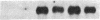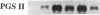Abstract
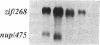
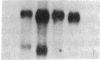
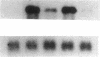
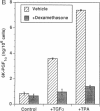
Growth factors and tumor promoters have been shown to play a role in intestinal epithelial growth regulation and transformation. In this study, transforming growth factor-alpha (TGF alpha) and the tumor promoter, tetradecanoyl phorbol acetate (TPA), are shown to stimulate the production of eicosanoids by rat intestinal epithelial (RIE-1) cells in culture. A 4.5-kb mRNA, which hybridizes to the mouse cyclooxygenase-2 cDNA probe, is elevated 18-fold within 30 min after TGF alpha or TPA treatment. Stimulation of RIE-1 cells with TGF alpha leads to the increase of a protein (M(r) approximately 69,000), which binds a monospecific antibody to the mouse cyclooxygenase-2 protein. Dexamethasone markedly inhibits the increase of the 4.5-kb mRNA. Pretreatment of TGF alpha or TPA-stimulated RIE-1 cells with dexamethasone or cyclooxygenase inhibitors prevents the increase in eicosanoid production by these cells. Treatment of quiescent RIE-1 cells with TGF alpha stimulates mitogenesis. This mitogenic activity is blocked by pretreating the cells with dexamethasone or cyclooxygenase inhibitors. A mitogen-inducible cyclooxygenase gene is thus shown to be regulated by TGF alpha and TPA in rat intestinal epithelial cells. We suggest that products of an intestinal growth factor-inducible cyclooxygenase may play a role in the regulation of mitogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blay J., Brown K. D. Functional receptors for epidermal growth factor in an epithelial-cell line derived from the rat small intestine. Biochem J. 1985 Jan 1;225(1):85–94. doi: 10.1042/bj2250085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R. Genes induced during the G0/G1 transition in mouse fibroblasts. Semin Cancer Biol. 1990 Feb;1(1):37–46. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clark B. J., Waterman M. R. The hydrophobic amino-terminal sequence of bovine 17 alpha-hydroxylase is required for the expression of a functional hemoprotein in COS 1 cells. J Biol Chem. 1991 Mar 25;266(9):5898–5904. [PubMed] [Google Scholar]
- DuBois R. N., McLane M. W., Ryder K., Lau L. F., Nathans D. A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. J Biol Chem. 1990 Nov 5;265(31):19185–19191. [PubMed] [Google Scholar]
- Dworski R., Sheller J. R., Wickersham N. E., Oates J. A., Brigham K. L., Roberts L. J., 2nd, Fitzgerald G. A. Allergen-stimulated release of mediators into sheep bronchoalveolar lavage fluid. Effect of cyclooxygenase inhibition. Am Rev Respir Dis. 1989 Jan;139(1):46–51. doi: 10.1164/ajrccm/139.1.46. [DOI] [PubMed] [Google Scholar]
- Giardiello F. M., Hamilton S. R., Krush A. J., Piantadosi S., Hylind L. M., Celano P., Booker S. V., Robinson C. R., Offerhaus G. J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993 May 6;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman M. J., Turk J., Shornick L. P. Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J Biol Chem. 1992 Oct 25;267(30):21438–21445. [PubMed] [Google Scholar]
- Kawahara R. S., Deng Z. W., Deuel T. F. Glucocorticoids inhibit the transcriptional induction of JE, a platelet-derived growth factor-inducible gene. J Biol Chem. 1991 Jul 15;266(20):13261–13266. [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Kujubu D. A., Herschman H. R. Dexamethasone inhibits mitogen induction of the TIS10 prostaglandin synthase/cyclooxygenase gene. J Biol Chem. 1992 Apr 25;267(12):7991–7994. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeDuc L. E., Needleman P. Regional localization of prostacyclin and thromboxane synthesis in dog stomach and intestinal tract. J Pharmacol Exp Ther. 1979 Oct;211(1):181–188. [PubMed] [Google Scholar]
- Lee D. Y., Lupton J. R., Chapkin R. S. Prostaglandin profile and synthetic capacity of the colon: comparison of tissue sources and subcellular fractions. Prostaglandins. 1992 Feb;43(2):143–164. doi: 10.1016/0090-6980(92)90083-6. [DOI] [PubMed] [Google Scholar]
- Marnett L. J. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992 Oct 15;52(20):5575–5589. [PubMed] [Google Scholar]
- Matin A., Cheng K. L., Suen T. C., Hung M. C. Effect of glucocorticoids on oncogene transformed NIH3T3 cells. Oncogene. 1990 Jan;5(1):111–116. [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Banion M. K., Winn V. D., Young D. A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Effect of piroxicam on primary intestinal tumors induced in rats by N-methylnitrosourea. Cancer Lett. 1984 Dec;25(2):117–121. doi: 10.1016/s0304-3835(84)80035-x. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Stiles C. D. Serum-inducible genes. Adv Cancer Res. 1989;53:1–32. doi: 10.1016/s0065-230x(08)60277-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Palmer J. R., Zauber A. G., Warshauer M. E., Stolley P. D., Shapiro S. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. J Natl Cancer Inst. 1991 Mar 6;83(5):355–358. doi: 10.1093/jnci/83.5.355. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Raynoschek C., Macdonald-Bravo H., Dorfman K., Mattéi M. G., Bravo R. Identification of an immediate early gene, pghs-B, whose protein product has prostaglandin synthase/cyclooxygenase activity. Cell Growth Differ. 1992 Jul;3(7):443–450. [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Sirois J., Levy L. O., Simmons D. L., Richards J. S. Characterization and hormonal regulation of the promoter of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Identification of functional and protein-binding regions. J Biol Chem. 1993 Jun 5;268(16):12199–12206. [PubMed] [Google Scholar]
- Thun M. J., Namboodiri M. M., Heath C. W., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991 Dec 5;325(23):1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Waddell W. R., Ganser G. F., Cerise E. J., Loughry R. W. Sulindac for polyposis of the colon. Am J Surg. 1989 Jan;157(1):175–179. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziober B. L., Willson J. K., Hymphrey L. E., Childress-Fields K., Brattain M. G. Autocrine transforming growth factor-alpha is associated with progression of transformed properties in human colon cancer cells. J Biol Chem. 1993 Jan 5;268(1):691–698. [PubMed] [Google Scholar]