Abstract
Plakophilin 3 (PKP3) belongs to the p120ctn family of armadillo-related proteins predominantly functioning in desmosome formation. Here we report that PKP3 is transcriptionally repressed by the E-cadherin repressor ZEB1 in metastatic cancer cells. ZEB1 physically associates with two conserved E-box elements in the PKP3 promoter and partially represses the activity of corresponding human and mouse PKP3 promoter fragments in reporter gene assays. In human tumours ZEB1 is upregulated in invasive cancer cells at the tumour–host interface, which is accompanied by downregulation of PKP3 expression levels. Hence, the transcriptional repression of PKP3 by ZEB1 contributes to ZEB1-mediated disintegration of intercellular adhesion and epithelial to mesenchymal transition.
Keywords: Epithelial to mesenchymal transition, Invasion, Transcription, Desmosomes, Cell adhesion
1. Introduction
The functional and structural integrity of epithelial tissues is based upon apico-basal polarity of epithelial cells, which in turn depends on a number of intercellular junctions, including gap junctions, tight junctions, adherens junctions and desmosomes [1]. Desmosomes, also known as maculae adherens, are intermediate filament-attached adhering junctions which contribute to strong intercellular adhesion and confer resistance to mechanical stress [2,3]. The organization of desmosomes closely resembles that of E-cadherin type adherens junctions. Desmosomes contain clusters of cadherin-type transmembrane proteins, the Desmogleins (Dsg) and Desmocollins (Dsc), which are heavily glycosylated and mediate intercellular adhesion by homo- and heterophilic interactions [4]. The cadherin-associated plaque region contains Plakoglobin and Desmoplakin, constitutive desmosomal components mediating the linkage to the intermediate filament network.
The Plakophilins (PKPs) represent an important group of proteins integral to the inner desmosomal plaque area. They belong to the p120ctn subfamily of armadillo-related proteins sharing a characteristic series of armadillo domain repeats of about 45 amino acids [5,6]. The different members designated as Plakophilin 1–3 (PKP1-3) interact with the desmosomal cadherins as well as with the cytoskeletal linker proteins Desmoplakin and Plakoglobin, and show distinct expression patterns and effects on desmosomal adhesion [6]. Their numerous interactions within the desmosomal plaque suggest that they act as scaffolding proteins required for proper desmosome assembly. In addition, Plakophilins may also be involved in transducing signals in response to stimuli from the cellular environment, since nuclear localization has been reported for all three Plakophilins [5,7-9]. Together, these data establish the Plakophilins as critical regulators of desmosome assembly and function. However, upstream signalling molecules that regulate the expression and/or function of the different Plakophilins in epithelial tissue homeostasis remain to be identified. Here we report that ZEB1, a potent transcriptional repressor of E-cadherin directly represses the expression of PKP3 in invasive human cancer cells. ZEB1 binds to the PKP3 promoter in vivo and strongly reduces corresponding promoter activities in reporter gene assays. In human colon cancer specimens membranous PKP3 is expressed mainly in the differentiated bulk tumour region but is downregulated in invasive cells upregulating ZEB1.
2. Materials and Methods
2.1. Cell culture and Affymetrix Gene Chip™ analyses
MDA-MB-231, MCF7, and EpH4 cells were cultivated as described previously [11]. For generation of E-cadherin expressing cell clones, MDA-MB-231 cells were transfected with E-cadherin-GFP and selected with 800 μg/ml Geneticin (G418; Invitrogen, Carlsbad, USA). RNA isolation, RNA quality control, RNA labeling, hybridization on Affymetrix Gene Chips™ (Human Genome U133 Plus 2.0) and data acquisition including fold-change analyses were done as described [10].
2.2. Reporter gene assays
Transient reporter experiments in human MCF7 and mouse EpH4 mammary epithelial cells were done as described previously [11].
2.3. RT–PCR
Total mRNA and cDNA was prepared as described previously [11]. PCR primers used were for PKP3: forward 5′-CTCGGAACGCTAGGAACAAG-3′, reverse 5′-AAGTCCTCCTTCCGATAGCC-3′; E-cadherin: forward 5′-TGGAGGAATTCTTGCTTTGC-3′, reverse 5′-CGTACATGTCAGCCAGCTTC-3′; Actin: forward 5′-ATCTGGCACCACACCTTCTAC-3′, reverse 5′-CAGCCAGGTCCAGACGCAGG-3′.
2.4. Antibodies
The following antibodies (Abs) were used: mouse monoclonal Ab to E-cadherin (BD Biosciences, Franklin Lakes, NJ, USA); goat polyclonal Ab to ZEB1 (ZEB-E20, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA); mouse monoclonal Ab to PKP3 (23E3/4, [28]; Zymed Laboratories, South San Francisco, CA, USA); rabbit polyclonal Ab against Actin (Sigma, St. Louis, USA); secondary Abs coupled to Alexa Fluor 488 (Molecular Probes Inc., Eugene, OR, USA), Texas Red or peroxidase (Jackson Laboratories, West-Grove, USA).
2.5. Immunofluorescence microscopy and immunoblotting
Cells were fixed in 2.5% formaldehyde (Merck Inc., Whitehouse Station, NJ, USA) and processed for immunofluorescence microscopy as described [12]. Immunoblotting of total cell lysates obtained from equal amounts of cells was performed as described previously [12].
2.6. Immunohistochemistry
Formalin-fixed, paraffin-embedded colorectal adenocarcinomas were obtained from surgical resection specimens. Tumour sections were deparaffinized in xylene, rehydrated in alcohols, and washed twice with water. Samples were boiled in 0.01 M citrate buffer (pH 6.0) for 30 min and subjected to immunohistochemistry employing the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA).
2.7. Chromatinimmunoprecipitations (ChIPs)
ChIP analyses were performed according to the instructions of the ChIP Assay Kit from Upstate Biotechnology (Lake Placid, NY, USA) as described previously [11]. Primers used were: PKP3 ChIP1 forward: 5′-CCCAAATTCCACCTTAAGCA-3′, reverse: 5′-CGCCTCCAAACCCAACTAT-3′; ChIP2 forward: 5′-ACCTGTTAGCAGCTGCAATTT-3′, reverse 5′-GAAGGGGGCGTCTGTCTG-3′.
2.8. RNA inhibition experiments
ZEB1-specific siRNA experiments were performed as described previously [11].
3. Results
3.1. Knock down of ZEB1 in invasive cancer cells causes re-expression of PKP3
Deconstruction of junctional complexes allows differentiated epithelial cells to convert to a motile fibroblastoid phenotype during embryonic development and physiological events in the adult organism [13,14]. This process, generally termed epithelial-mesenchymal transition (EMT), is also pathologically activated during cancer cell invasion and metastasis [15,16]. Recently, we have identified the transcription factor ZEB1 (δEF1, TCF8, AREB6) as a direct transcriptional repressor of E-cadherin and a potent inducer of EMT in human breast cancer cells [11] (see also Fig. 1B and C). In addition, depletion of ZEB1 in dedifferentiated and metastatic cancer cells by RNA interference not only restored E-cadherin expression but also caused the reestablishment of epithelial features including the partial formation of adherens and tight junctions [11]. These data suggest that ZEB1 may regulate various genes critically involved in the formation of epithelial adhesion complexes.
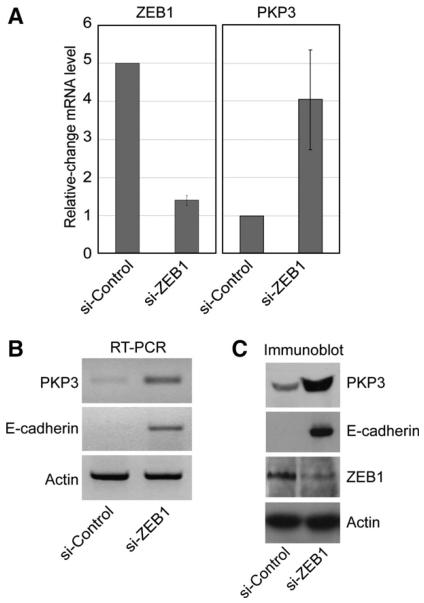
Fig. 1.
Knock down of ZEB1 causes upregulation of PKP3 expression. (A) RNAi-mediated knock down of ZEB1 in MDA-MB-231 breast cancer cells. Three days after treatment with either ZEB1-specific (si-ZEB1) or unspecific scrambled (si-Control) siRNAs total RNA was harvested and processed for Affymetrix Gene Chip analysis™. Diagram shows relative-change of mRNA levels of ZEB1 and PKP3 after ZEB1-specific knock down compared to cells treated with scrambled siRNA (si-Control). (B) PKP3 and E-cadherin mRNA levels after ZEB1 knock down. mRNA levels were analysed by semi-quantitative RT-PCR three days after ZEB1 knock down (see A). Actin was included for normalization. (C) Protein levels of PKP3, E-cadherin and ZEB1 after knock-down of ZEB1. For immunoblotting cells were treated with siRNAs as indicated in (A). Total MDA-MB-231 cell lysates were separated by SDS-PAGE and immunoblots were performed using antibodies to ZEB1, PKP3, E-cadherin and Actin (loading control).
In order to determine the function of ZEB1 during cancer progression, we specifically knocked down ZEB1 in invasive and dedifferentiated MDA-MB-231 cancer cells expressing high levels of endogenous ZEB1 and lacking all junctional adhesion complexes, and identified de-repressed genes by Affymetrix Gene Chip™ analyses. Among the genes whose transcript levels were significantly upregulated upon ZEB1 depletion, we detected the desmosmal protein PKP3 (Fig. 1A). RT-PCR experiments using cDNA samples from three independent siRNA knockdown experiments confirmed the PKP3 Gene Chip data. In all experiments only low amounts of PKP3 transcripts were detected in control cells treated with unspecific scrambled siRNAs, whereas strong expression was observed upon specific depletion of ZEB1 (Fig. 1B, one representative example is shown). Next we analysed whether the increase in PKP3 transcripts concomitantly led to the expression of functional protein products. MDA-MB-231 cells were treated with unspecific or ZEB1-specific siRNAs for three days and total cell lysates were probed for PKP3 protein by immunoblotting. Only minor amounts of PKP3 were detected in MDA-MB-231 control cells, whereas ZEB1 depletion caused strong upregulation of PKP3 protein levels (Fig. 1C).
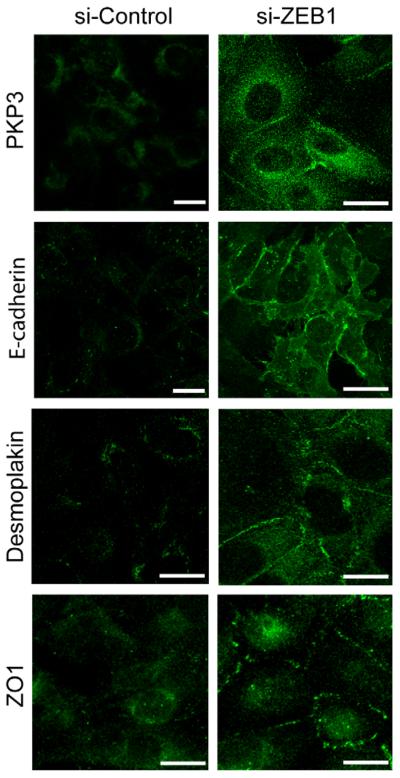
Immunofluorescence microscopy confirmed that PKP3 was upregulated in ZEB1-depleted MDA-MB-231 cells and located to spot-like structures in the cytoplasm or at the lateral plasma membrane (Fig. 2). In contrast, control cells exhibited only weak and diffuse cytoplasmic stainings (Fig. 2). However, defined dotted staining patterns along the whole lateral membrane, which are typical for desmosomal structures in fully polarized epithelia, could not be observed. Similar observations were made regarding the expression and intracellular localization of the desmosomal plaque protein Desmoplakin (Fig. 2). The lack of fully differentiated desmosomes might be due to the short-term inhibition of ZEB1 in the transient siRNA experiments (3 days siRNA treatment). At this stage the tight junction marker ZO1 only partially accumulated at the plasma membrane (Fig. 2). Alternatively, critical desmosomal components might still be missing and/or limiting in ZEB1-depleted MDA-MB-231 cells preventing complete assembly of desmosomes. Taken together, the knock down of ZEB1 caused strong derepression of PKP3 on both the mRNA and protein level and caused partial relocalization of PKP3 to the lateral plasma membrane.
Fig. 2.
PKP3 is partially relocalized to the plasma membrane upon ZEB1 knock-down. For immunofluorescence microscopy MDA-MB-231 cells were treated with ZEB1-specific (si-ZEB1) or unspecific scrambled (si-Control) si-RNAs for three days, fixed with formaldehyde and stained with PKP3, E-cadherin, Desmoplakin and ZO1 specific antibodies. Bars, 10 μm.
3.2. Expression of E-cadherin is not sufficient to induce PKP3 expression
Besides its major structural role in epithelial cell-cell adhesion E-cadherin can critically influence intracellular signalling pathways such as Wnt and receptor tyrosine kinase signalling [17]. Moreover, many reports demonstrated that ectopic E-cadherin expression is sufficient to confer epithelial differentiation to fibroblasts and dedifferentiated cancer cells [18]. As re-expression of functional E-cadherin is one of the hallmarks of ZEB1 knock down in MDA-MB-231 cancer cells [11], we investigated whether E-cadherin expression may be sufficient to induce PKP3 expression in a ZEB1 independent manner. We generated MDA-MB-231 cells stably expressing GFP-tagged E-cadherin, which has been shown previously to be functional and confer adhesion to E-cadherin-negative mesenchymal cells [19]. Ectopically expressed E-cadherin was enriched at sites of cell to cell contacts but accumulated also in the cytoplasm (Supplementary Fig. 1A). However, the cells did not display polarized and differentiated epithelial features, but formed only irregularly structured and weakly adhesive cell clusters (Supplementary Fig. 1A). In these cells PKP3 expression was induced neither at the mRNA nor at the protein level (Supplementary Fig. 1A and B). Expression of E-cadherin also did not alter ZEB1 expression levels in MDA-MB-231 cells (data not shown). Thus, expression of E-cadherin alone was not sufficient to upregulate PKP3 transcript or protein levels.
3.3. ZEB1 physically associates with the PKP3 promoter and represses its activity
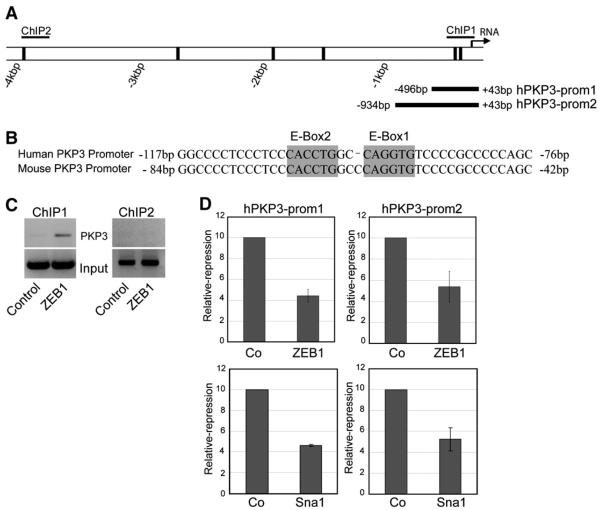
Next we analysed whether PKP3 represents a direct bona fide target gene of ZEB1 in MDA-MB-231 cancer cells. ZEB1 was previously shown to specifically interact with E-box elements in the proximal promoter region of target genes such as E-cadherin [11,20,21]. Hence, we searched the human and mouse proximal PKP3 promoter regions for the presence of E-box elements (5′-CACCTG-3′). Within the first 4 kbp upstream of the PKP3 transcription initiation site we detected six and seven E-box consensus sites in the human and mouse promoter, respectively, of which the two E-box elements closest to the initiation of transcription were highly conserved between both species (Fig. 3A and B; Fig. 4A; Supplementary Fig. 2). On the other hand two additional highly conserved stretches located further upstream did not contain E-box elements conserved in human and mouse (Supplementary Fig. 2). Hence, we performed chromatin immunoprecipitation (ChIP) assays to investigate whether ZEB1 can directly associate with the conserved E-box elements in the human PKP3 promoter in vivo. ZEB1-associated chromatin was immunoprecipitated from human MDA-MB-231 cells that express high levels of endogenous ZEB1 [11] using an antibody specifically recognizing the N-terminal domain of ZEB1 [11,22]. Chromatin fragments associated with ZEB1 were analysed for the presence of PKP3 promoter sequences by PCR using primers that either span the two conserved proximal E-boxes (−143/+43; ChIP1) or E-box 6 located further upstream (−3800/−3670; ChIP2) in the human PKP3 promoter (Fig. 3A). As depicted in Fig. 3C the ZEB1 antibody consistently pulled down chromatin fragments containing the two proximal E-boxes elements, whereas fragments containing the most upstream E-box could not be detected. In addition, unspecific control antibodies did not precipitate any PKP3 promoter sequences (Fig. 3C).
Fig. 3.
ZEB1 directly associates with the PKP3 promoter and represses its activity. (A) Scheme of the human PKP3 promoter. Relative positions of E-boxes are depicted as black bars along the first four kbp of the human PKP3 promoter. (B) The two proximal E-boxes (shaded) close to the transcription initiation site are conserved in the human and mouse PKP3 promoter. (C) ZEB1 physically interacts with the human PKP3 promoter. ZEB1 associated chromatin fragments derived from MDA-MB-231 cells were pulled down in chromatin immunoprecipitation (ChIP) experiments. PKP3 promoter regions were amplified by PCR (see ChIP1 and ChIP2 in A). Input confirms equal chromatin loading. Lane ZEB1: ZEB1-specific antibody; Lane Control: unspecific goat antibody. (D) ZEB1 represses the human PKP3 promoter activity. Human ZEB1 or Snail1 expression vector or control vector (p120ctn) were transfected into human epithelial MCF7 cells together with one of the two human PKP3 promoter constructs depicted in (A) (hPKP3-prom1 and hPKP3-prom2). Relative repression by ZEB1 and Snail1 (Sna1) is shown by mean values of four independent measurements compared to control vector. Bars represent standard errors on the mean.
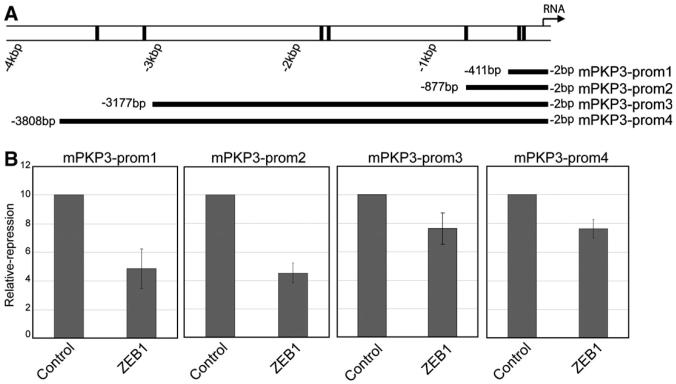
Fig. 4.
ZEB 1 represses the activity of the mouse PKP3 promoter. (A) Scheme of the first four kbp of the mouse PKP3 promoter. E-boxes are indicated by black bars. The position of the four promoter fragments are shown below the scheme (mPKP3-prom1-4). (B) Reporter assays were performed as indicated in Fig. 3 using a mouse full-length ZEB1 expression vector and EpH4 murine mammary epithelial cells.
To determine whether binding of ZEB1 to the proximal PKP3 promoter region in vivo is sufficient to repress the human PKP3 promoter activity, we generated two different but overlapping luciferase reporter constructs (−496/+43, hPKP3-prom1 and −934/+43, hPKP-prom2), both containing the proximal conserved E-boxes of the human promoter. These constructs were transfected into human MCF7 epithelial cells, which express high levels of endogenous PKP3 (Supplementary Fig. 1), together with a human ZEB1 expression vector or a control vector expressing an unrelated protein (p120ctn) as well as a β-galactosidase vector for normalization of transfection efficiency and viability. Compared to the control vector, expression of ZEB1 caused a significant repression of both human PKP3 promoter constructs (Fig. 3D). Similar to ZEB1 the transient overexpression of the E-box binding repressor Snail1 also interfered with the activity of the human PKP3 promoter.
In order to investigate whether the activity of the mouse PKP3 promoter is also affected by ZEB1, we tested four different mouse PKP3 promoter constructs in reporter gene assays (Fig. 4A). Two proximal shorter fragments encompassing the regions −411/−2 (with only the two conserved E-boxes; named mPKP3-prom1) or −877/−2 (with three E-boxes; named mPKP3-prom2), and two longer fragments ranging from −3177 to −2 (with six E-boxes; named mPKP3-prom3) and −3808 to −2 (with seven E-boxes; named mPKP3-prom4) were analysed in mouse mammary epithelial cells (EpH4) as described above using a full-length mouse ZEB1 expression construct [11]. Albeit ZEB1 repressed the activity of all four PKP3 promoter regions, repression was particularly effective with the two shorter reporter constructs containing mainly the proximal conserved E-boxes (Fig. 4B). These data indicate that in analogy to the human PKP3 promoter, the proximal E-boxes were necessary and sufficient to mediate repression by ZEB1. Only weak repressive effects were observed for the longer constructs (Fig. 4B). Hence, the longer promoter fragments might additionally contain regulatory elements that partially relieve the repressive effects of ZEB1. Taken together, our data demonstrate that endogenous ZEB1 can directly associate with the proximal PKP3 promoter in undifferentiated cancer cells and partially repress both human and mouse PKP3 promoter activities. Repression is mediated by conserved proximal E-box elements close to the transcription initiation site.
3.4. ZEB1 represses PKP3 during cancer cell invasion in vivo
To determine whether ZEB1 is able to repress PKP3 expression during cancer cell invasion in human tumours we performed double-immunohistochemical analyses of 10 paraffin-embedded human colon cancer specimens using antibodies to PKP3 and ZEB1. Colon cancer represents a suitable cancer type to study ZEB1 function in vivo, as ZEB1 has already been implicated in the repression of basal lamina components in colon tumours [23].
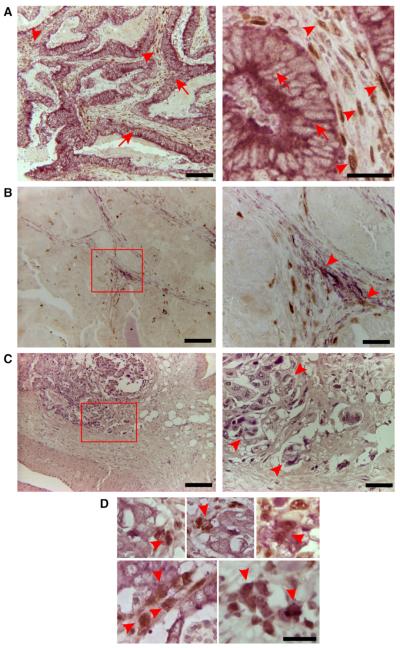
Immunohistochemistry revealed that within the differentiated bulk tumour area PKP3 localized primarily to the plasma membrane of cancer cells (Fig. 5A, violet stain, arrows). Accordingly, these cells express high amounts of E-cadherin and cytokeratin emphasizing their differentiated epithelial phenotype (data not shown). These differentiated cancer cells completely lacked detectable ZEB1 expression (Fig. 5A, no brown stain in cells indicated by arrows). However, ZEB1 accumulated in the nuclei of a large number of tumour-associated stroma cells (Fig. 5A, brown stain, arrowheads), whereas in normal colon stroma ZEB1 positive cells were only rarely detected (data not shown). Within the stroma ZEB1 might be specifically expressed in fibroblasts and partially in activated fibroblasts expressing Smooth Muscle Actin (SMA) (Fig. 5B, SMA, violet; ZEB1, brown. Arrowheads indicate colocalization of SMA and ZEB1). At present one can only speculate whether some of the ZEB1 positive stromal fibroblasts might have directly developed from epithelial cancer cells through a bona fide ZEB1-dependent EMT (compare also with [32]).
Fig. 5.
ZEB1 and PKP3 localization in human colon cancer. (A) Colon cancer specimens were double-stained for ZEB1 (brown stain) and PKP3 (violet stain). Left panel, overview of differentiated bulk tumour area. Right panel, higher magnification of differentiated area. PKP3 is expressed in differentiated regions of the tumour (violet stain, arrows), whereas ZEB1 is mostly detected in tumour-associated stroma cells (brown stain, arrowheads). (B) Double staining of ZEB1 (brown) and Smooth Muscle Actin (violet). Note that some of the activated tumour-associated fibroblasts express nuclear ZEB1. (C) Invasive area of colon tumour stained with Hematoxylin/Eosin. (D) Upregulation of ZEB1 in invasive cancer cells at the tumour–host interface (brown stain, arrowheads). ZEB1 expression is accompanied by downregulation of PKP3 (violet stain in cells marked with arrowheads). Note that residual PKP3-specific staining is still detectable in the cytoplasm of ZEB1 expressing cells. Bars, 120 μm (A, B, C left) or 30 μm (A, B, C, right and D).
Colon tumours showed defined areas of strong invasion, where loosely attached or single cancer cells invade the underlying stroma (Fig. 5C, Hematoxylin/Eosin stain of invasive colon cancer area). In contrast to the bulk tumour area, ZEB1 was strongly upregulated in distinct invading cancer cells at the tumour-host interface. Upregulation of ZEB1 was clearly visible in eight out of 10 cancer specimens (Fig. 5D, brown stain, arrowheads). Based on nuclear and cellular morphologies the ZEB1-positive cancer cells were clearly different from the ZEB1-expressing stroma cells (compare arrowhead marked cells in Fig. 5A, C and D). ZEB1 expression was always accompanied by defects in intercellular adhesion and loss of membrane associated PKP3. However, the cells still exhibited a rudimentary cytoplasmic PKP3 pool, which directly proved their epithelial origin (Fig. 5D).
In conclusion, significant levels of membrane-bound PKP3 can only be found in the differentiated main tumour mass of colorectal cancers barely expressing any ZEB1, whereas upregulation of ZEB1 at the invasive front was accompanied by downregulation of PKP3 protein levels and loss of intercellular adhesion.
4. Discussion
4.1. PKP3 is directly repressed by ZEB1 in human cancer cells
In the present study we demonstrate that ZEB1 directly represses the transcription of the desmosomal component PKP3. First, we found that knock down of ZEB1 by RNA interference (RNAi) was sufficient to upregulate expression of PKP3 at the mRNA and protein level. Derepression of PKP3 was independent of the expression and function of the major intercellular adhesion protein E-cadherin. Second, ZEB1 was able to physically associate with the proximal PKP3 promoter in vivo and to partially repress the activity of corresponding promoter fragments of the human and mouse PKP3 promoter in reporter gene assays. Third, upregulation of ZEB1 in human tumours correlated with cancer cell invasion and was accompanied by a significant downregulation of PKP3 protein levels.
Currently it is unclear which signalling pathways contribute to upregulation of ZEB1 in cancer cells at the tumour-host interface. Paracrine signalling mechanisms, such as release of tumour necrosis factor (TNF) α or transforming growth factor (TGF) β by tumour associated macrophages or other stroma cells might cause upregulation of ZEB1 in particular cancer settings [24-27].
4.2. ZEB1 disrupts desmosomal adhesion during EMT
PKP3 belongs to the armadillo repeat protein family and is broadly expressed in most simple and stratified epithelial tissues except for hepatocytes and hepatocellular carcinomas [5,6]. Regarding interactions with desmosomal components PKP3 exhibits the broadest repertoire among the plakophilins, as it directly interacts with Plakoglobin, the cytoskeletal linker protein Desmoplakin and all desmosomal cadherins [28]. Considering the extensive crosslinking capacity of PKP3 within the desmosmal plaque, downregulation of its expression by ZEB1 is likely to impair desmosome maturation and stability. In addition, upregulation of ZEB1 in cancer cells also affects the expression of other desmosomal components such as Desmoplakin, Desmogleins and Desmocollins (Aigner and Eger, unpublished results). Hence ZEB1 can simultaneously affect the expression of different desmosomal proteins, albeit it is unclear at present whether ZEB1 can repress the expression of all these desmosomal genes in a direct manner similar to PKP3.
Interestingly, the E-cadherin repressors Snail1 and SIP1 (ZEB2, a homologue of ZEB1) have also been reported to repress the expression of individual Plakophilins [29,30]. Upon overexpression in colon cancer cell lines Snail1 specifically repressed the transcription of PKP2, whereas SIP1 affected expression of PKP2 as well as Desmoplakin. Therefore, the multiple E-cadherin repressors may induce downregulation of distinct sets of epithelial target genes, which might depend on the cellular context and/or the environmental settings of malignant tissues. However, at present we cannot exclude the existence of a hierarchy of and/or crosstalk between the different E-cadherin repressors in the same cancer type.
In MDA-MB-231 cells the expression of Snail1 is significantly lower than the expression of ZEB1 [11] and knock-down of Snail in these cells is not accompanied by epithelial re-differentiation and upregulation of epithelial marker proteins such as PKP3 (our unpublished results). However, strong transient overexpression of Snail1 in MCF7 cells was sufficient to interfere with the promoter activity of PKP3. Hence, expression levels of the diverse E-cadherin repressors as well as the availability of cofactors might additionally be critical in determining their overall repressive capacity and target gene specificity.
Extensive expression profiling experiments in different cancer tissues and cell models are still needed to precisely unravel the gene expression networks established by the E-cadherin repressors ZEB1, SIP1/ZEB2, Snail1, Snail2 (Slug), and E47 in the course of EMT and cancer cell invasion.
In summary, ZEB1 not only affects the formation of adherens junctions by repressing its major constituent E-cadherin [11], but also induces the disassembly of desmosomal structures that normally provide epithelial sheets with additional adhesive strength and resistance to mechanical stress [2,31]. These data provide first experimental evidence of how ZEB1 simultaneously and coordinately remodels epithelial adhesion complexes in order to disrupt epithelial polarity and trigger EMT enabling individual tumour cells to escape from bulk tumour areas.
Acknowledgements
Grant Support: This study was supported by funds from the Hochschuljubiläumsstiftung of the City of Vienna to A.E. (H-703/2005), by Grants from the Austrian Science Research Fund (FWF) No. SFB 006 to R.F. (603) and H.B. (612), SFB-F28 to W.M., by funds of the Austrian Ministry of Education, Science, and the Arts (Austrian Genome Research Program GEN-AU) to M.S., W.S., N.S., A.W., and by Grants of the National Fund for Scientific Research – Flanders (FWO) to F.V.R. and S.B. A.S. is supported by the ÖAD-Pakistan Scholarship programme.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.febslet.2007.03.026.
References
- 1.Nelson WJ. Epithelial cell polarity from the outside looking in. News Physiol. Sci. 2003;18:143–146. doi: 10.1152/nips.01435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin. Cell. Dev. Biol. 2004;15:665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat. Rev. Mol. Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- 4.Garrod DR, Merritt AJ, Nie Z. Desmosomal cadherins. Curr. Opin. Cell Biol. 2002;14:537–545. doi: 10.1016/s0955-0674(02)00366-6. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt A, Jager S. Plakophilins – hard work in the desmosome, recreation in the nucleus? Eur. J. Cell Biol. 2005;84:189–204. doi: 10.1016/j.ejcb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Hatzfeld M. Plakophilins: multifunctional proteins or just regulators of desmosomal adhesion? Biochim. Biophys. Acta. 2007;1773:69–77. doi: 10.1016/j.bbamcr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Bonné S, van Hengel J, Nollet F, Kools P, van Roy F. Plakophilin-3, a novel armadillo-like protein present in nuclei and desmosomes of epithelial cells. J. Cell Sci. 1999;112(Pt 14):2265–2276. doi: 10.1242/jcs.112.14.2265. [DOI] [PubMed] [Google Scholar]
- 8.Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J. Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt A, Langbein L, Pratzel S, Rode M, Rackwitz HR, Franke WW. Plakophilin 3-a novel cell-type-specific desmosomal plaque protein. Differentiation. 1999;64:291–306. doi: 10.1046/j.1432-0436.1999.6450291.x. [DOI] [PubMed] [Google Scholar]
- 10.Pacher M, et al. Impact of constitutive IGF1/IGF2 stimulation on the transcriptional program of human breast cancer cells. Carcinogenesis. 2006;28:49–59. doi: 10.1093/carcin/bgl091. [DOI] [PubMed] [Google Scholar]
- 11.Eger A, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 12.Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J. Cell Biol. 2000;148:173–188. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 14.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- 15.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 16.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 17.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 18.Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A. The cadherin–catenin adhesion system in signaling and cancer. J. Clin. Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J. Cell Biol. 2001;154:1185–1196. doi: 10.1083/jcb.200104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda K, Kawakami K. DNA binding through distinct domains of zinc-finger-homeodomain protein AREB6 has different effects on gene transcription. Eur. J. Biochem. 1995;233:73–82. doi: 10.1111/j.1432-1033.1995.073_1.x. [DOI] [PubMed] [Google Scholar]
- 21.Remacle JE, Kraft H, Lerchner W, Wuytens G, Collart C, Verschueren K, Smith JC, Huylebroeck D. New mode of DNA binding of multi-zinc finger transcription factors: deltaEF1 family members bind with two hands to two target sites. Embo J. 1999;18:5073–5084. doi: 10.1093/emboj/18.18.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dohadwala M, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–5345. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 23.Spaderna S, et al. A Transient, EMT-Linked Loss of Basement Membranes Indicates Metastasis and Poor Survival in Colorectal Cancer. Gastroenterology. 2006;131:830–840. doi: 10.1053/j.gastro.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2006;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 25.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura G, et al. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev. Cell. 2006;11:93–104. doi: 10.1016/j.devcel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Jechlinger M, et al. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22:7155–7169. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- 28.Bonné S, Gilbert B, Hatzfeld M, Chen X, Green KJ, van Roy F. Defining desmosomal plakophilin-3 interactions. J. Cell Biol. 2003;161:403–416. doi: 10.1083/jcb.200303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandewalle C, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33:6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Craene B, Gilbert B, Stove C, Bruyneel E, van Roy F, Berx G. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 2005;65:6237–6244. doi: 10.1158/0008-5472.CAN-04-3545. [DOI] [PubMed] [Google Scholar]
- 31.Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J. Cell Sci. 2006;119:797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- 32.Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Ronnov-Jessen L. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am. J. Path. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]