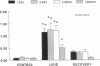Abstract
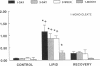
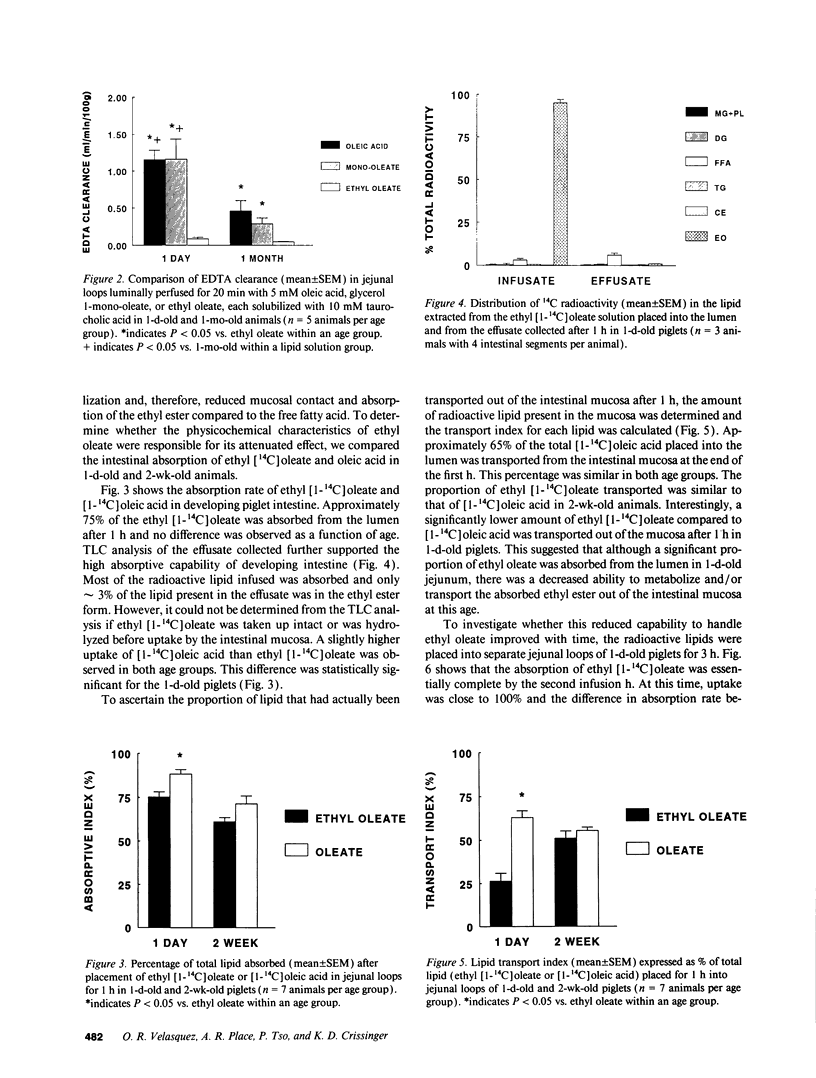
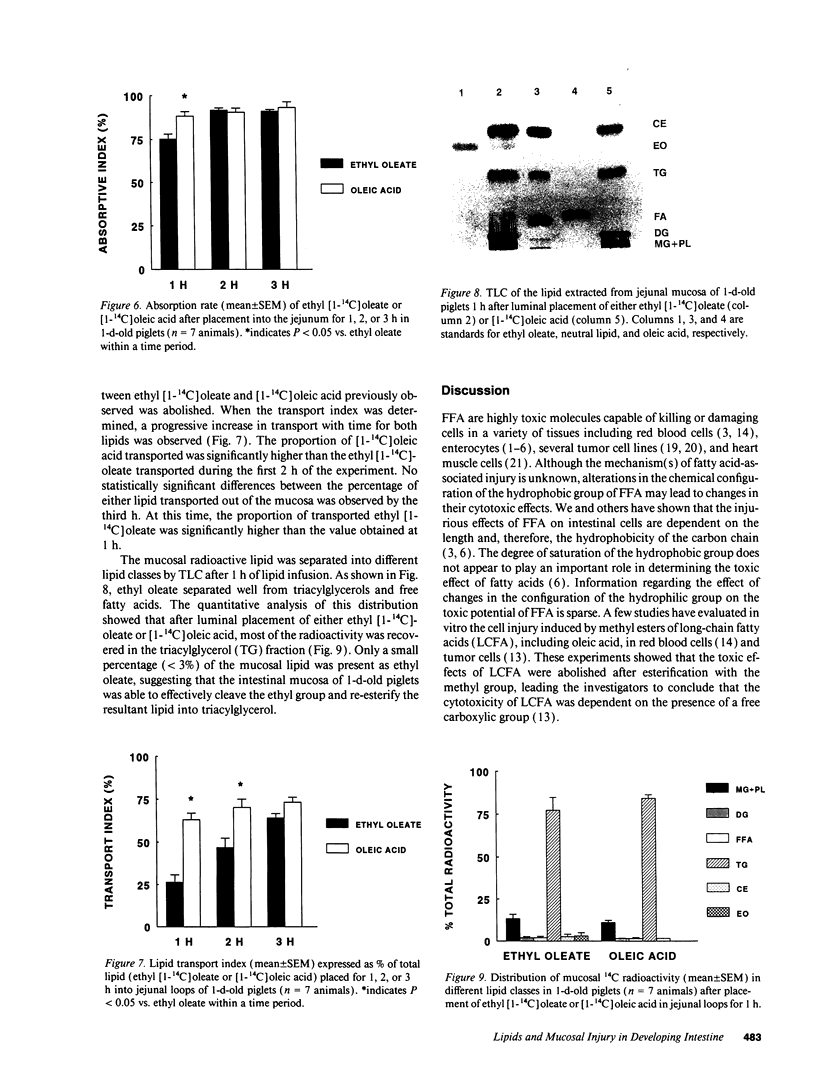
Although lipids are essential nutrients in the mammalian diet, we have shown that fatty acids are injurious to epithelial cells of developing piglet intestine during luminal perfusion. Furthermore, the intestine of young animals sustains greater injury than that of older piglets. In an effort to understand the mechanism for this developmental injury, we investigated whether changes in the chemical configuration of oleic acid would alter this damage. Mucosal permeability, as quantitated by the plasma-to-lumen clearance of 51chromium EDTA, was evaluated during luminal perfusion with oleic acid as compared with its ethyl (ethyl oleate) and glyceryl (glycerol-1-mono-oleate) esters, solubilized with taurocholic acid, in jejunum of 1-d-, 3-d-, 2-wk-, and 1-mo-old piglets. 51Chromium EDTA clearance increased significantly during oleic acid and glycerol-1-mono-oleate perfusion, but did not increase during perfusion with ethyl oleate or saline. This result was not secondary to failure of absorption of ethyl oleate, as [14C]oleic acid and ethyl [1-14C]oleate were absorbed to a similar extent. Furthermore, developing intestine was able to remove the ethyl group and then re-esterify the fatty acid to form triacyglycerol. These studies indicate that oleic acid-induced mucosal injury can be abolished when the carboxylic group of the fatty acid is esterified with an ethyl, but not a glycerol, group. Since the ethyl ester is also absorbed and metabolized similarly to the free fatty acid, this may provide a means of supplying long-chain fatty acids to developing intestine without causing mucosal damage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLANKENHORN D. H., AHRENS E. H., Jr Extraction, isolation, and identification of hydrolytic products of triglyceride digestion in man. J Biol Chem. 1955 Jan;212(1):69–81. [PubMed] [Google Scholar]
- Bergstedt S. E., Hayashi H., Kritchevsky D., Tso P. A comparison of absorption of glycerol tristearate and glycerol trioleate by rat small intestine. Am J Physiol. 1990 Sep;259(3 Pt 1):G386–G393. doi: 10.1152/ajpgi.1990.259.3.G386. [DOI] [PubMed] [Google Scholar]
- Borum P. R. Medium-chain triglycerides in formula for preterm neonates: implications for hepatic and extrahepatic metabolism. J Pediatr. 1992 Apr;120(4 Pt 2):S139–S145. doi: 10.1016/s0022-3476(05)81248-x. [DOI] [PubMed] [Google Scholar]
- Bégin M. E., Ells G., Horrobin D. F. Polyunsaturated fatty acid-induced cytotoxicity against tumor cells and its relationship to lipid peroxidation. J Natl Cancer Inst. 1988 Apr 6;80(3):188–194. doi: 10.1093/jnci/80.3.188. [DOI] [PubMed] [Google Scholar]
- Connor W. E., Neuringer M., Reisbick S. Essential fatty acids: the importance of n-3 fatty acids in the retina and brain. Nutr Rev. 1992 Apr;50(4 ):21–29. doi: 10.1111/j.1753-4887.1992.tb01286.x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Finley A. J., Davidson M. Bile acid excretion and patterns of fatty acid absorption in formula-fed premature infants. Pediatrics. 1980 Jan;65(1):132–138. [PubMed] [Google Scholar]
- Gaginella T. S., Phillips S. F. Ricinoleic acid (castor oil) alters intestinal surface structure. A scanning electronmiscroscopic study. Mayo Clin Proc. 1976 Jan;51(1):6–12. [PubMed] [Google Scholar]
- HOFMANN A. F. THE FUNCTION OF BILE SALTS IN FAT ABSORPTION. THE SOLVENT PROPERTIES OF DILUTE MICELLAR SOLUTIONS OF CONJUGATED BILE SALTS. Biochem J. 1963 Oct;89:57–68. doi: 10.1042/bj0890057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki T., Urakaze M., Makuta M., Ozawa A., Soda Y., Tatsumi H., Yano S., Kumagai A. Intake of different eicosapentaenoic acid-containing lipids and fatty acid pattern of plasma lipids in the rats. Lipids. 1987 Dec;22(12):994–998. doi: 10.1007/BF02536438. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- KRITCHEVSKY D., KIRK M. R. Detection of steroids in paper chromatography. Arch Biochem Biophys. 1952 Feb;35(2):346–351. doi: 10.1016/s0003-9861(52)80015-3. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Specian R. D., Grisham M. B., Tso P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol. 1991 Sep;261(3 Pt 1):G384–G391. doi: 10.1152/ajpgi.1991.261.3.G384. [DOI] [PubMed] [Google Scholar]
- Lapré J. A., Termont D. S., Groen A. K., Van der Meer R. Lytic effects of mixed micelles of fatty acids and bile acids. Am J Physiol. 1992 Sep;263(3 Pt 1):G333–G337. doi: 10.1152/ajpgi.1992.263.3.G333. [DOI] [PubMed] [Google Scholar]
- Lawson L. D., Hughes B. G. Absorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a high-fat meal. Biochem Biophys Res Commun. 1988 Oct 31;156(2):960–963. doi: 10.1016/s0006-291x(88)80937-9. [DOI] [PubMed] [Google Scholar]
- Lawson L. D., Hughes B. G. Human absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl esters. Biochem Biophys Res Commun. 1988 Apr 15;152(1):328–335. doi: 10.1016/s0006-291x(88)80718-6. [DOI] [PubMed] [Google Scholar]
- Lebenthal E., Lee P. C. Development of functional responses in human exocrine pancreas. Pediatrics. 1980 Oct;66(4):556–560. [PubMed] [Google Scholar]
- Mattson F. H., Volpenhein R. A. Relative rates of hydrolysis by rat pancreatic lipase of esters of C2-C18 fatty acids with C1-C18 primary n-alcohols. J Lipid Res. 1969 May;10(3):271–276. [PubMed] [Google Scholar]
- Morehouse J. L., Specian R. D., Stewart J. J., Berg R. D. Translocation of indigenous bacteria from the gastrointestinal tract of mice after oral ricinoleic acid treatment. Gastroenterology. 1986 Sep;91(3):673–682. doi: 10.1016/0016-5085(86)90638-4. [DOI] [PubMed] [Google Scholar]
- Nelson G. J., Ackman R. G. Absorption and transport of fat in mammals with emphasis on n-3 polyunsaturated fatty acids. Lipids. 1988 Nov;23(11):1005–1014. doi: 10.1007/BF02535644. [DOI] [PubMed] [Google Scholar]
- Nordøy A., Barstad L., Connor W. E., Hatcher L. Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am J Clin Nutr. 1991 May;53(5):1185–1190. doi: 10.1093/ajcn/53.5.1185. [DOI] [PubMed] [Google Scholar]
- Raz A., Livne A. Differential effects of lipids on the osmotic fragility of erythrocytes. Biochim Biophys Acta. 1973 Jun 22;311(2):222–229. doi: 10.1016/0005-2736(73)90269-1. [DOI] [PubMed] [Google Scholar]
- Reicks M., Hoadley J., Satchithanandam S., Morehouse K. M. Recovery of fish oil-derived fatty acids in lymph of thoracic duct-cannulated Wistar rats. Lipids. 1990 Jan;25(1):6–10. doi: 10.1007/BF02562420. [DOI] [PubMed] [Google Scholar]
- SARDA L., DESNUELLE P. Action de la lipase pancréatique sur les esters en émulsion. Biochim Biophys Acta. 1958 Dec;30(3):513–521. doi: 10.1016/0006-3002(58)90097-0. [DOI] [PubMed] [Google Scholar]
- Siegel I., Liu T. L., Yaghoubzadeh E., Keskey T. S., Gleicher N. Cytotoxic effects of free fatty acids on ascites tumor cells. J Natl Cancer Inst. 1987 Feb;78(2):271–277. [PubMed] [Google Scholar]
- Velasquez O. R., Henninger K., Fowler M., Tso P., Crissinger K. D. Oleic acid-induced mucosal injury in developing piglet intestine. Am J Physiol. 1993 Mar;264(3 Pt 1):G576–G582. doi: 10.1152/ajpgi.1993.264.3.G576. [DOI] [PubMed] [Google Scholar]
- Velasquez O. R., Tso P., Crissinger K. D. Fatty acid-induced injury in developing piglet intestine: effect of degree of saturation and carbon chain length. Pediatr Res. 1993 Jun;33(6):543–547. doi: 10.1203/00006450-199306000-00001. [DOI] [PubMed] [Google Scholar]
- Wenzel D. C., Hale T. W. Toxicity of free fatty acids for cultured rat heart muscle and endothelioid cells. II. Unsaturated long-chain fatty acids. Toxicology. 1978 Oct;11(2):119–125. doi: 10.1016/s0300-483x(78)90839-9. [DOI] [PubMed] [Google Scholar]
- Zhu Y. P., Su Z. W., Li C. H. Growth-inhibition effects of oleic acid, linoleic acid, and their methyl esters on transplanted tumors in mice. J Natl Cancer Inst. 1989 Sep 6;81(17):1302–1306. doi: 10.1093/jnci/81.17.1302. [DOI] [PubMed] [Google Scholar]
- el Boustani S., Colette C., Monnier L., Descomps B., Crastes de Paulet A., Mendy F. Enteral absorption in man of eicosapentaenoic acid in different chemical forms. Lipids. 1987 Oct;22(10):711–714. doi: 10.1007/BF02533970. [DOI] [PubMed] [Google Scholar]