Abstract
Background
African Americans generally have lower circulating levels of 25 hydroxyvitamin D (25(OH)D) than whites, attributed to skin pigmentation and dietary habits. Little is known about the genetic determinants of 25(OH)D levels, nor whether the degree of African ancestry associates with circulating 25(OH)D.
Methods
Using a panel of 276 ancestry informative genetic markers, we estimated African and European admixture for a sample of 758 African American and non-Hispanic white Southern Community Cohort Study participants. For African Americans, cutpoints of <85%, 85%–95%, and ≥95% defined “low”, “medium”, and “high” African ancestry. We estimated the association between African ancestry and 25(OH)D, and also explored whether vitamin D exposure (sunlight, diet) had varying effects on 25(OH)D levels dependent on ancestry level.
Results
Mean serum 25(OH)D levels among whites and among African Americans of low, medium, and high African ancestry were 27.2, 19.5, 18.3, and 16.5ng/mL, respectively. Serum 25(OH)D was estimated to decrease by 1.0–1.1ng/mL per 10% increase in African ancestry. The effect of high vitamin D exposure from sunlight and diet was 46% lower among African Americans with high African ancestry than among those with low/medium ancestry.
Conclusions
We found novel evidence that the level of African ancestry may play a role in clinical vitamin D status.
Impact
This is the first study to describe how 25(OH)D levels vary in relation to genetic estimation of African ancestry. Further study is warranted to replicate these findings and uncover the potential pathways involved.
Keywords: vitamin D, African Americans, health status disparities, genetics, epidemiology
INTRODUCTION
It is well documented that circulating levels of 25 hydroxyvitamin D (25(OH)D, the established clinical marker of vitamin D status) are lower among African Americans than whites in the United States (1). This is mediated largely by skin color, as melanin blocks the initial conversion of 7-dehydrocholesterol to cholecalciferol, the precursor of 25(OH)D, in the skin (1,2). Other possible contributors to the racial difference include diet, as African Americans tend to have diets lower in vitamin D and are less likely to take vitamin supplements (3). As vitamin D insufficiency is increasingly linked to a variety of chronic diseases, including cancer (1), this hormone is under scrutiny as a potential contributor to racial health disparities (3–5).
The extent to which racial differences in 25(OH)D levels are influenced by genetic variation tied to African ancestry is unknown. Evidence for a link between genetic markers of African ancestry and 25(OH)D levels might provide clues to investigate genetic determinants for vitamin D insufficiency, which could have implications for intervention strategies to ameliorate vitamin D insufficiency among African Americans and possibly other groups. There is significant variation in the level of African ancestry among African Americans, and this can now be estimated in fairly precise and quantitative terms (6–9). We therefore undertook a study to investigate the association between African ancestry and 25(OH)D levels within the Southern Community Cohort Study (SCCS), where we have previously shown evidence of striking racial disparities in 25(OH)D levels based on self-reported race (4).
MATERIALS AND METHODS
Study population
The SCCS is a prospective cohort study of cancer risk disparities related to race, socioeconomic status, and other factors (10). Men and women aged 40–79 were recruited in-person at community health centers and also by mail across 12 southeastern US states between 2002 and 2009 (11). With approximately 86,000 participants enrolled, African Americans comprise two-thirds of the cohort. From SCCS participants who enrolled from March 2002-October 2004 and donated a baseline blood sample (N=12,162), 792 were randomly selected using a 2×2×3×3 factorial design, with 22 individuals selected within each of the 36 strata defined by self-reported race (African American/white), sex, smoking status (current/former/never), and body mass index (18–24.99 kg/m2, 25–29.99 kg/m2, 30–45 kg/m2). This design provided a balanced distribution across these factors in consideration of other blood biomarkers being measured in addition to vitamin D.
SCCS participants provided written informed consent, and protocols were approved by Institutional Review Boards at Vanderbilt University and Meharry Medical College.
Baseline data and blood collection
Baseline information was collected using a computer-assisted, in-person interview conducted at the time of enrollment. The interview covered demographics, health history, anthropometrics, and a wide range of potential cancer risk factors (questionnaire available at SCCS website (11)). Dietary information was collected using a validated food frequency questionnaire including foods, beverages and nutritional supplements developed specifically for the SCCS (12). Race was self-reported by participants with use of a printed card and instructions to choose all racial/ethnic descriptors that applied. Venous blood samples (20mL) were collected during the baseline interview within the community health center, kept refrigerated and shipped cold overnight to Vanderbilt University where they were centrifuged the next day and stored at −80°C to await analysis.
Measurement of 25(OH)D
Blinded serum 25(OH)D measurements were performed in the lab of Dr. Bruce Hollis at the Medical University of South Carolina using a radioimmunoassay method associated with high intra-assay reliability (13). One half of the samples was assayed in 2005 (median 1.6 years after collection, and previously thawed once) and the second half assayed in the same lab using the same methods in 2009 (median 5.5 years after collection, and previously thawed once). Measured 25(OH)D is not known to be affected by storage times as long as 14 years nor by up to 10 freeze-thaw cycles (B. Hollis, personal communication). A comparison of vitamin D levels from the first and second assay batches indicated no systematic differences that would prevent the data from the two groups being combined. The average 25(OH)D values for self-reported African American subjects in batch 1 and batch 2 were 17.3 and 17.6ng/mL, respectively, and were 27.2 and 27.2ng/mL, respectively, for self-reported white subjects. A set of quintuplet identical samples was also included in each batch, four years apart, and their average measurement was the same in each batch: 13.2 ng/mL. The coefficients of variation on duplicate, blinded quality control samples in batches 1 and 2 were 6.7% and 7.4%, respectively.
Measures of vitamin D exposure
Dietary intake of vitamin D was estimated from the reported intake of the major dietary sources in the SCCS food frequency questionnaire: milk, cold cereal, tuna, eggs, multivitamins and calcium supplements. Individual measures of sun exposure were not obtained. As a surrogate, we used ground ultraviolet radiation (UVR) measurements from the National Oceanic and Atmospheric Administration UV station geographically closest to the subject’s residence as an estimate of ambient UVR exposure (14). These UVR measurements are converted to an index that estimates the erythemal intensity, with scores ranging from 0 (none) to 11+ (extreme). We used the average of the UVR scores recorded at the monitoring station during the 3-month period preceding each participant’s enrollment in the study.
Genetic analysis and ancestry estimation
Genomic DNA was extracted from buffy coat using Qiagen’s DNA Purification kits (Qiagen, Valencia, CA) according to manufacturer’s instructions, and genotyping was carried out using the Illumina GoldenGate genotyping platform (Illumina Inc., San Diego, CA). Laboratory personnel were blinded to information about the status of the samples. Blinded QC samples (N=29) and another 171 pairs of duplicate samples were included and the consistency rate was 99.9%.
We sought to construct a list of 300 ancestry informative markers (AIMs) from two sources, the first being a list of 360 single nucleotide polymorphisms (SNPs) we developed comparing frequencies between individuals of European (CEU) and African (YRI) descent in HapMap, using Chi-square values to rank the markers, and the second being a list of 1,509 AIM SNPs from an Illumina-designed panel for ancestry estimation, giving a total of 1,865 potential AIMs (after excluding duplicates). Because initial genotyping efforts for this project were designed for a sample of females, AIMs on the Y chromosome as well as those with an Illumina design score ≤0.5 were excluded (N=1,826 SNPs remaining). In addition, all potential AIMs within 40 candidate genes of interest (available upon request, and related to SCCS projects of obesity and cancer) or their 5Mb flanking regions were excluded (N=635 SNPs remaining). Potential AIMs were also excluded if they had a minor allele frequency of < 0.05 in both the CEU and YRI populations in HapMap (N=367 SNPs remaining). Finally, 300 of the remaining 367 potential AIMs were selected as the final set based on the highest allele frequency differences between the CEU and YRI populations in HapMap. Of these 300 AIMs, 292 passed the Illumina Scoring algorithm and were sent for genotyping. Of the 292 AIMs, 276 were successfully genotyped with call rates higher than 95% and were used to estimate African and European ancestry (see supplemental material for listing of the 276 SNPs).
Estimates of African and European ancestry were computed using a Bayesian clustering approach implemented using STRUCTURE software, version 2.2.3 (15,16). STRUCTURE identifies groups of individuals with similar allele frequency profiles and estimates the shared ancestry of individuals based solely on their genotypes under an assumption of Hardy-Weinberg equilibrium and linkage equilibrium in ancestral populations. It identifies the number (K) of ancestry population clusters (K=2 in this study) and assigns individuals admixture estimates for each, with the estimates summing to 1 across these clusters. An admixture estimate (from 0.00 to 1.00) for both African ancestry and European ancestry was thus generated for each participant.
Statistical Methods
Of the 792 subjects selected for this study, 34 (4.3%) were excluded because either 25(OH)D or African/European ancestry could not be estimated, or because ancestry estimates were highly discordant with self-reported race, implying potential data entry errors. This left 758 subjects (379 African American, 379 white) for analysis.
Ancestry estimates were used as continuous variables, and linear regression models were used to associate African ancestry (independent variable) with serum 25(OH)D levels (dependent variable). The distribution of serum 25(OH)D was approximately normal, so the values were included in the model untransformed. The ancestry variable was also categorized as “white”, and as “low”, “medium”, and “high” African ancestry using predetermined arbitrary cutpoints of <85%, 85% up to 95%, and ≥95% African ancestry. The use of alternate cutpoints (i.e., tertiles) resulted in the same overall findings. The linear regression models evaluating the association between ancestry and 25(OH)D were adjusted for vitamin D intake (IUs from food and supplements, continuous), sunlight exposure (UVR score, continuous), body mass index (continuous), current cigarette smoking (yes/no), education (<high school vs. not), alcohol drinking (average number of drinks/day, continuous), and current employment (yes/no). Season of blood collection was not used as a covariate because it was nearly interchangeable, and highly collinear, with UVR score, and we chose to use UVR score because it reflected both the time of year and the general geographical location of the participant.
To evaluate the influence of environmental exposures (sunlight and diet) on circulating 25(OH)D levels for African Americans of varying admixture, we defined high exposure as having a UVR score above the median and a dietary intake >400IU/day (current recommended daily intake), medium exposure as a UVR score above the median and a dietary intake ≤400IU/day, and low exposure as those with a UVR score below the median. In this categorization, sunlight exposure was given dominance over dietary intake given its greater impact on circulating vitamin D. We also evaluated effect modification by ancestry using regression models for African Americans containing interaction terms for (African ancestry × UVR score) and (African ancestry × dietary vitamin D intake), using the covariates in their continuous form.
RESULTS
African admixture in our self-reported white participants ranged from 0.001 to 0.171 (mean 0.009), and in our self-reported African American participants ranged from 0.505 to 0.999 (mean 0.929). Table 1 shows the distribution of the African American participants across the defined African ancestry categories (16% were classified as low, 28% medium, 56% high). SCCS methods of enrolling African Americans and whites from the same community health centers year-round resulted in only minor differences in residential UVR scores and season of enrollment by race. Among African Americans, neither UVR score nor dietary vitamin D intake was significantly associated with ancestry level, diminishing their ability to confound the relationship between ancestry and circulating 25(OH)D. Mean UVR scores were 5.5, 5.2, and 5.2 and mean vitamin D intake values were 208, 219, and 220 IUs/day for African Americans of low, medium, and high African admixture, respectively.
Table 1.
Ancestry estimation and other characteristics of 758 Southern Community Cohort Study participants.
| Characteristic | African American (N=379) | White (N=379) |
|---|---|---|
| Number of females/males | 187/192 | 187/192 |
| Median (IQR) percentage African ancestry | 0.971 (0.887–0.997) | 0.004 (0.003–0.009) |
| Median (IQR) percentage European ancestry | 0.029 (0.003–0.113) | 0.996 (0.991–0.997) |
| Ancestry categories | ||
| White/European | 0 | 379 (100%) |
| African – Low (<85%) | 60 (16%) | 0 |
| African – Medium (85%–94.99%) | 106 (28%) | 0 |
| African – High (≥95%) | 213 (56%) | 0 |
| Mean (S.D.) serum 25(OH)D levels, ng/mL | 17.5 (8.6) | 27.2 (11.1) |
| Mean (S.D.) age, years | 51.9 (9.0) | 54.1 (9.5) |
| Mean (S.D.) body mass index*, kg/m2 | 28.3 (5.6) | 28.4 (5.8) |
| Mean (S.D.) daily dietary intake of vitamin D†, IU | 218 (213) | 269 (236) |
| Mean (S.D.) residential UVR score | 5.2 (1.9) | 5.2 (2.0) |
| Season of enrollment‡ | ||
| % in winter | 17% | 22% |
| % in spring | 30% | 27% |
| % in summer | 25% | 26% |
| % in fall | 28% | 25% |
| % current smoker* | 34% | 34% |
| % currently working | 41% | 34% |
| % with less than high school education | 35% | 27% |
Selection/matching factor.
From food sources and multivitamin and calcium supplement sources.
Winter: December – February; Spring: March – May; Summer: June – August; Fall: September –November.
Abbreviations: IQR, interquartile range; S.D., standard deviation; IU, International Units; UVR, ultraviolet radiation
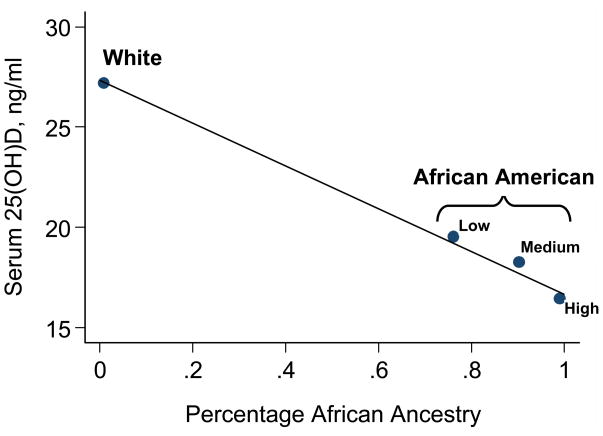
Average 25(OH)D levels decreased monotonically across categories of increasing African ancestry (Figure 1). In adjusted models using the entire population and also only African Americans, we found a significant 1.0–1.1 ng/mL decrease in 25(OH)D per 10% increase in African admixture (Table 2). In the fully adjusted model using the entire population, the r2 value increased from 0.16 to 0.35 with the addition of the ancestry variable, which would be expected given the ability of skin color differences between African Americans and whites to explain a good part of the variance in serum 25(OH)D. However, in models using only African Americans, the r2 value increased only slightly from 0.21 to 0.22 with the addition of the ancestry variable, despite the significant effect of ancestry noted in the model. In whites, the range of African admixture was too limited to reliably estimate the change in 25(OH)D per unit of African ancestry.
Figure 1.
Plot of the average serum level of 25(OH)D (y-axis) in relation to genetic estimation of African ancestry (x-axis). Points represent the average African ancestry estimate and the average 25(OH)D level for each predetermined category of subjects (white, and low, medium, and high African ancestry). Superimposed is the predicted line from a univariate linear regression model with African admixture (continuous) as the independent variable and the 25(OH)D level (continuous) as the dependent variable. Mean (standard error) 25(OH)D levels were 27.2 (0.6) ng/mL for whites, and 19.5 (1.3), 18.3 (0.8), and 16.5 (0.6) ng/mL for African Americans of low, medium and high African ancestry.
Table 2.
Linear regression-derived effect of African ancestry on circulating levels of 25(OH)D.
| African ancestry | Model including both African Americans and Whites | Model including African Americans only | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude change in 25(OH)D | Adjusted change in 25(OH)D* | Crude change in 25(OH)D | Adjusted change in 25(OH)D* | |||||
| beta† | P value | beta† | P value | beta† | P value | beta† | P value | |
| Continuous measure of African ancestry (per 0.10 increase in African ancestry) | −1.1 | <0.001 | −1.1 | <0.001 | −1.5 | 0.002 | −1.0 | 0.03 |
From a linear regression model adjusted for age at enrollment (continuous), sex, average daily dietary intake of vitamin D (continuous), mean residential UVR score (continuous), body mass index (continuous), currently employed (yes/no), current smoker (yes/no), number of alcoholic drinks per day (continuous), and education (less than high school vs. not).
Parameter estimate from linear regression model, interpreted as the average change in serum 25(OH)D level (ng/mL) associated with a 10% increase in African admixture.
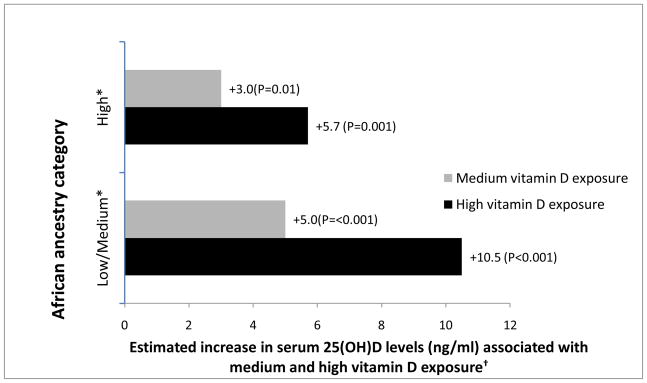
Among African Americans, both diet and UVR score were significant predictors of 25(OH)D: 2.1ng/mL increase per 200 IUs of intake, P< 0.001, and 1.6ng/mL increase per 1 UVR score, P<0.001). Having defined high, medium and low environmental exposure (sunlight and diet, see Methods) to vitamin D, linear regression models were used to examine the influence of environmental exposure on circulating 25(OH)D among African Americans of varying admixture (Figure 2). Those with low and medium African admixture were combined into one group because results for the two groups were similar. For each defined ancestry group, we observed the expected overall effect of serum 25(OH)D increasing with increasing environmental exposure. However, the effect diminished as African ancestry increased. Whereas high vitamin D exposure was associated with a serum 25(OH)D increase of 10.5 ng/mL for participants with low/medium African ancestry, it was associated with an increase only about half as high (5.7 ng/mL) for those with high African ancestry. This interaction between environmental exposures and African ancestry, however, did not reach statistical significance (p=0.39 using a likelihood ratio test to compare models with and without both interaction terms, p=0.19 for the model containing only the URV interaction term, and p=0.59 for the model containing only the dietary interaction term).
Figure 2.
Effect of environmental vitamin D exposure (sunlight and diet) on serum 25(OH)D levels among African Americans, based on African ancestry category (grouped as low/medium and high).
* Low African ancestry defined as African ancestry <85%, Medium as 85%–94.99%, High as ≥95%.
† Effects were estimated from a linear regression model adjusted for age, gender, and body mass index and are relative to low vitamin D exposure. High vitamin D exposure was defined as UVR score above the median and dietary intake >400IU/day; medium exposure as UVR score above the median and dietary intake ≤400IU/day; low exposure as UVR score below the median.
(Among those with Low/Medium African ancestry, mean UVR scores and dietary intakes for referent, medium, and high exposure were 3.7 and 224 IUs, 6.9 and 93 IUs, and 6.9 and 526 IUs, respectively. Among those with High African ancestry, mean UVR scores and dietary intakes for referent, medium, and high exposure were 3.5 and 226 IUs, 6.9 and 96 IUs, and 6.6 and 560 IUs, respectively.)
DISCUSSION
To our knowledge, this is the first study to describe how circulating 25(OH)D levels vary in relation to genetic estimation of African ancestry. We observed the expected difference in serum 25(OH)D between African Americans and whites. However, our findings also showed, within African Americans, an inverse relationship between African ancestry and 25(OH)D. Furthermore, among African Americans, vitamin D exposures had lesser effects on circulating 25(OH)D for those with higher African ancestry. Little is known about the genetic determinants of 25(OH)D levels (17). For environmental determinants, previous studies consistently show factors related to sunlight (e.g., ambient UVR, season, time spent outdoors, latitude, leisure time physical activity), diet, and other subject characteristics (e.g., age, adiposity, self-reported race, smoking, alcohol drinking) to account for no more than about 20–40% of the variability in circulating 25(OH)D (4,18–23). While error in measuring these external factors decreases the ability to fully explain 25(OH)D variability, it is also reasonable to assume that genetic factors are at play. For example, a recent report by Fu et al. (21) found 34% of the variability in circulating 25(OH)D to be explained by a multivariate model including environmental and genetic factors, and a common vitamin D binding protein gene (DBP) SNP was the second most explanatory factor, explaining 8.5% of the variance. This is among several studies that now show DBP SNPs (which have inter-ethnic differences in allele frequency) to be associated with 25(OH)D levels (20,21,23,24). The mechanism for this genetic association is unclear, but could involve changes to vitamin D binding protein levels or to the half-life of 25(OH)D (23). It has also been known for some time that there is substantial ethnic variation in the vitamin D receptor gene (VDR) (25), but the association between VDR genotypes and circulating 25(OH)D levels is equivocal (20,26). Further investigation of vitamin D pathway genes and their association with both African admixture and 25(OH)D levels may shed light on our findings.
It is unknown whether our findings reflect differences beyond skin color gradations along the spectrum of African ancestry, as skin pigmentation was not measured during the SCCS baseline interview. Others have reported a correlation between African admixture and measured skin pigmentation among African Americans (8,27). Thus, it is possible that the observed association between African ancestry and vitamin D level is mediated through skin shade. However, with few exceptions (i.e., a newly identified candidate gene GNG2 (28)), the AIM SNPs used in our analysis to assign African admixture were not on genes currently thought to affect skin pigmentation (8,28,29).
Limited research has addressed whether African Americans and whites may have inherently different capability to either synthesize (from UVR) or to absorb (from diet) vitamin D (30). Skin pigmentation has been an obvious focus of study, although the literature suggests that melanin’s interference in vitamin D synthesis is not insurmountable with sustained and controlled, and typically extended, UVR exposure (31–33). One small clinical study found similar serological response to an oral challenge of ergocalciferol (vitamin D2) in African Americans and whites (2). Still, in such studies, post-intervention 25(OH)D levels remained substantially lower in African Americans compared to whites (2,31).
We found that white SCCS participants had very little African admixture and African American SCCS participants had among the highest proportion of African ancestry reported from various US studies (6–9,34). A consequence of these participant characteristics is that we had limited data to describe the association between African ancestry and 25(OH)D for a wide range of intermediate ancestry levels. Thus, the relationship between ancestry level and 25(OH)D may be more complex than what is shown in Figure 2, which relies on points clustered near 0 and 1. Because of the generally high African admixture among African American SCCS participants, we find it all the more intriguing that we detected significant variation in circulating 25(OH)D within this admixture range.
In summary, this study provides novel evidence that the level of African admixture, even among African Americans, is associated with clinical vitamin D status. Further study is warranted to replicate these findings and uncover the potential pathways involved.
Supplementary Material
Acknowledgments
Financial support: The Southern Community Cohort Study is funded by a grant from the National Cancer Institute (R01 CA092447). Genotyping work was partially supported by a grant from the Komen for the Cure Foundation (OP05-0927-DR1). Sample preparation was conducted at the Survey and Biospecimen Shared Resources that is supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA68485).
The authors wish to thank Regina Courtney and the late Qing Wang for serum and DNA sample preparation, and Kevin M. Bradley and Joan P. Breyer for genotyping assistance, including contributing to the design of gene targets. We thank Charles Matthews for his oversight of activities related to SNP selection and genotyping, and Sarah Cohen for coordination, statistical review, and construction of genetic datasets.
References
- 1.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 2.Matsuoka LY, Wortsman J, Chen TC, Holick MF. Compensation for the interracial variance in the cutaneous synthesis of vitamin D. J Lab Clin Med. 1995;126:452–7. [PubMed] [Google Scholar]
- 3.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–9. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 4.Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: Implications for cancer disparities (United States) Cancer Causes Control. 2008;19:527–35. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 5.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. 2010;8:11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–51. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parra EJ, Kittles RA, Argyropoulos G, et al. Ancestral proportions and admixture dynamics in geographically defined African Americans living in South Carolina. Am J Phys Anthopol. 2001;114:18–29. doi: 10.1002/1096-8644(200101)114:1<18::AID-AJPA1002>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Shriver MD, Parra EJ, Dios S, et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum Genet. 2003;112:387–99. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- 9.Zakharia F, Basu A, Absher D, et al. Characterizing the admixed African ancestry of African Americans. Genome Biology. 2009;10:R141. doi: 10.1186/gb-2009-10-12-r141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Signorello LB, Hargreaves MK, Steinwandel MD, et al. Southern Community Cohort Study: Establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972–9. [PMC free article] [PubMed] [Google Scholar]
- 11.http://www.southerncommunitystudy.org
- 12.Buchowski MS, Schlundt DG, Hargreaves MK, Hankin JH, Signorello LB, Blot WJ. Development of a culturally sensitive food frequency questionnaire for use in the Southern Community Cohort Study. Cell Mol Biol. 2003;49:1295–304. [PubMed] [Google Scholar]
- 13.Hollis BW, Kamerud JQ, Selvaag SR, et al. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 14.http://www.cpc.ncep.noaa.gov/products/stratosphere/uv_index/
- 15.http://pritch.bsd.uchicago.edu/structure.html
- 16.Pritchard JK, Stephens M, Donnelly P. Inference of Population Structure Using Multilocus Genotype Data. Genetics. 2000;155:945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn J, Yu K, Stolzenberg-Solomon, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010 May 7; doi: 10.1093/hmg/ddq155. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 19.Tseng M, Giri V, Bruner DW, Giovannucci E. Prevalence and correlates of vitamin D status in African American men. BMC Public Health. 2009;9:191. doi: 10.1186/1471-2458-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–8. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L, Yun F, Oczak M, Wong BYL, Vieth R, Cole DEC. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvimtain D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42:1174–7. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Chan J, Jaceldo-Siegl K, Fraser GE. Determinants of serum 25 hydroxyvitamin D levels in a nationwide cohort of blacks and non-Hispanic whites. Cancer Causes Control. 2010;21:501–11. doi: 10.1007/s10552-009-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinotte M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hyroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009;89:634–40. doi: 10.3945/ajcn.2008.26445. [DOI] [PubMed] [Google Scholar]
- 24.Abbas S, Linseisen J, Slanger T, et al. The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast cancer risk, independent of the vitamin D status. Cancer Epidemiol Biomarkers Prev. 2008;17:1339–43. doi: 10.1158/1055-9965.EPI-08-0162. [DOI] [PubMed] [Google Scholar]
- 25.Uitterlinden AG, Fang Y, van Meurs JBJ, Pols HAP, van Leeuwen JPTM. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–56. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Lopez E, Jansen T, Ivaskevicius V, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann NY Acad Sci. 2006;1079:327–34. doi: 10.1196/annals.1375.050. [DOI] [PubMed] [Google Scholar]
- 27.Parra EJ, Kittles RA, Shriver MD. Implications of correlations between skin color and genetic ancestry for biomedical research. Nat Genet. 2004;36:S54–60. doi: 10.1038/ng1440. [DOI] [PubMed] [Google Scholar]
- 28.Ganesan AK, Ho H, Bodemann B, et al. Genome-wide siRNA-based functional genomics of pigmentation identified novel genes and pathways that impact melanogenesis in human cells. PloS Genet. 2008;4:e1000298. doi: 10.1371/journal.pgen.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009;18:R9–17. doi: 10.1093/hmg/ddp003. [DOI] [PubMed] [Google Scholar]
- 30.Dawson-Hughes B. Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr. 2004;80(suppl):1763S–6S. doi: 10.1093/ajcn/80.6.1763S. [DOI] [PubMed] [Google Scholar]
- 31.Brazerol WF, McPhee AJ, Mimouni F, Specker BL, Tsang RC. Serial ultraviolet B exposure and serum 25hydroxyvitamin D response in young adult American blacks and whites: no racial differences. J Am Coll Nutr. 1988;7:111–8. doi: 10.1080/07315724.1988.10720227. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigmentation is not an essential regulator. Science. 1981;211:590–3. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 33.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 34.Destro-Bisol G, Maviglia R, Caglia A, et al. Estimating European admixture in African Americans by using microsatellites and a microsatellite haplotype (CD4/Alu) Hum Genet. 1999;104:149–57. doi: 10.1007/s004390050928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.