Abstract
TGF-β plays a key role in upregulating matrix production in injury-induced renal fibrosis, but how TGF-β signaling in distinct compartments of the kidney, such as specific segments of the nephron, affects the response to injury is unknown. In this study, we determined the role of TGF-β signaling both in development of the renal collecting system and in response to injury by selectively deleting the TGF-β type II receptor in mice at the initiation of ureteric bud development. These mice developed normally but demonstrated a paradoxic increase in fibrosis associated with enhanced levels of active TGF-β after unilateral ureteral obstruction. Consistent with this observation, TGF-β type II receptor deletion in cultured collecting duct cells resulted in excessive integrin αvβ6-dependent TGF-β activation that increased collagen synthesis in co-cultured renal interstitial fibroblasts. These results suggest that inhibiting TGF-β receptor–mediated function in collecting ducts may exacerbate renal fibrosis by enhancing paracrine TGF-β signaling between epithelial and interstitial cells.
TGF-β, one of the most important promoters of fibrosis in all organs, primarily mediates scarring by inducing collagen synthesis by fibroblasts. TGF-β exists in three isoforms, TGF-β1, -β2, and -β3, which have both redundant and nonredundant physiologic effects. All three isoforms bind to the TGF-β type II receptor (TβRII), which leads to the formation of a heterotetrameric signaling complex comprising both type I and type II TGF-β receptors. The type I receptor activates Smad signaling by phosphorylating Smads 2/3, which then bind to Smad4 and accumulate in the nucleus to modulate gene transcription or it signals through Smad-independent pathways.1–3
TGF-β mediates multiple cellular events within its microenvironment, thus requiring tight local control of its activity. TGF-β ligands are secreted in an inactive form as a result of noncovalent binding to the latency-associated peptide (LAP).4 Most TGF-β is sequestered in the matrix as the latent form, so activation is the key step in determining TGF-β bioactivity. The mature TGF-β homodimer is activated by heat, acidification, oxidation, and proteolytic cleavage from the LAP by proteases such as matrix metalloproteinases and plasmin. In addition, thrombospondin 1 (TSP-1) and integrins are physiologically important activators that act by inducing conformational changes in the LAP/TGF-β complex.5 Specifically, integrin αvβ6, expressed on epithelial cells, binds to the RGD sequence present in the LAP of TGF-β1 and -β3 to liberate mature TGF-β upon integrin activation.6
TGF-β plays a crucial role in both renal development and the progression of fibrosis after kidney injury. TGF-β2 is the major isoform required for renal development. TGF-β2 null mice have severe renal dysplasia with renal tubular dilation and epithelial degeneration, and exogenous TGF-β2 modulates branching morphogenesis in organ cultures.7–11 Furthermore, mouse chimeras with reduced TβRII expression develop cystic kidneys.12 In contrast, TGF-β1 is the primary mediator of TGF-β–dependent profibrotic effects. Overexpression of active TGF-β1 in mice induced both tubulointerstitial fibrosis and glomerulosclerosis in the kidney.13,14 Moreover, inhibiting TGF-β signaling, either pharmacologically or genetically, attenuated tubulointerstitial fibrosis in renal injury models.15,16 An important limitation of those studies is that they did not target specific cellular compartments within the kidney because the inhibitors were given systemically, and genetic studies were performed on global knockout mice. In vitro studies have implicated interstitial fibroblasts as the principal mediators of TGF-β–induced tubulointerstitial fibrosis in vivo, and the contribution of TGF-β activity in renal tubular epithelial cells is not known.
To determine the potential role of TGF-β signaling in the renal collecting system on both development and disease, we deleted TβRII at the initiation of ureteric bud (UB) development by crossing Tgfbr2flox/flox mice with Hoxb7Cre mice. This approach allowed us to study TGF-β signaling while avoiding the functional redundancy of the ligands and signaling pathways. Contrary to expectations, the UB in the Hoxb7Cre;Tgfbr2flox/flox mice developed normally. Even more surprising, these mice developed increased fibrosis in response to unilateral ureteric obstruction (UUO), a model of tubulointerstitial injury. Deleting TβRII in collecting duct (CD) cells in vitro resulted in increased integrin αvβ6–dependent TGF-β activation that increased collagen synthesis in co-cultured renal interstitial fibroblasts. Our finding that deleting TβRII in renal CD cells increases TGF-β activation and exacerbates renal fibrosis has important implications for pharmacologic strategies that target TβRII to decrease fibrosis.
Results
Deleting TβRII in the Collecting System Worsens Renal Injury after UUO
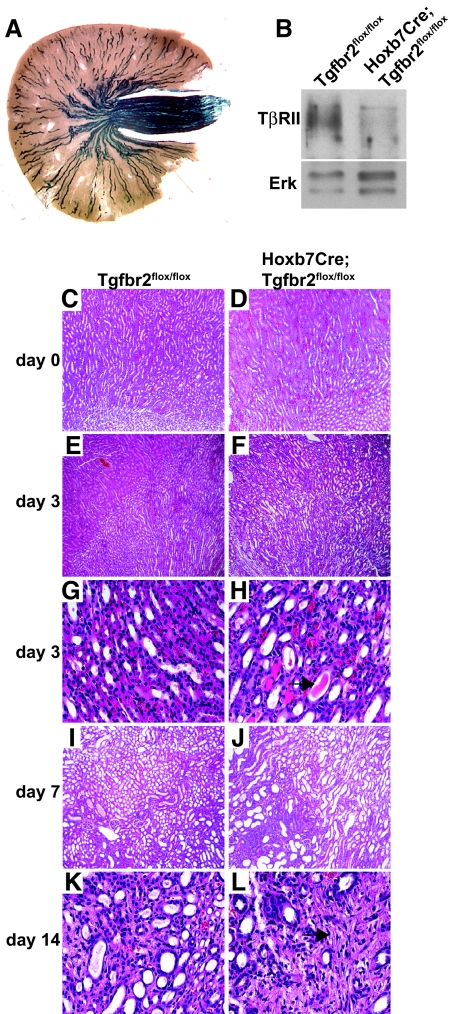
To define the role of TβRII in development of the renal collecting system, we deleted TβRII at the initiation of UB development (embryonic day 10.5) by crossing the Tgfbr2flox/flox mouse on a ROSA26 reporter background with the Hoxb7Cre mouse. Strong β-galactosidase staining was present throughout the collecting system of Hoxb7Cre;Tgfbr2flox/flox mice (Figure 1A), and TβRII immunoblots of renal papillae confirmed that the receptor was deleted (Figure 1B). No abnormalities in branching morphogenesis or renal architecture were noted in adult Hoxb7Cre;Tgfbr2flox/flox mice (Figure 1, C and D), which have normal life spans and reproductive capabilities. Thus, UB-derived TβRII does not play a significant role in renal development.
Figure 1.
Hoxb7Cre;Tgfbr2flox/flox mice develop normally but sustain greater injury after UUO. (A) β-gal staining of Hoxb7Cre;Tgfbr2flox/flox mice with the ROSA26 reporter demonstrates Cre expression in the collecting system. (B) Tissue lysates of renal papillae from adult mice are immunoblotted with antibodies directed against TβRII. Each blot shows a representative kidney from a total of three mice per genotype. (E through L) Kidney tissue from uninjured mice (C and D) and mice injured by UUO (E through L) is stained with hematoxylin and eosin. Three days after UUO injury, hematoxylin and eosin staining reveals tubular dilation (E and F) as well as increased epithelial flattening (G and H) and tubular casts (black arrow). Seven days after UUO, tubular damage worsens and fibrosis develops (I and J); this is further exacerbated at 14 days (K and L; black arrow). Magnifications: ×100 in C through F, I, and J; ×400 in G, H, K, and L
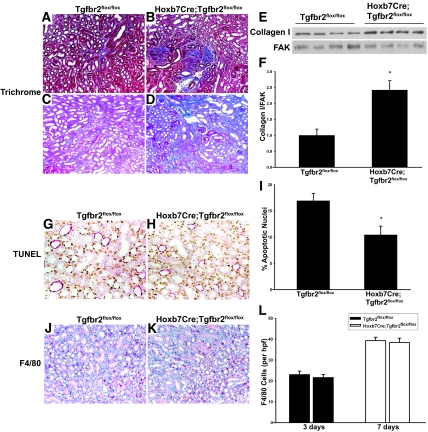
Because the role of TβRII in the renal collecting system in renal fibrosis after injury is unknown, we determined how Hoxb7Cre;Tgfbr2flox/flox mice respond to UUO. Unexpectedly, tubular injury was increased in the Hoxb7Cre;Tgfbr2flox/flox mice compared with Tgfbr2flox/flox mice at 3, 7, and 14 days after UUO (Figure 1, E through L). By day 3, the Hoxb7Cre;Tgfbr2flox/flox mice displayed increased tubular dilation, flattening of the CD epithelium, proteinaceous casts in the collecting system, and increased interstitial cellularity in the corticomedullary region (Figure 1, E through H). At day 7, the Hoxb7Cre;Tgfbr2flox/flox mice had greater tubular epithelial cell dropout and evidence of increased fibrosis (Figure 1, I and J), which persisted at day 14 (Figure 1, K and L). The injury was scored (as described in the Concise Methods section) at days 3 and 7 after UUO, and the Hoxb7Cre;Tgfbr2flox/flox mice had a consistently greater injury score than did the Tgfbr2flox/flox mice: 3.00 ± 0.25 (SE) versus 2.00 ± 0.00 (SE) at 3 days; 4.00 ± 0.00 (SE) versus 3.00 + 0.25 (SE) at 7 days. The increased fibrosis in the Hoxb7Cre;Tgfbr2flox/flox mice was confirmed by trichrome staining on kidney sections 7 and 14 days after UUO (Figure 2, A through D). Immunoblots performed on kidney lysates at this time point also demonstrated a statistically significant increase of collagen I in the Hoxb7Cre;Tgfbr2flox/flox mice (Figure 2, E and F). When terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining was performed to investigate whether apoptosis accounted for the increased injury in the Hoxb7Cre;Tgfbr2flox/flox mice, less tubular apoptosis was seen in the CD epithelium 3 days after UUO (Figure 2, G through I), and there was no difference between genotypes at days 7 and 14 (data not shown). Furthermore, the discrepancy in injury between the genotypes was not due to increased inflammation, because there was no difference in the number of macrophages, as shown by F4/80 staining (Figure 2, J through L), or T cells as determined by CD3 IHC (data not shown). There were also no differences in inflammatory cytokines between the genotypes as assessed by protein microarrays of tissue lysates 3 days after UUO (data not shown). Thus, deleting TβRII in the collecting system worsened tubular injury and increased fibrosis after UUO, and these findings were not accounted for by differences in apoptosis or inflammation.
Figure 2.
Hoxb7Cre;Tgfbr2flox/flox mice have increased collagen I expression that is not attributed to changes in apoptosis or inflammation. (A through D) Fibrillar collagen is detected 7 (A and B) and 14 (C and D) days after UUO by Trichrome staining. (E) Lysates of corticomedullary tissue of obstructed kidney are immunoblotted for collagen I. (F) Collagen I bands from four mice per genotype are quantified by densitometry, normalized to focal adhesion kinase (FAK), and reported as means ± SEM. *P < 0.05. (G and H) TUNEL staining shows apoptotic nuclei (brown) in Hoxb7Cre;Tgfbr2flox/flox and Tgfbr2flox/flox mice 3 days after UUO. CD localization of apoptosis is determined by co-staining with AQP2 (red). (I) TUNEL-positive cells from 10 high-powered fields per mouse are counted in a blinded manner using four mice per genotype. *P < 0.05. (J and K) F4/80 staining is performed for macrophage infiltration. (L) F4/80-positive cells are quantified and expressed as described for TUNEL staining. Magnifications: ×100 in A through D, J, and K; ×400 in G and H.
Increased Active TGF-β Is Present in Hoxb7Cre;Tgfbr2flox/flox Mice after UUO
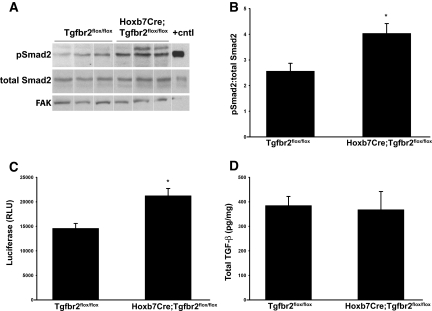
Because of the paradoxic increased fibrosis in Hoxb7Cre;Tgfbr2flox/flox mice, we hypothesized that deleting TβRII altered production or activation of TGF-β. To test this hypothesis, we assessed the levels of Smad2 phosphorylation in tissue lysates of obstructed kidneys. Hoxb7Cre;Tgfbr2flox/flox mice had increased pSmad2 3 days after UUO (Figure 3, A and B). Because increased Smad phosphorylation could reflect compensatory alterations in non–TGF-β signaling pathways (e.g., activins, angiotensin), we confirmed that increased levels of active TGF-β were present in kidneys from injured Hoxb7Cre;Tgfbr2flox/flox mice using the plasminogen activator inhibitor 1 promoter and a luciferase reporter (PAI/L) assay, which measures all bioactive TGF-β isoforms (Figure 3C). The amount of total TGF-β1, as measured by ELISA, was similar in the Hoxb7Cre;Tgfbr2flox/flox and Tgfbr2flox/flox mice (Figure 3D). Thus, there was increased active TGF-β in the kidneys of the Hoxb7Cre;Tgfbr2flox/flox mice compared with the Tgfbr2flox/flox mice.
Figure 3.
Hoxb7Cre;Tgfbr2flox/flox mice have increased active TGF-β compared with Tgfbr2flox/flox mice. (A) pSmad2 immunoblots are performed on obstructed kidney lysates from Hoxb7Cre;Tgfbr2flox/flox and Tgfbr2flox/flox mice 3 days after UUO. TGF-β–treated cells are used as a positive control. Three representative blots per genotype are shown, and the vertical white line separates samples that are rearranged from two blots performed simultaneously and developed for equivalent times. (B) Band intensity is quantified as a ratio of pSmad2 to total Smad2 using densitometry. Data are derived from five mice per genotype and reported as the mean ± SEM. *P < 0.05. (C) Active TGF-β is determined by the PAI/L assay, as described in the Concise Methods section. Composite values for six mice per genotype at 3 days after UUO are reported as the mean in random luciferase units (RLU) ± SE. *P < 0.05. (D) Total TGF-β levels from kidney lysates 3 days after UUO are measured by an ELISA assay. The data represent the average values of TGF-β (pg) per mg of protein for five mice in each genotype.
Conditioned Medium from TβRII−/− CD Cells Has More Active TGF-β
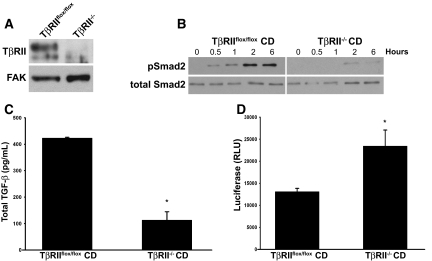
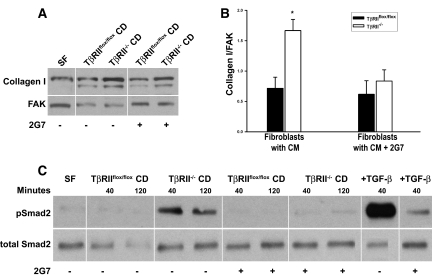
On the basis of the in vivo results, we hypothesized that deleting TβRII in the CD epithelium would increase TGF-β activation, resulting in collagen I synthesis by renal interstitial cells. To test this hypothesis, we generated CD cells from papillae of the Tgfbr2flox/flox mice and subsequently deleted TβRII in vitro by adeno-Cre infection. Deletion of the receptor was confirmed by immunoblotting cell lysates (Figure 4A). As expected, TβRII−/− CD cells were unable to phosphorylate Smad2 in response to TGF-β stimulation (Figure 4B), indicating that Smad-dependent signaling was abrogated with TβRII deletion.
Figure 4.
Conditioned media from TβRII−/− CD cells have greater levels of active TGF-β than TβRIIflox/flox CD cells. (A) TβRII−/− and TβRIIflox/flox CD cell lysates are immunoblotted with a TβRII antibody. (B) TβRIIflox/flox and TβRII−/− CD cells are stimulated with TGF-β (5 ng/ml) for various periods, and then immunoblots for pSmad2 are performed. (C and D) Total and active levels of TGF-β from media of TβRIIflox/flox and TβRII−/− CD cells collected over 72 hours are determined by ELISA (C) and PAI/L assays (D). Total TGF-β (C) is reported as pg of TGF-β per mg of protein, and active TGF-β (D) is measured by random luciferase units (RLU). The values reported in C and D reflect averages ± SE from three separate experiments. *P < 0.05.
We then assessed whether deleting TβRII would alter the amount of active TGF-β in CD cell–conditioned medium. When we measured total and active TGF-β, 75% less total TGF-β1 was produced by TβRII−/− CD cells; however, there was approximately twice as much active TGF-β (all isoforms) in the medium (Figure 4, C and D). To determine whether the conditioned medium from TβRII−/− CD cells stimulated collagen I production by renal interstitial cells, we generated renal inner medullary fibroblasts and characterized them by Oil Red-O staining, as described previously.17 When the interstitial cells were incubated with TβRII−/− or TβRIIflox/flox CD cell–conditioned medium for 48 hours, significantly more collagen I was produced by cells exposed to TβRII−/− medium (Figure 5, A and B), which was inhibited by addition of a blocking pan–TGF-β antibody (clone 2G7) that blocks all TGF-β isoforms. Increased TGF-β signaling in the interstitial cells incubated with TβRII−/− CD medium was confirmed by increased pSmad2 levels 40 minutes and 2 hours after stimulation (Figure 5C). To exclude the possibility that TβRII−/− CD cell–conditioned medium had increased bioactive TGF-β solely as a result of its lack of TβRII binding and internalization, we co-cultured fibroblasts and the TβRII−/− or TβRIIflox/flox CD cells in a transwell system. Under these conditions, the fibroblasts co-cultured with TβRII−/− CD cells still expressed increased collagen I relative to those cultured with TβRIIflox/flox CD cells, and this difference was also significantly inhibited by a blocking pan–TGF-β antibody (data not shown).
Figure 5.
Conditioned medium from TβRII−/− CD cells stimulates increased collagen I production by interstitial cells. (A) Fibroblasts are incubated with conditioned medium from both TβRIIflox/flox and TβRII−/− CD cells and immunoblotted for collagen I expression in the presence or absence of 2G7 (a blocking pan–TGF-β antibody at 20 μg/ml). Fibroblasts are incubated with serum-free (SF) medium as a negative control. The white lines separating samples represent where the order of samples within the same blot was changed. (B) Densitometry of collagen I/focal adhesion kinase (FAK) is performed using three separate experiments and reported as means ± SEM. *P < 0.05. (C) Conditioned medium from CD cells with and without TβRII is placed on fibroblasts in the presence or absence of 2G7 (blocking pan–TGF-β antibody) for varying periods, after which the fibroblasts are lysed and immunoblotted for pSmad2. The vertical white line separating samples indicates where the order of samples from the same experiment on two gels developed for equivalent times is changed.
Integrin αvβ6 Mediates the Increased TGF-β Activation in TβRII−/− CD Conditioned Medium
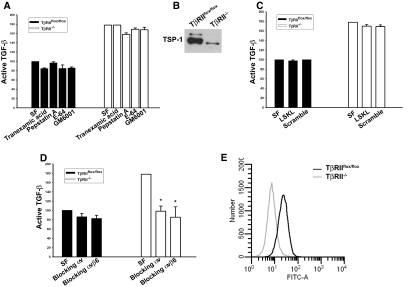
To determine why TβRII−/− CD cells have increased active TGF-β in the conditioned medium, we inhibited several known activators of TGF-β, including plasmin, metalloproteinases, and aspartic and cysteine proteases, using tranexamic acid, GM6001, pepstatin A, and E64, respectively.18 None of these compounds affected the levels of active TGF-β (Figure 6A). Also, TSP-1, a well-established TGF-β activator,19 did not mediate the increased TGF-β activity, because TβRII−/− CD cell medium expressed less soluble TSP-1 and a TSP-1 blocking peptide did not alter active TGF-β levels (Figure 6, B and C); however, a blocking integrin αvβ6 antibody reduced active TGF-β in TβRII−/− CD cell–conditioned medium to levels similar to those found in TβRIIflox/flox CD cell medium (Figure 6D). The effect of blocking integrin αvβ6 was comparable to that observed with an integrin αv–blocking antibody (Figure 6D). FACS analysis (Figure 6E) showed no differences in integrin αvβ6 expression between TβRII−/− and TβRIIflox/flox CD cells, suggesting that TβRII−/− CD cells have increased integrin αvβ6 activation rather than expression.
Figure 6.
Increased TGF-β activation in TβRII−/− CD cells is mediated by integrin αvβ6. (A) Effects of the protease inhibitors tranexamic acid (plasmin, plasminogen), pepstatin A (aspartyl), E-64 (cysteine), and GM6001 (matrix metalloproteinase) on TGF-β activation by TβRII−/− CD cells are determined by the PAI/L assay. The levels of active TGF-β in A, C, and D are expressed as a percentage of active TGF-β in TβRIIflox/flox CD cell serum-free (SF) conditioned medium. (B) TSP-1 levels in conditioned medium from TβRIIflox/flox and TβRII−/− CD cells are determined by immunoblotting. (C) LSKL, a TSP-1–blocking peptide, as well as a scramble peptide are added to conditioned medium to assess the effect on active TGF-β levels. (D) Blocking integrin αv and αvβ6 antibodies are added to the conditioned medium of TβRII−/− and TβRIIflox/flox CD cells. (E) Integrin αvβ6 expression on TβRIIflox/flox and TβRII−/− CD cells is measured by FACS analysis. Experiments in A through E were performed three times with data reported as means ± SEM. *P < 0.05. The concentrations used in these experiments are as follows: 10 mM tranexamic acid, 10 μM GM6001, 1 μg/ml integrin αv, 100 μg/ml integrin αvβ6, 10 μM TSP-1 blocking and scramble peptides, 100 μg/ml E-64, and 2.5 μg/ml pepstatin A.
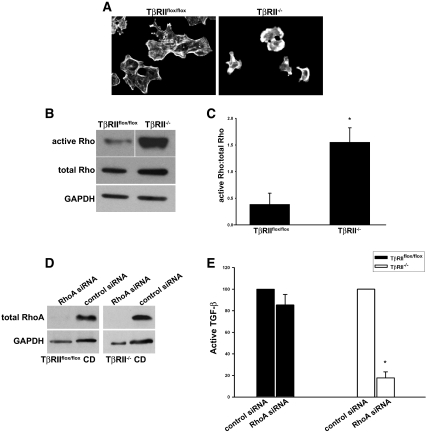
Previous studies showed that activation of the small GTPase RhoA is necessary for integrin αvβ6–mediated activation of TGF-β in vitro.20,21 Rhodamine phalloidin staining demonstrated that TβRII−/− cells have increased stress fiber formation compared with TβRIIflox/flox cells, a finding consistent with increased RhoA activation (Figure 7A).22 This was confirmed when Rho activity was measured (Figure 7, B and C). To assess whether the augmented Rho activity in TβRII−/− CD cells contributed to the increased activation of TGF-β, we depleted RhoA in both TβRIIflox/flox and TβRII−/− CD cells using small interfering RNA (siRNA; Figure 7D). Reducing RhoA protein expression did not alter viability or morphology of cells; however, it did have a significant suppressive effect on active TGF-β formation in TβRII−/− CD cells (Figure 7E). These findings suggest that increased Rho activation, at least in part, accounts for the increased TGF-β activity seen in the TβRII−/− CD cells.
Figure 7.
TβRII−/− CD cells have increased active RhoA. (A) Rhodamine phalloidin staining of actin cytoskeleton in TβRIIflox/flox CD and TβRII−/− CD cells. (B) Rho activity in cell lysates is measured as described in the Concise Methods section, and a representative experiment (a total of three performed) is shown. (C) A ratio of the densitometry of active Rho to total Rho from these three experiments is expressed as mean ± SEM. *P < 0.05. (D) Lysates of CD cells transfected with either pooled RhoA siRNA or control siRNA are immunoblotted with an antibody to RhoA. (E) Active TGF-β in the conditioned medium of cells transfected with RhoA siRNA is measured using the PAI/L assay and expressed as a percentage of active TGF-β measured in control siRNA-treated CD cells. This experiment was performed three times with error bars depicting the SE. *A significant (P < 0.05) drop in active TGF-β in RhoA siRNA-treated TβRII−/− compared with TβRIIflox/flox CD cells.
Discussion
On the basis of studies in which TGF-β activity was blocked systemically, TGF-β ligands play an important role in kidney development and promotion of renal fibrosis after injury. In contrast, our studies specifically investigated the role of TGF-β signaling in the renal collecting system. When we deleted TβRII in the UB, renal development was normal, but fibrosis, which was associated with increased TGF-β activation, was exacerbated after UUO. The increased TGF-β activation was recapitulated in vitro, where it was shown to be dependent on RhoA and integrin αvβ6 activation. These results highlight the cell-specific differences in TGF-β signaling in the kidney and suggest that inhibiting epithelial TβRII may cause a paradoxic increase in renal fibrosis after certain forms of injury.
Lack of a developmental renal phenotype in the Hoxb7Cre;Tgfbr2flox/flox mice was surprising given that cystic kidneys were observed in both the chimeric Tgfbr2−/− and the TGF-β2 null mice and that exogenous TGF-β ligands significantly decreased UB branching in kidney cultures7,9–12; however, our results are consistent with the normal developmental phenotype observed when Smad4 was deleted in the UB using a similar Hoxb7Cre promoter.23 These findings suggest either that TGF-β signaling specifically in the UB is not necessary for renal development or that such signaling pathways are independent of Smad4 and TβRII.
Given the well-documented strong profibrotic effects of TGF-β, the increased tubular injury and fibrosis sustained by the Hoxb7Cre;Tgfbr2flox/flox mice were unexpected. We inhibited TGF-β signaling in a cell-specific manner, allowing us to differentiate its effects on epithelial cells relative to fibroblasts. Consistent with our studies, increased pancreatic but not hepatic fibrosis was noted when a dominant negative TβRII was expressed specifically in the pancreatic and hepatic epithelium.24 These findings emphasize that blocking TβRII results in cell type–specific effects in different organs.
The reasons for increased tubular injury and fibrosis in the Hoxb7Cre;Tgfbr2flox/flox mice are unclear. Neither apoptosis nor inflammation played a role, which contrasts with previous studies suggesting that TGF-β mediates protection against apoptosis in proximal tubular epithelial cells.25,26 It is possible that deleting TβRII weakens the structural integrity of the epithelial cell, thereby predisposing the Hoxb7Cre;Tgfbr2flox/flox mice to greater tubular dilation and injury. The lack of a difference in inflammation between the Hoxb7Cre;Tgfbr2flox/flox and Tgfbr2flox/flox mice directly contrasts with the decreased inflammation observed in Smad3 null mice after UUO,27 suggesting that specifically inhibiting epithelial signaling was not sufficient to reduce the intense macrophage infiltration induced by UUO.
Our in vitro data showing that the excessive activated TGF-β in TβRII−/− CD cell–conditioned medium enhanced collagen I production by interstitial fibroblasts suggest that the increased fibrosis in Hoxb7Cre;Tgfbr2flox/flox mice is due to the increased levels of active TGF-β produced by the TβRII−/− CD cells. Furthermore, this effect is due to greater activation rather than production of latent TGF-β. Similar epithelial–stromal interactions between proximal tubular TGF-β production and myofibroblasts have been implicated in renal fibrosis.28
Increased TGF-β activation by TβRII−/− CD cells was not dependent upon proteases or TSP-1 but was mediated by integrin αvβ6. Because integrin αvβ6 expression was not changed in TβRII−/− CD cells, changes in integrin activation accounted for integrin-mediated TGF-β activation. Integrin αvβ6 is expressed only by epithelial cells, where it is present at low basal levels; however, it is greatly upregulated after epithelial injury, resulting in increased fibrosis by activating TGF-β in the kidney as well as other organs.6,29,30 Blocking integrin αvβ6 with antibodies or genetically deleting it attenuated fibrosis in multiple injury models purportedly through effects on fibroblasts.31 This suggests that integrin αvβ6–mediated activation of TGF-β can alter fibrosis through paracrine effects on surrounding cells, which is consistent with our data.
Rho activity has been shown to be critical for integrin αvβ6–dependent activation of TGF-β.20,21 The increased Rho activity in TβRII−/− CD cells was unexpected on the basis of previous studies showing that increased TGF-β signaling stimulates Rho activation32,33; however, one study demonstrating that ligand binding to TβRII causes Par6 phosphorylation and subsequent RhoA degradation in epithelial cells34 may provide insight into how deleting TβRII results in increased RhoA. Although we have not defined the mechanisms whereby TβRII regulates RhoA activity, our results suggest that TβRII expression decreases Rho activity, which in turn decreases the activation state of integrin αvβ6. Extrapolating these in vitro data to our in vivo model might suggest that Hoxb7Cre;Tgfbr2flox/flox mice would have increased fibrosis even without injury; however, integrin αvβ6 expression is thought to be minimally expressed in the adult kidney tubules but upregulated after injury,6,29,30 which may explain why the increased fibrosis in Hoxb7Cre;Tgfbr2flox/flox mice occurred only after UUO.
In summary, we demonstrated that deleting TβRII in the UB did not alter normal renal development; however, it led to increased fibrosis and active TGF-β after injury. Our in vitro data suggest this increased TGF-β activation, which resulted in increased collagen production by fibroblasts, was mediated by RhoA-dependent integrin αvβ6 activation. This paradoxic increase in fibrosis illustrates the importance of understanding how epithelial TGF-β signaling modulates the response to injury. These findings have clear clinical implications because both TGF-β and TβRII are being pursued as pharmacologic targets for the treatment of fibrosis in the kidney as well as other organs.
Concise Methods
Generation of Hoxb7Cre;Tgfbr2flox/flox Mice
Tgfbr2flox/flox mice containing the ROSA26 reporter with the Cre-sensitive lacZ gene were crossed with the Hoxb7Cre mice (gift of Dr. A. McMahon).35–37 Floxed Tgfbr2 mice lacking Cre were used as negative controls, and all mice were generation 10 on the C57BL/6 background.
Obstructive Kidney Model
UUO was performed on mice aged 8 to 12 weeks by exposing the right kidney through a flank incision and ligating the ureter just distal to the renal pelvis. At least five mice per genotype were killed at 3, 5, 7, and 14 days after UUO. All experiments were approved by the Vanderbilt University Institutional Animal Use and Care Committee.
Immunohistochemistry
Mouse kidneys were fixed in 4% paraformaldehyde, embedded in paraffin, and incubated with the following primary antibodies: Collagen IV (Biodesign), F4/80 (Serotec), CD3 (AbD Serotec), and FSP-1 (a gift from Dr. Eric Neilson, Vanderbilt Medical Center). Trichrome staining was performed according to the kit's instructions (Sigma-Aldrich), and TUNEL staining was performed as described previously.38 Cells with positive staining for TUNEL and F4/80 were counted from 10 high-power fields in a blinded manner using four to five mice per genotype. Kidney injury was scored by a renal pathologist, who calculated the percentage of tubules with cell necrosis, loss of brush border, cast formation, and tubular dilation as follows: 0, none; 1, ≤10%; 2, 11 to 25%; 3, 26 to 45%; 4, 46 to 75%; and 5, >76%. At least 10 fields (×200) were reviewed for each slide in a blinded manner.
Immunoblots
Protein from obstructed kidney tissue was extracted using a lysis buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 2% SDS, 1% Triton, phosphatase inhibitors, and protease inhibitor cocktail) and homogenized by sonication. Lysates were clarified by centrifugation, and protein was electrophoresed onto SDS-PAGE and subsequently transferred to nitrocellulose membranes. Membranes were blocked in 5% milk and then incubated with various primary antibodies followed by the appropriate horseradish peroxidase–conjugated secondary antibodies. Immunoreactive bands were identified using enhanced chemiluminescence according to the manufacturer's instructions and quantified with densitometry. Primary antibodies included collagen I (MDBiosciences), pSmad2 (Cell Signaling), total Smad2 (Cell Signaling), TβRII (Santa Cruz Biotechnology), focal adhesion kinase (Santa Cruz Biotechnology), and extracellular signal–regulated kinase (Cell Signaling).
Measurement of Total and Active TGF-β
Protein was extracted from medullary and inner cortical regions of kidneys 3 days after UUO in a detergent-free lysis buffer as described previously.39 Serum-free conditioned medium, with or without various inhibitors, was collected from TβRIIflox/flox or TβRII−/− CD cells after 72 hours. Latent TGF-β in tissue and medium was activated by acidification, allowing for quantification of total TGF-β using the Quantikine TGF-β1 ELISA kit (R&D Systems). Equivalent amounts of protein were used, and values were reported as pg/mg protein. Bioactive TGF-β was determined by incubating the tissue lysates or conditioned medium with mink lung epithelial cells containing a TGF-β–responsive element in the PAI/L, as described previously.40,41
Generation of Cell Populations
CD cells were generated from TβRII floxed adult mice based on previously described methods.42 These cells were cultured in DMEM/F12 supplemented with 10% FBS and immortalized with sv40 large T antigen. TβRII was deleted by adeno-Cre infection. Primary medullary fibroblasts from TβRII floxed mice were generated as described previously43 and characterized by Oil Red-O staining.
Co-culture Experiments of CD Cells and Interstitial Fibroblasts
Serum-free medium with and without a pan–TGF-β blocking antibody (20 μg/ml clone 2G7) was collected after 48 hours from either TβRIIflox/flox or TβRII−/− CD cells and placed on interstitial fibroblasts for an additional 48 hours. In a separate experiment, the CD cell–conditioned medium was placed on interstitial fibroblasts for shorter periods for determination of Smad2 activation. Lysates of interstitial cells and the underlying matrix were made using a 2× laemmli lysis buffer.
For CD/fibroblast co-cultures, either TβRIIflox/flox or TβRII−/− CD cells were plated on 0.4-μ transwells and, when 90% confluent, placed on top of the fibroblasts in the presence or absence of a blocking pan–TGF-β antibody (20 μg/ml clone 2G7) for 48 hours. Afterward, the transwells were removed but the fibroblasts continued to incubate with the conditioned medium for an additional 48 hours before the cells were lysed, as described already.
Inhibitors of TGF-β Activation
The following reagents were used to test TGF-β activation: TSP-1 blocking and scramble peptides (LSKL and SLLK, respectively), TSP-1 antibody (Lab Vision), blocking integrin αv (PharMingen) and integrin αvβ6 (Chemicon) antibodies, E-64 (Sigma-Aldrich), pepstatin A (Boehringer-Mannheim), GM6001 (Chemicon/Millipore), and tranexamic acid (Sigma-Aldrich).
Rhodamine Phalloidin Staining of CD Cells
CD cells were plated onto chamber slides coated with collagen I (10 μg/ml). Three hours later, cells were gently washed with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.1% BSA, and incubated with rhodamine phalloidin (1:400) for 1 hour for visualization of the actin cytoskeleton.
Rho Activation Assay
The amount of active, GTP-bound Rho was measured according to kit instructions (cat. no. 17-294; Upstate) and as described previously.44 Briefly, cells were lysed with provided buffer and cleared by centrifugation, and the supernatants were rotated for 1 hour with the GST-RBD fusion protein containing the Rho-binding domain of rhotekin bound to glutathione-Sepharose beads. Samples were washed in buffer, subjected to SDS-PAGE, and immunoblotted with an anti-Rho antibody (clone 55).
RhoA Inhibition by siRNA
TβRIIflox/flox and TβRII−/− CD cells were transfected with either Dharmacon's mixture of four siRNAs targeting RhoA (On-Target plus SMART pool) or Ambion's Silencer negative control siRNA using Lipofectamine 2000 reagent (Invitrogen). Forty-eight hours after transfection, the cells were trypsinized and replated on a six-well plate. After an additional 48 hours, some cells were used for lysates to confirm knockdown of RhoA by immunoblots (mouse monoclonal RhoA antibody; Santa Cruz Biotechnology), and others were serum-starved for 72 hours to generate conditioned medium. The PAI/L assay was then used to measure active TGF-β in this conditioned medium.
Statistical Analysis
The t test with unequal variance was used to compare two sets of data. P ≤ 0.05 was considered statistically significant. All in vitro experiments were repeated three times, and the results were reported as means ± SEs.
Disclosures
None.
Acknowledgments
This work was supported by a National Kidney Foundation fellowship award and a Vanderbilt Physician Scientist Development Award (L.G.), a Merit Review from the Department of Veterans Affairs (A.P., R.Z., and R.C.H.), National Institute of Diabetes and Digestive and Kidney Diseases grants 2P01DK065123 (A.P. and R.Z.), DK075594 (R.Z.), DK65123 (R.Z.), and P30DK79341-01 (A.P., R.Z., and R.C.H.), and an American Heart Association established investigator award (R.Z.).
We thank Raymond Mernaugh and Heping Yan for providing the pan-blocking TGF-β antibodies (clone 2G7), Joanne Murphy-Ullrich for helpful suggestions, and Eric Neilson for the FSP-1 antibody. We also appreciate Linda Davis's work with the β-gal staining and generous technical assistance given by Heloisa Colleta.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “TGF-β Signaling and the Renal Tubular Epithelial Cell: Too Much, Too Little, and Just Right,” on pages 1241–1243.
REFERENCES
- 1.Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J: TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71: 1003–1014, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Yamashita H, ten Dijke P, Franzen P, Miyazono K, Heldin CH: Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-beta. J Biol Chem 269: 20172–20178, 1994 [PubMed] [Google Scholar]
- 3.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC: Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev 17: 19–27, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Annes JP, Munger JS, Rifkin DB: Making sense of latent TGFbeta activation. J Cell Sci 116: 217–224, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C: Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int 65: 459–468, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D: The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO: TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development 128: 1045–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Sakurai H, Nigam SK: Transforming growth factor-beta selectively inhibits branching morphogenesis but not tubulogenesis. Am J Physiol 272: F139–F146, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK: TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol 266: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T: TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124: 2659–2670, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sims-Lucas S, Caruana G, Dowling J, Kett MM, Bertram JF: Augmented and accelerated nephrogenesis in TGF-beta2 heterozygous mutant mice. Pediatr Res 63: 607–612, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Oshima M, Oshima H, Taketo MM: TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol 179: 297–302, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Isaka Y, Fujiwara Y, Ueda N, Kaneda Y, Kamada T, Imai E: Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest 92: 2597–2601, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottinger EP, Kopp JB: Lessons from TGF-beta transgenic mice. Miner Electrolyte Metab 24: 154–160, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D: Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E: Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature 346: 371–374, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Grupp C, Muller GA: Renal fibroblast culture. Exp Nephrol 7: 377–385, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Jenkins G: The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol 40: 1068–1078, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Schultz-Cherry S, Murphy-Ullrich JE: Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol 122: 923–932, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D: Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest 116: 1606–1614, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G: Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q). Am J Pathol 174: 1264–1279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzi A, Coffa S, Bulus N, Zhu W, Chen D, Chen X, Mernaugh G, Su Y, Cai S, Singh A, Brissova M, Zent R: H-Ras, R-Ras, and TC21 differentially regulate ureteric bud cell branching morphogenesis. Mol Biol Cell 17: 2046–2056, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oxburgh L, Chu GC, Michael SK, Robertson EJ: TGFbeta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development 131: 4593–4605, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Bottinger EP, Jakubczak JL, Roberts IS, Mumy M, Hemmati P, Bagnall K, Merlino G, Wakefield LM: Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J 16: 2621–2633, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HT, Chen SW, Doetschman TC, Deng C, D'Agati VD, Kim M: Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol 295: F128–F136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HT, Kim M, Kim J, Kim N, Emala CW: TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol 27: 416–424, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbate M, Zoja C, Rottoli D, Corna D, Tomasoni S, Remuzzi G: Proximal tubular cells promote fibrogenesis by TGF-beta1-mediated induction of peritubular myofibroblasts. Kidney Int 61: 2066–2077, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, et al. : Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 108: 2241–2251, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB: Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am J Pathol 163: 1261–1273, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, 3rd, Sheppard D, Gardner HA, Violette SM: Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170: 110–125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL: Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell 12: 27–36, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A: Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol 284: F911–F924, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL: Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307: 1603–1609, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Chytil A, Magnuson MA, Wright CV, Moses HL: Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis 32: 73–75, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Kress C, Vogels R, De Graaff W, Bonnerot C, Meijlink F, Nicolas JF, Deschamps J: Hox-2.3 upstream sequences mediate lacZ expression in intermediate mesoderm derivatives of transgenic mice. Development 109: 775–786, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Srichai MB, Hao C, Davis L, Golovin A, Zhao M, Moeckel G, Dunn S, Bulus N, Harris RC, Zent R, Breyer MD: Apoptosis of the thick ascending limb results in acute kidney injury. J Am Soc Nephrol 19: 1538–1546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule J, Chatziantoniou C, Ronco P, Boffa J: Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-β activation and cell infiltration. Am J Pathol 173: 631–642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Waarde MA, van Assen AJ, Kampinga HH, Konings AW, Vujaskovic Z: Quantification of transforming growth factor-beta in biological material using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 247: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB: An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Husted RF, Hayashi M, Stokes JB: Characteristics of papillary collecting duct cells in primary culture. Am J Physiol 255: F1160–F1169, 1988 [DOI] [PubMed] [Google Scholar]
- 43.Dunn MJ, Staley RS, Harrison M: Characterization of prostaglandin production in tissue culture of rat renal medullary cells. Prostaglandins 12: 37–49, 1976 [DOI] [PubMed] [Google Scholar]
- 44.Arthur WT, Ellerbroek SM, Der CJ, Burridge K, Wennerberg K: XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J Biol Chem 277: 42964–42972, 2002 [DOI] [PubMed] [Google Scholar]