SUMMARY
Apoptotic death of hepatocytes, a feature and contributing factor of many chronic and acute liver diseases, can be a consequence of over-activation of the immune system. Injection with lipopolysaccharide (LPS) plus the transcriptional inhibitor D(+)-galactosamine (GalN) or mitogenic T cell activation cause fatal hepatocyte apoptosis in mice. In both settings hepatocyte killing is mediated by TNFα/TNF-R signaling but the effector mechanisms remain unclear. Our analysis of gene-targeted mice showed that caspase-8 is essential for hepatocyte killing in both settings. Loss of Bid, the pro-apoptotic BH3-only protein activated by caspase-8, and essential for Fas ligand-induced hepatocyte killing, resulted only in a minor reduction of liver damage. However, combined loss of Bid and another BH3-only protein, Bim, activated by JNK, protected mice from LPS+GalN-induced hepatitis. These observations identify caspase-8 and the BH3-only proteins Bid and Bim as potential therapeutic targets for treatment of inflammatory liver diseases.
Keywords: caspase-8, Bid, apoptosis, TNF, hepatitis, Bim
INTRODUCTION
Abnormal apoptotic death of hepatocytes is thought to be a cause or contributing factor in many chronic as well as acute liver diseases (reviewed in (Bradham et al., 1998; Ding and Yin, 2004). TNFα, FasL and related members of the TNF cytokine family have been implicated in hepatocyte killing but the signaling pathways contributing to development and progression of these diseases are presently unclear. The molecular basis of hepatocyte destruction in hepatitis can be studied using mouse models. For example, injection of low doses of bacterial lipopolysaccharide (LPS) in the presence of the liver-specific transcriptional inhibitor D-(+)-galactosamine (GalN) or treatment with the T cell mitogen concanavalin A (ConA) cause fatal hepatocyte destruction. In both settings, TNFα is essential for hepatocyte killing and animal mortality (independently confirmed by us for both treatments, see Figure S1 available online). Secreted TNFα appears to be critical in LPS+GalN injected mice, whereas both secreted and membrane-bound TNFα contribute to hepatocyte destruction in ConA-injected animals (Grivennikov et al., 2005; Nowak et al., 2000; Pfeffer et al., 1993; Rothe et al., 1993). The mechanisms by which TNFα kills hepatocytes are still unclear. TNF-R1 has an intra-cellular ‘death domain’ and therefore belongs to the ‘death receptor’ sub-group within the TNF-R family, which also includes Fas (APO-1/CD95), DR3, and the TRAIL receptors DR4 and DR5 (Ashkenazi and Dixit, 1998). Genetic and biochemical studies, using fibroblasts from gene-targeted mice, have shown that TNF-R1 triggers apoptosis by binding the adaptor proteins TRADD and FADD, which facilitate recruitment and activation of the aspartate-specific cysteine protease, caspase-8 (Ashkenazi and Dixit, 1998). Signaling through the kinase JNK has also been implicated in TNFα-induced apoptosis signaling (Baud et al., 1999; Kamata et al., 2005; Wang et al., 2006), but the death effectors acting downstream of JNK in this process have not yet been identified. Caspase-8 can proteolytically activate the pro-apoptotic BH3-only Bcl-2 family member Bid (Li et al., 1998; Luo et al., 1998), which triggers apoptosis by activating Bax/Bak (members of the second pro-apoptotic subgroup within the Bcl-2 family), either directly or indirectly by binding to pro-survival Bcl-2 family members (e.g. Bcl-xL, Mcl-1) (Zha et al., 2000). Experiments with gene-targeted mice have shown that caspase-8 and Bid are both essential for Fas-induced apoptosis in hepatocytes, although only caspase-8 but not Bid is required for killing of T lymphoid cells (Kang et al., 2004; Kaufmann et al., 2007b; Salmena et al., 2003; Yin et al., 1999).
The roles of caspase-8 and Bid in TNFα-induced apoptosis are still unclear, although Bid deficiency was shown to afford a minor degree of protection in TNFα-treated mouse embryo fibroblasts (MEF) in vitro and in hepatocytes of mice injected with LPS+GalN (Chen et al., 2007; Zhao et al., 2001). Notably, most studies on TNFα-induced apoptosis have been performed with cells in culture and very little is known about the mechanisms by which over-activation of the immune system causes TNFα-mediated immuno-pathological tissue destruction. Our experiments with gene-targeted mice demonstrated that TNFα-mediated hepatocyte apoptosis requires caspase-8 and involves the pro-apoptotic BH3-only proteins Bid, activated by caspase-8, and Bim, activated by JNK, respectively. These cell death inducers and effectors can therefore be considered potential therapeutic targets for immuno-pathological liver disorders.
RESULTS
Caspase-8 Is Essential for LPS plus GalN-Induced Hepatocyte Destruction
Experiments with gene-targeted mice demonstrated that expression of caspase-8 within hepatocytes is essential for anti-Fas-antibody induced hepatocyte killing and fatal hepatitis (Kang et al., 2004). It is, however, not clear whether caspase-8 is also essential for pathological killing of hepatocytes by TNFα. In fact, several studies with cultured cells have indicated that TNFα kills cells by caspase-independent, perhaps even non-apoptotic mechanisms (reviewed in (Ding and Yin, 2004)). When mice lacking caspase-8 selectively in hepatocytes (caspase-8 loxP homozygotes expressing the Cre recombinase under control of the hepatocyte-specific albumin promoter) were challenged with LPS plus GalN, they showed only minor elevation of serum ALT and AST levels (Figure 1A), retained normal liver structure (Figure 1B) and all mice survived long-term (Figure 1C). In contrast, all littermate controls succumbed to this treatment within 8–10 h (Figure 1C), presenting at autopsy with abnormally elevated serum levels of ALT and AST (Figure 1A; Alb-Cre/caspase-8fl/fl vs control mice: p<0.015 for ALT, p<0.0015 for AST) and extensive disruption of liver architecture (Figure 1B). Consistent with these observations, Western blot analysis of liver extracts from LPS+GalN treated control animals revealed processing of Bid (p22) into its active p15 form tBid as well as extensive processing of caspase-3 and -7, whereas no Bid-cleavage and no activation of effector caspases could be detected in Alb-Cre/caspase-8fl/fl mice (Figure S2).
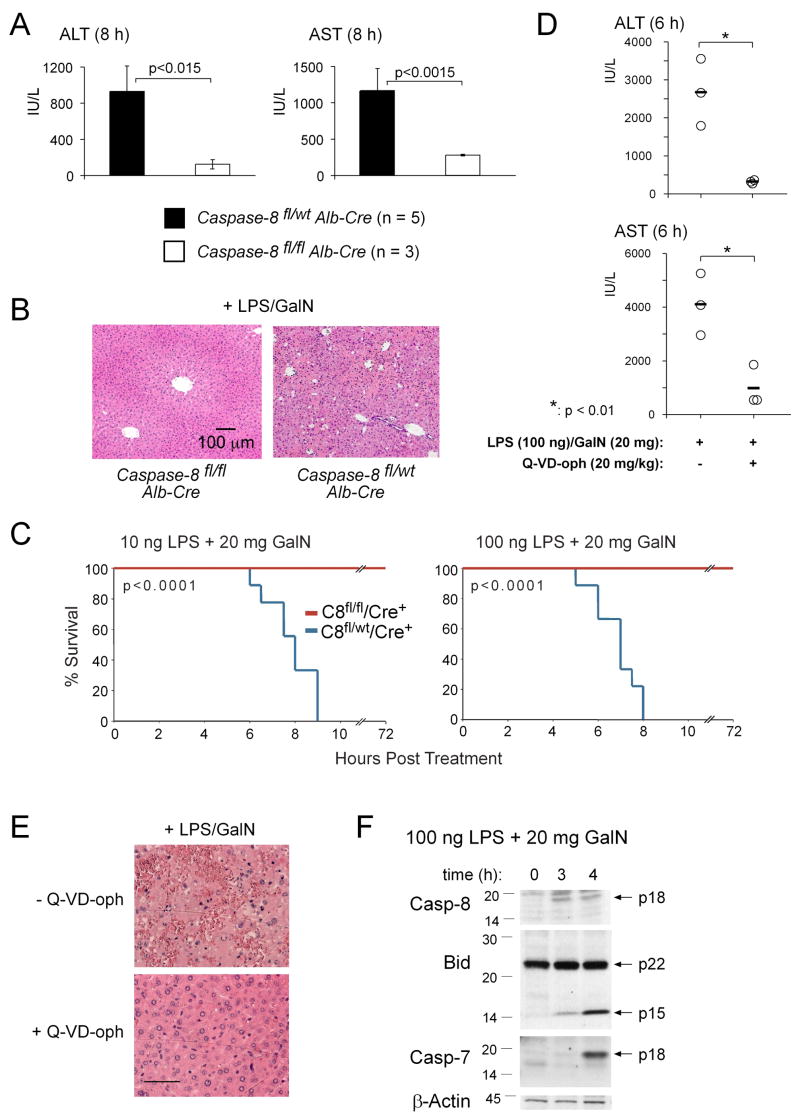
Figure 1. LPS plus GalN-Induced Hepatitis Requires the Initiator Caspase, Caspase-8, Is Inhibited by a Pan-Caspase Inhibitor and Involves Cleavage of the Pro-Apoptotic BH3-Only Bcl-2 Family Member Bid.
(A) Mice lacking caspase-8 in hepatocytes (albumin Cre transgenic caspase-8flox/flox) and littermate controls (albumin Cre transgenic caspase-8flox/wt mice) were injected with 100 ng LPS plus 20 mg GalN. At the time when the wt mice were terminally ill (~8 h), all animals were sacrificed and serum levels of the liver transaminases ALT and AST measured. Data shown represent means +/−SD of 3–5 mice for each genotype. (B) H&E stained histological liver sections from mice treated as in (A). Pictures shown are representative of the analysis of at least 3 mice for each treatment and genotype. (C) Nine mice of the indicated genotypes in each group were injected i.p. with LPS+GalN and followed over 72 h post injection. P values were calculated using a time to event analysis with a log rank test. No mortality was observed in mice of either genotype injected with 20 mg GalN alone (n = 3 per genotype). (D) Three control wt mice and 3 wt mice pre-treated with 20 mg/kg of the pan-caspase inhibitor Q-VD-oph (i.p. injection 30 min prior to treatment) were injected with 100 ng LPS plus 20 mg GalN. All animals were sacrificed after 6 h and serum levels of ALT and AST measured. (E) H&E stained histological liver sections from the same mice treated in (D). Pictures shown are representative of the analysis of 3 mice for each treatment. (F) Mice (wt) were injected with 100 ng LPS plus 20 mg GalN and sacrificed after the indicated time points. Processing of caspase-8, Bid and caspase-7 was examined by Western blot analysis. Probing with a monoclonal antibody to β-actin served as a loading control.
Consistent with the experiments using mice lacking caspase-8 in their hepatocytes, treatment of C57BL/6 (wt) mice with the pan-caspase inhibitor Q-VD-oph resulted in a highly significant protection from LPS+GalN induced hepatitis, as assessed by serum levels of ALT/AST and histological examination (Figures 1D, 1E and S3A). However, administration of Q-VD-oph, even at multiple dosages, afforded less protection than loss of caspase-8 (compare Figures S3B and 1C), presumably because this treatment did not achieve complete blockade of this enzyme. Similar to Fas-activation (Li et al., 1998; Luo et al., 1998), injection of wt mice with LPS+GalN caused rapid processing of pro-caspase-8 to produce the active p18 fragment, cleavage of Bid (p22) into its active truncated p15 form (tBid) as well as processing and activation of effector caspases, such as caspase-7 (p17) (Figure 1F). No processing of caspase-8, Bid or effector caspases was seen in liver extracts from LPS+GalN injected mice lacking TNFα (Figure S4). Collectively, these results demonstrate that upon LPS+GalN injection, activation of caspase-8 within hepatocytes is required for TNFα-mediated liver destruction and fatal hepatitis.
Bid Is a Minor Contributor to LPS plus GalN-Induced Hepatocyte Apoptosis
Caspase-8-mediated activation of Bid is essential for anti-Fas antibody induced liver destruction (Yin et al., 1999). We confirmed this observation (Kaufmann et al., 2007b) and found that bid−/− mice are also resistant to FasL (Figure S5). This is an important finding because anti-Fas antibodies do not always mimic the physiological ligand, FasL (Huang et al., 1999).
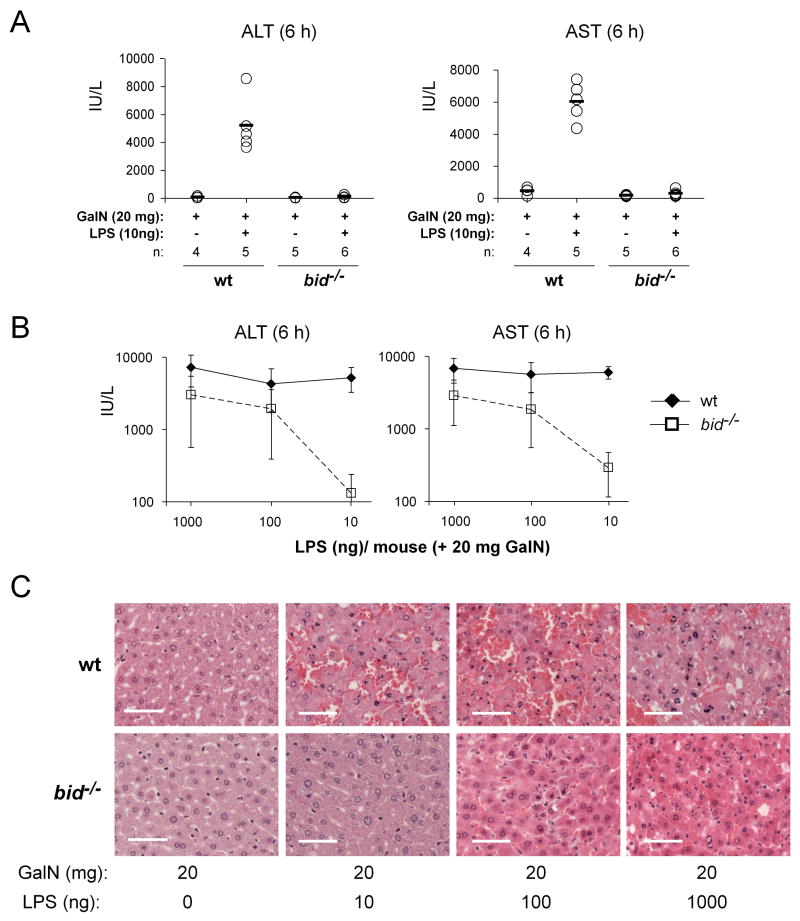
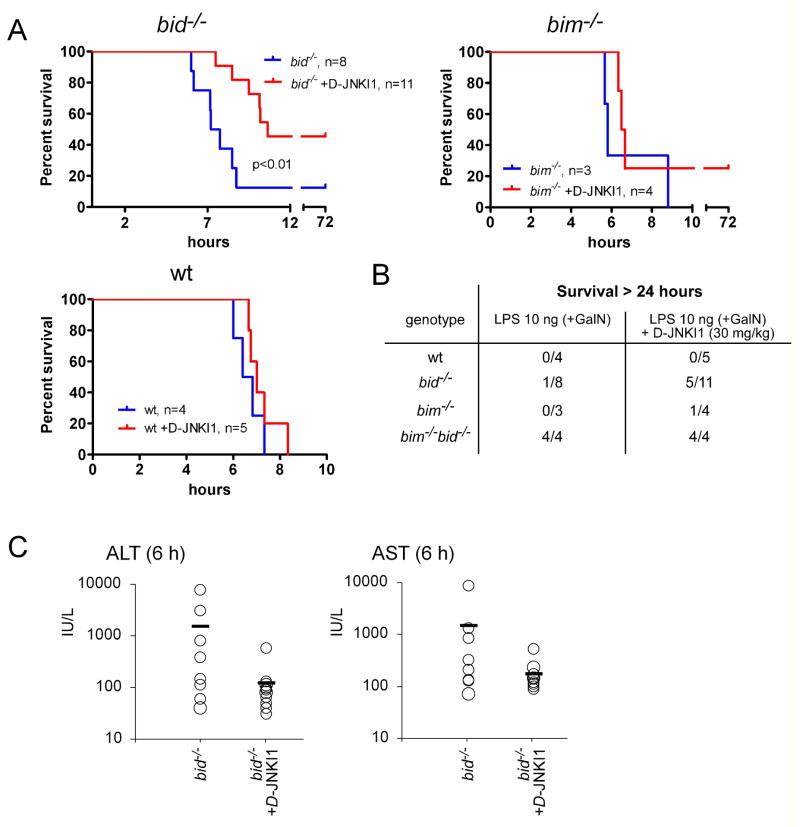
Although Bid-cleavage is readily detectable in the livers of LPS+GalN-treated animals (Figure 1F), recently published data indicate that, in contrast to Fas-signaling (Kaufmann et al., 2007a; Yin et al., 1999), TNFα>TNF-R1-induced hepatocyte apoptosis may not be entirely dependent on Bid (Chen et al., 2007). In order to evaluate the role of Bid in the in vivo killing of hepatocytes by TNFα, wt and bid−/− mice were challenged with various doses of LPS plus GalN. Upon injection of very low doses of LPS (10 ng) plus GalN, bid−/− mice were clearly less severely affected than wt animals. All wt mice succumbed within 6–8 h of treatment, whereas all bid−/− mice remained alive and healthy at that time point, presenting with nearly normal ALT and AST serum levels (Figure 2A; bid−/− vs. wt: p<0.0002 for ALT, p<0.0001 for AST) and only minor liver destruction (Figure 2C). However, only 3 out of 7 bid−/− mice survived this treatment for 5 days (experiment stopped thereafter); the other 4 became terminally ill within 24 h (Figure 3C). Moreover, when the dose of LPS was increased to 100 or 1000 ng, not only the wt but also all bid−/− animals succumbed to this treatment within 12 h, although the serum levels of ALT and AST remained significantly lower in the bid−/− mice compared to the wt littermates (Figure 2B; p<0.02 for ALT and p<0.002 for AST). Consistent with the measurements of the serum levels of ALT and AST, histological examination of liver sections taken after 6 h showed extensive hepatocyte destruction in bid−/− mice (albeit slightly less than in wt controls) after injection of 100 or 1000 ng LPS plus GalN (Figure 2C). These results demonstrate that Bid contributes to LPS+GalN induced hepatocyte apoptosis, but its loss can only delay but not prevent the fatal outcome of this TNFα-mediated process.
Figure 2. Bid Contributes to LPS plus GalN-Induced Hepatitis.
(A) Bid-deficient mice and wt littermate controls were injected i.p. with 10 ng of LPS plus GalN (20 mg per mouse) or with GalN alone as control. Mice were sacrificed after 6 h, bled and sera analyzed for the liver transaminases ALT and AST. (B) Bid-deficient mice and wt littermate controls were injected i.p. with increasing doses (10, 100 and 1000 ng) of LPS, all in the presence of 20 mg GalN, and analyzed after 6 h as in (A). Data are presented as means +/−SD. (C) Histological examination of H&E stained liver sections of bid−/− and wt mice subjected to the treatments indicated (scale bars = 50μm). Pictures shown are representative of the analysis of at least 3 mice for each treatment and genotype.
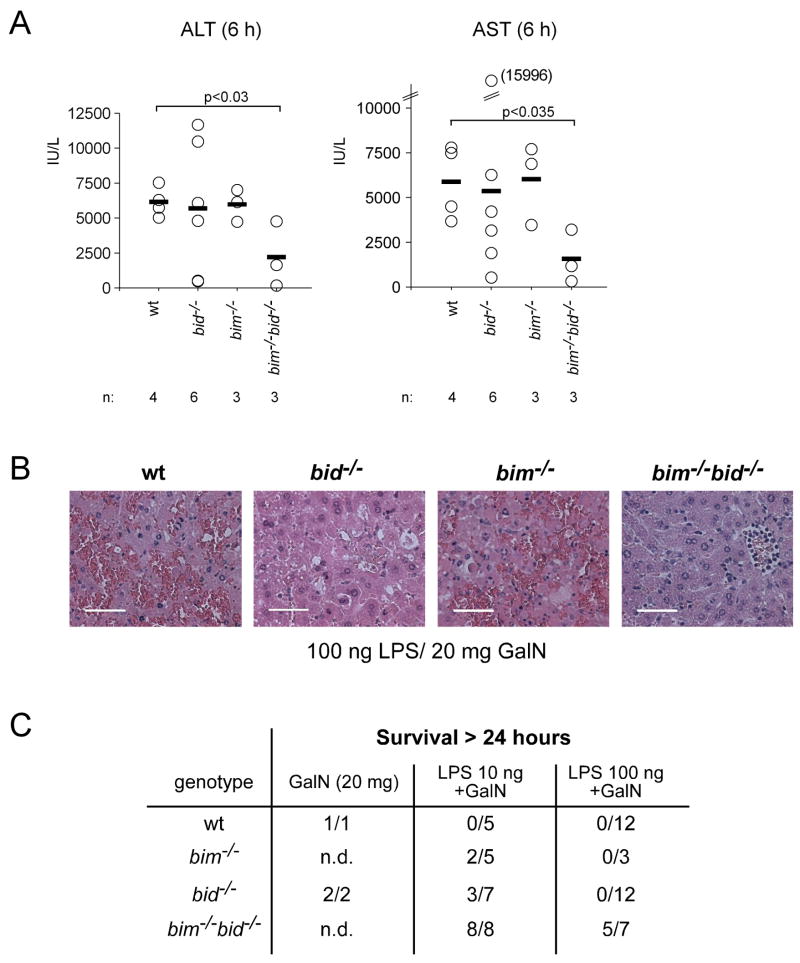
Figure 3. Loss of Bim Cooperates with Loss of Bid to Protect Mice from LPS plus GalN-Induced Hepatitis.
(A) Mice lacking both Bid and Bim (bim−/− bid−/−) and control animals (wt, bid−/− or bim−/−) were injected with 100 ng LPS plus 20 mg GalN. At the time when the wt mice were sick (6 h), all animals were sacrificed and serum levels of ALT and AST measured. Data shown represent means +/−SD of 3–6 mice for each genotype and each treatment. (B) Histological examination of H&E stained liver sections of wt, bid−/−, bim−/−, and bim−/− bid−/− mice injected 6 h earlier with 100 ng LPS plus 20 mg GalN (scale bars = 50 μm). Pictures shown are representative of the analysis of at least 3 mice for each treatment and genotype. (C) Table summarizing the long-term (followed up to 5 days) survival of the indicated genotypes of mice treated with 10 ng or 100 ng LPS plus 20 mg GalN. Mice that did not survive this treatment all succumbed within 24 h.
Combined Loss of Bid and Bim Potently Protects Mice from LPS plus GalN-Induced Fatal Hepatitis
Not only Bid (Li et al., 1998; Luo et al., 1998), but also the related BH3-only protein Bim was reported to be activated by caspase-mediated proteolysis (Chen and Zhou, 2004). Therefore, and because Bim plays a major role in apoptosis in many cell types (Bouillet et al., 1999; Huang and Strasser, 2000) and binds avidly to all pro-survival Bcl-2 family members (Chen et al., 2005; Kuwana et al., 2005), we examined whether Bid and Bim cooperate in TNFα-mediated hepatocyte killing in vivo. Interestingly, bim−/− mice were less sensitive than control (wt) animals to injection with 10 ng LPS+GalN (2/5 surviving long-term), although no protection was evident when they were challenged with 100 ng LPS+GalN (Figure 3C). Remarkably, bim−/− bid−/− mice were significantly more resistant than wt, bim−/− or bid−/− mice to both 10 as well as 100 ng LPS+GalN (Figure 3). At the time when all wt, bid−/− and bim−/− mice were ill, serum ALT and AST levels of bim−/− bid−/− mice were significantly lower than those found in the control animals (Figure 3A; bim−/− bid−/− vs. wt mice, p<0.03 for ALT, p<0.035 for AST). Histological analysis confirmed reduced liver destruction in bim−/− bid−/− mice compared to all control animals (Figure 3B). Furthermore, 8 out of 8 bim−/− bid−/− mice did not get sick when treated with 10 ng LPS+GalN and survived long-term (followed up to 5 days); a dose at which all wt mice succumbed within 6–8 h and only 3 out of 7 bid−/− mice and 2 out of 5 bim−/− mice survived longer than 24 h (Figure 3C). Remarkably, even at 100 ng LPS+GalN, a dose at which all wt (12/12), all bid−/− (12/12) and all bim−/− (3/3) mice succumbed within 12 h, 5 out of 7 bim−/− bid−/− mice survived long-term (followed up to 5 days), even though some of these surviving mice went through phases of sickness during the first 24 h of treatment (Figure 3C and Figure S6). Consistent with the finding that Bid and Bim have overlapping functions in LPS+GalN-induced hepatocyte killing, Western blot analysis (Figure S7A) and fluorogenic enzyme activity assays (Figure S7B) showed that loss of Bid and to a lesser extent loss of Bim both diminished activation of effector caspases and that loss of both of these BH3-only proteins caused significantly greater reduction, particularly at later time points. Synergy between loss of Bid and loss of Bim appears to be specific to LPS+GalN-induced (TNF-mediated) hepatocyte killing, since fibroblasts and T lymphocytes from bim−/− bid−/− mice were either normally sensitive or no more resistant than bim−/− cells to a diverse range of apoptotic stimuli ((Willis et al., 2007) and TK, PB and AS, unpublished observations). These results demonstrate that the two pro-apoptotic BH3-only proteins Bid and Bim cooperate in TNFα-mediated hepatocyte apoptosis induced by treatment with LPS+GalN.
Mechanisms of Activation of Bid and Bim during LPS plus GalN-Induced Hepatitis
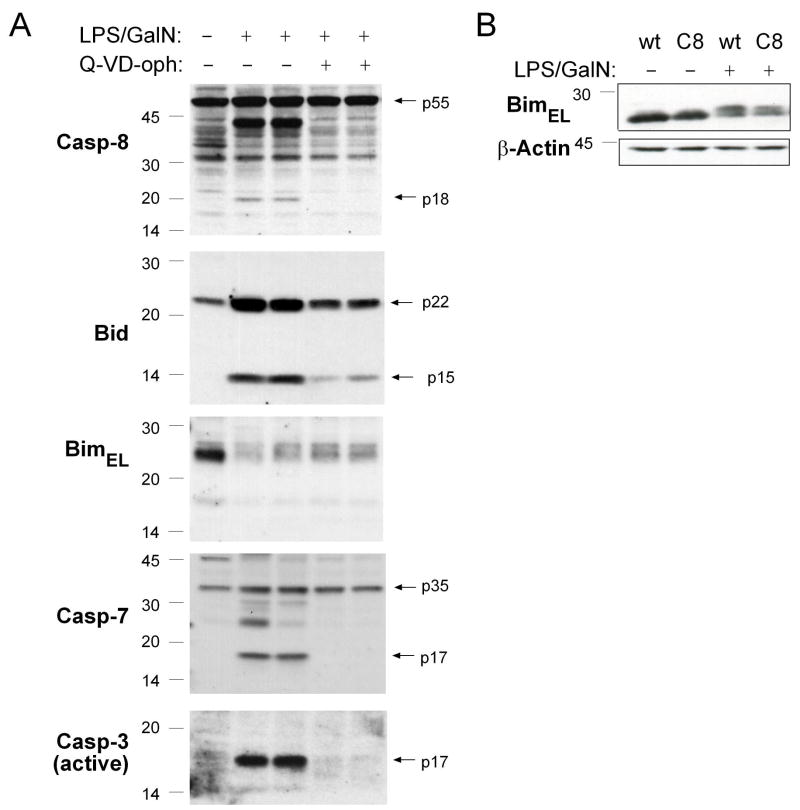
Since Bid and Bim play critical overlapping roles in TNFα-induced apoptosis, we investigated how they are activated in hepatocytes from animals injected with LPS+GalN. This treatment caused rapid processing of Bid into the potently pro-apoptotic truncated tBid form (Figures 1F and 4A). Loss of caspase-8 or pre-treatment of wt mice with Q-VD-oph, which affords substantial protection from LPS plus GalN induced hepatitis (Figures 1D, 1E and S3), significantly decreased the production of tBid in LPS+GalN injected mice and this correlated with a lack of pro-caspase-8 processing and a complete lack of effector caspase activation (Figures 4A and S2).
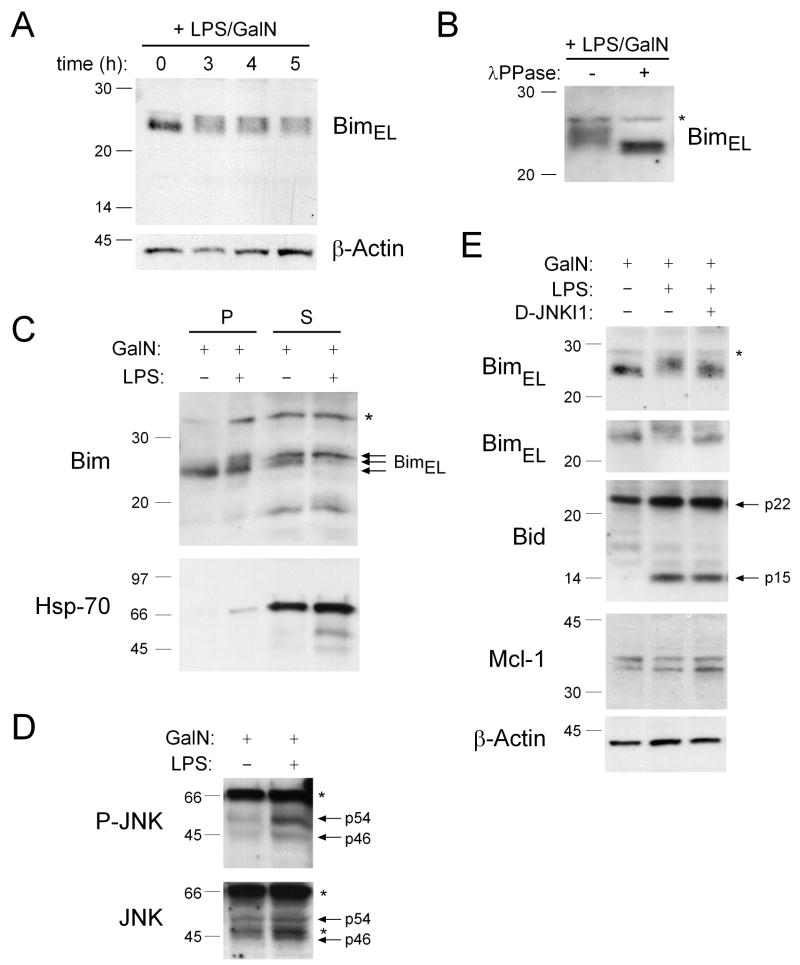
Figure 4. Treatment with LPS plus GalN Causes a Post-Translational Modification of Bim that Does not Require Caspase-8 or other Caspases.
(A) Mice (wt), with or without pre-treatment with 20 mg/kg of the pan-caspase inhibitor Q-VD-oph, were injected with LPS (100 ng) plus GalN (20 mg) and sacrificed 4 h later. Total protein extracts from the livers of these animals were probed by Western blotting for Bim, Caspase-8, Bid, Caspase-7 and active Caspase-3. (B) Mice lacking caspase-8 in hepatocytes (C8: albumin Cre transgenic caspase-8flox/flox) and littermate controls (albumin Cre transgenic caspase-8flox/wt) were injected with 100 ng LPS plus 20 mg GalN and sacrificed after 4 h. Total protein extracts derived from the livers of these animals were probed by Western blotting for Bim or β-actin (loading control).
Although caspase-mediated activation of BimEL (the most abundantly expressed isoform of Bim in the liver and other tissues (O’Reilly et al., 2000)) has been reported (Chen and Zhou, 2004), we found no evidence for BimEL cleavage in livers of LPS+GalN-injected mice (Figures 4A and 5A). JNK-mediated phosphorylation has also been implicated in the activation of Bim (Lei and Davis, 2003). Notably, a mobility shift in BimEL in liver extracts was observed soon after injection with LPS+GalN (Figures 4A, 5A and S4). Treatment of these extracts with λ-phosphatase demonstrated that the modification of Bim was due to a change in phosphorylation (Figure 5B) and examination of livers from LPS+GalN injected tnfα−/− mice showed that it was TNFα dependent (Figure S4). The LPS+GalN-induced phosphorylation of Bim does not require caspase activity, as the shift in Bim mobility on SDS-PAGE was not affected by prior administration of the pan-caspase inhibitor Q-VD-oph (Figure 4A) or loss of caspase-8 in hepatocytes (Figure 4B). The pro-apoptotic activity of Bim can be regulated by its sequestration to microtubules by binding to the dynein motor complex (Puthalakath et al., 1999) and JNK-mediated phosphorylation was reported to cause release and activation of Bim in response to certain stress stimuli (Lei and Davis, 2003; Putcha et al., 2003). We therefore performed sub-cellular fractionation to examine whether the phosphorylation of Bim seen after LPS+GalN injection affected its sequestration to the microtubular-associated dynein motor complex. Western blot analysis of the microtubule-enriched pellet and soluble fractions revealed that the LPS+GalN treatment-induced phosphorylated form of Bim was released from the microtubular dynein motor complex into the cytosol whereas the unmodified form of Bim was preferentially detected in the dynein motor complex enriched fraction (Figure 5C). Western blot analysis also revealed an increase of phopshorylated (i.e. activated) JNK in livers of LPS+GalN-injected mice (Figure 5D) and the mobility shift in Bim elicited by this treatment was prevented by prior administration of the synthetic JNK-inhibitory D-peptide, D-JNKI1 (Figure 5E). In contrast, D-JNKI1 injection did not affect processing of Bid to tBid (Figure 5E), demonstrating that this inhibitor did not interfere with the LPS+GalN-induced production of TNFα or activation of caspase-8. Importantly, injection of this JNK inhibitor reduced LPS+GalN induced hepatocyte destruction in bid−/− mice but not in those lacking Bim (Figures 6A, B and C), consistent with the notion that JNK specifically regulates the pro-apoptotic activity of Bim, which cooperates with Bid in hepatocyte killing. Collectively, these results show that caspase-8-dependent activation of Bid plus JNK-induced activation of Bim cooperate in TNFα-mediated hepatocyte destruction in LPS+GalN injected mice.
Figure 5. Bim Is Phosphorylated by JNK Kinase in the Livers of Mice Injected with LPS plus GalN.
(A) Mice (wt) were injected with LPS (100 ng) plus GalN (20 mg) and sacrificed at the time points indicated. Levels and post-translational modifications of Bim were investigated in liver-derived total protein extracts by Western blotting. Probing with an antibody to β-actin was used as a loading control. (B) Mice (wt) were injected with LPS (100 ng) plus GalN (20 mg) and sacrificed after 3 h. Total protein extracts were prepared from the liver in the absence of phosphatase inhibitors and left untreated or treated in vitro with λ-phosphatase prior to analyzing Bim modifications by Western blotting. (C) Mice were treated with GalN (20 mg) alone (controls) or with LPS (100 ng) plus GalN (20 mg) and livers harvested after 4 h. Protein extracts were prepared and then subjected to subcellular fractionation into the dynein enriched pellet fraction (P), containing BimEL/L sequestered to microtubules, and a soluble fraction (S) containing free cytosolic Bim. Fractions were analyzed by Western blotting using antibodies to Bim or HSP70 (control for the purity of the fractions). (D) Liver extracts from wt mice treated with 100 ng LPS plus 20 mg GalN for 4 h were probed by Western blotting with an antibody specific for phosphorylated (activated) JNK and the membrane re-probed with an antibody to total JNK (loading control). (E) Wt mice that had either been left untreated or pre-treated for 30 min with the JNK inhibitory peptide D-JNKI1 (20 mg/kg, i.p.) were treated for 4 h with either 20 mg GalN or with 100 ng LPS plus 20 mg GalN. Total liver extracts were probed by Western blotting with antibodies to Bim (two blots from independent experiments shown), Bid, Mcl-1 and β-actin (loading control). Asterix indicate non-specific bands.
Figure 6. Inhibition of JNK Enhances Resistance of Bid−/− Mice to LPS plus GalN-Induced Hepatocyte Destruction.
(A) Bid−/−, bim−/− or wt mice were injected with JNK inhibitory peptide D-JNKI1 (30 mg/kg, i.p.) 30 min prior to treatment with 10 ng LPS plus GalN and were then monitored for survival for up to 72 h. Mice injected with LPS+GalN served as controls. P values were calculated using a time to event analysis using a log-rank test. (B) Table summarizing the long-term (followed up to 3 days) survival of wt, bid−/−, bim−/− and bim−/− bid−/− mice treated with 10 ng LPS+GalN, with or without pre-treatment with D-JNKI1. Mice that did not survive, all succumbed to the treatment within 24 h. (C) Serum levels of ALT and AST in bid−/− mice treated with 10 ng LPS plus 20 mg GalN, with or without pre-treatment with D-JNKI1 (30 mg/kg), were measured after 6 h. P-values: wt vs. bid−/−: p (ALT) = 0.09; p (AST) = 0.16. Data shown represent means +/−SD of 8–11 mice for each genotype and are derived from three independent experiments.
Concanavalin A-Induced Hepatitis Requires Caspase-8 and Involves JNK-Mediated Activation of Bim
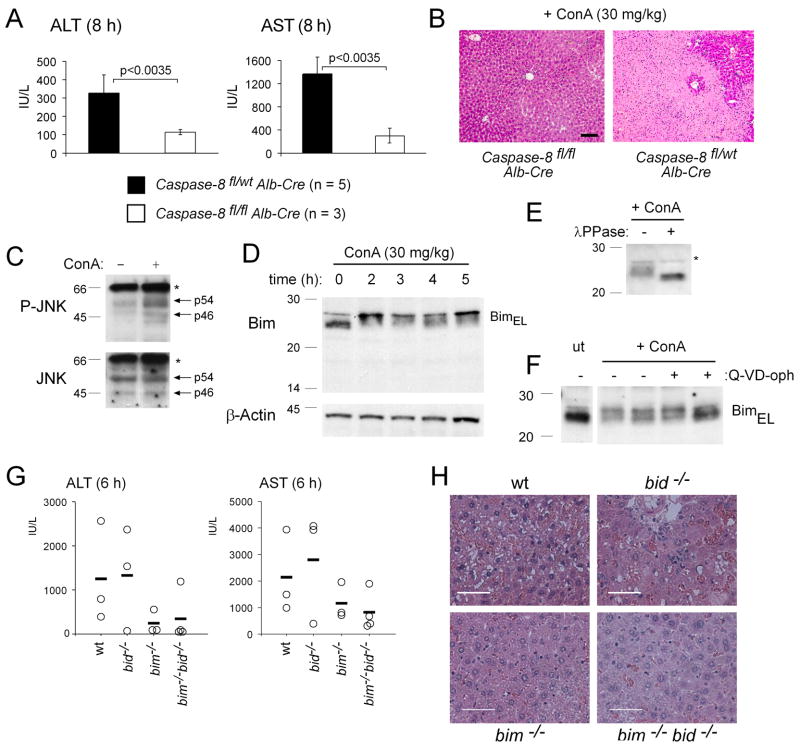
We next tested whether caspase-8 and the BH3-only proteins Bid and Bim are also critical for hepatocyte killing and hepatitis induced in different pathological settings. Injection of ConA into mice causes systemic activation of T lymphocytes resulting in fatal hepatitis that is mediated by TNFα (Kusters et al., 1997; Trautwein et al., 1998). When mice lacking caspase-8 selectively in hepatocytes were challenged with ConA, they survived this treatment and showed only minor elevation of serum ALT and AST levels (Figure 7A) and retained normal liver structure (Figure 7B). In contrast, all littermate controls succumbed to this treatment within 6–8 h, presenting at autopsy with abnormally elevated serum levels of ALT and AST (Figure 7A; caspase-8 deficient vs control mice: p<0.0035 for both ALT and AST) and extensive disruption of liver architecture (Figure 7B).
Figure 7. Caspase-8 is Essential and Bim a Contributor in ConA-Induced Hepatitis.
(A) Mice lacking caspase-8 in hepatocytes (albumin Cre transgenic caspase-8flox/flox) and littermate controls (albumin Cre transgenic caspase-8flox/wt mice) were injected with 30 mg/kg ConA. At the time when the wt mice were sick (8 h), all animals were sacrificed and serum levels of ALT and AST measured. Data shown represent means +/−SD of 3–5 mice for each genotype and each treatment. (B) H&E stained histological liver sections from mice treated as in (A). Pictures shown are representative of the analysis of at least 3 mice for each treatment and genotype. (C) Liver extracts from wt mice treated with 30 mg/kg ConA for 4 h were probed by Western blotting with an antibody specific for phosphorylated (activated) JNK and the membrane re-probed with an antibody to total JNK (loading control). (D) Mice (wt) were injected with ConA (30 mg/kg) and sacrificed at the time points indicated. Bim levels and possible post-translational modifications were analyzed by Western blotting of liver extracts as described in Figure 5A. (E) Mice (wt) were injected with 30 mg/kg ConA and sacrificed after 3 h. Total protein extracts were prepared from the liver in the absence of phosphatase inhibitors and left untreated or treated in vitro with λ-phosphatase prior to analyzing Bim modifications by Western blotting. (F) Mice (wt), with or without pre-treatment with 20 mg/kg of the pan-caspase inhibitor Q-VD-oph, were injected with ConA (30 mg/kg) and sacrificed 5 h later. Total protein extracts from the livers of these animals were probed by Western blotting for Bim. (G) Mice lacking both Bid and Bim (bim−/− bid−/−) and control animals (wt, bid−/− or bim−/−) were injected with 30 mg/kg ConA. At the time when the wt mice were sick (6 h), all animals were sacrificed and serum levels of ALT and AST measured. Data shown represent means +/−SD (n=3 for wt, bid−/−, bim−/− and n=4 for bid−/− bim−/− mice). (H) Histological examination of H&E stained liver sections of mice of the indicated genotypes subjected for 6 h to treatment with 30 mg/kg ConA (bars = 50 μm). Pictures shown are representative of the analysis of at least 3 mice for each genotype.
In contrast to treatment with LPS+GalN, loss of Bid did not confer significant protection against ConA-induced hepatocyte killing. However, similarly to LPS+GalN injection, we observed rapid increases of phosphorylated (i.e. activated) JNK and phosphorylated Bim in livers of mice challenged with ConA (Figures 7C, D and E). This modification in Bim could not be blocked by pre-administration of the pan-caspase inhibitor Q-VD-oph (Figure 7F) or specific loss of caspase-8 in hepatocytes (data not shown). Although mice lacking Bim or both Bid and Bim had lower serum levels of ALT and AST compared to ConA-injected wt or bid−/− mice, these enzymes were still abnormally elevated (Figure 7G). Futhermore, disruption of liver architecture in bim−/− and bim−/− bid−/− mice after six hours of treatment, although less severe compared to wt or bid−/− mice (Figure 7H), eventually led to the death of all bim−/− as well as bim−/− bid−/− mice. Collectively, these results show that caspase-8 is essential and Bim a contributor in ConA-induced hepatitis, another widely used model of TNFα-mediated liver destruction.
DISCUSSION
Over-stimulation of the immune system plays a major role in immuno-pathology of the liver as well as certain other tissues and this is mediated to a large extent through the pro-apoptotic activity of TNFα (Bradham et al., 1998; Ding and Yin, 2004). The mechanisms by which TNFα kills cells under these conditions remain unclear. Using the widely employed LPS+GalN as well as the ConA injection models of fatal hepatitis, we defined an essential role for caspase-8 and important contributory roles for the two pro-apoptotic BH3-only Bcl-2 family members Bid and Bim in TNFα-induced tissue destruction. When higher levels of LPS (1000 ng) were administered or with ConA injection, BH3-only proteins were no longer limiting for fatal hepatitis. In contrast, in another model of ‘death receptor’ induced fatal hepatitis, mediated by Fas activation, Bid is essential for hepatocyte apoptosis (Kaufmann et al., 2007b; Yin et al., 1999). What could be the reasons for these differences? We speculate that differences in the requirement for Bid, Bim and possibly additional BH3-only proteins for hepatocyte destruction induced by different death receptor signals may arise from differences in the levels of caspase-8 activity. Higher levels of caspase-8 activity would be expected to cause more direct effector caspase activation and may also increase apoptosis signaling through direct cleavage of certain vital targets, thereby rendering this pathway to hepatocyte apoptosis less dependent on Bid and other BH3-only proteins (Figure S8). It appears likely that the reduction in the caspase-8 inhibitor c-FLIP (Figure S9A), probably caused by GalN-mediated blockade of its transcriptional inducer NF-κB (Geisler et al., 2007), contributes to the finding that caspase-8 can suffice for LPS+GalN-induced (TNFα-mediated) hepatocyte killing even in the absence of Bid (and Bim). It cannot be excluded that additional differences between TNFα-versus FasL-induced hepatocyte killing may arise from the ability of TNF-R1 and TNF-R2 to trigger pro-apoptotic pathways in addition to caspase-8 activation that are not activated by Fas (Figure S8).
Our genetic studies show that Bim and Bid can both contribute to TNFα-induced apoptosis signaling. The BH3-only proteins Bim and Bid initiate apoptosis signaling through activation of caspase-9 and consequent activation of effector caspases by activating Bax/Bak, either directly and/or indirectly by blocking the pro-survival Bcl-2 family members that keep Bax/Bak in check (Youle and Strasser, 2008). In response to TNFα, Bid is activated by caspase-8-mediated proteolysis (Li et al., 1998; Luo et al., 1998). Although caspase-mediated activation of BimEL has been reported (Chen and Zhou, 2004), we found no evidence for BimEL cleavage in livers of LPS+GalN injected mice. Caspase-8 might theoretically contribute to Bim activation indirectly, such as by cleaving a protein that controls its sequestration to microtubules, a critical mode of Bim regulation (Puthalakath et al., 1999), but we found no evidence for this (data not shown). Bim does, however, not necessarily have to be activated by caspase-8 to contribute to TNFα-induced apoptosis signaling. Any TNFα-induced signal that activates Bim (see below) would be expected to promote activation of caspase-9 and effector caspases (either on its own or with tBid), because Bim is a potent activator of Bax/Bak (Figure S8; (Youle and Strasser, 2008)).
JNK has been implicated in TNFα-induced apoptosis signaling and JNK-mediated phosphorylation has been implicated in the activation of the pro-apoptotic BH3-only protein Bim by triggering its release from sequestration to the microtubular dynein motor complex (Lei and Davis, 2003; Putcha et al., 2003). Remarkably, a mobility shift in BimEL, which was prevented by addition of a highly specific JNK inhibitor, and a redistribution of the post-translationally modified Bim from microtubules to the cytosol were observed in livers from mice injected with LPS+GalN or ConA. Importantly, injection of the JNK inhibitor reduced LPS+GalN induced hepatocyte killing in bid−/− mice but not in bim−/− mice, which lack the pro-apoptotic effector activated by JNK. We therefore conclude that JNK contributes to TNF-R1-induced apoptosis signaling in hepatocytes of LPS+GalN injected mice by unleashing the pro-apoptotic activity of Bim. ConA injection causes hepatocyte killing through both secreted as well as membrane-bound TNFα and therefore involves both TNF-R1 and TNF-R2, whereas LPS+GalN acts mainly through secreted TNFα, which signals only through TNF-R1 (Grivennikov et al., 2005; Nowak et al., 2000; Pfeffer et al., 1993; Rothe et al., 1993). The fact that membrane-bound TNFα can activate JNK not only through TNF-R1 but also TNF-R2 may therefore explain why Bim is more important than Bid in ConA-induced hepatocyte killing (Figure S8).
In contrast to caspase-8 deficiency, combined loss of Bid and Bim did not afford complete protection against LPS+GalN- or ConA-induced hepatocyte destruction. Some bim−/− bid−/− mice succumbed when injected with higher doses of LPS (e.g. 100 ng) plus GalN, and even some of the long-term survivors went through phases of sickness. Moreover, all bim−/− and bim−/− bid−/− mice injected with ConA got sick and had to be sacrificed, although they exhibited less liver damage than wt or bid−/− animals. This may indicate that pro-apoptotic proteins in addition to Bid and Bim (perhaps other BH3-only proteins) contribute to LPS+GalN- and ConA-induced fatal hepatitis. Theoretically, TNFα might also promote apoptosis by causing a reduction in the levels of pro-survival Bcl-2 family members, but we found no significant changes in Mcl-1 or Bcl-xL (the most highly expressed ones in hepatocytes), in animals treated with LPS+GalN or ConA (Figures S2, S7, S9B and not shown). As discussed above, we prefer the hypothesis that with increased caspase-8 activation (achieved by increased TNFα-signaling due to increased levels of LPS or injection of ConA), leading to increased direct activation of effector caspases and cleavage of vital proteins, hepatocyte killing may become independent of the BH3-only protein>Bax/Bak>Apaf-1/Caspase-9 amplification mechanism for effector caspase activation (Figure S8). It is noteworthy that it has been speculated that at high doses of LPS, other TNFα-mediated or perhaps even TNFα-independent non-apoptotic death pathways might also be activated in hepatocytes (Kamata et al., 2005). Our experiments with mice lacking caspase-8 in hepatocytes imply, however, that such processes would have to be caspase-8 dependent.
In conclusion, our results show that caspase-8 is essential and the BH3-only proteins Bid and Bim are critical contributors in the pathological killing of hepatocytes that is caused by TNFα in vivo. These observations may have implications for diseases characterized by hepato-cellular destruction, indicating that they might respond to treatment with enzymatic inhibitors of caspase-8 or compounds that can antagonize both Bid and Bim.
EXPERIMENTAL PROCEDURES
Mice
The generation and genotyping of Bid-deficient mice on an inbred C57BL/6 background has been described (Kaufmann et al., 2007b). Bim−/− mice (Bouillet et al., 1999) were originally generated on a mixed C57BL/6x129SV background by homologous recombination in 129SV-derived ES cells but have been backcrossed for >10 generations onto the C57BL/6 background. The bim−/− bid−/− mice were generated by serially intercrossing the two parental strains. Mice lacking caspase-8 selectively in hepatocytes were generated by crossing mice with a loxP targeted caspase-8 gene, generated on a mixed C57BL/6x129SV background and backcrossed with C57BL/6 mice for 3 generations (Salmena et al., 2003) with transgenic mice expressing the Cre recombinase under control of the hepatocyte-specific albumin promoter (backcrossed onto C57BL/6 background for 6 generations). TRAIL−/− (Cretney et al., 2002) and bim−/− mice (Bouillet et al., 1999) were generated by homologous recombination in 129SV-derived ES cells and have been backcrossed for >10 generations onto the C57BL/6 background. TNF−/− mice (generated using C57BL/6 ES cells) (Korner et al., 1997) were obtained from Dr. Heinrich Korner, the Centenary Institute of Cancer Medicine and Cell Biology, Sydney, Australia. C57BL/6 pfp−/− mice (Kägi et al., 1994) were generated using C57BL/6 ES cells. C57BL/6 GrzABM−/− mice were established by intercrossing C57BL/6 GrzM−/− mice with C57BL/6 GrzAB−/− mice (Pao et al., 2005) (both strains generated using 129Sv ES cells and backcrossed to C57BL/6 for >12 and 8 generations, respectively). All experiments with mice were performed according to the guidelines of the animal ethics committees of the Melbourne Health Research Directorate or the Ontario Cancer Institute.
In vivo Models of Hepatitis
For Fas-mediated hepatitis, mice were injected intravenously (i.v.) with 0.25 μg/g body weight recombinant soluble Fas ligand (FLAG® tagged, Apotech) that had been crosslinked with 2 μg anti-FLAG® antibody (M2, SIGMA) per μg of FasL. For the LPS+GalN model, mice were injected intra-peritoneally (i.p.) with 10, 100 or 1000 ng LPS (DIFCO) in the presence of 20 mg of the liver-specific transcriptional inhibitor D(+)-galactosamine (GalN, SIGMA). For T cell activation mediated hepatitis, mice were injected i.v. with 30 mg/kg body weight of ConA (SIGMA). For biochemical studies, at the time when wt mice succumbed to these treatments, all mice of an experimental group were sacrificed, bled (for serum analysis of liver enzymes) and the livers removed for histological analysis. For survival analysis, mice were treated as indicated and monitored until becoming terminally ill. Statistical analyses were performed by applying a two-tailed unpaired t test. The pan-caspase inhibitor Q-VD-oph (MP Biomedicals) was administered i.p. at 20 mg/kg, 30 min prior to LPS+GalN injection. The D-JNKI1 inhibitory peptide (H-Gly-D-Arg-D-Lys-D-Lys-D-Arg-D-Arg-D-Gln-D-Arg-D-Arg-D-Arg-D-Pro-D-Pro-D-Arg-D-Pro-D-Lys-D-Arg-D-Pro-D-Thr-D-Thr-D-Leu-D-Asn-D-Leu-D-Phe-D-Pro-D-Gln-D-Val-D-Pro-D-Arg-D-Ser-D-Gln-D-Asp-D-Thr-NH2) (GL Biochem Shanghai Ltd) was administered i.p. at 30 mg/kg, 30 min prior to LPS/GalN injection.
Western Blotting and Caspase Activity Assays
Mouse livers were surgically removed and cell suspensions prepared by passing them through a stainless steel sieve. Red blood cells were lysed in a hypotonic buffer and hepatocyte lysates prepared in a buffer containing 20 mM Tris/HCl pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1% Triton X-100, 10% glycerol, 500 μg/mL Pefabloc (AEBSF), 1 μg/mL each of Leupeptin, Aprotinin and Pepstatin, 100 μg/mL soybean trypsin inhibitor and 2 μg/mL E64. Proteins (40 μg) in cell lysates were size-separated on pre-cast 12% SDS PAGE gradient gels (Invitrogen). Membranes were probed with a rat anti-Bid monoclonal antibody (clone 2D1, Alexis; (Kaufmann et al., 2007b)), a polyclonal rabbit anti-Bim antibody (Stressgen), a rat anti-mouse caspase-8 monoclonal antibody (clone 1G12, Alexis; (O’Reilly et al., 2004)), a rabbit polyclonal antibody to active caspase-3 (Cell Signaling), a mouse monoclonal antibody to caspase-7 (gift from Y. Lazebnik), a rat monoclonal antibody to c-FLIP (clone Dave-2, Alexis), a rabbit polyclonal antibody to Mcl-1 (Rockland), a mouse monoclonal antibody to phospho-JNK or a rabbit polyclonal antibody to JNK (both from Cell Signaling). Probing with a mouse monoclonal antibody to β-actin (SIGMA, AC-40) served as a loading control.
A fluorogenic assay for effector caspase activity in liver extracts was performed according to the manufaturer’s (Bachem, Switzerland) protocols.
Sub-cellular Fractionation
Sub-cellular fractionation was performed as described earlier (Puthalakath et al., 1999). In brief, ~5×106 liver cells from mice injected with either PBS, GalN or LPS+GalN were lysed in 500 μL of extraction buffer (0.05 M PIPES-NaOH, 0.05 M HEPES pH 7.0, 2 mM MgCl2, 1 mM EDTA, 1 mM DTT plus protease and phosphatase inhibitors) containing 1% Triton X-100. Cell debris and nuclei were removed by centrifugation at 500 × g. The supernatant was spun at 125’000 ×g for 60 min at 4° C and the filamentous actin-enriched pellet (P1) discarded. The remaining supernatant was incubated for 13 min at 37° C with 20 μM taxol and 5 U apyrase (Sigma). This mixture was gently laid on top of 0.5 mL 7.5% sucrose (in extraction buffer) and centrifuged for 30 min at 125’000 × g at 30° C. The sedimented pellet was saved as the dynein-enriched P2 fraction and the supernatant as the S fraction.
Supplementary Material
Acknowledgments
We are grateful to Drs J Adams, S Cory, D Huang, K Rajewski, H Koerner and Y Lazebnik for gifts of gene-targeted mice or antibodies. We thank K Vella, N Iannarella and G Siciliano for animal care, C Young for excellent technical assistance, M Robati and B Helbert for genotyping, the Biochemistry Department of the Royal Melbourne Hospital for ALT/AST measurements and Drs J Adams, S Cory, D Huang, D Vaux and L O’Reilly for advice and critical comments on the manuscript. This work was supported by grants (program #257502) and fellowships from the NHMRC (Canberra), the NCI (NIH, USA; # CA 80188 and #CA 43540), the Leukemia and Lymphoma Society of America (SCOR grant #7015), the JDRF/NHMRC, the Swiss National Science Foundation, the Roche Research Foundation, the Novartis Foundation (formerly Ciba-Geigy-Jubilaeumsstiftung; postdoctoral fellowships to TK) and the Mildred-Scheel Stiftung/Deutsche Krebshilfe (postdoctoral fellowship to PJJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Baud V, Liu ZG, Bennett B, Suzuki N, Xia Y, Karin M. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes and Development. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bradham CA, Plumpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhou Q. Caspase cleavage of BimEL triggers a positive feedback amplification of apoptotic signaling. Proc Natl Acad Sci U S A. 2004;101:1235–1240. doi: 10.1073/pnas.0308050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chen X, Ding WX, Ni HM, Gao W, Shi YH, Gambotto AA, Fan J, Beg AA, Yin XM. Bid-independent mitochondrial activation in tumor necrosis factor alpha-induced apoptosis and liver injury. Mol Cell Biol. 2007;27:541–553. doi: 10.1128/MCB.01166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J Cell Mol Med. 2004;8:445–454. doi: 10.1111/j.1582-4934.2004.tb00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler F, Algul H, Paxian S, Schmid RM. Genetic inactivation of RelA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology. 2007;132:2489–2503. doi: 10.1053/j.gastro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Forster I, Clausen BE, et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-xL. Proc Natl Acad Sci U S A. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DCS, Strasser A. BH3-only proteins – essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- Kägi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Gugasyan R, Gerondakis S, Dixit VM, Strasser A. Loss of the BH3-only protein Bid does not rescue RelA-deficient embryos from TNF-R1-mediated fatal hepatocyte destruction. Cell Death Differ. 2007a;14:637–639. doi: 10.1038/sj.cdd.4402061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, Johnstone RW, Dixit VM, Strasser A. The BH3-Only Protein Bid Is Dispensable for DNA Damage- and Replicative Stress-Induced Apoptosis or Cell-Cycle Arrest. Cell. 2007b;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Korner H, Cook M, Riminton DS, Lemckert FA, Hoek RM, Ledermann B, Kontgen F, Fazekas de St Groth B, Sedgwick JD. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol. 1997;27:2600–2609. doi: 10.1002/eji.1830271020. [DOI] [PubMed] [Google Scholar]
- Kusters S, Tiegs G, Alexopoulou L, Pasparakis M, Douni E, Kunstle G, Bluethmann H, Wendel A, Pfizenmaier K, Kollias G, et al. In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur J Immunol. 1997;27:2870–2875. doi: 10.1002/eji.1830271119. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 Domains of BH3-Only Proteins Differentially Regulate Bax-Mediated Mitochondrial Membrane Permeabilization Both Directly and Indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Luo X, Budlhardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, MacKay SL, Edwards CK, 3rd, Moldawer LL. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1202–R1209. doi: 10.1152/ajpregu.2000.278.5.R1202. [DOI] [PubMed] [Google Scholar]
- O’Reilly LA, Cullen L, Visvader J, Lindeman GJ, Print C, Bath ML, Huang DC, Strasser A. The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am J Pathol. 2000;157:449–461. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly LA, Divisekera U, Newton K, Scalzo K, Kataoka T, Puthalakath H, Ito M, Huang DC, Strasser A. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell Death Differ. 2004;11:724–736. doi: 10.1038/sj.cdd.4401408. [DOI] [PubMed] [Google Scholar]
- Pao LI, Sumaria N, Kelly JM, van Dommelen S, Cretney E, Wallace ME, Anthony DA, Uldrich AP, Godfrey DI, Papadimitriou JM, et al. Functional analysis of granzyme M and its role in immunity to infection. J Immunol. 2005;175:3235–3243. doi: 10.4049/jimmunol.175.5.3235. [DOI] [PubMed] [Google Scholar]
- Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DCS, O’Reilly LA, King SM, Strasser A. The pro-apoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au B, Berry DM, Tamblyn L, Shehabeldin EM, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes and Development. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein C, Rakemann T, Brenner DA, Streetz K, Licato L, Manns MP, Tiegs G. Concanavalin A-induced liver cell damage: activation of intracellular pathways triggered by tumor necrosis factor in mice. Gastroenterology. 1998;114:1035–1045. doi: 10.1016/s0016-5085(98)70324-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li S, Childs EE, Kuharsky DK, Yin XM. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-α-induced liver injury. Journal of Biological Chemistry. 2001;276:27432–27440. doi: 10.1074/jbc.M102465200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.