Abstract
Background
Malignant mesothelioma is a highly aggressive tumor with poor prognosis. Conventional therapies for mesothelioma are generally non-curative, and new treatment paradigms are urgently needed. We hypothesized that the tumor-specific midkine (Mdk) promoter could confer transcriptional targeting to oncolytic adenoviruses for effective treatment of malignant mesothelioma.
Methods
We analyzed Mdk expression by quantitative RT-PCR in six human mesothelioma cell lines, and tested Mdk promoter activity by luciferase reporter assay. Based on these data, we constructed a replication-selective oncolytic adenovirus, designated AdMdk-E1-iresTK, which contains an Mdk promoter-driven adenoviral E1 gene and HSV-thymidine kinase (TK) suicide gene, and CMV promoter-driven green fluorescent protein (GFP) marker gene. Selectivity of viral replication and cytolysis were characterized in normal vs. mesothelioma cells in vitro, and intratumoral spread and antitumor efficacy were investigated in vivo.
Results
Mdk promoter activity was restricted in normal cells, but highly activated in mesothelioma cell lines. AdMdk-E1-iresTK was seen to efficiently replicate, produce viral progeny, and spread in multiple mesothelioma cell lines. Lytic spread of AdMdk-E1-iresTK mediated efficient killing of these mesothelioma cells, and its in vitro cytocidal effect was significantly enhanced by treatment with the prodrug, ganciclovir. Intratumoral injection of AdMdk-E1-iresTK caused complete regression of MESO4 and MSTO human mesothelioma xenografts in athymic mice. In vivo fluorescence imaging demonstrated intratumoral spread of AdMdk-E1-iresTK-derived signals, which vanished after tumor eradication.
Conclusions
These data indicate that transcriptional targeting of viral replication by the Mdk promoter represents a promising general strategy for oncolytic virotherapy of cancers with upregulated Mdk expression, including malignant mesothelioma.
Keywords: oncolytic adenovirus, suicide gene therapy, molecular imaging, malignant mesothelioma
Introduction
Malignant mesothelioma is an aggressive cancer that arises from the mesothelial cells of serous membranes lining the pleural, peritoneal, and pericardial cavities. Pleural mesothelioma is one of the most lethal cancers, and in most cases is caused by previous exposure to asbestos [1, 2]. Conventional therapies for this malignancy include surgical resection, chemotherapy, and irradiation, but these measures are generally non-curative [2, 3]. Recent randomized studies with combined chemotherapy using cisplatin and antifolate drugs have demonstrated some survival benefit for patients with mesothelioma [4], but none of these treatments significantly impacts the progression and outcome of mesothelioma. Moreover, this disease is increasing in frequency throughout the world [1, 2], with the incidence of pleural mesothelioma predicted to peak over the next 10–20 years [1, 2, 5]. As a result, new therapeutic paradigms and alternative treatment options are urgently needed for more effective treatment of this aggressive and currently incurable malignancy.
Most pleural mesotheliomas spread by local invasion [3, 29], and therefore they may be well suited for gene therapy owing to their accessibility, which allows both intrapleural and intratumoral gene delivery [3, 30]. A number of gene therapy strategies for mesothelioma are being tested in clinical trials, and suicide gene therapy, in particular, has experienced some success [31]. Among the 34 patients receiving replication-defective adenoviral vector-mediated HSV-thymidine kinase (TK) suicide gene therapy in one clinical trial, four achieved considerable tumor regression, and two patients achieved complete tumor regression for more than seven years after treatment.
Pleural mesothelioma is usually widely spread over a large area, and to date, the inefficiency of gene delivery to solid tumors has been a major obstacle to the successful clinical application of this therapeutic approach. Human clinical trials have documented that even high-titer adenoviral vectors only achieve limited transduction into tumor cells and this is confined to areas surrounding the needle track. Notably, even in patients who showed responses to suicide gene therapy using conventional adenoviral vectors, the extent and duration of the response was proportionally greater than the level of transduction actually achieved, suggesting that immune responses triggered by the vector itself contributed significantly to antitumoral efficacy.
An emerging technology that shows considerable promise as a novel treatment option is the use of oncolytic viruses that are capable of tumor-selective replication [6, 7]. The primary approach to achieve tumor-selective viral replication has been to replace endogenous viral regulatory sequences with a tissue- or tumor-specific promoter. This will restrict the sites of viral replication and expression of therapeutic transgenes. Here, we examine the utility of a highly promising tumor-selective promoter, derived from the midkine gene, in targeting mesothelioma.
Midkine (Mdk) is a basic heparin-binding growth factor, and is a developmentally important retinoic acid-responsive protein that is strongly induced during mid-gestation [8]. Mdk has various biological activities that are closely linked to neural cell survival and development [9]. Mdk is also implicated in the development of cancer because of its mitogenic effect [10, 11], promotion of angiogenesis [12], anti-apoptotic activity [13, 14], fibrinolytic activity [15], and transforming activity [16]. Mdk is overexpressed in various human cancers, including esophageal [17], gastrointestinal [18], hepatocellular [19], lung [20], breast [21], pancreatic carcinoma [22, 23], Wilm's tumor [24], and neuroblastoma [25], when compared with adjacent normal tissues. In contrast, Mdk expression in normal human adult tissues is quite limited, with moderate expression in the kidneys and weak expression in lungs, colon, and thyroid gland [8, 18, 21, 24].
In the present study, we found that the Mdk promoter is highly active in mesothelioma cells. Consequently, we used the Mdk promoter to drive expression of the adenoviral E1 gene and develop a transcriptionally-targeted oncolytic adenovirus. Here, we demonstrate that this oncolytic adenovirus shows selective replication and cytolysis in multiple established mesothelioma cell lines in vitro, and achieved complete regression of human malignant mesothelioma xenografts in athymic nude mice in vivo.
Materials and Methods
Cell lines
Normal human adult mesothelial cells (NMC) were purchased from Zen Bio, Inc. (Research Triangle Park, NC, USA) and maintained in Mesothelial Cell Growth Medium (Zen-Bio, Inc.). Non-malignant transformed human pleural mesothelial cells (Met5A) were originally established by transfecting normal human mesothelial cells with a plasmid containing the SV40 large T antigen gene, but are non-tumorigenic. Met5A and four human mesothelioma cell lines, MSTO-211H (MSTO), NCI-H2052 (H2052), NCI-H2452 (H2452), and NCI-H28 (H28), were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Other human mesothelioma cell lines, ACC-MESO-1 (MESO1) and ACC-MESO-4 (MESO4) were purchased from the RIKEN BioResearch Center (Tsukuba, Ibaraki, Japan). These cells were grown in RPMI 1640 (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal calf serum (FCS; HyClone, Logan, UT, USA). The human embryonic kidney cell lines 293 [26] (Microbix, Toronto, Canada) and 293T were cultured in Dulbecco's modified Eagle's medium (Nacalai Tesque) supplemented with 10% FCS. Primary human coronary artery smooth muscle cells (SMC) and human dermal fibroblasts and their specific media were purchased from Cell Systems (Kirkland, WA, USA). All the cells were grown in 5% CO2 at 37°C.
Mdk and CAR mRNA expression profile analysis
The expression of Mdk mRNA in cell lines was analyzed by reverse transcription and the polymerase chain reaction (RT-PCR). Total RNA was extracted from semiconfluent cell cultures on 10 cm dishes using the ISOGEN RNA extraction solution (Nippon Gene, Tokyo, Japan), whereupon it was treated with DNase to remove genomic DNA contamination. The RNA was analyzed by quantitative real-time PCR for expression of Mdk, coxsackievirus and adenovirus receptor (CAR), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA with the Taqman One-step RT-PCR master mix reagent kit (Applied Biosystems Japan Ltd., Tokyo, Japan) as described by the manufacturer. The primers and TaqMan probe for Mdk (Hs00171064_m1), CAR (Hs00154661_m1) and GAPDH (Hs99999905_m1) were purchased from Applied Biosystems Japan (Taqman gene expression assays). Briefly, 40 ng of total RNA was added to the reaction mixture (TaqMan one-step RT-PCR master mix reagents), containing 18 pmol of each of the primers and 5 pmol of the probe, and amplified for 1 cycle of 30 min at 48°C and 10 min at 95°C, followed by 50 cycles of 15 sec at 95°C and 1 min at 60°C.
Assessment of Mdk promoter activity
The relative luciferase activities of promoter constructs were quantitated in cell lines transfected with a reporter gene plasmid containing firefly luciferase driven by the Mdk or SV40 promoter, together with Renilla luciferase-expressing plasmid (pRL-TK, Promega, Madison, USA). The cells were lysed 48 h after transfection, and assayed for luciferase activity using the Dual-Glo luciferase assay system (Promega). Data shown are the mean ± standard deviation (SD) calculated from triplicates.
Vector plasmids and virus production
The recombinant adenoviral vectors used in these experiments include an HSV-TK suicide gene expression cassette, and were constructed using the AdEasy XL system (Stratagene, La Jolla, CA, USA). Firstly, a marker gene cassette, containing the enhanced green fluorescent protein (EGFP) gene driven by the human cytomegalovirus (CMV) promoter [27], was subcloned into the pShuttle backbone (Stratagene) to generate the intermediate construct, pShuttleG. For replication-competent adenoviruses, the Mdk-E1-ires-TK and CMV-E1-ires-TK gene cassettes were inserted into pShuttleG, to generate pShuttleG-Mdk-E1-ires-TK and pShuttleG-CMV-E1-ires-TK, respectively. Similarly, for replication-defective adenoviruses, insertion of the Mdk-TK and CMV-TK gene cassettes into pShuttleG generated pShuttleG-Mdk-TK and pShuttleG-CMV-E1-ires-TK, respectively. A detailed description of this subcloning strategy can be provided on request.
These shuttle plasmids were then transformed into Escherichia coli BJ5183-AD (Stratagene) for homologous recombination in order to generate pAdMdk-E1-iresTK (Ad881), pAdCMV-E1-iresTK (Ad883), pAdMdk-TK (Ad884) and pAdCMV-TK (Ad885).
All vectors were propagated in 293 cells and purified by CsCl ultracentrifugation, followed by dialysis against 10 mM Tris-HCL buffer (pH 8.0) with 10% glycerol. The titers of these vectors were determined by conventional limiting dilution on 293 cells and showed similar viral yield. The particle to plaque-forming unit (pfu) ratios of these vectors were within the range of 10.7 to 24.8 particles/pfu. Titers were also determined by EGFP expression using a FACScalibur flow cytometer (Becton Dickinson Japan, Tokyo, Japan) as EGFP-transducing units (TU) per mL.
Viral progeny production assay
To assay the production of virus progeny, 5 × 105 cells/well were seeded in 6-well plates and were infected with adenoviruses at a multiplicity of infection (MOI) of 100 viral particles per cell in 2 mL of 5% FCS medium. After 3 h, the infection medium was replaced with 2 mL of growth medium. Forty hours later, cells and media were harvested, freeze/thawed three times, centrifuged, and serial dilutions of the virus supernatants were titered on 293 cells by EGFP expression using flow cytometry.
In vitro cytotoxicity assay
For screening the oncolytic activity of each adenovirus in various cell lines, cells were cultured on duplicate 24-well plates (1 × 105 cells/well) and infected with MOIs of 0, 0.1, 1, 10, 100, or 1000. Half of the supernatant medium volume was replaced with fresh medium containing 4% FCS every other day. On Day 8, the cells were fixed with 10% buffered formalin containing 1% crystal violet for 30 min, followed by three washes in tap water and air-dried.
For quantitative analysis of the cytocidal effect of each adenovirus, five sets of 96-well tissue-culture plates containing Met5A, MESO4, or MSTO cells (1 × 104 cells/well) were infected with Ad881, Ad883, Ad884, or Ad885 at MOIs of 0, 0.01, 0.1, 1, 10, or 100. Half of the supernatant medium volume was replaced with fresh medium containing 4% FCS every other day. On Day 6, one set of plates was treated with ganciclovir (GCV) (1 mg/mL) (Toronto Research Chemicals Inc., North York, Ontario, Canada). On Days 1, 2, 4, and 8 post-infection, the viable cell numbers of triplicate cultures were measured by the Alamar Blue method according to the manufacturer's instructions (Alamar Biosciences, Inc., Sacramento, CA, USA). Briefly, 40 μL of Alamar Blue was aseptically added to the cultures, which were then returned to the incubator for 3 h; fluorescence was measured by an ARVO X4 multilabel plate reader with a 544 nm excitation wavelength and a 590 nm emission wavelength (PerkinElmer Japan, Tokyo, Japan). The percentage of viable cells was determined by calculation of the fluorescence of viable cells as measured against wells containing no viral vectors.
Subcutaneous human malignant mesothelioma xenograft models and in vivo fluorescence imaging analysis
All animal experiments were conducted according to institutional guidelines under an approved protocol. Human malignant mesothelioma xenografts were established in 6- to 7-week-old female BALB/c-nu/nu (nude) mice (Charles River Japan Co., Yokohama, Japan) by subcutaneous inoculation of 1 × 106 MESO4 cells into the right dorsal flank. When tumors reached a diameter of ~5 mm, mice were injected intratumorally with 100 μL of PBS, Ad881 or Ad884 (5 × 108 TU) on Day 0. Over sequential days, mice were anesthetized with sodium pentobarbital and whole body images (0.05- to 0.5-second exposure) were taken and analyzed with the Maestro in vivo fluorescence imaging system (CRi, Woburn, MA, USA). All images were analyzed using the Maestro software provided by the manufacturer. In a separate set of experiments, MESO4 cells were grown subcutaneously in nude mice to a diameter of 5–6 mm as above, and five groups of mice (n = 8 per group) were then injected intratumorally with 100 μL of PBS, Ad881 or Ad884 (5 × 108 TU) on Day 0, followed by intraperitoneal administration of either GCV (100 mg/kg/day) or saline (0.9% NaCl) from Day 14 to Day 28 (n = 8 per group). The mice were observed closely and the tumors were measured twice a week. The tumor volume was calculated as a × b2 × 0.5, where a and b were the large and small diameters, respectively. This experiment was repeated using MSTO cells, and tumors were injected with 100 μL of PBS, Ad881 or Ad884 (5 × 108 TU) on Day 0 and Day 6, followed by intraperitoneal administration of either GCV or saline from Day 24 to Day 48 (n = 8 per group).
To compare the antitumor efficacy between Mdk-driven Ad 881 and CMV-driven Ad 883, subcutaneous MSTO tumors in nude mice were injected intratumorally with 5 × 108 TU of either Ad881 (n = 4), Ad883 (n = 4) or Ad884 (n = 3), or PBS (n = 3) on Day 0, followed by intraperitoneal administration of GCV from Day 14 to Day 28.
To compare the potential for hepatotoxicity caused by targeted vs. non-targeted adenovirus, 6- to 7-week-old female nude mice were intravenously injected with 5 × 108 TU of Ad881, Ad883, or PBS (n = 3 per group) via tail vein. Sera were collected from mice sacrificed on Day 3, and the serum levels of alanine aminotransferase (ALT) were measured by the Transaminase CII-test (Wako Pure Chemical Industries, Osaka, Japan) to assess hepatotoxicity.
Statistical analysis
The results are presented as mean ± SD. Statistical significance of differences was calculated using Student's t-test, and a P value of <0.01 was considered significant.
Results
Tumor-specific Mdk mRNA expression and Mdk promoter activities in human cell lines
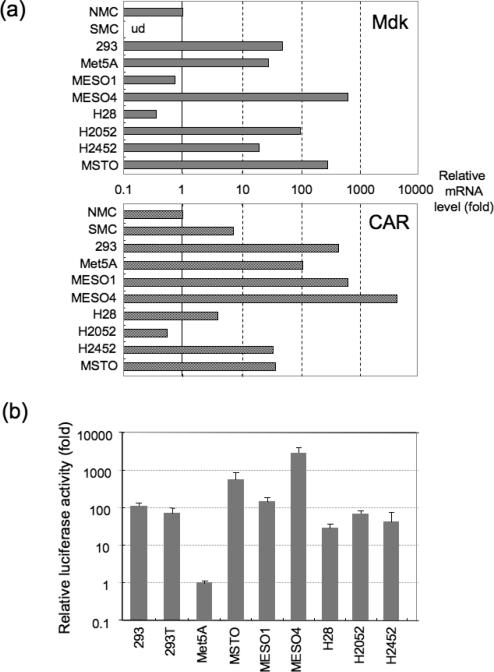
To evaluate whether the Mdk promoter could be useful for targeting mesothelioma in the context of a transcriptionally targeted oncolytic adenoviruses, firstly we analyzed the expression levels of Mdk and CAR in human mesothelioma cell lines by quantitative RTPCR. Mdk mRNA was expressed in all of the six mesothelioma cell lines tested (MESO1, MESO4, H28, H2052, H2452, and MSTO), but was low in NMC and undetectable in SMC (Figure 1a). Among the mesothelioma cells, MESO4 and MSTO showed the highest expression (>100 fold more than NMC). CAR mRNA expression was also confirmed in these mesothelioma cell lines.
Figure 1. Evaluation of tumor-specific Mdk promoter activities in vitro.
(a) Relative mRNA levels of Mdk and CAR in cell lines by quantitative RT-PCR. Total RNA was extracted from various human cells, including normal mesothelial cells (NMC), smooth muscle cells (SMC), 293 human embryonic kidney cells, non-malignant transformed human pleural mesothelial cells (Met5A), and malignant mesothelioma cell lines (MESO1, MESO4, H28, H2052, H2452, and MSTO). The RNA was reverse-transcribed and amplified by PCR with specific primers for Mdk, CAR, and GAPDH. GAPDH was used as an endogenous RNA control to normalize for differences in the amount of total RNA.
(b) Mdk promoter activities in cell lines. Relative luciferase activities in various cell lines transfected with a firefly luciferase-expressing reporter plasmid driven by the Mdk or SV40 promoter, together with Renilla luciferase-expressing plasmid. At 48 h after transfection, the cells were lysed and assayed for luciferase activity using the Dual-Glo luciferase assay system. Data were normalized to the baseline value in Met5A, and shown as the mean ± SD calculated from triplicates.
To analyze Mdk promoter activity, cells were transfected with an Mdk promoter-driven luciferase expression plasmid. The luciferase activities were normalized to the activity in Met5A non-malignant transformed human pleural mesothelial cells. The Mdk promoter was found to be highly activated in all of the six mesothelioma cells lines tested (Figure 1b), as well as in human embryonic kidney 293 and 293T cell lines, as previously reported. These findings suggest that Mdk is a promising tumor-specific promoter for transcriptional targeting of malignant mesothelioma cells, and could be employed to effectively regulate conditional replication of oncolytic adenoviruses.
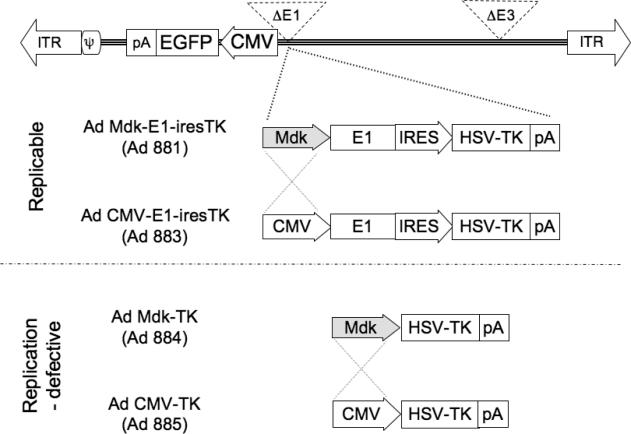
Design and production of the oncolytic and control adenoviruses
As shown in Figure 2, four different adenoviruses were constructed and tested. Ad881 is a conditionally replicating adenovirus, in which the adenoviral E1 gene is driven by the Mdk promoter. Ad881 is armed with the HSV-TK suicide gene, and also carries the EGFP marker gene. Ad883 serves as a replication-competent virus control, in which the Mdk promoter was replaced with the constitutively active CMV promoter. Ad884 and Ad885 serve as replication-defective virus controls, in which HSV-TK transgene expression is driven by Mdk or CMV promoters, respectively.
Figure 2. Schematic structure of recombinant adenovirus vectors.
Replication-competent adenoviruses were constructed by insertion of Mdk promoter (Ad881) and human CMV promoter (Ad883)-driven E1 expression cassettes linked to the HSV-TK suicide gene via an internal ribosome entry site. Non-replicative adenoviruses were constructed by insertion of an HSV-TK cassette driven by the Mdk promoter (Ad884) and human CMV promoter (Ad885).
ITR: adenovirus inverted terminal repeat sequence, ψ: packaging signal, CMV: cytomegalovirus promoter, EGFP: enhanced green fluorescent protein, pA: polyadenylation signal, ires: internal ribosome entry site, HSV-TK; Herpes simplex virus-thymidine kinase suicide gene.
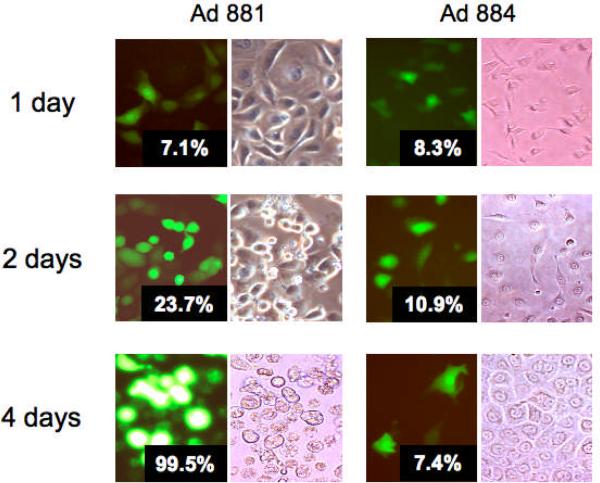
Virus spread and cytotoxicity of oncolytic adenovirus in mesothelioma cells
To determine whether the Mdk promoter can confer tumor-selective replication and cytolytic properties in mesothelioma cells, MESO4 cells were infected with the Mdk-driven oncolytic adenovirus Ad881 or the corresponding replication-defective control virus Ad884, at a low MOI. A low percentage (<10%) of EGFP-positive cells was detected 24 h post-infection in both cultures, but a rapid increase in the EGFP-positive population was seen only in the Ad881-infected culture over the next four days, eventually reaching almost 100% (Figure 3). This reflects robust spread of the oncolytic adenovirus in the mesothelioma cells. In addition, Ad881-infected cells display an increasing cytopathic effect (CPE) over time, while CPE was not observed in Ad884-infected cells (Figure 3). Similar results were obtained using other mesothelioma cell lines (i.e., MESO1, MSTO, and H2452) (data not shown), indicating that lytic spread of the Ad881 oncolytic adenovirus mediates efficient killing in multiple established mesothelioma cell lines.
Figure 3. Virus spread and cytotoxicity of oncolytic adenovirus in mesothelioma cells.
MESO4 cells were infected with Ad881 or Ad884, at an MOI of 1. Photomicrographs were obtained at the indicated times after infection (200× magnification). Inset numbers indicate quantitation of EGFP-positive cells by flow cytometry.
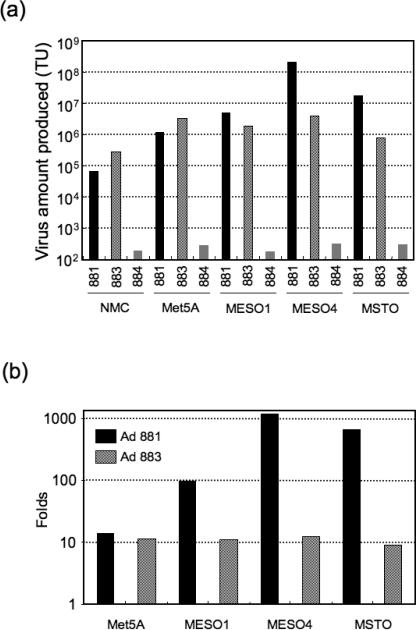
Tumor-selective propagation of Mdk promoter-based oncolytic adenoviruses in malignant mesothelioma cell lines
To assess oncolytic adenovirus propagation in mesothelial cells, normal mesothelial cells (NMC), the non-malignant transformed mesothelial cell line Met5A, and the malignant mesothelioma cell lines MESO1, MESO4, and MSTO, were infected with Mdk-driven replication-competent Ad881, CMV-driven replication-competent Ad883, or Mdk-driven replication-defective Ad884 at an MOI of 100. After 48 h, cells and medium were harvested to assay adenovirus progeny production in the cell lines. In both NMC and Met5A cells, the Mdk promoter-driven Ad881 showed 3–4-fold less virus production than the CMV promoter-driven Ad883 (Figure 4a). In contrast, in malignant mesothelioma cells Ad881 showed much higher levels of virus production compared to Ad883: in MESO1, MESO4, and MSTO cells, Ad881 showed, respectively, 2.6-fold, 51.3-fold, and 21.2-fold higher virus production than Ad883 (Figure 4a). When normalized to the baseline value in NMC cells, Mdk-driven Ad881 oncolytic adenovirus replicates selectively, and efficiently produces viral progeny in these malignant cells (Figure 4b). This suggests that the strength and selectivity of the Mdk-promoter in these malignant cells may outweigh their differences in CAR expression.
Figure 4. Tumor-selective propagation of Mdk promoter-based oncolytic adenoviruses in malignant mesothelioma cell lines.
(a) Human malignant mesothelioma cell lines (MESO1, MESO4, and MSTO), normal mesothelial cells (NMC), and non-malignant transformed human pleural mesothelial cells (Met5A) were infected with Ad881, Ad883, or Ad884 at an MOI of 100. Forty-eight hours after infection, cells and media were harvested to determine the viral titer in transducing units (TU) by EGFP expression using flow cytometry. (b) Virus production levels as determined above, normalized to the baseline value in NMC cells.
Oncolytic efficiency of an Mdk promoter-based oncolytic adenovirus in untransformed and mesothelioma cell cultures
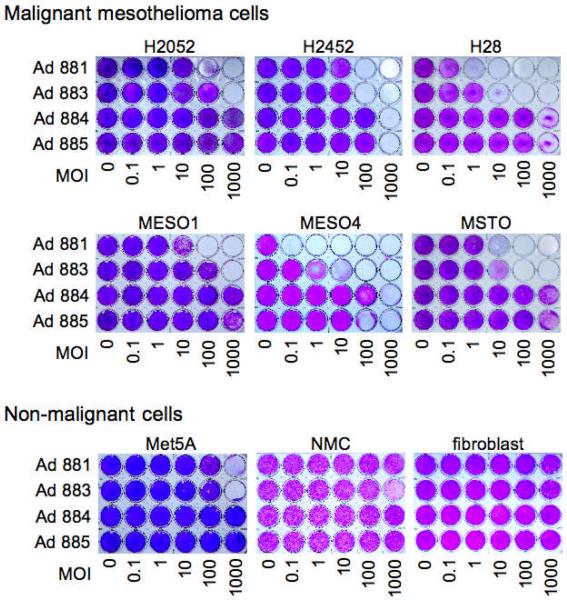
To investigate tumor-specific cytotoxicity, malignant mesothelioma cell lines (MESO1, MESO4, H28, H2052, H2452, and MSTO), Met5A non-malignant transformed mesothelial cells, and untransformed cell lines (NMC and fibroblast), either remained uninfected or were infected with Ad881, Ad883, Ad884, or Ad885 at various MOIs. After eight days, oncolytic efficiency of these viruses was examined by staining adherent viable cells with crystal violet. All of the mesothelioma cell lines tested showed cytolysis in a dose-dependent manner by the replication-competent adenoviruses, Ad881 and Ad883, although the sensitivity varied among different cell lines (Figure 5). As expected from the virus propagation assay results, Ad881 showed cytotoxicity at levels comparable to Ad883 in Met5A non-malignant transformed mesothelial cells. In most malignant mesothelioma cell lines, Mdk-driven Ad881 caused more extensive cell death at lower MOIs than CMV-driven Ad883, which is consistent with the higher level of virus propagation seen in most of these cell lines (Figures 4a and 4b). Notably, in NMC cells, Ad883 was observed to cause CPE at an MOI of 1000. In contrast, Ad881 showed no apparent CPE in NMC or fibroblasts even at MOI 1000, representing a therapeutic window ranging between 2–5 orders of magnitude for Ad881 cytotoxicity in the various mesothelioma cell lines compared to normal cells. These findings indicate that the Mdk promoter confers highly tumor-specific cytotoxicity to oncolytic Ad881 in mesothelioma cell lines.
Figure 5. Oncolytic efficiency of Mdk promoter-driven oncolytic adenovirus in normal and mesothelioma cell cultures.
Human malignant mesothelioma cell lines (H2052, H2452, H28, MESO1, MESO4, and MSTO), non-malignant transformed human pleural mesothelial cells (Met5A), and the human normal cell lines (fibroblast and NMC), were infected with Ad881, Ad883, Ad884, or Ad885, at MOIs from 0.1 to 1000. Crystal violet staining of viable cells was used to evaluate oncolytic activity eight days post-infection. MOI: multiplicity of infection.
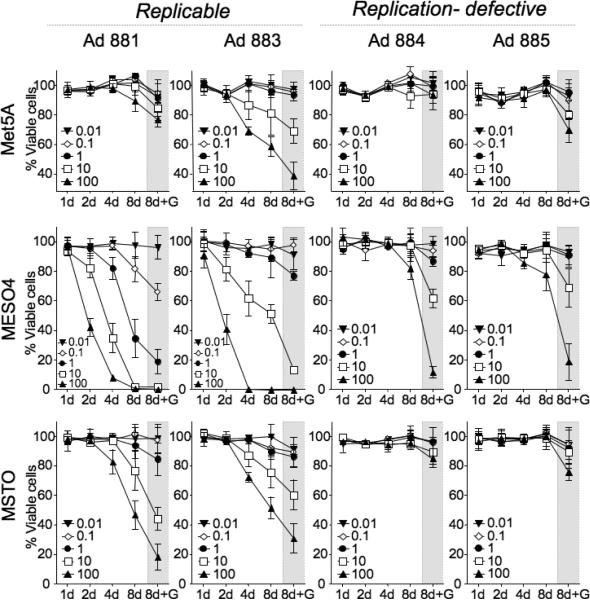
Quantitative in vitro cytotoxicity assay
The cytotoxicity of the adenoviruses was assayed by infecting replicate cultures of Met5A, MESO4, and MSTO cells with Ad881, Ad883, Ad884, or Ad885 at various MOIs. At serial time points thereafter, the number of surviving cells was analyzed using a colorimetric method. In one set of replicate cultures, ganciclovir (GCV), the prodrug for HSV-TK, was added to the cultures on Day 6 post-infection. As shown in Figure 6, the replication-defective vectors, Ad884 (with an Mdk-driven HSV-TK expression cassette) and Ad885 (with a CMV-driven HSV-TK cassette), showed cytotoxicity only in the presence of GCV. On the other hand, both the Mdk-driven Ad881 and the CMV-driven Ad883 replication-competent adenoviruses showed time- and dose-dependent cytotoxicity in MESO4 and MSTO cells, in which the Mdk promoter is highly activated. These cytocidal effects were significantly enhanced by GCV treatment. As expected, CMV-driven Ad883 was also cytotoxic to Met5A non-malignant transformed mesothelial cells, even without GCV treatment. In contrast, Mdk-driven Ad881 did not show significant cytotoxicity in the absence of prodrug, consistent with its inability to propagate at high levels in Met5A cells (Figure 4a), which have low levels of Mdk promoter activity (Figure 1b). These findings confirm the selective cytotoxicity of Mdk-driven oncolytic Ad881 in malignant mesothelioma cells.
Figure 6. Quantitative in vitro cytotoxicity assay.
Met5A, MESO4, and MSTO cells (1 × 104/well) were cultured in multiple replicate 96-well plates and infected with Ad881, Ad883, Ad884, or Ad885, at MOIs from 0.01 to 100. On the indicated days, the number of surviving cells was analyzed by a colorimetric methods using Alamar blue. One set of cultures was treated with GCV (1 mg/mL) during the last three days prior to the colorimetric assay (8d+G). Data shown are the mean ± SD calculated from triplicates.
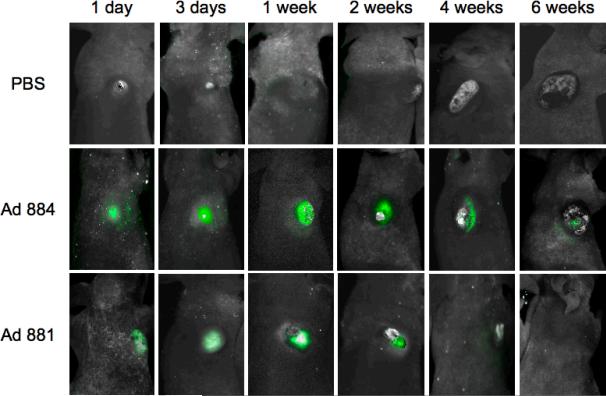
In vivo imaging of the spread of oncolytic adenovirus in malignant mesothelioma xenografts
The presence of the EGFP marker gene in the adenoviral backbone, enabled the spread of the oncolytic adenovirus to be monitored in vivo by fluorescence imaging in a non-invasive manner. Athymic nude mice with subcutaneous MESO-4 human malignant mesothelioma xenografts received an intratumoral injection with 5 × 108 TU of either Ad881 or Ad884, or PBS control on Day 0. Virus replication was monitored by in vivo imaging at serial time points thereafter.
In mice injected with replication-defective Ad884, intratumoral EGFP expression was first observed on Day 1, reached a peak level by Day 3, maintained a plateau level for up to 4 weeks, and then decreased, while tumors kept growing (Figure 7). In contrast, tumors injected with Mdk-driven oncolytic adenovirus Ad881 showed a robust increase in EGFP expression during the first week of infection. The peak EGFP fluorescence intensity during this time was approximately three-fold higher than in Ad884-injected tumors. Subsequently, EGFP expression was observed to diminish gradually by week 4, correlating with tumor regression. These data indicate that Mdk-driven oncolytic adenovirus was capable of efficient replication and progressive spread through mesothelioma tumors in vivo. Ultimately, virus propagation could not be sustained due to lack of host cells in the regressing tumor.
Figure 7. In vivo imaging of the spread of oncolytic adenovirus in malignant mesothelioma xenograft tumors.
MESO4 tumors were grown subcutaneously in nude mice to approximately 5–6 mm in diameter, and injected intratumorally with 5 × 108 TU of either Ad881 or Ad884, or PBS control on Day 0 (n = 3 per group). At different time points indicated in the figure, whole body images (0.05- to 0.5-second exposure) were taken and analyzed by in vivo fluorescence imaging. Representative images are shown from each group.
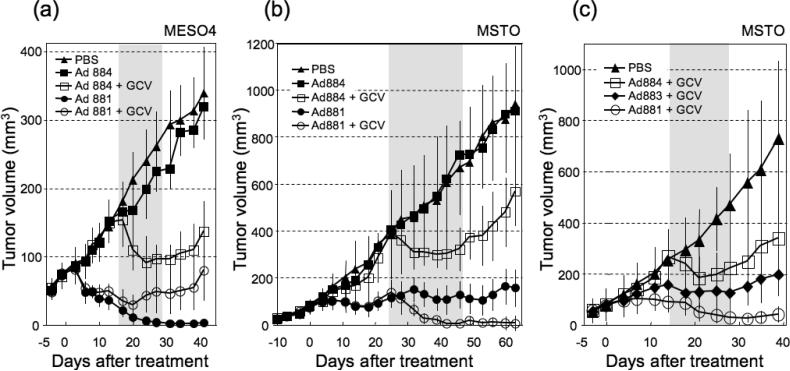
Therapeutic efficacy of recombinant adenoviruses in malignant mesothelioma xenograft models
We examined the antitumoral therapeutic efficacy in vivo, and assessed whether suicide gene activation would augment the effectiveness of viral oncolysis. To do this, nude mice bearing established MESO4 subcutaneous tumors were treated with a single intratumoral injection of 5 × 108 TU of Ad881, Ad884, or PBS control on Day 0, followed by intraperitoneal administration of either GCV or saline from Day 14 to Day 28. Tumors treated with replication-defective adenovirus Ad884 showed partial regression (40.9% reduction in tumor volume) only in the presence of GCV, and tumor regrowth was observed (Figure 8a). Without GCV treatment, the Mdk-driven oncolytic adenovirus, Ad881, caused complete regression of subcutaneous tumors, which were barely palpable by 3 weeks post-infection. The absence of residual viable tumor tissue was confirmed by necropsy and pathological examination of these tumor-free animals. In the presence of GCV, the Ad881-treated group showed a 64.2% reduction in tumor volume by Day 20, and tumor regrowth was observed thereafter, suggesting that further viral replication and spread was inhibited by suicide gene activation.
Figure 8. In vivo antitumor effect of recombinant adenoviruses in a malignant mesothelioma xenograft model.
(a) MESO4 tumors were grown subcutaneously in nude mice to approximately 5–6 mm in diameter, and injected intratumorally with 5 × 108 TU of either Ad881 or Ad884, or PBS control on Day 0. This was followed by intraperitoneal administration of either GCV or saline from Day 14 to Day 28 (gray zone; n = 8 per group). Tumor volumes were measured twice a week, and data shown are the mean ± SD.
(b) Subcutaneous MSTO tumors established in nude mice were injected intratumorally with 5 × 108 TU of either Ad881 or Ad884, or PBS control on Day 0 and Day 6, followed by intraperitoneal administration of either GCV or saline from Day 24 to Day 48 (gray zone; n = 8 per group).
(c) Subcutaneous MSTO tumors established in nude mice were injected intratumorally with 5 × 108 TU of either Ad881 (n = 4), Ad883 (n = 4) or Ad884 (n = 3), or PBS control (n = 3) on Day 0, followed by intraperitoneal administration of GCV from Day 14 to Day 28 (gray zone).
We then compared the antitumor efficacy of Mdk-targeted replication-competent Ad881 vs. Mdk-targeted replication-defective Ad884 in the MSTO mesothelioma model, in which both Mdk and CAR are expressed at lower levels than in MESO4. Subcutaneous MSTO tumors were established in nude mice, and injected intratumorally with PBS, Ad881 or Ad884 (5 × 108 TU) on Day 0 and Day 6, followed by intraperitoneal administration of either GCV or saline from Day 24 to Day 48. Tumors treated with Ad884 showed partial regression (23.2% reduction in tumor volume) only in the presence of GCV, and tumor regrowth was observed (Figure 8b). Without GCV treatment, Ad881 yielded significant growth inhibition compared with PBS control, but did not achieve complete tumor regression. In contrast, in the presence of GCV, the Ad881-treated group showed complete regression of subcutaneous tumors, and no tumor regrowth was observed up to 4 weeks after discontinuation of GCV.
Next, we compared the anti-tumor efficacy of targeted Mdk-driven Ad881 vs. non-targeted CMV-driven Ad883 replication-competent adenoviruses in the subcutaneous MSTO tumor model. Mdk-targeted but non-replicating Ad884 was also used as a control. Nude mice bearing MSTO tumors established as above were injected intratumorally with PBS, Ad881, Ad883 or Ad884 (5 × 108 TU) on Day 0, followed by intraperitoneal administration of GCV from Day 14 to Day 28. Prior to GCV administration, Mdk-driven Ad881 but not CMV-driven Ad883 showed significant inhibition of tumor growth compared to the PBS-treated group. Additionally, when combined with GCV treatment, Mdk-targeted replication-competent Ad881 yielded a significant reduction in tumor volume (77.1%) in comparison to the non-targeted CMV-driven replication competent control virus Ad883 (21.3%), as well as the Mdk-targeted non-replicating Ad884 (31.2%) (Figure 8c). Tumor regrowth was observed in Ad884- and Ad883- treated groups after GCV discontinuation, but not in the Ad881-treated group.
We next compared hepatotoxicity in mice after intravenous injection with PBS, targeted Mdk-driven Ad881, or non-targeted CMV-driven Ad883 (5 × 108 TU). Sera were collected on Day 3 after virus administration, and assayed for ALT activity. We found that serum ALT levels in mice injected with the non-targeted Ad883 virus were significantly higher (225.5 ± 39.52 U/L) compared to those injected with Mdk-targeted Ad881 (29.70 ± 14.98 U/L) (P = 0.0098). Notably, no significant difference was observed in serum ALT values between Ad881-injected mice (29.70 ± 14.98 U/L) and the PBS-injected negative control group (15.43 ± 1.981 U/L) (P = 0.3986). These results suggest that transcriptional targeting may be able to reduce potential adverse effects of oncolytic adenovirus on extra-tumoral organs, particularly the liver.
Discussion
Tumor specificity and viral infectivity are critical parameters for the success of oncolytic virotherapy. In this study, we asked whether the Mdk promoter could confer tumor selectivity to oncolytic adenovirus vectors and enable transcriptional targeting of malignant mesothelioma. Significantly, we found that Mdk mRNA was expressed to some extent in all six of the human mesothelioma cell lines we tested, although its expression was restricted in normal cells. Consistent with the Mdk expression data, we confirmed that the Mdk promoter is highly activated in these human mesothelioma cell lines. We also found that CAR mRNA was expressed to some extent in all of the six human mesothelioma cell lines we tested, indicating that they were susceptible to adenoviral infection.
On this basis, we proceeded to develop a transcriptionally-targeted oncolytic adenovirus controlled by the Mdk promoter, armed with the HSV-TK suicide gene, that also carries the EGFP marker gene. This Mdk-regulated virus (Ad881) demonstrates tumor cell-specific replication and cytotoxicity as well as potent therapeutic efficacy against malignant mesothelioma cells both in vitro and in vivo.
The Mdk-driven oncolytic adenovirus achieves robust tumor-specific cytotoxicity in MESO4 human mesothelioma cells (Figure 6) and complete regression of subcutaneous tumor xenografts in athymic mice, following a single intratumoral injection (Figure 8a). This striking result may reflect the high permissivity of MESO4 cells for Mdk promoter-driven adenovirus infection, as reflected by MESO4 cells having the highest observed levels of Mdk and CAR expression (Figure 1a). However, in MSTO cells, which show high Mdk expression but low CAR expression (Figure 1a), oncolytic effects were subtherapeutic compared to MESO4 cells.
Further enhancement of therapeutic efficacy has been reported by the synergistic combination of radiation and chemotherapy with oncolytic virotherapy [32], particularly when the oncolytic viruses are armed with suicide genes or immunomodulatory genes [7]. Accordingly, we tested the effect of arming the Mdk promoter-driven adenovirus with an HSV-TK suicide gene expression cassette. As expected, the in vitro cytocidal effects were significantly enhanced by treatment with the prodrug, GCV, (Figure 6), and in vivo antitumor activity was enhanced, resulting in complete tumor regression in the MSTO-xenograft mouse model (Figure 8b). However, in the MESO4-xenograft mouse model, GCV treatment was associated with a significant reduction of antitumoral efficacy in vivo, compared to the effectiveness of oncolytic virotherapy without GCV (Figure 8a). This reduction of antitumoral efficacy may result from inhibition of viral replication and from premature induction of tumor cell death due to suicide gene activation by GCV. Although most studies combining oncolytic virotherapy with suicide gene therapy have reported enhancement of therapeutic efficacy [33–36], there has been at least one study reporting that intracellular conversion of prodrug to active toxin can result in complete inhibition of oncolytic vaccinia virus replication [37]. It is likely that suicide gene function will be most useful in situations where oncolytic activity is subtherapeutic, and the timing of prodrug administration may be critical for optimal therapeutic synergy. On the other hand, suicide gene-mediated termination of virus replication may also be useful as a safety precaution to eliminate any residual virus remaining after tumor eradication.
There have only been two prior studies reporting transcriptional targeting of oncolytic adenovirus to mesothelioma cells. These studies used tumor-specific promoters derived from the survivin [28] and CREBBP/EP300 inhibitory protein 1 (CRI1) genes [38]. It is difficult to make a direct comparison to these studies as the survivin-targeted adenovirus employed a different panel of mesothelioma cell lines. In addition, in luciferase and viral replication assays the survivin promoter generally showed 10- to 100-fold lower activity than the constitutively active CMV promoter [28]. In contrast, we found that the Mdk promoter-driven oncolytic adenovirus generally showed 10-fold higher replication levels and cytolytic activity in mesothelioma cells than the equivalent control vector driven by CMV, while still maintaining reduced cytotoxicity to normal mesothelial cells.
In this context, it is interesting to note that the survivin-targeted adenovirus gave up to a 5-fold enhancement of infectivity in mesothelioma cell lines with fiber modifications, to increase CAR-independent binding [28]. While we found adequate levels of CAR expression in our mesothelioma cell panel, it is likely that oncolytic virotherapy with Mdk-driven adenoviruses can be further improved by combining tumor-targeted transcriptional control with re-targeted binding tropism via adenovirus fiber modification.
The previously reported CR11-driven adenovirus was tested in a similar panel of mesothelioma cells (H2452, H2052, MSTO) to those used here, but in this case, there was no CMV-driven control vector, and therefore it is difficult to draw a direct comparison. Notably, in that study the wild type CR11 promoter showed up to 10-fold higher luciferase induction, while a synthetic version containing four tandem repeats of the CR11 promoter showed up to 30-fold higher induction, compared to normal mesothelial cells [38]. Hence, additional engineering of the Mdk promoter may also result in further gains in promoter activity while maintaining selectivity.
In conclusion, we have identified the Mdk promoter as a tumor-specific regulatory element that is highly useful for targeting oncolytic adenovirus to malignant mesothelioma. As the Mdk gene is highly upregulated in a variety of other cancers, this may represent a highly promising general strategy for transcriptionally-targeted oncolytic virotherapy.
Acknowledgments
We thank members of the Joint-Use Research Facilities of the Hyogo College of Medicine for their technical assistance. This work was supported by the Osaka Cancer Research Foundation (SK); Grant-in-Aid for Researchers, Hyogo College of Medicine (SK); and Grant-in-Aid for Promotion of Technical Seeds in Advanced Medicine, Hyogo College of Medicine (SK).
REFERENCES
- 1.Ismail-Khan R, Robinson LA, Williams CC, Jr., et al. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006;13:255–263. doi: 10.1177/107327480601300402. [DOI] [PubMed] [Google Scholar]
- 2.Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–2090. doi: 10.1200/JCO.2008.19.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Most RG, Robinson BW, Nelson DJ. Gene therapy for malignant mesothelioma: beyond the infant years. Cancer Gene Ther. 2006;13:897–904. doi: 10.1038/sj.cgt.7700935. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 5.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 6.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 7.Liu TC, Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–884. doi: 10.1038/gt.2008.72. [DOI] [PubMed] [Google Scholar]
- 8.Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–143. doi: 10.1016/S0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- 9.Li YS, Milner PG, Chauhan AK, et al. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science. 1990;250:1690–1694. doi: 10.1126/science.2270483. [DOI] [PubMed] [Google Scholar]
- 10.Muramatsu H, Muramatsu T. Purification of recombinant midkine and examination of its biological activities: functional comparison of new heparin binding factors. Biochem Biophys Res Commun. 1991;177:652–658. doi: 10.1016/0006-291x(91)91838-4. [DOI] [PubMed] [Google Scholar]
- 11.Mashour GA, Ratner N, Khan GA, et al. The angiogenic factor midkine is aberrantly expressed in NF1-deficient Schwann cells and is a mitogen for neurofibroma-derived cells. Oncogene. 2001;20:97–105. doi: 10.1038/sj.onc.1204026. [DOI] [PubMed] [Google Scholar]
- 12.Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57:1814–1819. [PubMed] [Google Scholar]
- 13.Owada K, Sanjo N, Kobayashi T, et al. Midkine inhibits caspase-dependent apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase in cultured neurons. J Neurochem. 1999;73:2084–2092. [PubMed] [Google Scholar]
- 14.Qi M, Ikematsu S, Ichihara-Tanaka K, et al. Midkine rescues Wilms' tumor cells from cisplatin-induced apoptosis: regulation of Bcl-2 expression by Midkine. J Biochem. 2000;127:269–277. doi: 10.1093/oxfordjournals.jbchem.a022604. [DOI] [PubMed] [Google Scholar]
- 15.Kojima S, Inui T, Muramatsu H, et al. Midkine is a heat and acid stable polypeptide capable of enhancing plasminogen activator activity and neurite outgrowth extension. Biochem Biophys Res Commun. 1995;216:574–581. doi: 10.1006/bbrc.1995.2661. [DOI] [PubMed] [Google Scholar]
- 16.Kadomatsu K, Hagihara M, Akhter S, et al. Midkine induces the transformation of NIH3T3 cells. Br J Cancer. 1997;75:354–359. doi: 10.1038/bjc.1997.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyauchi M, Shimada H, Kadomatsu K, et al. Frequent expression of midkine gene in esophageal cancer suggests a potential usage of its promoter for suicide gene therapy. Jpn J Cancer Res. 1999;90:469–475. doi: 10.1111/j.1349-7006.1999.tb00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aridome K, Tsutsui J, Takao S, et al. Increased midkine gene expression in human gastrointestinal cancers. Jpn J Cancer Res. 1995;86:655–661. doi: 10.1111/j.1349-7006.1995.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato M, Shinozawa T, Kato S, Awaya A, Terada T. Increased midkine expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2000;124:848–852. doi: 10.5858/2000-124-0848-IMEIHC. [DOI] [PubMed] [Google Scholar]
- 20.Garver RI, Jr., Chan CS, Milner PG. Reciprocal expression of pleiotrophin and midkine in normal versus malignant lung tissues. Am J Respir Cell Mol Biol. 1993;9:463–466. doi: 10.1165/ajrcmb/9.5.463. [DOI] [PubMed] [Google Scholar]
- 21.Garver RI, Jr., Radford DM, Donis-Keller H, Wick MR, Milner PG. Midkine and pleiotrophin expression in normal and malignant breast tissue. Cancer. 1994;74:1584–1590. doi: 10.1002/1097-0142(19940901)74:5<1584::aid-cncr2820740514>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.Kaname T, Kadomatsu K, Aridome K, et al. The expression of truncated MK in human tumors. Biochem Biophys Res Commun. 1996;219:256–260. doi: 10.1006/bbrc.1996.0214. [DOI] [PubMed] [Google Scholar]
- 23.Maeda S, Shinchi H, Kurahara H, et al. Clinical significance of midkine expression in pancreatic head carcinoma. Br J Cancer. 2007;97:405–411. doi: 10.1038/sj.bjc.6603879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsutsui J, Kadomatsu K, Matsubara S, et al. A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms' tumor and other human carcinomas. Cancer Res. 1993;53:1281–1285. [PubMed] [Google Scholar]
- 25.Fiegel HC, Kaifi JT, Wachowiak R, et al. Midkine is highly expressed in neuroblastoma tissues. Pediatr Surg Int. 2008;24:1355–1359. doi: 10.1007/s00383-008-2263-0. [DOI] [PubMed] [Google Scholar]
- 26.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 27.Kubo S, Seleme MC, Soifer HS, et al. L1 retrotransposition in nondividing and primary human somatic cells. Proc Natl Acad Sci U S A. 2006;103:8036–8041. doi: 10.1073/pnas.0601954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu ZB, Makhija SK, Lu B, et al. Targeting mesothelioma using an infectivity enhanced survivin-conditionally replicative adenoviruses. J Thorac Oncol. 2006;1:701–711. doi: 10.1097/01243894-200609000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson DJ, Robinson BW, Allan J, van der Most R. Gene therapy of mesothelioma. Expert Opin Biol Ther. 2005;5:1039–1049. doi: 10.1517/14712598.5.8.1039. [DOI] [PubMed] [Google Scholar]
- 30.Albelda SM, Wiewrodt R, Sterman DH. Gene therapy for lung neoplasms. Clin Chest Med. 2002;23:265–277. doi: 10.1016/s0272-5231(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 31.Sterman DH, Recio A, Vachani A, et al. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res. 2005;11:7444–7453. doi: 10.1158/1078-0432.CCR-05-0405. [DOI] [PubMed] [Google Scholar]
- 32.Adusumilli PS, Chan MK, Chun YS, et al. Cisplatin-induced GADD34 upregulation potentiates oncolytic viral therapy in the treatment of malignant pleural mesothelioma. Cancer Biol Ther. 2006;5:48–53. doi: 10.4161/cbt.5.1.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–413. [PubMed] [Google Scholar]
- 34.Nanda D, Vogels R, Havenga M, Avezaat CJ, Bout A, Smitt PS. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001;61:8743–8750. [PubMed] [Google Scholar]
- 35.Porosnicu M, Mian A, Barber GN. The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene. Cancer Res. 2003;63:8366–8376. [PubMed] [Google Scholar]
- 36.Barton KN, Paielli D, Zhang Y, Koul S, Brown SL, Lu M, Seely J, Kim JH, Freytag SO. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol Ther. 2006;13:347–356. doi: 10.1016/j.ymthe.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Puhlmann M, Gnant M, Brown CK, Alexander HR, Bartlett DL. Thymidine kinase-deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor-directed gene therapy. Hum Gene Ther. 1999;10:649–657. doi: 10.1089/10430349950018724. [DOI] [PubMed] [Google Scholar]
- 38.Fukazawa T, Matsuoka J, Naomoto Y, Maeda Y, Durbin ML, Tanaka N. Malignant pleural mesothelioma-targeted CREBBP/EP300 inhibitory protein 1 promoter system for gene therapy and virotherapy. Cancer Res. 2008;68:7120–7129. doi: 10.1158/0008-5472.CAN-08-0047. [DOI] [PubMed] [Google Scholar]