Abstract
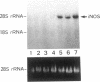
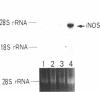
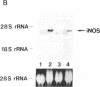
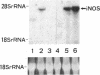
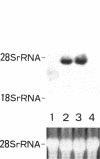
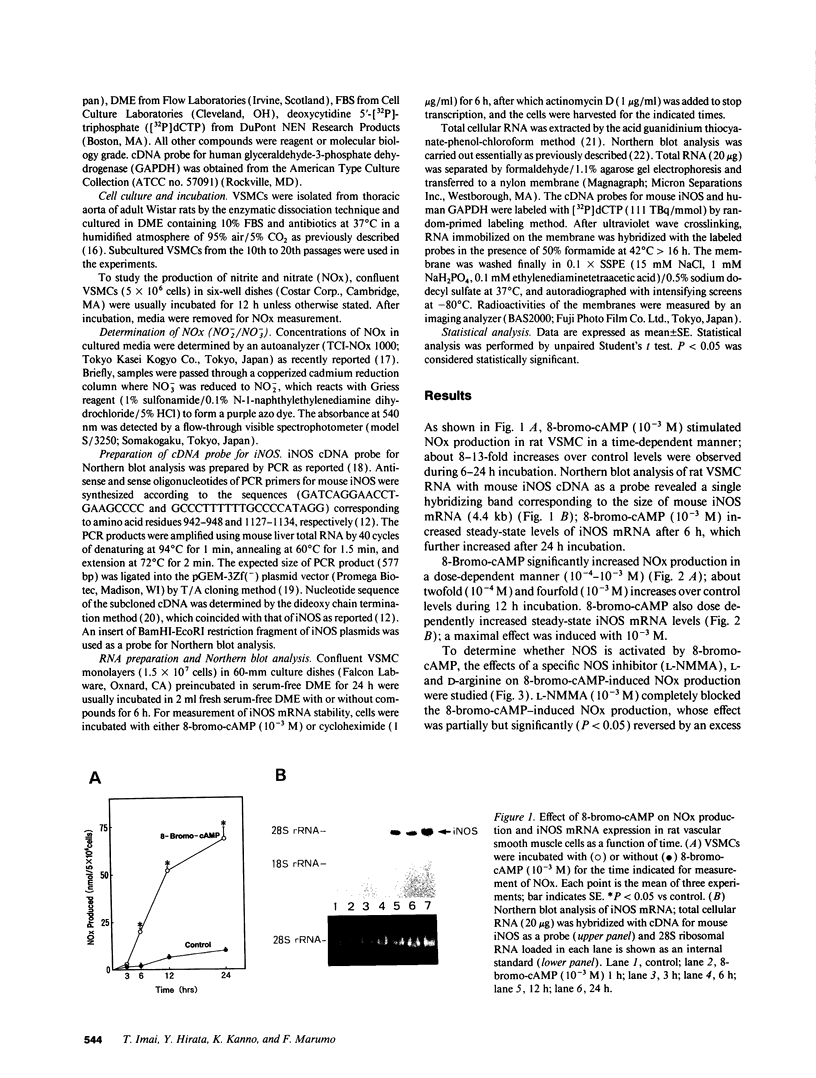
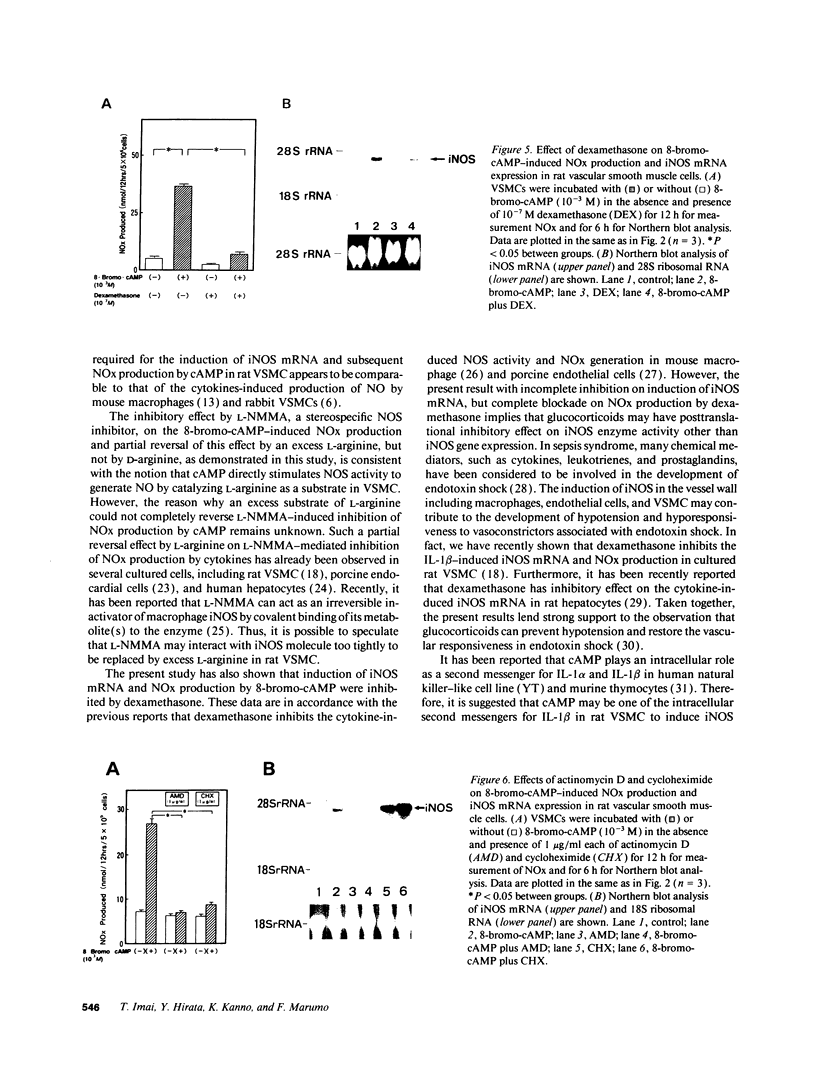
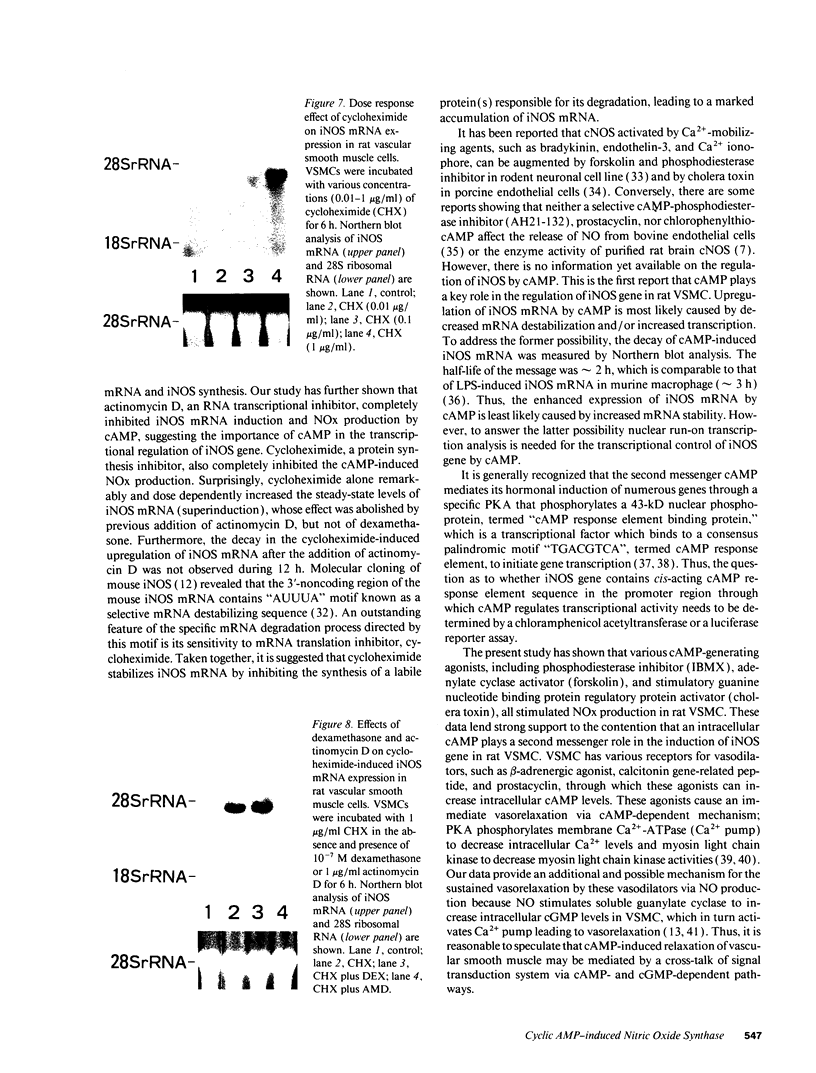
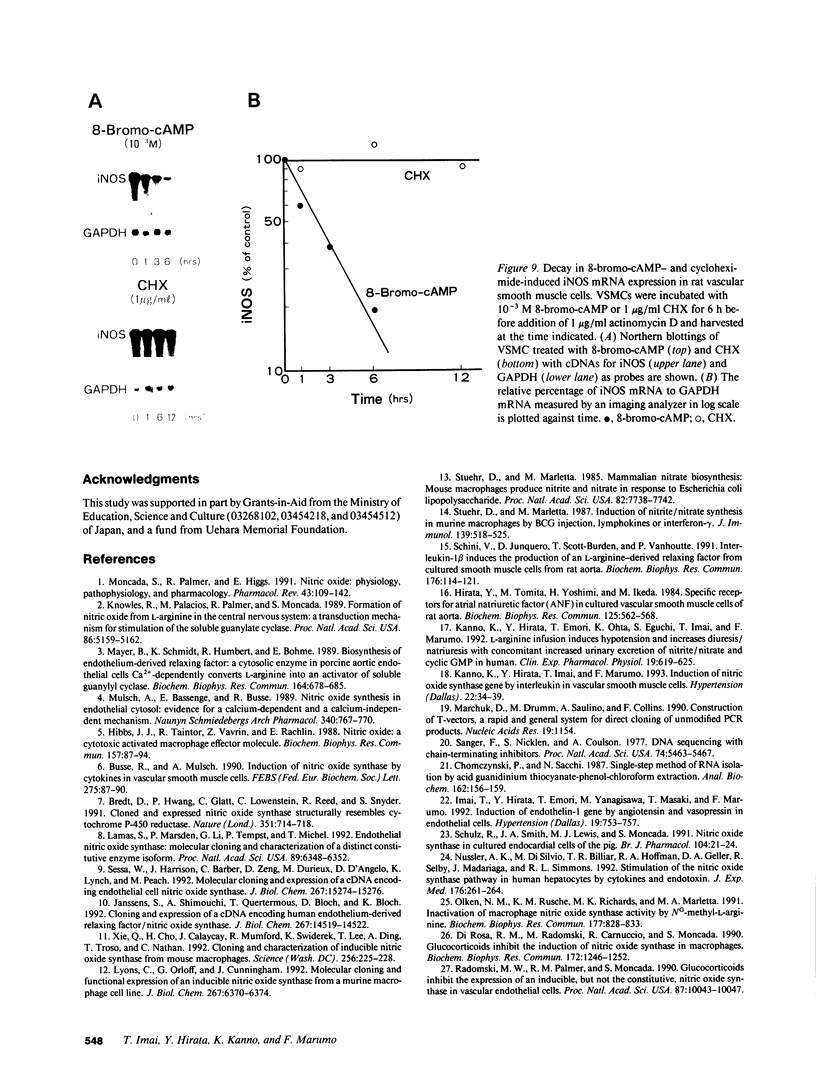
By measurements of NO2-/NO3- (NOx) production and Northern blot analysis, we studied the effects of a membrane-permeable cAMP derivative, 8-bromo-cAMP, on the expression of inducible nitric oxide synthase (iNOS) gene and the synthesis of NOx in cultured rat vascular smooth muscle cells (VSMCs). 8-bromo-cAMP stimulated NOx production and increased steady-state levels of iNOS mRNA in rat VSMC in a time- and dose-dependent manner. NG-monomethyl-L-arginine, a NOS inhibitor, completely blocked the 8-bromo-cAMP-induced NOx production, whose effect was partially, but significantly reversed by an excess L-arginine, but not by D-arginine. Compounds that increase intracellular cAMP levels (cholera toxin, forskolin, and 3-isobutyl-1-methylxanthine), all stimulated NOx production. Dexamethasone inhibited the stimulated NOx production, as well as the induction of iNOS mRNA by cAMP. Both actinomycin D and cycloheximide completely blocked the stimulated NOx production by cAMP. Actinomycin D abolished the cAMP-induced iNOS mRNA, whereas cycloheximide remarkably increased iNOS mRNA levels in the presence and absence of 8-bromo-cAMP (superinduction). Actinomycin D, but not dexamethasone, completely abolished the cycloheximide-induced iNOS mRNA. The half-life of cAMP-induced iNOS mRNA was approximately 2 h, whereas no decay in the cycloheximide-induced iNOS mRNA was observed during 12 h. These results demonstrate that iNOS gene is upregulated by cAMP and the superinduction of iNOS mRNA is attributable to increased mRNA stability in rat VSMC.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone R. C. The pathogenesis of sepsis. Ann Intern Med. 1991 Sep 15;115(6):457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Boulanger C. M., Vanhoutte P. M. Cholera toxin augments the release of endothelium-derived relaxing factor evoked by bradykinin and the calcium ionophore A23187. Gen Pharmacol. 1992 Jan;23(1):27–31. doi: 10.1016/0306-3623(92)90042-i. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Radomski M., Carnuccio R., Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Nussler A. K., Di Silvio M., Lowenstein C. J., Shapiro R. A., Wang S. C., Simmons R. L., Billiar T. R. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Tomita M., Yoshimi H., Ikeda M. Specific receptors for atrial natriuretic factor (ANF) in cultured vascular smooth muscle cells of rat aorta. Biochem Biophys Res Commun. 1984 Dec 14;125(2):562–568. doi: 10.1016/0006-291x(84)90576-x. [DOI] [PubMed] [Google Scholar]
- Imai T., Hirata Y., Emori T., Yanagisawa M., Masaki T., Marumo F. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension. 1992 Jun;19(6 Pt 2):753–757. doi: 10.1161/01.hyp.19.6.753. [DOI] [PubMed] [Google Scholar]
- Janssens S. P., Shimouchi A., Quertermous T., Bloch D. B., Bloch K. D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992 Jul 25;267(21):14519–14522. [PubMed] [Google Scholar]
- Kanno K., Hirata Y., Emori T., Ohta K., Eguchi S., Imai T., Marumo F. L-arginine infusion induces hypotension and diuresis/natriuresis with concomitant increased urinary excretion of nitrite/nitrate and cyclic GMP in humans. Clin Exp Pharmacol Physiol. 1992 Sep;19(9):619–625. doi: 10.1111/j.1440-1681.1992.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Kanno K., Hirata Y., Imai T., Marumo F. Induction of nitric oxide synthase gene by interleukin in vascular smooth muscle cells. Hypertension. 1993 Jul;22(1):34–39. doi: 10.1161/01.hyp.22.1.34. [DOI] [PubMed] [Google Scholar]
- Karaki H., Sato K., Ozaki H., Murakami K. Effects of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. Eur J Pharmacol. 1988 Nov 1;156(2):259–266. doi: 10.1016/0014-2999(88)90329-9. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Otten A., Frölich J. C., Förstermann U. Endothelial cyclic GMP and cyclic AMP do not regulate the release of endothelium-derived relaxing factor/nitric oxide from bovine aortic endothelial cells. J Pharmacol Exp Ther. 1991 Feb;256(2):677–682. [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsbach R. B., Murphy W. J., Lowenstein C. J., Snyder S. H., Russell S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993 Jan 25;268(3):1908–1913. [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B., Schmidt K., Humbert P., Böhme E. Biosynthesis of endothelium-derived relaxing factor: a cytosolic enzyme in porcine aortic endothelial cells Ca2+-dependently converts L-arginine into an activator of soluble guanylyl cyclase. Biochem Biophys Res Commun. 1989 Oct 31;164(2):678–685. doi: 10.1016/0006-291x(89)91513-1. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olken N. M., Rusche K. M., Richards M. K., Marletta M. A. Inactivation of macrophage nitric oxide synthase activity by NG-methyl-L-arginine. Biochem Biophys Res Commun. 1991 Jun 14;177(2):828–833. doi: 10.1016/0006-291x(91)91864-9. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser G. Nitric oxide formation caused by Ca2+ release from internal stores in neuronal cell line is enhanced by cyclic AMP. Eur J Pharmacol. 1992 Sep 1;227(1):89–93. doi: 10.1016/0922-4106(92)90147-n. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer C. F., Brackett D. J., Wilson M. F. The benefits of corticosteroid given after the onset of hypotension during endotoxin shock in the conscious rat. Adv Shock Res. 1983;10:183–194. [PubMed] [Google Scholar]
- Scheid C. R., Honeyman T. W., Fay F. S. Mechanism of beta-adrenergic relaxation of smooth muscle. Nature. 1979 Jan 4;277(5691):32–36. doi: 10.1038/277032a0. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Junquero D. C., Scott-Burden T., Vanhoutte P. M. Interleukin-1 beta induces the production of an L-arginine-derived relaxing factor from cultured smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1991 Apr 15;176(1):114–121. doi: 10.1016/0006-291x(91)90897-g. [DOI] [PubMed] [Google Scholar]
- Schulz R., Smith J. A., Lewis M. J., Moncada S. Nitric oxide synthase in cultured endocardial cells of the pig. Br J Pharmacol. 1991 Sep;104(1):21–24. doi: 10.1111/j.1476-5381.1991.tb12378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa W. C., Harrison J. K., Barber C. M., Zeng D., Durieux M. E., D'Angelo D. D., Lynch K. R., Peach M. J. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem. 1992 Aug 5;267(22):15274–15276. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Yamashita U., Chedid M., Mizel S. B. Cyclic AMP--an intracellular second messenger for interleukin 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8201–8205. doi: 10.1073/pnas.85.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull J. T., Blumenthal D. K., Cooke R. Regulation of contraction by myosin phosphorylation. A comparison between smooth and skeletal muscles. Biochem Pharmacol. 1980 Oct 1;29(19):2537–2543. doi: 10.1016/0006-2952(80)90063-5. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]