/barttin channels.
Patch excision modified fast gating of ClC-K/barttin channels and caused long-lasting common closures of both protopores (Figures 4 and 5). We do not know the mechanistic origin of the “rundown”-associated common closures. The effect of barttin on the slow common gate might require an intact cytoskeleton. Alternatively, common closures in excised patches might occur by mechanisms that are completely different from slow gating events in ClC-0 and ClC-1.
ClC-K channels lack the “gating” glutamate at the amino-terminal end of the F helix that is thought to be responsible for fast protopore gating.15–17 In contrast to our expectations, WT rClC-K1 and WT hClC-Ka/barttin display such gating processes (Figures 1, 3, and 5). We conclude that fast gating is possible also in the absence of a protonable residue at the position of the gating glutamate. Moreover, V166E changes rClC-K1 gating in a different way, as expected. This mutation does not implement an additional gating component but inverts the voltage dependence of the pre-existing fast and slow gating processes; i.e., the fast gate is activated by hyperpolarization in WT and by depolarization in V166E rClC-K1 (Figure 2). Moreover, time constants of activation and deactivation remain comparable for WT and mutant channels. These findings argue against E166 acting as the fast gate in this ClC isoform and suggest that the amino acid at position 166 determines only the voltage dependence of fast and slow gating (Figures 1 through 3). The effect of barttin on slow gating of WT and V166E rClC-K1 is very similar, although V166E inverts the voltage dependence of this particular process (Figure 2). However, barttin modifies fast gating and the unitary conductance of V166E, but not of WT channels.
All known ClC chloride channels exhibit voltage-dependent gating processes.3,23 For most ClC channels, the physiologic significance of these gating processes is unclear. Especially, cooperative gating processes that mediate joint opening and closing of the two identical protopores have attracted much biophysical interest,22,24 but the functional effect of these processes has yet to be clarified. This study establishes that barttin modifies the function of the ClC-K channel by modifying cooperative slow gating. Gating of ClC-K channels and its modulation by barttin thus serve optimization of epithelial transport. Barttin is the first identified protein that modifies slow gating of ClC channels. It might represent a paradigm for new therapeutic approaches to modify renal NaCl resorption or to treat certain chloride channelopathies.
Concise Methods
Channel Expression
tsA201 cells were transfected with 0.4 to 7 μg of a bicistronic vector that contains the coding regions of WT or V166E rClC-K1 and of the CD8 antigen (pSVL-rClC-K1-IRES-CD8), using a calcium phosphate precipitation method.11 When indicated, a plasmid encoding human barttin was cotransfected.11 Electrophysiological experiments were performed typically 1 to 2 days after transient transfection. For experiments in which ClC-K was expressed without barttin, channel cDNA amounts and time periods between transfection and experiment were increased. Cells were incubated for at least 5 minutes before use together with polystyrene microbeads precoated with anti-CD8 antibodies (Dynabeads M-450 CD 8; Dynal, Great Neck, NY). Only cells decorated with microbeads were used for electrophysiological recordings.11 In some of our experiments fluorescence protein-tagged constructs (YFP-rClC-K1, YFP-hClC-Ka, and barttin-CFP13) were used.
Electrophysiology
Standard whole-cell and excised inside-out patch-clamp recordings were performed using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) as described.25,26 Pipettes were pulled from borosilicate glass and had resistances between 1.0 and 2.0 MΩ for whole-cell recordings and 2 to 26 MΩ for single-channel recordings. For noise analyses and single-channel experiments, pipettes were covered with dental wax to reduce their capacitance. More than 80% of the series resistance was compensated by an analog procedure, resulting in calculated voltage errors <5 mV. Cells/patches were clamped to 0 mV for at least 5 seconds between test sweeps. The standard extracellular solution contained the following (in mM): NaCl (140), KCl (4), CaCl2 (2), MgCl2 (1), HEPES (5), pH 7.4. The standard intracellular solution contained the following (in mM): NaCl (120), MgCl2 (2), EGTA (5), HEPES (10), pH 7.4. For cell-attached patches KCl (140 mM) substituted for NaCl in the bath solution and pipettes were filled with standard extracellular solution. Liquid potentials were calculated and subtracted a priori.
Data Analysis
The data were analyzed with a combination of the pClamp9/10 (Molecular Devices) and SigmaPlot (Jandel Scientific, San Rafael, CA) programs. Isochronal current amplitudes were measured immediately after capacitive current relaxation, i.e., usually 0.5 milliseconds after the voltage step. All values are given as mean ± SEM.
To determine the time course of macroscopic current activation and deactivation (Figures 1 and 2), the sum of a monoexponential or biexponential function and a time-independent value (I(t) = a1 exp(−t/τfast) + a2 exp(−t/τslow) + c) was fit to data recorded during a series of voltage steps from different holding potentials. For WT rClC-K1 without barttin, fast deactivation time constants at positive potentials were determined after a hyperpolarizing prepulse. Fast deactivation time constants of V166E rClC-K1 at hyperpolarizing holding potentials were determined from monoexponential fits to fast current relaxations after a short (5 milliseconds) voltage step to +180 mV. Slow deactivation at positive potentials was studied after a hyperpolarizing prepulse to −135 mV.
To obtain the relative probabilities of channel openings, isochronal current amplitudes 500 microseconds after stepping to −125 mV (+105 mV for WT rClC-K1/barttin) were plotted versus the voltage of a preceding 300- to 600-millisecond pulse and fit with a modified Boltzmann equation, exhibiting a voltage-independent minimum open probability (Pmin) and a voltage-dependent term: P(V) = (Pmax − Pmin)/(1 + ezδF(V − V0.5)/RT) + Pmin, with zδ being the apparent gating charge, Pmax the maximum open probability, and V0.5 the midpoint of activation. Open probabilities of V166E rClC-K1 were normalized to maximum values obtained from the Boltzmann fit. WT channels without barttin exhibit a very shallow voltage dependence, preventing extrapolation of the maximum open probability. We therefore arbitrarily normalized slow gate activation curves to a relative open probability of 0.5 at +125 mV and fast gate activation curves to 0.5 at −125 mV (Figure 1C). To determine the voltage dependence of the slow gate open probability in V166E rClC-K1, we inserted a short step to +180 mV (2 to 5 milliseconds) that fully activated the fast gate (Figure 2, C and F).
Single-Channel Analysis
Single-channel recordings were performed in the inside-out configuration with pipettes containing the standard extracellular solution and cells bathed in the standard intracellular solution. Currents were sampled at 50 kHz and filtered at 200 to 500 Hz. We found ClC-K channels in 34 out of 74 patches from cells expressing ClC-Ka/barttin, in 17 out of 41 patches expressing rClC-K1 alone, and in 21 out of 41 patches with rClC-K1/barttin. For V166E rClC-K1, 27 out of 100 patches contained the channel alone and 36 out of 94 patches coexpressing barttin contained V166E rClC-K1/barttin channels. Single channels were identified using the following criteria: (1) Comparable single-channel behavior was repeatedly observed only in patches from cells expressing the particular isoform; (2) unitary current amplitudes resemble results obtained from nonstationary noise analysis; and (3) the voltage dependence of absolute open probabilities determined from single-channel recordings were similar to the voltage dependence of relative open probabilities obtained from tail current analyses.
Single-channel current amplitudes were determined from all-points amplitude histograms of single- or multiple-channel recordings at various potentials and fit by Gaussian distributions. The relative areas of the different Gaussian components were used to estimate absolute open probabilities. Log-binned dwell-time data were fitted monoexponentially using maximum likelihood estimation.27 For hClC-Ka/barttin the number of pores per patch (Figure 5) was determined by counting the maximum number of conductance levels in long-lasting recordings at positive potentials.28
Noise Analysis
Nonstationary noise analysis was performed as described.25 Currents were filtered at 10 kHz and digitized with a sampling rate of 50 kHz. A series of 50 to 200 records were recorded by pulsing to a certain voltage, and pairs of subsequent records were then subtracted using the Analysis software (kindly provided by Dr. F. Bezanilla, University of Chicago, Chicago, IL) to compute the experimental nonstationary ensemble variance.29 The variance points were sorted into evenly spaced current bins, and the statistical errors caused by averaging were superimposed as error bars. For some experiments, the analysis was repeated after digital filtering at 1 kHz without any difference in outcome.
Disclosures
None.
Supplementary Material
Acknowledgments
These studies were supported by the Deutsche Forschungsgemeinschaft (FA301/10-1 to C.F.).
Parts of this study were reported as abstracts (Acta Physiol 189: 99, 2007 and Acta Physiol 192: 53, 2008).
We thank Drs. A. L. George and S. Uchida for providing the expression constructs for barttin and rClC-K1, Drs. Alexi Alekov, Patricia Hidalgo, and Ute Scholl for helpful discussions, and Birgit Begemann and Toni Becher for excellent technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Modulation of ClC-K Channel Function by the Accessory Subunit Barttin,” on pages 1238–1239.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Uchida S, Sasaki S: Function of chloride channels in the kidney. Annu Rev Physiol 67: 759–778, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Sile S, Vanoye CG, George AL, Jr: Molecular physiology of renal ClC chloride channels/transporters. Curr Opin Nephrol Hypertens 15: 511–516, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Jentsch TJ: CLC chloride channels and transporters: From genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 43: 3–36, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Uchida S, Sasaki S, Furukawa T, Hiraoka M, Imai T, Hirata Y, Marumo F: Molecular cloning of a chloride channel that is regulated by dehydration and expressed predominantly in kidney medulla. J Biol Chem 268: 3821–3824, 1993 [PubMed] [Google Scholar]
- 5.Adachi S, Uchida S, Ito H, Hata M, Hiroe M, Marumo F, Sasaki S: Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle's loop and collecting ducts of rat kidney. J Biol Chem 269: 17677–17683, 1994 [PubMed] [Google Scholar]
- 6.Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ: Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci U S A 91: 6943–6947, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlingmann KP, Konrad M, Jeck N, Waldegger P, Reinalter SC, Holder M, Seyberth HW, Waldegger S: Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med 350: 1314–1319, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Rickheit G, Maier H, Strenzke N, Andreescu CE, De Zeeuw CI, Muenscher A, Zdebik AA, Jentsch TJ: Endocochlear potential depends on Cl− channels: Mechanism underlying deafness in Bartter syndrome IV. EMBO J 27: 2907–2917, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ: Barttin is a Cl− channel ß subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature 414: 558–561, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Waldegger S, Jeck N, Barth P, Peters M, Vitzthum H, Wolf K, Kurtz A, Konrad M, Seyberth HW: Barttin increases surface expression and changes current properties of ClC-K channels. Pflugers Arch 444: 411–418, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Scholl U, Hebeisen S, Janssen AG, Muller-Newen G, Alekov A, Fahlke Ch: Barttin modulates trafficking and function of ClC-K channels. Proc Natl Acad Sci U S A 103: 11411–11416, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayama A, Rai T, Sasaki S, Uchida S: Molecular mechanisms of Bartter syndrome caused by mutations in the BSND gene. Histochem Cell Biol 119: 485–493, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Janssen AG, Scholl U, Domeyer C, Nothmann D, Leinenweber A, Fahlke Ch: Disease-causing dysfunctions of barttin in Bartter syndrome type IV. J Am Soc Nephrol 20: 145–153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riazuddin S, Anwar S, Fischer M, Ahmed ZM, Khan SY, Janssen AG, Zafar AU, Scholl U, Husnain T, Belyantseva IA, Friedman PL, Riazuddin S, Friedman TB, Fahlke Ch: Molecular basis of DFNB73: mutations of BSND can cause nonsyndromic deafness or Bartter syndrome. Am J Hum Genet 85: 273–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutzler R, Campbell ED, Cadene M, Chait MB, MacKinnon R: X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415: 287–294, 2002 [DOI] [PubMed] [Google Scholar]
- 16.de Santiago JA, Nehrke K, Arreola J: Quantitative analysis of the voltage-dependent gating of mouse parotid ClC-2 chloride channel. J Gen Physiol 126: 591–603, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niemeyer MI, Cid LP, Zuniga L, Catalan M, Sepulveda FV: A conserved pore-lining glutamate as a voltage- and chloride-dependent gate in the ClC-2 chloride channel. J Physiol 553: 873–879, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFelice LJ: Introduction to Membrane Noise, New York, Plenum Press, 1981 [Google Scholar]
- 19.Sigworth FJ: The variance of sodium current fluctuations at the node of Ranvier. J Physiol (London) 307: 97–129, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez O, Gonzalez C, Latorre R: Counting channels: A tutorial guide on ion channel fluctuation analysis. Adv Physiol Educ 26: 327–341, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Miller C: Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci 299: 401–411, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Lin YW, Lin CW, Chen TY: Elimination of the slow gating of ClC-0 chloride channel by a point mutation. J Gen Physiol 114: 1–12, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen TY, Hwang TC: CLC-0 and CFTR: Chloride channels evolved from transporters. Physiol Rev 88: 351–387, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Lisal J, Maduke M: The ClC-0 chloride channel is a ‘broken’ Cl−/H+ antiporter. Nat Struct Mol Biol 15: 805–810, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebeisen S, Heidtmann L, Cosmelli D, Gonzalez C, Poser B, Latorre R, Alvarez O, Fahlke Ch: Anion permeation in human ClC-4 channels. Biophys J 84: 2306–2318, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melzer N, Biela A, Fahlke Ch: Glutamate modifies ion conduction and voltage-dependent gating of excitatory amino acid transporter-associated anion channels. J Biol Chem 278: 50112–50119, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Sigworth FJ, Sine SM: Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J 52: 1047–1054, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn R: Estimating the number of channels in patch recordings. Biophys J 60: 433–439, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinemann SH, Conti F: Nonstationary noise analysis and application to patch clamp recordings. Method Enzymol 207: 131–148, 1992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.