Abstract
Background
Endemic transmission of measles continues in many countries that have a high human immunodeficiency virus (HIV) burden. The effects that HIV infection has on immune responses to measles and to measles vaccine can impact measles elimination efforts. Assays to measure antibody include the enzyme immunoassay (EIA), which measures immunoglobulin G (IgG) to all measles virus (MV) proteins, and the plaque reduction neutralization (PRN) assay, which measures antibody to the hemagglutinin and correlates with protection. Antibody avidity may affect neutralizing capacity.
Methods
HIV-infected and HIV-uninfected Zambian children were studied after measles vaccination (n = 44) or MV infection (n = 57). Laboratory or wild-type MV strains were used to infect Vero or Vero/signaling lymphocyte-activation molecule (SLAM) cells in PRN assays. IgG to MV was measured by EIA, and avidity was determined by ammonium thiocyanate dissociation.
Results
HIV infection impaired EIA IgG responses after vaccination and measles but not PRN responses measured using laboratory-adapted MV. Avidity was lower among HIV-infected children 3 months after vaccination and 1 and 3 months after measles. Neutralization of wild-type MV infection of Vero/SLAM cells correlated with IgG avidity.
Conclusion
Lower antibody quality and quantity in HIV-infected children after measles vaccination raise challenges for assuring the long-term protection of these children. Antibody quality in children receiving antiretroviral therapy requires assessment.
Until the recent acceleration of measles control efforts, measles was a leading cause of vaccine-preventable mortality in children <5 years of age in low-income countries [1]. Many deaths due to measles occurred in sub-Saharan Africa, where almost 90% of global pediatric HIV infections occur [2, 3]. Although measles deaths in Africa have been greatly reduced, sustaining these reductions requires maintaining high levels of vaccine coverage and vaccine effectiveness. Infants born to HIV-infected women have lower levels of measles virus (MV)–specific transplacental antibody and often become susceptible to infection before administration of the live attenuated measles vaccine at 9 months of age [3, 4]. In addition, HIV infection is associated with a greater severity of measles [5], higher measles mortality [6], and prolonged MV RNA shedding [7]. As antiretroviral therapy becomes more available, the quality of the immune responses of HIV-infected individuals to measles vaccine and measles will become increasingly important for measles control efforts [8].
Neutralizing antibody provides the best correlate of protection from MV infection [9]. In a study in Zambia, the quantity of neutralizing antibody initially produced in response to measles vaccination at age 9 months, as measured by the standard plaque reduction neutralization (PRN) assay, did not appear to differ between HIV-infected and HIV-uninfected children, but titers waned rapidly in HIV-infected children, suggesting that B cells failed to mature into long-lived plasma cells [10]. A study in Malawi using an enzyme immunoassay (EIA) for measurement of MV-specific immunoglobulin G (IgG) after vaccination at age 6 and 9 months with the same vaccine used in Zambia showed no significant difference in response to the first dose but lower rates of seroconversion after the second dose in HIV-infected children [11]. A study in the United States found lower titers and lower avidity [12] in HIV-infected children. It is not known whether differences between these results reflect differences in the vaccines delivered, the populations studied, assay sensitivity, or the types of antibodies being measured by each assay.
EIA measures IgG to many MV proteins, including nonprotective antibody to the abundant nucleocapsid (N) protein, whereas the PRN assay measures protective antibody to the hemagglutinin (H) protein [9]. H has 2 overlapping binding sites that interact variably with the 2 known cellular receptors, the signaling lymphocyte-activation molecule (SLAM; CD150) and the membrane cofactor protein (CD46) [13–16]. Wild-type MV strains that cause natural disease preferentially bind to SLAM, which is expressed on activated T cells, B cells, and antigen-presenting cells, whereas laboratory-adapted MV strains used in PRN assays can also bind to CD46, which is expressed on all nucleated cells [17, 18].
MV H binds to SLAM with higher affinity than CD46, so antibody with higher avidity may be required to neutralize the wild-type MV interaction with SLAM than to neutralize the interaction between laboratory-adapted MV and CD46 [13, 19]. Higher-avidity antibodies will bind at lower concentrations and are more likely to be protective [20]. Avidity maturation occurs in the germinal centers of secondary lymphoid tissue and is correlated with the development of long-lived antibody-secreting plasma cells [21], so impaired avidity maturation in response to vaccination may contribute to failure of protection. Furthermore, low-avidity antibody may predispose to formation of immune complexes in the event of wild-type MV infection, as observed for atypical measles after immunization with a formalin-inactivated vaccine [22].
To better understand the effect of HIV infection on antibody responses to MV and to determine the influence of assay type on the results, we studied the development of antibody avidity, IgG isotypes, specificity for MV proteins, and neutralizing capacity after vaccination or natural measles.
METHODS
Study populations
Samples were collected during a study of the immunogenicity of the Edmonston-Zagreb measles vaccine (Berna Biotec) delivered to Zambian children at 9 months of age from 2000 through 2002 [10]. A questionnaire was administered and blood was collected at the time of vaccination and 3 months after vaccination. Plasma was available from 44 vaccinated children (23 boys), including 29 HIV-uninfected and 15 HIV-infected children.
Samples were also available from a study of children with measles admitted to the University Teaching Hospital in Lusaka, Zambia, from 1998 through 2000 [23]. A questionnaire was administered and blood was collected at hospital admission, at discharge, and at 1- and 3-month follow-up visits. Plasma samples were available from 57 hospitalized children (26 boys), of which 33 were from HIV-uninfected children and 24 were from HIV-infected children. Measles was confirmed by detection of MV-specific immunoglobulin M by EIA (Wampole Laboratories). For analysis, samples were grouped on the basis of the number of days after the onset of rash: entry (0–7 days; n = 47), discharge (8–21 days; n = 27), first follow-up (22–63 days; n = 53), and second follow-up (64–175 days; n = 21).
For both groups, HIV infection was confirmed by detection of plasma HIV RNA using a reverse-transcriptase polymerase chain reaction assay (Amplicor HIV-1 Monitor, version 1.5; Roche Molecular Systems). At the time of the study, children were not receiving antiretroviral treatment in Zambia. The percentage of CD4+ T cells in whole blood was determined using a FACScan flow cytometer and CellQuest software (version 1.2; Becton Dickinson). For all children, written informed consent was obtained from parents or guardians before enrollment. Studies were approved by the institutional review boards of the University of Zambia, the London School of Hygiene and Tropical Medicine, and the Johns Hopkins Bloomberg School of Public Health.
MV strains
The Zambia strain of wild-type MV (D2 genotype) was recovered by inoculating B95a cells with a nasopharyngeal swab sample collected in 2001 from an 11-month-old boy. Stocks of Zambia wild-type virus were grown in Vero cells expressing SLAM [24]. Chicago-1 MV stocks were grown by infecting Vero cells at a multiplicity of 0.001 which provided a high titer stock by 5 days after infection. Edmonston MV stocks (Center for Biologics Evaluation and Research, Food and Drug Administration) were prepared and used as described previously [10, 25].
Assays for MV-specific neutralizing antibody
A modified PRN assay was used [10]. Plasma samples were tested in parallel with the Second International World Health Organization Serum Standard 66/202 (5000 mIU/mL). For samples from vaccinated children, the assay was previously performed using the Edmonston strain of MV for infection of Vero cells [10]. For samples from patients with measles, the assay was performed using both the Chicago-1 and Zambia strains of MV for infection of Vero or Vero/SLAM cells. Efficiency of plaque formation by Chicago-1 MV is similar on Vero and Vero/SLAM cells, and the uncorrected PRN titers for the international standard serum are similar for Chicago-1 on Vero (2310) and Vero/SLAM (2244) cells. The standard serum has a PRN titer of 1872 for Zambia MV on Vero/SLAM cells.
Assays for MV-specific EIA antibody and avidity
MV-specific IgG binding antibody, avidity, and isotypes were measured by EIA, as described elsewhere [26]. Briefly, 96-well Maxisorp plates (Nalgene Nunc) were coated overnight at 4°C with 1 µg of Edmonston MV-infected Vero cell lysate (Advanced Biotechnologies) per well diluted in NaHCO3 (pH 9.6), incubated with plasma diluted 1:100 in blocking buffer (2% skim milk in phosphate-buffered saline [PBS]), and detected with alkaline phosphatase–conjugated goat anti–human IgG (Accurate Chemicals). The substrate used was p-nitrophenyl phosphate (Sigma). Absorbance was read at 405 nm using SOFTmax PRO software (version 3.1.1; Molecular Devices), and data are expressed as optical density (OD) values. A PBS negative control and a laboratory standard positive control (OD > 2.5) were included in each assay.
Avidity was measured as described elsewhere [26]. Briefly, plates were incubated with plasma samples as described above, washed and incubated at room temperature for 15 min with 50 µL of increasing concentrations of ammonium thiocyanate (NH4SCN) (0–3 mol/L) in 0.5 mol/L increments. The plates were washed, and IgG was detected as described above. The avidity index (AI) for each sample was defined as the concentration of NH4SCN required to reduce antibody binding by 50% [27, 28]. Samples with absorbance readings of <0.3 in the absence of NH4SCN were not analyzed for avidity.
MV protein–specific IgG was measured using the above-described protocol except that plates were coated with lysates of L cells expressing Edmonston MV H (1:20) or F (1:10) proteins [29] or with N protein expressed in baculovirus (0.5 µg/mL) [30]. L cell lysates were stored in PBS with 100 mmol/L glycine, 1% Triton X-100 and protease inhibitors. For determination of IgG isotypes, horseradish peroxidase–conjugated sheep anti–human IgG1, IgG2, IgG3, and IgG4 (The Binding Site) were used as secondary antibodies. The substrate used was 3,3′,5,5′-tetra-methylbenzidine (Sigma), and absorbance was read at 655 nm.
Statistical analysis
The Wilcoxon signed-rank test was used to compare values at vaccination and 3 months after vaccination and to compare changes in values in hospitalized children with time. The Wilcoxon rank-sum test was used to compare unpaired samples between HIV-uninfected and HIV-infected children. The Spearman rank correlation coefficient was used to determine correlations between results of different assays. All comparisons were performed using Stata software (release 10.0, StataCorp).
RESULTS
Responses to measles vaccine
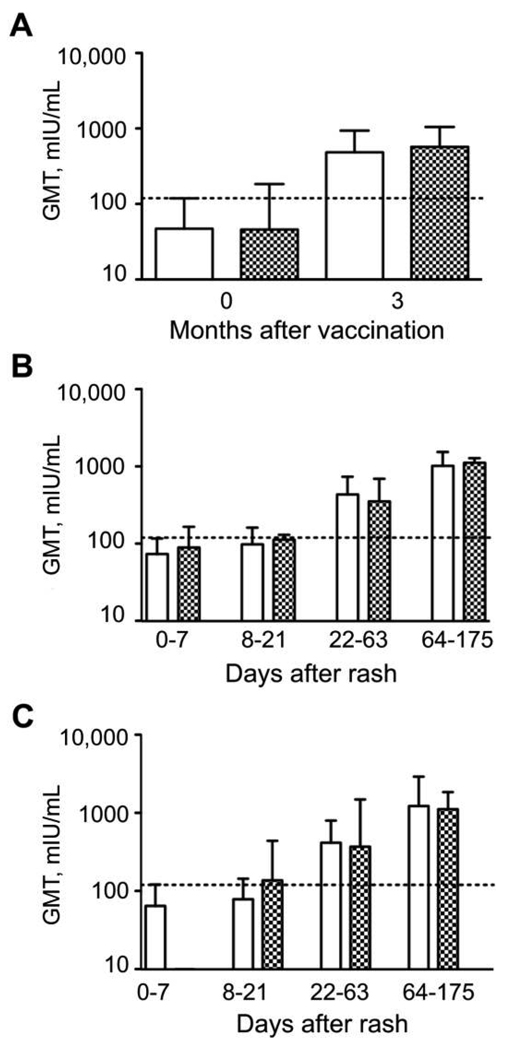
To assess the neutralizing capacity of plasma samples, PRN assays were performed using the Edmonston MV strain to infect Vero cells [10] (Figure 1). The geometric mean titer (GMT) for all children increased from 47 (95% confidence interval [CI], 23–98) at vaccination to 517 (95% CI, 333–803) 3 months after vaccination (n = 33). PRN antibody levels at 3 months did not differ between HIV-infected and HIV-uninfected children (P = .78). For HIV-uninfected children the GMT increased from 48 at vaccination to 485 three months after vaccination (n = 20; P < .001), and for HIV-infected children the GMT increased from 46 at vaccination to 570 three months after vaccination (n = 13; P = .02) (Figure 1A). Small amounts of PRN maternal antibody present at the time of vaccination were below the level predicted to interfere with vaccination and did not differ between groups [4].
Figure 1.
Geometric mean titers (GMTs) of measles virus (MV)–specific neutralizing antibodies after vaccination and after natural infection in human immunodeficiency virus (HIV)–infected and HIV-uninfected children. Plaque reduction neutralization (PRN) after vaccination was measured using the Edmonston strain of MV to infect Vero cells [10] (A). PRN after natural MV infection was measured using the Chicago-1 strain of MV to infect Vero (B) or Vero/signaling lymphocyte-activation molecule (C) cells. White bars indicate HIV-uninfected children, checkered bars indicate HIV-infected children, error bars indicate interquartile ranges, and dashed lines indicate the generally accepted protective level of PRN antibody (120 mIU/mL).
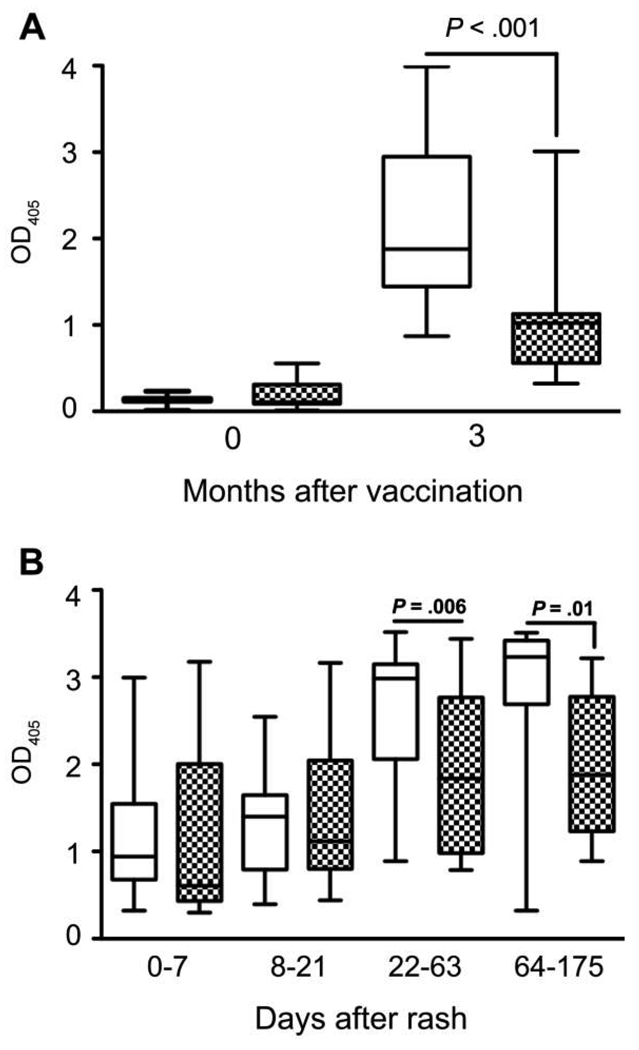
MV-specific IgG to MV proteins was measured by EIA (Figure 2). The median OD for all children increased from 0.1 at vaccination to 1.5 three months after vaccination (n = 44; P < .001). The median IgG OD increased from 0.1 to 1.9 for HIV-uninfected children (n = 29; P < .001) and from 0.1 to 1.0 for HIV-infected children (n = 15; P < .001) (Figure 2A). The median IgG OD 3 months after vaccination was lower for HIV-infected children than for HIV-uninfected children (P < .001). Three-month PRN antibody levels and EIA IgG levels were not strongly correlated (for HIV-infected children, ρ = −0.10 and P = .73; for HIV-uninfected children, ρ = 0.4 and P = .08).
Figure 2.
Measles virus–specific immunoglobulin G antibody responses, determined by enzyme immunoassay after vaccination (A) and infection (B) in human immunodeficiency virus (HIV)–uninfected (white boxes) and HIV-infected (checkered boxes) children. Boxes indicate upper and lower quartiles, lines inside boxes indicate medians, and whisker bars indicate the 10th and 90th percentiles. P values were determined by the Wilcoxon signed-rank test or the Wilcoxon rank-sum test. OD405, optical density read at 405 nm.
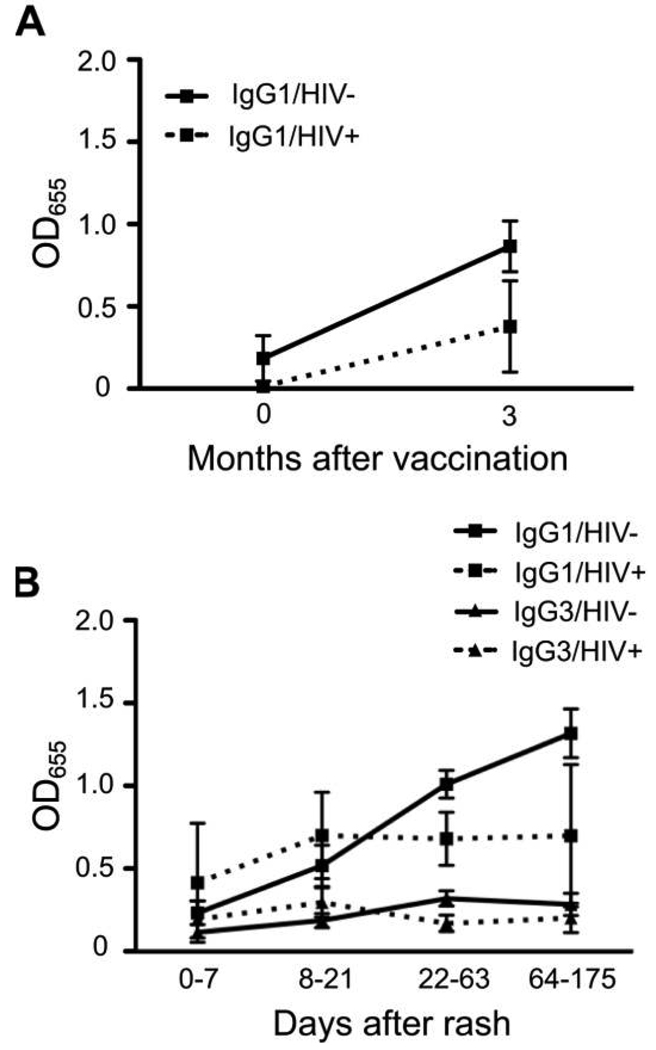
IgG1 was the predominant isotype, and IgG1 levels appeared to increase more after vaccination among HIV-uninfected children (0.1 to 0.8; P = .06) than among HIV-infected children (0 to 0.3; P = .5) children, but differences were not significant (P = .9) (Figure 3A). MV-specific IgG2, IgG3, and IgG4 were not detected.
Figure 3.
Isotypes of measles virus–specific immunoglobulin G (IgG) responses after vaccination (A) and infection (B), as determined by enzyme immunoassay. OD655, optical density read at 655 nm.
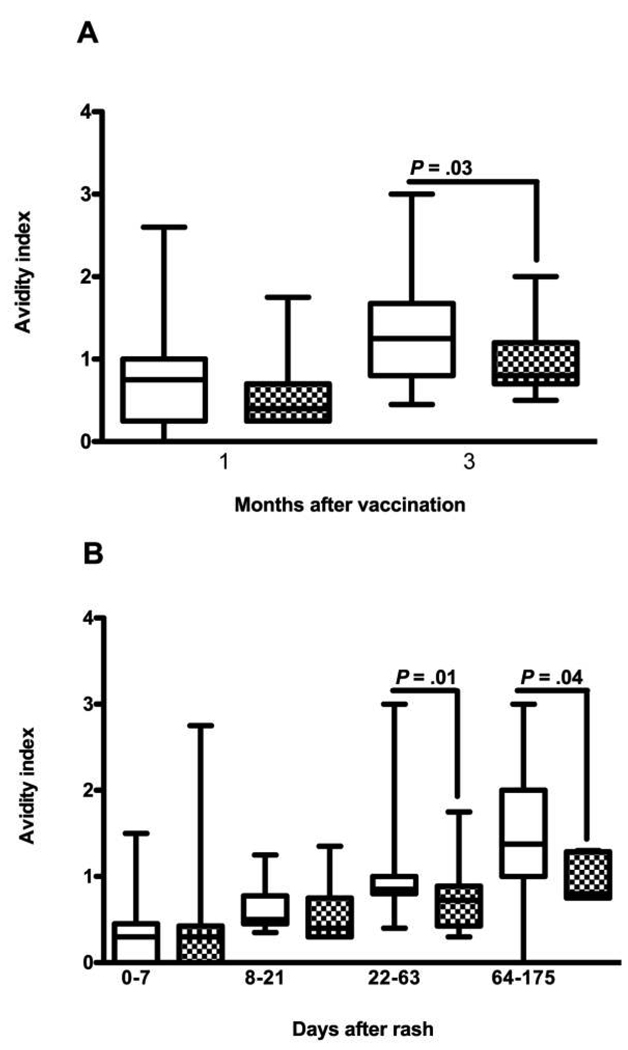
To assess differences in the quality of antibody produced, the avidity of MV-specific IgG was measured. The median AI 3 months after vaccination was 1.25 for HIV-uninfected children and was 0.8 for HIV-infected children (P = .03) (Figure 4A). The IgG OD values were correlated with AI for both HIV-uninfected children (ρ = 0.58; P = .001) and HIV-infected children (ρ = 0.59; P = .02).
Figure 4.
Measles virus–specific immunoglobulin G (IgG) avidity maturation after vaccination (A) and infection (B) in human immunodeficiency virus (HIV)–uninfected (white boxes) and HIV-infected (checkered boxes) children. Boxes indicate upper and lower quartiles, lines inside boxes indicate median values, and whisker bars indicate 10th and 90th percentiles. P values were determined by the Wilcoxon-signed rank test or the Wilcoxon rank-sum test.
Responses to wild-type MV infection
HIV-infected and HIV-uninfected children did not differ significantly by sex (P = .11), and the median age was 14 months for uninfected children and 10 months for infected children (P = .63). Standard PRN assays were performed on Vero cells (expressing CD46) to assess the neutralization capacity of plasma samples for the Chicago-1 MV strain (Figure 1). The GMT for all children with measles increased after infection, from 80 (95% CI, 56–115) at entry (n = 37) to 105 (95% CI, 81–136) at discharge (n = 30; P = .09 for entry vs discharge), to 392 (95% CI, 262–588) at first follow-up (n = 39; P < .001 for discharge vs first follow-up), and to 1058 (95% CI, 854–1312) at second follow-up (n = 20; P = .003 for first vs second follow-up). There was no evidence of any difference between HIV-infected and uninfected children at any time (P = .53 for all time points) (Figure 1B).
To identify potential differences in neutralization capacity when both MV receptors are available for MV entry, assays were performed on Vero/SLAM cells (Figure 1C). The GMT for all children with measles was 71 (95% CI, 55–142) at entry (n = 17), 92 (95% CI, 58–146) at discharge (n = 11; P = .63 for entry vs discharge), 402 (95% CI, 234–690) at first follow-up (n = 21; P = .02 for discharge vs first follow-up), and 1192 (95% CI, 646–2200) at second follow-up (n = 12; P = .06 for first vs second follow-up). There was no evidence of any difference between HIV-infected and HIV-uninfected children in PRN values or between values as measured on Vero or Vero/SLAM cells at any time (P = .93 for all time points) (Figure 1C).
MV-specific IgG to all MV proteins was measured by EIA (Figure 2). The median MV-specific IgG ODs for all children were 0.8 at entry (n = 47), 1.2 at discharge (n = 27; P = .74), 2.4 at first follow-up (n = 53; P < .001), and 3.2 at second follow-up (n = 21; P = .014). There was no evidence of a difference in IgG OD values between HIV-infected and HIV-uninfected children at entry (P = .33) or discharge (P = .79), but HIV-infected children had lower IgG OD values at first (1.8 vs 3.0; P = .006) and second (1.9 vs 3.2; P = .017) follow-up (Figure 2B). MV-specific neutralizing antibody and IgG levels were not strongly correlated for either HIV-infected or HIV-uninfected children at discharge or either follow-up visit.
MV-specific IgG1 was the predominant isotype produced in response to wild-type MV infection. MV-specific IgG1 levels increased through the second follow-up visit for HIV-uninfected children but plateaued after discharge for HIV-infected children (Figure 3B). However, there was no statistically significant difference in MV-specific IgG1 or IgG3 levels between HIV-infected and HIV-uninfected children at entry (P = .4 and P = .45), discharge (P = .6 and P = .2), or first (P = .08 and P = .11) or second (P = .3 and P = .2) follow-up, perhaps because of limited power to detect a significant difference. MV-specific IgG2 and IgG4 antibodies were not detected.
To determine whether there were differences in the MV proteins recognized by HIV-infected and HIV-uninfected children, IgG antibodies specific for MV N, H, and F were measured (Table 1). N-specific responses accounted for the largest proportion of MV-specific IgG among both HIV-infected and HIV-uninfected children. Both N and H OD values were lower among HIV-infected children, and F-specific responses were not detected.
Table 1.
Specificity of Immunoglobulin G for Measles Virus Hemagglutinin (H), Fusion (F), and Nucleocapsid (N) Proteins, as Determined by Enzyme Immunoassay after Natural Measles in Human Immunodeficiency Virus (HIV)–Uninfected and HIV-Infected Zambian Children
| H specific | F specific | N specific | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after rash | HIV uninfected |
No. | HIV infected |
No. | HIV uninfected |
No. | HIV infected |
No. | HIV uninfected |
No. | HIV infected |
No. |
| 0–7 (entry) | 0.12 (0.1) | 10 | 0.0 (0.1) | 3 | 0.02 (0.1) | 10 | 0 | 3 | 0.46 (0.6) | 10 | 0.4 (0.7) | 4 |
| 8–21 (discharge) | 0.12 (0.1) | 6 | 0.1 (0.0) | 3 | 0.02 (0.1) | 2 | 0 | 3 | 0.80 (0.8) | 6 | 0.3 (0.3) | 6 |
| 22–63 (first follow-up) | 0.15 (0.2) | 16 | 0.1 (0.1) | 2 | 0.04 (0.1) | 4 | 0 | 2 | 1.14 (1.1) | 15 | 0.5 (0.4) | 4 |
| 64–175 (second follow-up) | 0.46 (0.7) | 4 | 0.1 (0.1) | 2 | 0.27 (0.7) | 8 | 0 | 2 | 1.38 (1.6) | 3 | 0.5 (0.1) | 2 |
NOTE. Data are the mean (standard deviation) optical density value at 405 nm for each group.
To determine the maturation of antibody avidity after natural MV infection, AIs were determined (Figure 4). The median AI for all children was 0.3 (n = 48) at entry, 0.5 (n = 27) at discharge, 0.8 at first follow-up (n = 52), and 1.25 at second follow-up (n = 22). The median AI for HIV-uninfected children was 0.3 at entry (n = 29), 0.5 at discharge (n = 13; P = .008 for entry vs discharge), 0.85 at first follow-up (n = 32; P = .003 for discharge vs first follow-up), and 1.38 at second follow-up (n = 14; P = .006 for first vs second follow-up). The median AI for HIV-infected children was 0.3 at entry (n = 19), 0.38 at discharge (n = 14; P = .07 for entry vs discharge), 0.75 at first follow-up (n = 20; P = .05 for discharge vs first follow-up), and 0.8 at second follow-up (n = 8; P = .05 for first vs second follow-up) (Figure 4B). AI values were lower for HIV-infected children at first follow-up (P = .014) and at second follow-up (P = 0.04) than for HIV-uninfected children. AI did not correlate with the percentage of CD4+ T cells at entry for HIV-infected or HIV-uninfected children.
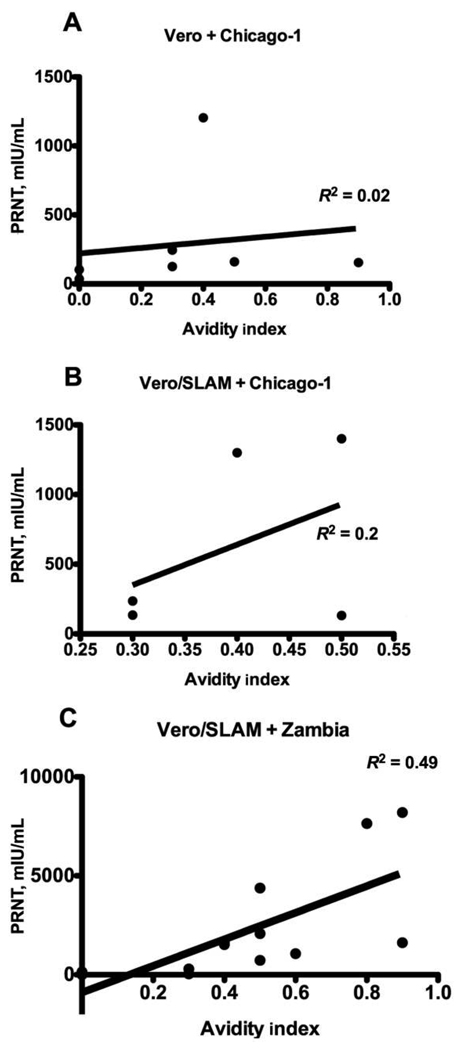
To assess the potential relevance of AI to protection from reinfection, correlation between AI and PRN titers was assessed (Figure 5). There was no strong evidence of a correlation with AI when PRN was measured using the Chicago-1 strain of MV on Vero cells (ρ = 0.618; P = .139) (Figure 5A) or Vero/SLAM cells (ρ = 0.158; P = .783) (Figure 5B). However, AI and neutralization were correlated when PRN was measured using the wild-type Zambia MV strain for infection of Vero/SLAM cells (ρ = 0.78; P = .002) (Figure 5C).
Figure 5.
Linear regression analysis showing correlation between avidity index and plaque reduction neutralization for plasma samples from children with measles tested on Vero (A) and Vero/signaling lymphocyte-activation molecule (SLAM) (B) cells using Chicago-1 measles virus and on Vero/SLAM cells using a wild-type measles virus isolate from Zambia (C).
DISCUSSION
In the present study, we have shown that HIV infection influences both the quantity and quality of the antibody produced in response to measles vaccination and to measles. Identification of these defects depends on the tests used to assess MV-specific antibody. We found that HIV infection does not affect production of neutralizing antibody, as measured by the standard PRN assay, during the first 3 months after immunization at age 9 months or after natural infection. However, vaccine-induced neutralizing antibody wanes rapidly, suggesting that a defect in the quality of the response exists [10].
EIA, using a lysate of MV-infected cells as antigen, measures IgG binding to many MV proteins but is dominated by the response to N, and EIA is less sensitive than the PRN assay for detecting immunity to MV when antibody levels are low [31, 32]. Our analysis by EIA showed that both the amount and the avidity of MV-specific IgG produced were impaired by HIV infection. The development of high-avidity antibody correlated with the ability to neutralize the infection of Vero/SLAM cells with a wild-type strain of MV. These experiments suggest that HIV-infected children are less well protected from MV infection by vaccination than are HIV-uninfected children. This suggestion is supported by the observation that HIV-infected children hospitalized with measles in Zambia are significantly more likely than HIV-uninfected children to have a history of vaccination [6]. Furthermore, specific aspects of the antibody tests chosen to measure the response to measles need to be considered in designing studies to assess immune responses in this population.
These experiments also confirm previous observations that EIA responses to vaccination are lower in HIV-infected children than in HIV-uninfected children [11, 12]. Prior experiments also showed that antibody levels waned over time and were not augmented by repeat vaccination [12]. It is presumed that the differences in the results obtained by the 2 methods are due to the differences in the specificities being analyzed. EIA antibody was primarily IgG1 and was directed against N, whereas PRN antibody can be of any subclass and is primarily directed against specific epitopes on H. Understanding the isotype profile is likely to be important because IgG subclasses differ in many biologic properties—including half-life, Fc receptor binding, and complement activation—that alter effectiveness.
The mechanisms by which HIV infection impairs IgG responses and avidity maturation have yet to be fully elucidated. Because most children are infected with HIV during the perinatal period before encountering MV antigens, avidity maturation occurs in the context of an already-impaired immune system. Avidity maturation is T cell dependent and requires the activation-induced, cytidine deaminase–mediated process of somatic hypermutation of the variable regions of antibody genes [33, 34], all of which are impaired in HIV-infected individuals [35–37]. We did not observe a correlation between avidity and CD4+ T cell counts, but it is likely that HIV-induced functional alteration of CD4+ T cells contributes to impaired antibody responses.
HIV can also have direct effects on the B cell compartment [38]. Ongoing HIV replication is associated with B cell dysregulation, increased B cell activation and turnover, and an increase in immature and transitional B cells [39, 40]. There is loss of memory B cells and decreases in levels of previously induced antibody [39, 41–43]. HIV Nef protein can penetrate bystander B cells and inhibit class-switch recombination and IgG production [44]. In addition, chemokine and chemokine receptor expression important for B cell migration within lymphoid tissues, for germinal center formation, and for B cell homing to bone marrow is decreased by HIV infection [39]. Germinal center abnormalities are seen throughout the course of HIV infection as HIV uptake by follicular dendritic cells and can lead to overload and network disruption [39, 45]. It is likely that HIV-induced effects on T and B cells, on germinal center integrity, and on antigen presentation all contribute to the reduced quantity and quality of MV-specific antibody observed in Zambian children.
The observation that neutralization and avidity were not correlated when laboratory-adapted strains of MV were used to infect Vero or Vero/SLAM cells but were correlated when a wild-type field isolate was used to infect Vero/SLAM cells indicates the importance of the high-affinity interaction between SLAM and the H protein of field isolates. Furthermore, it suggests that the avidity of the antibody induced by vaccination is important for protection against wild-type MV infection.
Our findings show that HIV impairs qualitative features of the antibody response to MV vaccination and infection. Increased access to antiretroviral treatment—with particular emphasis on reducing mother-to-child transmission—should reduce the prevalence of HIV among children. For children who are infected, treatment is increasingly available, and the response of HIV-infected children upon treatment to measles vaccination and revaccination requires evaluation. The present study underscores the need for a variety of assays to effectively measure the immunogenicity of measles vaccine in HIV-infected persons and for the additional information provided by measurement of avidity.
Acknowledgments
We thank Judy Beeler and Susette Audet of the Food and Drug Administration for performing PRN assays after vaccination and Brandyn Lau for expert technical assistance.
Financial support: National Institute of Allergy and Infectious Diseases (grant AI23047 to D.E.G. and Division of Intramural Research support to T.C.Q.); Wellcome Trust–Burroughs Fund Infectious Disease Initiative (grant GR059114MA to W.J.M.); Bill and Melinda Gates Foundation (grant 3522 to D.E.G.).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting, Washington, DC, 25–28 October 2008 (abstract 810).
References
- 1.Wolfson LJ, Strebel PM, Gacic-Dobo M, Hoekstra EJ, McFarland JW, Hersh BS. Has the 2005 measles mortality reduction goal been achieved? A natural history modelling study. Lancet. 2007;369:191–200. doi: 10.1016/S0140-6736(07)60107-X. [DOI] [PubMed] [Google Scholar]
- 2.Cutts FT, Henao-Restrepo A, Olive JM. Measles elimination: progress and challenges. Vaccine. 1999;17 Suppl 3:S47–S52. doi: 10.1016/s0264-410x(99)00309-6. [DOI] [PubMed] [Google Scholar]
- 3.Moss WJ, Cutts F, Griffin DE. Implications of the HIV epidemic for control and eradication of measles. Clin Infect Dis. 1999;29:106–112. doi: 10.1086/520136. [DOI] [PubMed] [Google Scholar]
- 4.Scott S, Moss WJ, Cousens S, et al. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis. 2007;45:1417–1424. doi: 10.1086/522989. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Measles in HIV-infected children, United States. MMWR Morb Mortal Wkly Rep. 1988;37:183–186. [PubMed] [Google Scholar]
- 6.Moss WJ, Fisher C, Scott S, et al. HIV type 1 infection is a risk factor for mortality in hospitalized Zambian children with measles. Clin Infect Dis. 2008;46:523–527. doi: 10.1086/526525. [DOI] [PubMed] [Google Scholar]
- 7.Permar SR, Moss WJ, Ryon JJ, et al. Prolonged measles virus shedding in human immunodeficiency virus–infected children, detected by reverse transcriptase–polymerase chain reaction. J Infect Dis. 2001;183:532–538. doi: 10.1086/318533. [DOI] [PubMed] [Google Scholar]
- 8.Scott S, Mossong J, Moss WJ, Cutts FT, Cousens S. Predicted impact of the HIV-1 epidemic on measles in developing countries: results from a dynamic age-structured model. Int J Epidemiol. 2008;37:356–367. doi: 10.1093/ije/dyn007. [DOI] [PubMed] [Google Scholar]
- 9.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 10.Moss WJ, Scott S, Mugala N, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–355. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 11.Helfand RF, Witte D, Fowlkes A, et al. Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J Infect Dis. 2008;198:1457–1465. doi: 10.1086/592756. [DOI] [PubMed] [Google Scholar]
- 12.Brunell PA, Vimal V, Sandu M, Courville TM, Daar E, Israele V. Abnormalities of measles antibody response in human immunodeficiency virus type 1 (HIV-1) infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:540–548. [PubMed] [Google Scholar]
- 13.Hashiguchi T, Kajikawa M, Maita N, et al. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc Natl Acad Sci U S A. 2007;104:19535–19540. doi: 10.1073/pnas.0707830104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 15.Naniche D, Varior-Krishnan G, Cervoni F, et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–898. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 17.Yanagi Y, Takeda M, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87:2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- 18.Erlenhofer C, Duprex WP, Rima BK, ter Meulen V, Schneider-Schaulies J. Analysis of receptor (CD46, CD150) usage by measles virus. J Gen Virol. 2002;83:1431–1436. doi: 10.1099/0022-1317-83-6-1431. [DOI] [PubMed] [Google Scholar]
- 19.Santiago C, Bjorling E, Stehle T, Casasnovas JM. Distinct kinetics for binding of the CD46 and SLAM receptors to overlapping sites in the measles virus hemagglutinin protein. J Biol Chem. 2002;277:32294–32301. doi: 10.1074/jbc.M202973200. [DOI] [PubMed] [Google Scholar]
- 20.Olszewska W, Obeid OE, Steward MW. Protection against measles virus-induced encephalitis by anti-mimotope antibodies: the role of antibody affinity. Virology. 2000;272:98–105. doi: 10.1006/viro.2000.0285. [DOI] [PubMed] [Google Scholar]
- 21.Phan TG, Paus D, Chan TD, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med. 2003;9:1209–1213. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 23.Moss WJ, Monze M, Ryon JJ, Quinn TC, Griffin DE, Cutts F. Prospective study of measles in hospitalized, human immunodeficiency virus (HIV)-infected and HIV-uninfected children in Zambia. Clin Infect Dis. 2002;35:189–196. doi: 10.1086/341248. [DOI] [PubMed] [Google Scholar]
- 24.Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa HY. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J Virol. 2001;75:4399–4401. doi: 10.1128/JVI.75.9.4399-4401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen BJ, Audet S, Andrews N, Beeler J. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26:59–66. doi: 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Nair N, Gans H, Lew-Yasukawa L, Long-Wagar AC, Arvin A, Griffin DE. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J Infect Dis. 2007;196:1339–1345. doi: 10.1086/522519. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald RA, Hosking CS, Jones CL. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods. 1988;106:191–194. doi: 10.1016/0022-1759(88)90196-2. [DOI] [PubMed] [Google Scholar]
- 28.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 29.Beauverger P, Buckland R, Wild F. Establishment and characterisation of murine cells constitutively expressing the fusion, nucleoprotein and matrix proteins of measles virus. J Virol Methods. 1993;44:199–210. doi: 10.1016/0166-0934(93)90055-v. [DOI] [PubMed] [Google Scholar]
- 30.Hummel KB, Erdman DD, Heath J, Bellini WJ. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol. 1992;30:2874–2880. doi: 10.1128/jcm.30.11.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den HS, Gageldonk-Lafeber AB, Van Binnendijk RS, van Gageldonk PG, Berbers GA. Comparison of measles virus-specific antibody titres as measured by enzyme-linked immunosorbent assay and virus neutralisation assay. Vaccine. 2003;21:4210–4214. doi: 10.1016/s0264-410x(03)00490-0. [DOI] [PubMed] [Google Scholar]
- 32.Cohen BJ, Dobias D, Andrews N. Comparison of plaque reduction neutralisation test (PRNT) and measles virus-specific IgG ELISA for assessing immunogenicity of measles vaccination. Vaccine. 2008;26:6392–6397. doi: 10.1016/j.vaccine.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 33.Cozine CL, Wolniak KL, Waldschmidt TJ. The primary germinal center response in mice. Curr Opin Immunol. 2005;17:298–302. doi: 10.1016/j.coi.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Dudley DD, Chaudhuri J, Bassing CH, Alt FW. Mechanism and control of V(D)J recombination versus class switch recombination: similarities and differences. Adv Immunol. 2005;86:43–112. doi: 10.1016/S0065-2776(04)86002-4. [DOI] [PubMed] [Google Scholar]
- 35.Scamurra RW, Miller DJ, Dahl L, et al. Impact of HIV-1 infection on VH3 gene repertoire of naive human B cells. J Immunol. 2000;164:5482–5491. doi: 10.4049/jimmunol.164.10.5482. [DOI] [PubMed] [Google Scholar]
- 36.Wisnewski A, Cavacini L, Posner M. Human antibody variable region gene usage in HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:31–38. doi: 10.1097/00042560-199601010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Poli G, Pantaleo G, Fauci AS. Immunopathogenesis of human immunodeficiency virus infection. Clin Infect Dis. 1993;17:S224–S229. [PubMed] [Google Scholar]
- 38.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cagigi A, Nilsson A, De MA, Chiodi F. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine. 2008;26:3016–3025. doi: 10.1016/j.vaccine.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 40.Malaspina A, Moir S, Ho J, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci USA. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moir S, Malaspina A, Pickeral OK, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–599. [PubMed] [Google Scholar]
- 42.De MA, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 43.Titanji K, De MA, Cagigi A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 44.Qiao X, He B, Chiu A, Knowles DM, Chadburn A, Cerutti A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol. 2006;7:302–310. doi: 10.1038/ni1302. [DOI] [PubMed] [Google Scholar]
- 45.Taruishi M, Terashima K, Dewan Z, et al. Role of follicular dendritic cells in the early HIV-1 infection: in vitro model without specific antibody. Microbiol Immunol. 2004;48:693–702. doi: 10.1111/j.1348-0421.2004.tb03480.x. [DOI] [PubMed] [Google Scholar]