Abstract
Background
Extracts of the medicinal plant Tripterygium wilfordii Hook F (TwHF) have been used in China for centuries to treat a spectrum of inflammatory diseases.
Objective
To compare the benefits and side effects of TwHF extract with those of sulfasalazine for the treatment of active rheumatoid arthritis.
Design
Randomized, controlled trial. A computer-generated code with random, permuted blocks was used to assign treatment.
Setting
2 U.S. academic centers (National Institutes of Health, Bethesda, Maryland, and University of Texas, Dallas, Texas) and 9 rheumatology subspecialty clinics (in Dallas and Austin, Texas; Tampa and Fort Lauderdale, Florida; Arlington, Virginia; Duncanville, Pennsylvania; Wheaton and Greenbelt, Maryland; and Lansing, Michigan).
Patients
121 patients with active rheumatoid arthritis and 6 or more painful and swollen joints.
Intervention
TwHF extract, 60 mg 3 times daily, or sulfasalazine, 1 g twice daily. Patients could continue stable doses of oral prednisone or nonsteroidal anti-inflammatory drugs but had to stop taking disease-modifying antirheumatic drugs at least 28 days before randomization.
Measurements
The primary outcome was the rate of achievement of 20% improvement in the American College of Rheumatology criteria (ACR 20) at 24 weeks. Secondary end points were safety; radiographic scores of joint damage; and serum levels of interleukin-6, cholesterol, cortisol, and adrenocorticotropic hormone.
Results
Outcome data were available for only 62 patients at 24 weeks. In a mixed-model analysis that imputed data for patients who dropped out, 65.0% (95% CI, 51.6% to 76.9%) of the TwHF group and 32.8% (CI, 21.3% to 46.0%) of the sulfasalazine group met the ACR 20 response criteria (P = 0.001). Patients receiving TwHF also had significantly higher response rates for ACR 50 and ACR 70 in mixed-model analyses. Analyses of only completers showed similar significant differences between the treatment groups. Significant improvement was demonstrated in all individual components of the ACR response, including the Health Assessment Questionnaire disability score. Interleukin-6 levels rapidly and significantly decreased in the TwHF group. Although not statistically significant, radiographic progression was lower in the TwHF group. The frequency of adverse events was similar in both groups.
Limitations
Only 62% and 41% of patients continued receiving TwHF extract and sulfasalazine, respectively, during the 24 weeks of the study. Long-term outcome data were not collected on participants who discontinued treatment.
Conclusion
In patients who continued treatment for 24 weeks and could also use stable oral prednisone and nonsteroidal anti-inflammatory drugs, attainment of the ACR 20 response criteria was significantly greater with TwHF extract than with sulfasalazine.
Primary Funding Source
National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Rheumatoid arthritis is characterized by chronic inflammation of the joint lining (synovial membrane) (1), which causes pain and swelling of diarthrodial joints. Over time, uncontrolled disease results in progressive joint damage, disability, and increased mortality (2). The evolving understanding of the immune mechanisms that perpetuate the inflammatory response has led to effective targeted therapies, including inhibitors of inflammatory cytokines (tumor necrosis factor, interleukin-1, and interleukin-6), modulators of activation of CD4+ T cells and dendritic cells, and agents that deplete B cells (3, 4). Despite the clinical efficacy of these therapies, many patients have no clinically meaningful response or discontinue treatment because of adverse events. Furthermore, the limited availability of effective biologics in developing countries, the need for parenteral administration of the biologics, and the relatively high cost all restrict access to these therapies in many patients with rheumatoid arthritis around the world (5).
In traditional Chinese medicine, extracts of the roots of the medicinal vine Tripterygium wilfordii Hook F (TwHF) (known in China as “lei gong teng” or “thunder god vine”) have shown therapeutic promise in treating autoimmune and inflammatory conditions as well as cancer (6–8). More recently, different extracts of TwHF have been used in Chinese allopathic medicine for the treatment of autoimmune and inflammatory diseases, and small controlled trials reported good responses with TwHF extracts in patients with cadaveric kidney transplants (9, 10) and Crohn disease (11).
Context
In Chinese medicine, extracts of Tripterygium wilfordii Hook F (TwHF, known as “lei gong teng” or “thunder god vine”) are used to treat autoimmune and inflammatory conditions. Small clinical trials suggest that TwHF may benefit patients with rheumatoid arthritis.
Contribution
This trial compared TwHF extract with sulfasalazine in 121 patients with active rheumatoid arthritis who could continue oral prednisone and nonsteroidal anti-inflammatory drugs but not disease-modifying antirheumatic drugs. Among patients who continued treatment for 24 weeks, achievement of 20% improvement in American College of Rheumatology criteria was greater with TwHF than with sulfasalazine. Adverse event rates were similar.
Caution
Only 62% and 41% of patients continued TwHF and sulfasalazine treatment, respectively, and provided 24 weeks of data.
—The Editors
Of the approximately 380 metabolites isolated from the plant, 95% are terpenoids (12, 13). Three diterpenoids—triptolide, tripdiolide, and triptonide (13)—are the most abundant and account for the immunosuppressive and anti-inflammatory effects observed with the root extracts in both in vitro and in vivo studies (6). In 2 previous single-center trials of patients with rheumatoid arthritis, the extract was standardized by the content of triptolide and tripdiolide (14). This made it possible to use optimal doses identified in an open-label trial (15) for the design of a subsequent small placebo-controlled study (16). Although the number of patients was small, the apparent clinical impact and experimental results indicating potent inhibition of the expression of proinflammatory genes both in vitro and in vivo in animal models (17–21) provided the rationale for our multicenter, double-blind, active comparator trial of a standardized TwHF extract in patients with active rheumatoid arthritis.
METHODS
Design Overview
This randomized, controlled, 24-week study was conducted between March 2004 and October 2005. All participants provided written informed consent to enter the trial, and the institutional review boards at the participating sites approved the protocol. All investigators and outcome assessors were blinded to group assignment of the patients. Our objective was to determine whether therapy with TwHF extract, 180 mg/d, was statistically significantly better than therapy with sulfasalazine, 2 g/d, over 24 weeks in patients with rheumatoid arthritis by using standard outcome measures.
Setting and Participants
Our study was conducted at 11 U.S. centers: 2 academic centers (National Institutes of Health, Bethesda, Maryland, and University of Texas, Dallas, Texas) and 9 rheumatology subspecialty clinics (1 each in Dallas and Austin, Texas; Tampa and Fort Lauderdale, Florida; Arlington, Virginia; Duncanville, Pennsylvania; Wheaton and Greenbelt, Maryland; and Lansing, Michigan). Eligible patients had to be at least 18 years of age and have established rheumatoid arthritis, defined by the American College of Rheumatology (ACR) classification criteria (22) as rheumatoid arthritis lasting longer than 6 months. Eligible patients had active disease, defined as 6 or more painful and swollen joints, a visual analogue scale score for pain of at least 3 (on a scale of 1 to 10, with 1 being mild), and a C-reactive protein (CRP) level of 57.14 nmol/L or greater (≥0.6 mg/dL) or an erythrocyte sedimentation rate (ESR) greater than 25 mm/h. Patients who were taking any disease-modifying antirheumatic drug at screening underwent a 28-day washout period. The use of oral prednisone, at stable doses up to 7.5 mg/d, and nonsteroidal anti-inflammatory drugs were allowed as long as the dose was not changed for 28 days before randomization and the patient agreed to continue to take the medication during the study. Table 1 lists baseline patient characteristics.
Table 1.
Patient Characteristics at Baseline
| Characteristic | TwHF Group (n = 60) |
Sulfasalazine Group (n = 61) |
|---|---|---|
| Mean age (SD), y | 54 (11) | 52 (12) |
| Women, n (%) | 44 (73) | 54 (87) |
| Race, n (%)* | ||
| White | 35 (58) | 31 (51) |
| Black | 9 (15) | 15 (25) |
| Latino | 14 (23) | 12 (20) |
| Other | 2 (4) | 3 (4) |
| Medications at randomization, n (%)† | ||
| Oral prednisone | 17 (28) | 17 (28) |
| Methotrexate† | 8 (13) | 10 (16) |
| Other DMARD† | 7 (12) | 6 (10) |
| Hydroxychloroquine | 5 | 3 |
| Leflunomide | 1 | 3 |
| Minocycline | 1 | 0 |
| Adalimumab | 0 | 1 |
| Mean tender joints (SD), n ‡ | 34 (16) | 33 (17) |
| Mean swollen joints (SD), n ‡ | 24 (12) | 22 (13) |
| Mean pain score (SD)§ | 71 (18) | 72 (17) |
| Mean physical function score (SD)‖ | 1.65 (0.58) | 1.73 (0.63) |
| Mean global assessment score (SD)§ | ||
| Assessed by patient | 67 (18) | 69 (19) |
| Assessed by physician | 70 (18) | 69 (17) |
| Mean DAS 28 (SD) | 6.91 (1.01) | 6.95 (1.00) |
| Mean total radiographic score at baseline (SD)¶ | 27.3 (51.0) | 21.4 (31.2) |
| Erosive disease, %¶ | 67.4 | 60.5 |
| Mean total radiographic score at baseline (SD)¶** | 40.0 (59.6) | 34.0 (34.7) |
| Mean ESR (SD), mm/h | 49 (29) | 51 (23) |
| Mean CRP level (SD) | ||
| nmol/L | 255.2 (307.6) | 236.2 (272.4) |
| mg/dL | 2.68 (3.23) | 2.48 (2.86) |
CRP = C-reactive protein; DAS 28 = Disease Activity Score 28; DMARD = disease-modifying antirheumatic drug; ESR = erythrocyte sedimentation rate; TwHF = Tripterygium wilfordii Hook F.
Self-reported.
Patients receiving methotrexate and other DMARDs underwent a 4-week washout before randomization.
68 joints were assessed for tenderness, and 66 joints were assessed for swelling.
A 100-mm visual analogue scale was used, in which higher values indicate more severe pain or impairment of overall well-being.
Scores on the modified Health Assessment Questionnaire range from 0 to 3, with higher scores indicating greater disease severity.
Baseline erosions and radiographic scores were obtained from 43 participants in each group for whom 2 sets of radiographs were available. Values range from 0 to 440, with higher scores indicating more articular damage on radiographic evaluation.
In patients with erosive disease only.
Randomization and Interventions
We used a computer-generated, pseudo-random code (with random, permuted blocks) to assign patients to treatment groups across all centers. We assigned eligible patients at a 1:1 ratio to receive either TwHF extract, 180 mg/d, or sulfasalazine, 2 g/d. In the event of gastrointestinal intolerance, the protocol allowed for temporary dose reduction of 50%. As described elsewhere (15, 16), the triptolide and tripdiolide content of the ethanol and ethyl-acetate extract (measured by high-performance liquid chromatography [22]) was used to standardize the drug preparation for this study. On the basis of data on in vitro activity and in vivo toxicity, 30 mg of TwHF extract were formulated per capsule. Our study was conducted under the U.S Food and Drug Administration–approved Investigational New Drug application 39191.
Outcomes and Measurements
Patients were evaluated clinically and by laboratory measures at baseline, 2 weeks, and every 4 weeks for a total of 24 weeks. A rheumatologist or trained staff member masked to treatment allocation assessed the patients. Serum or plasma specimens were obtained from the patients at baseline, 4 weeks, and 24 weeks and stored at −80 °C until analysis. Radiographs of hands and feet were obtained at baseline and 24 weeks or at study discontinuation.
The primary end point was a 20% improvement at 24 weeks, as defined by ACR criteria (ACR 20) (23). To meet criteria, a patient must have 20% or greater improvement in both tender and swollen joints (68 tender and 66 swollen joints were assessed) and 20% or greater improvement in 3 or more of the following: the physician’s or patient’s assessment of global health status, the patient’s assessment of pain on a visual analogue scale, the patient’s assessment of function (using a modified version of the Health Assessment Questionnaire [HAQ]), and the serum CRP level.
Secondary end points included the efficacy of TwHF in achieving ACR 50 and ACR 70 responses at 24 weeks, the improvement in the European League Against Rheumatism Disease Activity Score 28 (DAS 28) measure, and a change in the Sharp–van der Heijde score of the hand and foot radiographs (24). Radiographs were obtained at baseline and at the end of the study and were scored by 2 independent readers who were blinded to the randomization schedule and the radiograph sequence. Drug adherence was assessed by using a daily diary and by pill counts. Body weight, blood pressure, and serum glucose level were measured at each visit.
Laboratory assessments included ESR (Westergren method); high-sensitivity CRP with normal levels up to 38.1 nmol/L (0.4 mg/dL), which was analyzed in a central laboratory; and interleukin-6 levels, which were measured at baseline, 4 weeks, and 24 weeks by using high-sensitivity enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota). Rheumatoid factor was measured by immunonephelometry with a BNII analyzer (Siemens Medical Solutions Diagnostics, Newark, Delaware), cortisol and adrenocorticotropic hormone levels by immunochemiluminescence methods with an Immulite 2500 (Siemens Medical Solutions Diagnostics, Los Angeles, California), and plasma lipids by Synchron LX-20 automated analyzers (Beckman Coulter, Brea, California).
Safety assessments consisted of all patients marking adverse events in their drug diaries, which were reviewed on each visit. Vital signs and safety laboratory measures, including a complete blood count and a chemistry profile (electrolyte and liver and kidney function tests), were recorded at each visit. Adverse events were graded by severity according to the National Cancer Institute Common Toxicity Criteria guidelines. An electrocardiogram (ECG) was obtained from all patients at baseline, 2 weeks, and the end of study. After 24 weeks, no follow-up was conducted.
Statistical Analysis
We designed our study to detect differences in the primary end point with greater than 90% power at a 2-sided level of significance of 0.05. To properly account for missing end point data due to dropouts, we used mixed-effects analyses to predict each patient’s ACR response at the end of study visit and to properly account for uncertainty in that prediction. The response was categorized according to the ACR 20, ACR 50, and ACR 70 criteria. In a similar manner, we compared changes in DAS 28 from baseline visit between treatment groups. We modeled the treatment group, visit number (2 random-effect terms for visit number and visit number squared), and their interaction as fixed effects. We added linear and quadratic random-effect terms to more realistically model patient efficacy trajectories and patient as a random intercept. We did not add a quadratic term for visit number in the fixed-effects model specification because visit was already modeled there more flexibly than as a linear or quadratic functional specification. For the fixed effect, we specified visit number as a categorical variable. In all models, the random effect was highly statistically significant, indicating the importance of including it (25). The treatment groups were compared with respect to the primary end point, the proportion of ACR 20 responders at the end of the study, and the secondary efficacy variables of ACR 50 response, ACR 70 response, and moderate or good improvement of the DAS 28 using the exact test for stratified 2 × 2 tables, stratifying for study center. For inter- or intragroup comparisons of continuous variables, including erosion data, the 2-sample t test or t test for paired data, respectively, were used, except where noted otherwise.
For the safety evaluation, summary statistics were used to compare adverse events in the 2 treatment groups. All analyses were computed by using Stata, version 10 (Stata-Corp, College Station, Texas), or StatXact, version 6.0 (Cytel, Cambridge, Massachusetts), for the exact stratified contingency table analyses. All reported P values are 2-sided and have not been adjusted for multiple comparisons.
A protocol-specified, last-observation-carried-forward approach for handling missing data was also done.
Role of the Funding Source
This study was funded by the intramural research program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), which was responsible for design of the study, analysis of the data, and preparation of the manuscript and also served as 1 of the patient recruiting centers, and Phytomedics, which provided funds to support the costs of the contract research organization that monitored the sites. Staff at NIAMS identified and purchased the TwHF roots in Fujian and Hunan provinces, China, and Phytomedics provided funds to make the extract and formulate the study medication under the supervision of NIAMS investigators. NIAMS investigators analyzed the extract for content of diterpenoids and biological activity, and Phytomedics provided funds to test the extract for toxins and adulterants.
RESULTS
Patient Characteristics
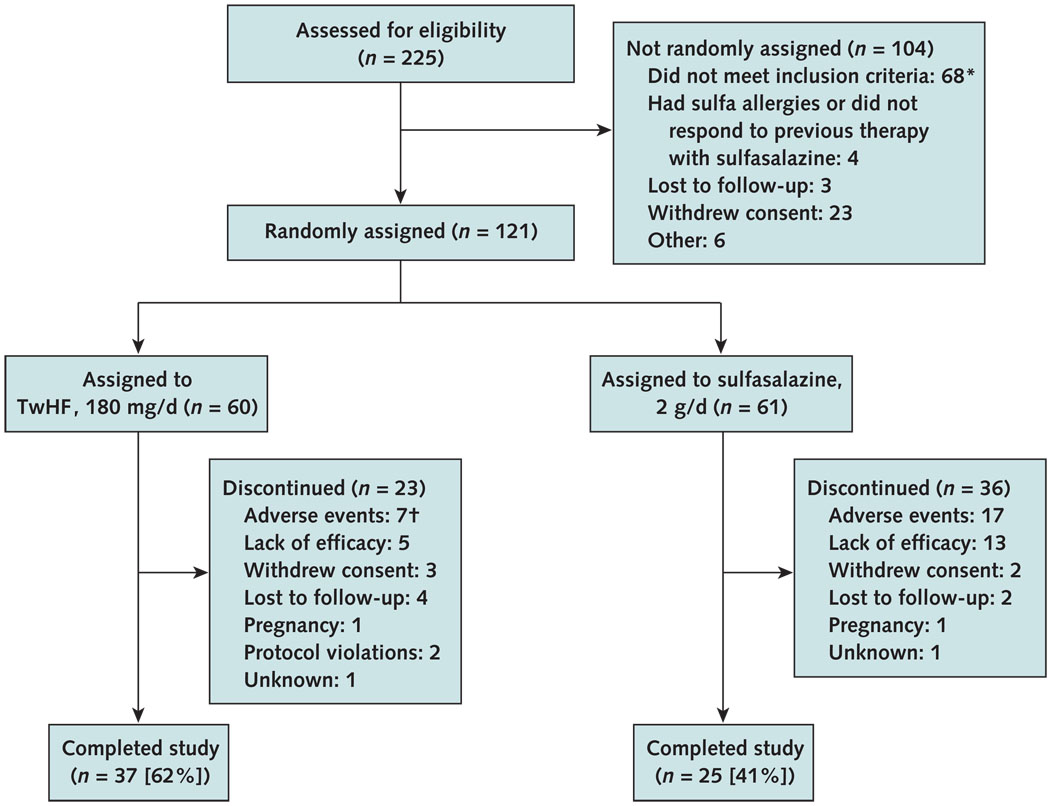
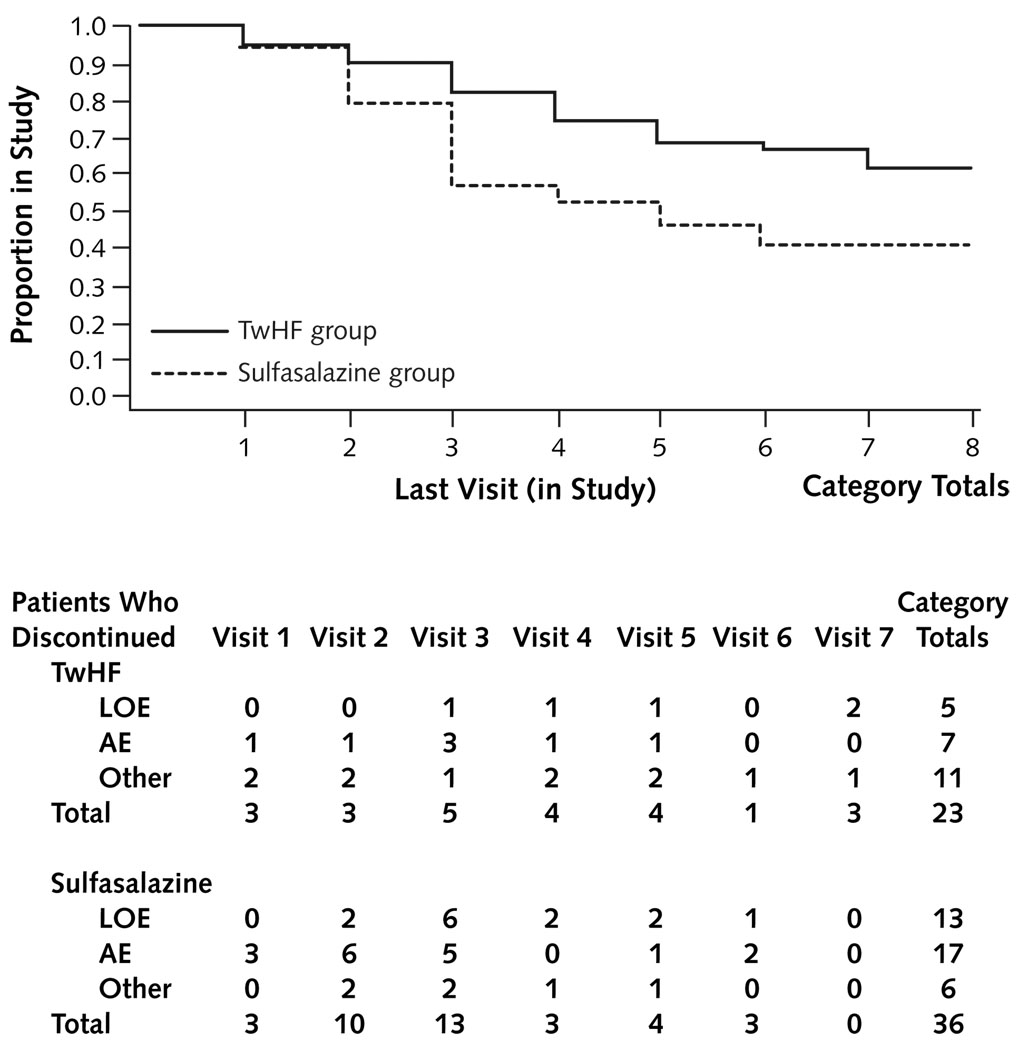
Table 1 summarizes the baseline demographic and clinical characteristics, by treatment group. All patients had active disease, as reflected by the number of tender and swollen joints, ESR and CRP values, and high DAS 28 scores. Of patients receiving TwHF extract, 62% completed 24 weeks of evaluation, compared with 41% of patients who received sulfasalazine (P = 0.029). Significantly more patients in the sulfasalazine group discontinued study participation because of adverse events or lack of efficacy than patients in the TwHF group (P < 0.001) (Figures 1 and 2). During the study, participants in the TwHF group took 92.5% of their pills and participants in the sulfasalazine group took 87.9% of their pills (Appendix Table 1, available at www.annals.org).
Figure 1. Study flow diagram.
TwHF = Tripterygium wilfordii Hook F.
* 62 patients were not included because disease activity was too low and 6 patients because of health issues.
† Significant difference (P = 0.039) between the sulfasalazine group and the TwHF group.
Figure 2. Time trajectory of withdrawals.
Values below the trajectory are the numbers of patients in the TwHF and sulfasalazine groups who discontinued treatment because of AEs, LOE, or other reasons. AE = adverse event; LOE = lack of effect; TwHF = Tripterygium wilfordii Hook F.
Appendix Table 1.
Study Adherence
| Variable | TwHF Group (n = 60) |
Sulfasalazine Group (n = 61) |
P Value |
|---|---|---|---|
| Mean days of study (SE) | 129.1 ± 7.5 | 98.2 ± 8.8 | 0.008 |
| Mean pills taken (SE), %* | 92.5 ± 1.7 | 87.9 ± 2.3 | 0.120 |
TwHF = Tripterygium wilfordii Hook F.
Calculated from the number of pills actually taken (per diary assessment) relative to the number of pills the patients were supposed to take while in the study. By using a daily pill diary and blister card checks, the percentage of pills taken was corrected for time in the study. Although the time in the study was longer in patients receiving TwHF, adherence to medication intake in both groups was good and not significantly different.
Clinical Efficacy
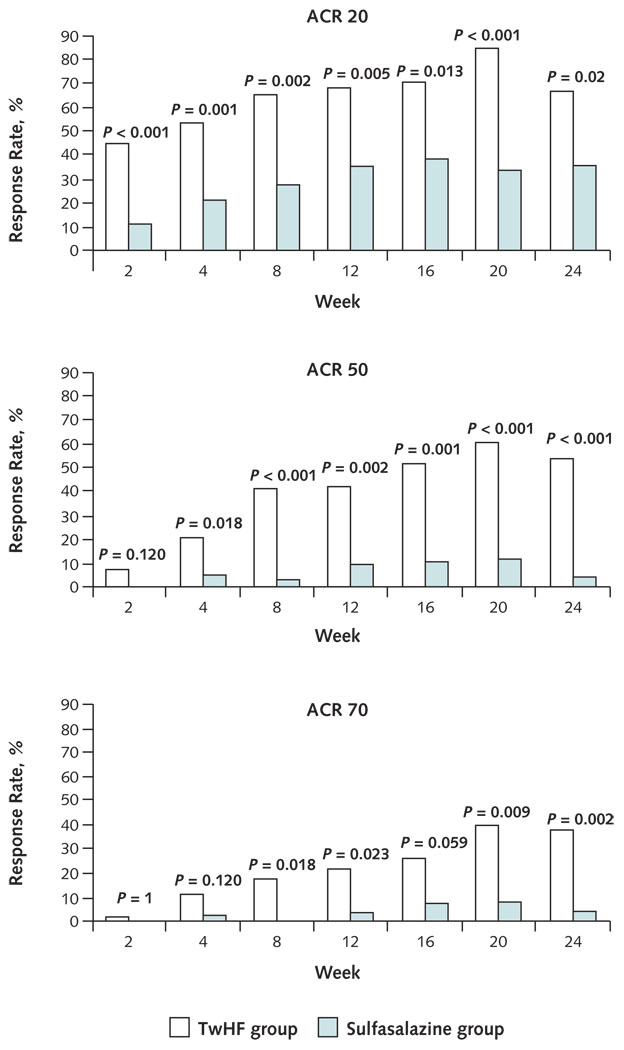
After 24 weeks of treatment, 67.57% (95% CI, 50.2% to 82.0%) of patients who received TwHF and completed the study and 36.00% (CI, 18.0% to 57.5%) of patients who received sulfasalazine and completed the study achieved at least a 20% improvement in disease activity as determined by the ACR 20 response. A similar improvement in the ACR 50 and ACR 70 responses was observed. In the TwHF group, ACR 50 responses were observed in 54.05% (CI, 39.9% to 70.5%) and ACR 70 responses in 37.84% (CI, 22.5% to 55.2%). In the sulfasalazine group, both ACR 50 and ACR 70 responses were observed in 4% (CI, 0.1% to 20.4%). P values were 0.02 for the ACR 20 comparison, less than 0.001 for the ACR 50 comparison, and 0.002 for the ACR 70 comparison (Figure 3). Figure 3 also shows the ACR 20, ACR 50, and ACR 70 responses for all patients in the study at the respective visits.
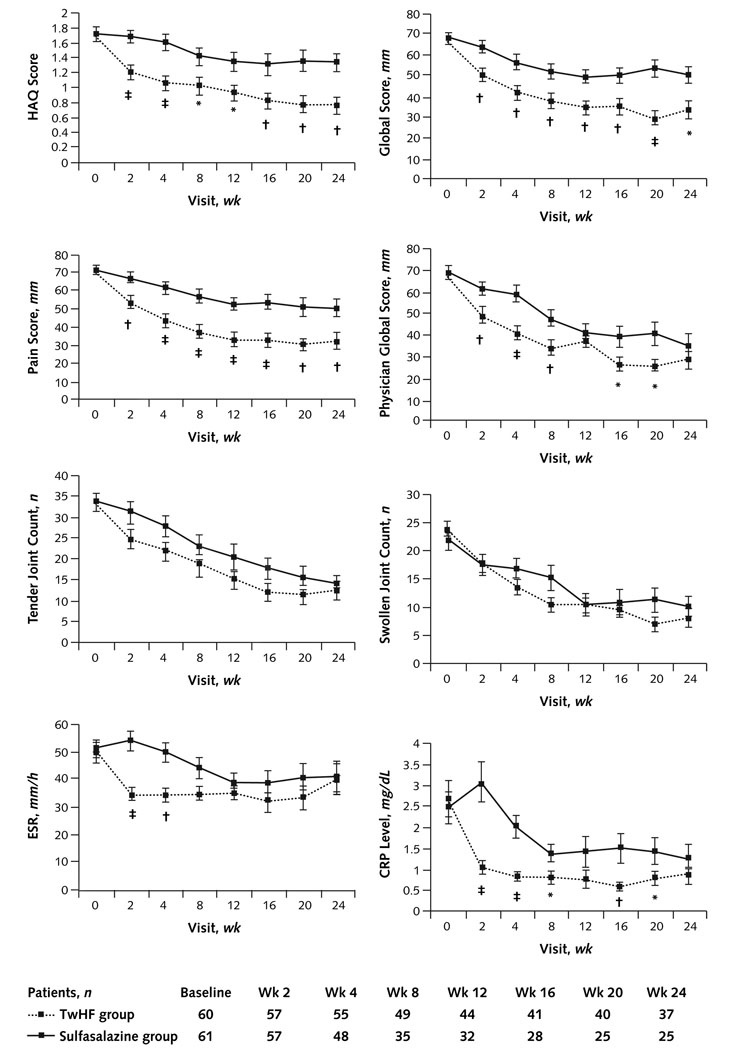
Figure 3. Clinical outcomes at 2 to 24 weeks.
Group comparisons were made at each visit. Data are shown only for patients who had the actual visit. Another analysis included all participants who were present at the respective visit. The number of participants in each group at a given visit is stated at the bottom of Figure 4. This analysis confirms the rapid onset of the clinical and laboratory response. A significant group difference between treatment groups is already seen early in the study, at a time when the withdrawal rate was much lower. ACR = American College of Rheumatology; TwHF = Tripterygium wilfordii Hook F.
In an intention-to-treat, mixed-model analysis that accounted for all randomly assigned patients, ACR 20 responses were modeled to be as high as 65.0% (CI, 51.6% to 76.9%) in patients who received TwHF and 32.8% (CI, 21.3% to 46.0%) in patients who received sulfasalazine (P = 0.001). ACR 50 responses were modeled to be 33.3% (CI, 21.7% to 46.7%), and ACR 70 responses were modeled to be 16.7% (CI, 8.3% to 28.5%) in patients receiving TwHF. In patients receiving sulfasalazine, these responses were modeled to be 4.9% (CI, 1.0% to 13.7%; P < 0.001) and 1.6% (CI, 0.04% to 8.8%; P = 0.004), respectively. Similar differences were noted when the protocol-specified, last-observation-carried-forward analysis was used (Appendix Figure 1, available at www.annals.org). The mean improvement in the DAS 28 was 2.40 points (CI, 2.07 to 2.73 points) in the TwHF group and 1.50 points (CI, 1.16 to 1.85 points) in the sulfasalazine group (P < 0.001). The individual objective and subjective measures of disease activity rapidly changed from baseline after administration of the TwHF extract. Significant differences from baseline and significantly larger improvements in the TwHF group compared with the sulfasalazine group were apparent at 2 weeks of therapy and persisted throughout the study for HAQ disability assessment, pain, the patient’s and physician’s global assessment of health, ESR, and CRP level. Improvements in number of swollen and tender joints were statistically significantly greater in the TwHF group than in the sulfasalazine group starting from 8 weeks of therapy (Figure 4). The largest improvement in CRP and ESR occurred within the first 2 weeks of treatment with TwHF.
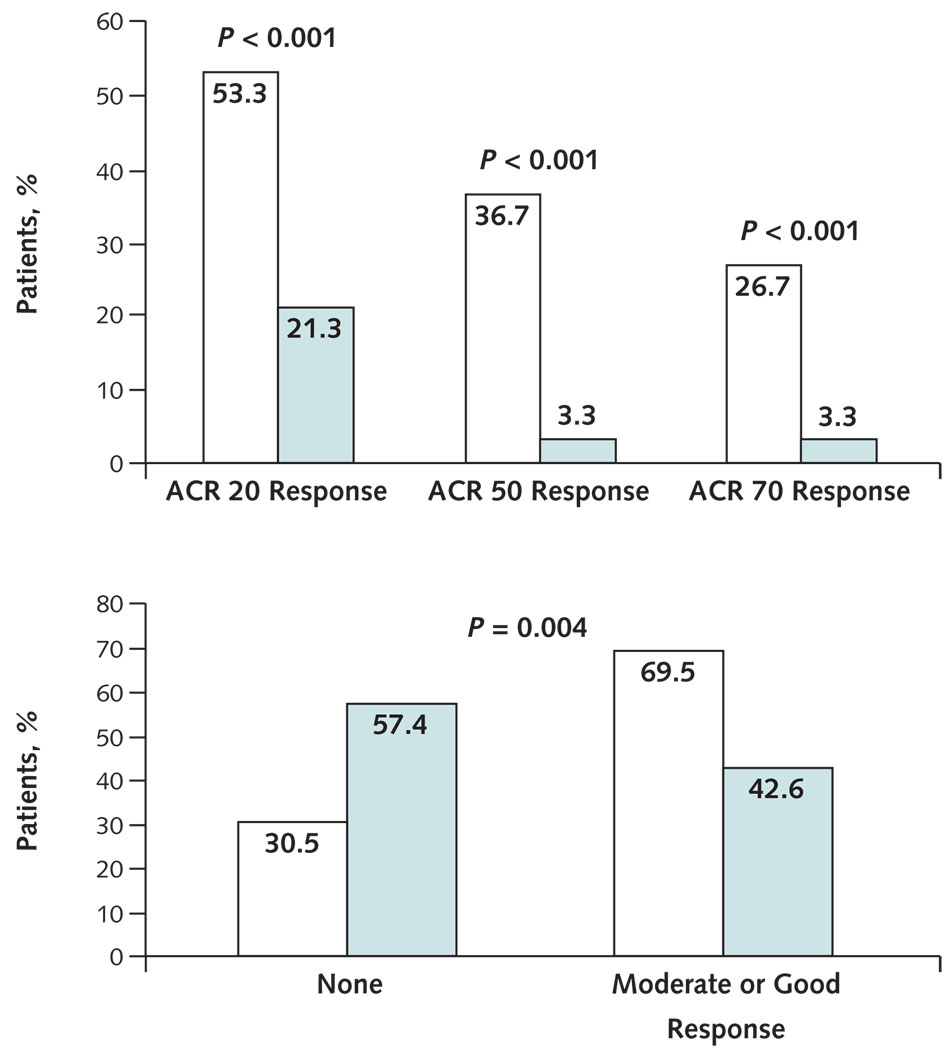
Appendix Figure 1. ACR responses based on last-observation-carried-forward analysis.
Green bars represent the sulfasalazine group, and white bars represent the TwHF group. ACR = American College of Rheumatology; TwHF = Tripterygium wilfordii Hook F. Top. Percentages of patients achieving responses defined by the ACR 20, ACR 50, and ACR 70 criteria at 24 weeks. Bottom. Percentages of patients with moderate or good European League Against Rheumatism responses at 24 weeks. A moderate European League Against Rheumatism response is a decrease (improvement) of >0.6 and ≤1.2, and a good response is a decrease of >1.2.
Figure 4. Comparisons of clinical responses by American College of Rheumatology criteria.
CRP = C-reative protein; ESR = erythrocyte sedimentation rate; HAQ = Health Assessment Questionnaire; TwHF = Tripterygium wilfordii Hook F. Outcomes from all patients who were evaluated on the respective visit are depicted. The actual number of patients evaluated at the respective visit is listed at the bottom. The HAQ score; patient assessment of global disease activity and pain and physician assessment of disease activity, both measured on a visual analogue scale from 0 to 10 mm (with higher numbers indicating greater severity); the number of painful and swollen joints on physical examination out of a total of 68 tender joints and 66 swollen joints (hips excluded); ESR; and CRP were assessed at each study visit.
* P < 0.05.
† P < 0.01.
‡ P < 0.001.
Appendix Table 2 (available at www.annals.org) shows a worst-case scenario analysis. Even in the setting of the most extreme (and implausible) assumptions, which put bounds on the relative effectiveness, the TwHF response is better than the sulfasalazine response (ACR 20, 41.7% vs. 24.6%; P = 0.055).
Appendix Table 2.
Worst-Case Scenario Analysis*
| Outcome† | Response Rate (95% CI),% | P Value | |
|---|---|---|---|
| TwHF Group | Sulfasalazine Group |
||
| ACR 20 | 41.7 (29.1–55.1) | 24.6 (14.5–37.3) | 0.055 |
| ACR 50 | 33.3 (21.7–46.7) | 11.5 (4.7–22.2) | 0.005 |
| ACR 70 | 23.3 (13.4–36.0) | 11.5 (4.7–22.2) | 0.098 |
ACR = American College of Rheumatology; TwHF = Tripterygium wilfordii Hook F.
A worst-case scenario analysis with regard to biasing against the superior effect of TwHF was performed. Each patient who withdrew from the TwHF group for any reason was considered not to have achieved an ACR 20, ACR 50, or ACR 70 response. Patients in the sulfasalazine group who withdrew were considered ACR responders except for those who withdrew for lack of efficacy and for adverse events; they were considered to have had no ACR response. The results indicate that, even under these artificial assumptions, the TwHF response is better than the sulfasalazine response.
121 patients total.
The mean improvement in patient function, as assessed by a decrease in HAQ score at 6 months, was 0.60 (SD, 0.69) in the TwHF group versus 0.22 (SD, 0.42) in the sulfasalazine group (P < 0.001). An improvement greater than 0.3 points on the HAQ (considered clinically meaningful [26]) was observed in 58% of patients receiving TwHF and 29% of patients receiving sulfasalazine (P = 0.002), whereas an improvement of 0.6 points was observed in 47% of patients receiving TwHF and 18% of patients receiving sulfasalazine (P = 0.001).
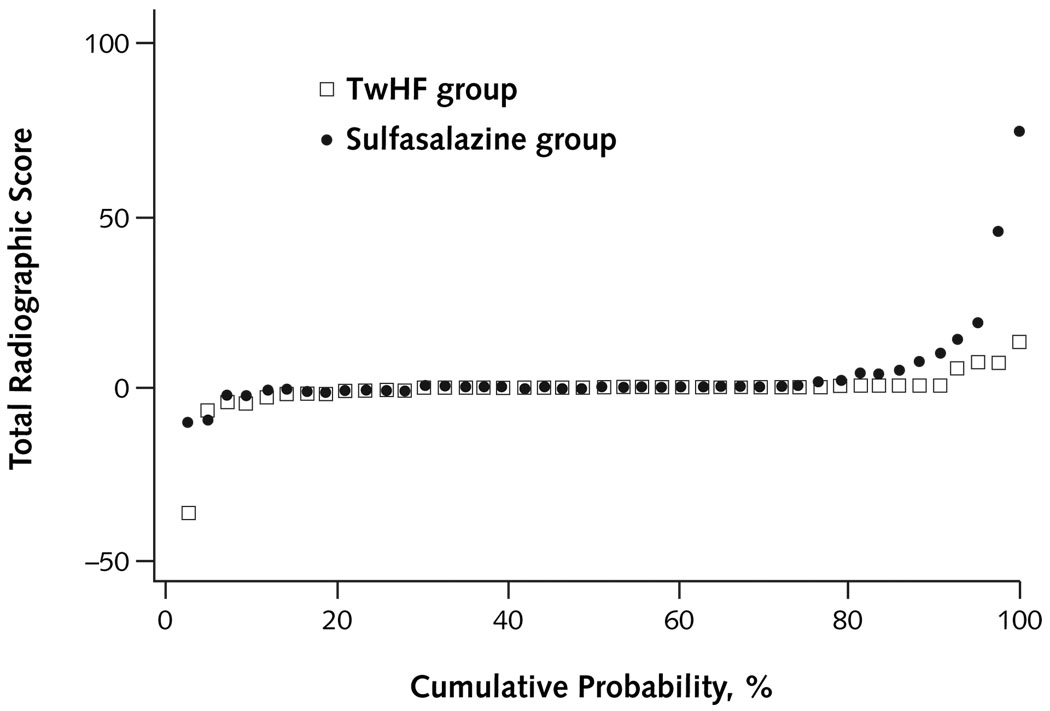
Radiographic assessment indicated that patients receiving TwHF extract had no progression of mean joint space narrowing and erosion scores, compared with respective progression scores of 1.65 (SD, 0.84) and 2.17 (SD, 1.47) in the sulfasalazine group (Table 2 and Appendix Figure 2, available at www.annals.org); this finding was not statistically significant.
Table 2.
Changes From Baseline in Inflammatory Markers, Radiographic Scores, Metabolic Measures, and Other Measures*
| Variable | Baseline | Change From Baseline at Week 4 | Change From Baseline at Week 24 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TwHF Group (n = 37) |
Sulfasalazine Group (n = 25) |
P Value | TwHF Group (n = 37) |
Sulfasalazine Group (n = 25) |
P Value | TwHF Group (n = 37) |
Sulfasalazine Group (n = 25) |
P Value | |
| Inflammatory markers | |||||||||
| Interleukin-6 level, pg/mL | 36.20 ± 7.02 [17.20] | 22.02 ± 6.60 [6.04] | 0.160 | −23.80 ± 6.87† | −6.91 ± 5.10 | 0.070 | −24.81 ± 6.31‡ | −4.63 ± 6.82 | 0.037 |
| Rheumatoid factor level, IU/mL§ | 788.15 ± 398.57 [138.00] | 365.94 ± 99.13 [170.00] | 0.83 | −336.38 ± 219.35‡ | −27.59 ± 25.91 | <0.001 | −483.77 ± 253.42‡ | −152.59 ± 48.74† | 0.46 |
| Radiographic scores‖ | |||||||||
| Total joint score | 27.26 ± 7.78 [6.25] | 21.39 ± 4.75 [7.50] | 0.52 | – | – | NA | −0.23 ± 0.97 | 3.82 ± 2.09 | 0.083 |
| Joint narrowing score | 14.95 ± 3.77 [3.00] | 13.32 ± 3.05 [3.50] | 0.74 | – | – | NA | −0.20 ± 0.43 | 1.65 ± 0.84 | 0.053 |
| Erosion score | 12.31 ± 4.16 [1.50] | 8.07 ± 1.97 [1.75] | 0.36 | – | – | NA | −0.03 ± 0.59 | 2.17 ± 1.47 | 0.167 |
| Metabolic measures | |||||||||
| Cortisol level, nmol/L | 283.05 ± 23.45 [248.29] | 212.70 ± 20.42 [193.12] | 0.037 | −11.31 ± 19.04 | 62.07 ± 22.90¶ | 0.017 | −33.38 ± 26.76 | 3.59 ± 20.42 | 0.31 |
| ACTH level, pmol/L | 2.14 ± 0.26 [1.32] | 1.77 ± 0.17 [1.43] | 0.28 | −0.04 ± 0.20 | 0.37 ± 0.25 | 0.21 | −0.18 ± 0.20 | −0.12 ± 0.22 | 0.84 |
| Total cholesterol level | 0.26 | <0.001 | <0.001 | ||||||
| mmol/L | 3.73 ± 0.20 [3.57] | 4.07 ± 0.20 [4.0] | 1.01 ± 0.15‡ | 0.18 ± 0.16 | 1.64 ± 0.19‡ | 0.48 ± 0.18¶ | |||
| mg/dL | 144.12 ± 7.90 [138.00] | 157.16 ± 7.74 [157.00] | 39.09 ± 5.91‡ | 7.08 ± 6.27 | 63.32 ± 7.48‡ | 18.56 ± 6.88¶ | |||
| HDL cholesterol level | 0.21 | <0.001 | <0.001 | ||||||
| mmol/L | 0.88 ± 0.04 [0.87] | 0.97 ± 0.06 [0.91] | 0.34 ± 0.04‡ | −0.05 ± 0.04 | 0.47 ± 0.06‡ | 0.11 ± 0.03† | |||
| mg/dL | 34.00 ± 1.57 [33.50] | 37.52 ± 2.47 [35.00] | 13.12 ± 1.54‡ | −1.76 ± 1.55 | 18.09 ± 2.24‡ | 4.44 ± 1.32† | |||
| LDL cholesterol level | 0.22 | <0.001 | <0.001 | ||||||
| mmol/L | 1.72 ± 0.12 [1.67] | 1.96 ± 0.14 [1.92] | 0.51 ± 0.10‡ | −0.03 ± 0.06 | 0.82 ± 0.12‡ | 0.14 ± 0.08 | |||
| mg/dL | 66.59 ± 4.68 [64.50] | 75.64 ± 5.58 [74.00] | 19.68 ± 3.75‡ | −1.20 ± 2.45 | 31.59 ± 4.50‡ | 5.52 ± 3.06 | |||
| Other measures | |||||||||
| Body weight, lb | 178.62 ± 6.56 [179.00] | 182.31 ± 10.04 [164.00] | 0.75 | 0.73 ± 0.43 | 1.16 ± 0.59 | 0.55 | −0.18 ± 1.27 | 1.57 ± 1.18 | 0.34 |
| Systolic blood pressure, mm Hg | 123.86 ± 2.89 [120.00] | 128.80 ± 3.59 [124.00] | 0.29 | 0.43 ± 2.79 | −1.64 ± 2.44 | 0.60 | 1.22 ± 2.76 | 0.68 ± 2.37 | 0.89 |
| Diastolic blood pressure, mm Hg | 75.08 ± 2.09 [75.00] | 76.20 ± 2.10 [77.00] | 0.72 | 1.49 ± 1.49 | 1.12 ± 2.08 | 0.88 | 2.32 ± 1.81 | 2.28 ± 2.05 | 0.99 |
| Serum glucose level | 0.58 | 0.70 | 0.32 | ||||||
| mmol/L | 6.38 ± 0.52 [5.22] | 5.95 ± 0.54 [5.33] | −0.19 ± 0.34 | −0.02 ± 0.24 | −0.84 ± 0.48 | −0.22 ± 0.28 | |||
| mg/dL | 114.92 ± 9.31 [94.00] | 107.16 ± 9.80 [96.00] | −3.46 ± 6.07 | −0.28 ± 4.39 | −15.16 ± 8.62 | −3.88 ± 5.09 | |||
ACTH = adrenocorticotropic hormone; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NA = not applicable; TwHF = Tripterygium wilfordii Hook F.
All measurements, except radiographic scores, are reported only for patients who completed the entire 24-week study. Data are presented as means ± SEs; medians are given in square brackets.
Statistically significant change from baseline to weeks 4 or 24 within the same treatment group (P < 0.01).
Statistically significant change from baseline to weeks 4 or 24 within the same treatment group (P < 0.001).
Rheumatoid factor titers were compared only in patients who had a positive baseline titer >20 IU/mL. Nonparametric analysis was used for analysis.
Radiographs were evaluated in all patients who had a complete set of radiographs (not just study completers): 43 patients receiving TwHF and 43 patients receiving sulfasalazine.
Statistically significant change from baseline to weeks 4 or 24 within the same treatment group (P < 0.05).
Appendix Figure 2. Probability plot of change in radiographic score from baseline to end of study.
The probability plot shows changes in total radiographic score from baseline to follow-up ranked for magnitude of change and organized by treatment group of all participants with available data. The graph shows that more patients in the sulfasalazine group than in the TwHF group have an increase in radiographic scores and that the magnitude of the increase is also larger in the sulfasalazine group than in the TwHF group. The graph also shows that most patients in both treatment groups have no radiographic progression. All of these patients are graphed at or around zero. TwHF = Tripterygium wilfordii Hook F.
Laboratory Response to Treatment With TwHF Extract
Plasma interleukin-6 levels were significantly lower after 4 weeks of treatment with TwHF and remained low at 24 weeks. At 6 months, interleukin-6 levels in the TwHF group had decreased by 24.81 pg/mL (SD, 6.31) compared with 4.63 pg/mL (SD, 6.82) in the sulfasalazine group (P = 0.037). In addition, reductions in rheumatoid factor levels were more pronounced at 4 and 24 weeks in patients who received TwHF (P < 0.001 for both comparisons) compared with patients who received sulfasalazine, in whom a significant decrease was seen only at 24 weeks (P = 0.003) (Table 2). Pearson correlations were used to assess whether a relationship existed between changes in interleukin-6 levels from baseline to week 24 and to assess the corresponding changes in DAS 28 for each treatment. We did not observe a significant relationship in either the TwHF group (R2 = 0.002; P = 0.84) or the sulfasalazine group (R2 = 0.072; P = 0.22). Because of the proposed binding of the major active components of the TwHF extract, triptolide and tripdiolide, to the glucocorticoid receptor (13), we assessed metabolic variables that would be expected to change with alterations in glucocorticoid metabolism. However, body weight, systolic and diastolic blood pressure, and plasma levels of cortisol and adrenocorticotropic hormone did not significantly change with treatment (Table 2). Of note, total cholesterol levels increased significantly (P < 0.001) in the patients receiving TwHF, with both high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol levels increasing by 53% and 48%, respectively (Table 2). Nevertheless, the ratio of LDL cholesterol to HDL cholesterol remained unchanged (data not shown).
Safety and Tolerability
While receiving the study drug, patients reported at least 1 adverse event with similar frequency in both treatment groups (Table 3). Significantly more patients in the sulfasalazine group than the TwHF group experienced adverse events classified as moderate to severe by the investigator (P = 0.039). Table 3 lists adverse events that occurred at a frequency of 5% or more, and these adverse events were similar in both treatment groups. About 60% of all patients with adverse events experienced gastrointestinal symptoms. Despite the high frequency of gastrointestinal events, 59% of patients receiving TwHF and 49% of patients receiving sulfasalazine completed the study, and gastrointestinal symptoms resolved in 69% of the TwHF group and 70% of the sulfasalazine group.
Table 3.
Adverse Events
| Adverse Event | TwHF Group | Sulfasalazine Group | P Value* | ||
|---|---|---|---|---|---|
| All Patients (n = 60) |
Patients Who Withdrew (n = 7) |
All Patients (n = 61) |
Patients Who Withdrew (n = 17) |
||
| Total events, n (%) | |||||
| Any event | 53 (88.3) | 55 (90.2) | 0.78 | ||
| Related to study drug† | 34 (56.7) | 37 (60.7) | 0.71 | ||
| Serious adverse events‡ | 3 (5) | 7 (11.5) | 4 | 0.32 | |
| Most frequent adverse events, n (%)§ | |||||
| Gastrointestinal adverse events | |||||
| Nausea | 13 (22) | 3‖ | 21 (34) | 4 | 0.157 |
| Vomiting | 9 (15) | 1‖ | 9 (15) | 1 | 1.00 |
| Diarrhea | 15 (25) | 1 | 11 (18) | 0.38 | |
| Constipation | 5 (8) | 8 (13) | 0.56 | ||
| Dyspepsia | 13 (22) | 1 | 5 (8) | 0.044 | |
| Abdominal distention | 6 (10) | 1‖ | 2 (3) | 0.163 | |
| Abdominal pain | 11 (18) | 6 (10) | 0.20 | ||
| Gastroenteritis | 0 (0) | 4 (7) | 0.119 | ||
| Infectious adverse events | |||||
| Upper respiratory tract infection | 11 (18) | 6 (10) | 0.20 | ||
| Influenza | 2 (3) | 4 (7) | 0.68 | ||
| Pneumonia | 1 (2) | 0 (0) | 0.50 | ||
| Urinary tract infection | 2 (3) | 6 (10) | 0.27 | ||
| Other infections¶ | 2 (3) | 7 (11) | 1 | 0.163 | |
| Other adverse events | |||||
| Headache | 5 (8) | 13 (21) | 0.072 | ||
| Rash | 7 (12) | 7 (11) | 7 | 1.00 | |
| Fatigue | 5 (8) | 1‖ | 10 (16) | 0.27 | |
| Peripheral edema | 4 (7) | 3 (5) | 0.72 | ||
| Cough | 4 (7) | 2 (3) | 0.44 | ||
| Hypertension | 2 (3) | 4 (7) | 0.68 | ||
| Hypercholesterolemia | 4 (7) | 1 (2) | 0.21 | ||
| Blurred vision | 4 (7) | 0 (0) | 0.057 | ||
| Hot flush | 3 (5) | 2 (3) | 0.68 | ||
| Amenorrhea | 3 (5) | 1 (2) | 0.37 | ||
| Dry mouth | 3 (5) | 0 (0) | 0.119 | ||
| Anemia | 0 (0) | 5 (8) | 0.057 | ||
| Chest pain | 3 (5) | 1 (2) | 0.37 | ||
| Myocardial infarction | 0 (0) | 1 (2) | 1.00 | ||
| Deep venous thrombosis | 1 (2) | 0 (0) | 0.50 | ||
| Thrombocytopenia | 2 (3) | 1 | 0 (0) | 0.24 | |
| Neutropenia | 1 (2) | 0 (0) | 0.50 | ||
| ECG changes on study | 4 (7) | 5 (8) | 0.98 | ||
ECG = electrocardiography; TwHF = Tripterygium wilfordii Hook F.
Based on the Fisher exact test.
None of the serious adverse events were thought to be related to study drug (see Appendix Table 3, available at www.annals.org, for list of serious adverse events).
Frequency ≥5% in either treatment group, except for infections and cardiac, hematologic, and hepatic events, which were included independent of frequency.
Determined by the site physician.
Patient had both events as reasons for withdrawal.
Included fungal infection (2 events) and 1 event each of cellulitis, herpes zoster infection, oral candidiasis, otitis externa, postoperative infection, tooth abscess, and viral infection.
Seventeen patients who received sulfasalazine and 8 patients who received TwHF discontinued the study because of adverse events (P = 0.071). Adverse events that led to study discontinuation in the TwHF group included gastrointestinal events in 6 patients, thrombocytopenia in 1 patient, and 1 serious adverse event—a femoral fracture—in 1 patient. In the sulfasalazine group, 4 patients who discontinued treatment experienced serious adverse events (1 had cholecystitis with cholecystectomy, an incarcerated inguinal hernia, and gastroparesis and partial small-bowl obstruction; 1 had atrial fibrillation and pancreatitis; 1 had a viral infection; and 1 had exacerbation of asthma and hypertension), 5 patients had nausea, and 8 patients had an allergic drug reaction.
Fifteen serious adverse events were observed in 10 patients (Appendix Table 3, available at www.annals.org), 3 of whom received TwHF (3 events) and 7 of whom received sulfasalazine (12 events). Of those, only 4 patients in the sulfasalazine group discontinued the study drug. Despite consenting to effective birth control, 2 patients became pregnant while receiving TwHF or sulfasalazine and subsequently delivered healthy babies. Mild prolongation of the corrected QT interval on ECG was seen in patients receiving TwHF, without an increase in arrhythmias on ECG or an increase in corrected QT intervals above the normal range (Appendix Table 4, available at www.annals.org). Reversible amenorrhea was observed in 3 patients receiving TwHF, and permanent amenorrhea was observed in 1 patient receiving sulfasalazine.
Appendix Table 3.
Serious Adverse Events
| Serious Adverse Events | TwHF Group (n = 3) |
Sulfasalazine Group (n = 7) |
|---|---|---|
| Deep venous thrombosis | 1 | 0 |
| Femoral fracture | 1 | 0 |
| Cholecystitis with cholecystectomy | 1 | 1*† |
| Atrial fibrillation and pancreatitis (2 separate events) | 0 | 1† |
| Incarcerated inguinal hernia, gastroparesis, and partial small-bowel obstruction (3 separate events) | 0 | 1*† |
| Incarcerated ventral hernia | 0 | 1 |
| Failure of knee prosthesis | 0 | 1 |
| Viral infection | 0 | 1† |
| Exacerbation of asthma and hypertension (2 events) | 0 | 1† |
| Patellar fracture | 0 | 1 |
TwHF = Tripterygium wilfordii Hook F.
The same patient had cholecystitis and an incarcerated inguinal hernia, gastro-paresis, and partial small-bowel obstruction at the same time after receiving sulfasalazine for 1 day (2 doses).
The patient permanently stopped receiving the study drug.
Appendix Table 4.
Changes From Baseline in QTc on ECG
| Variable | Baseline | Change From Baseline at Week 2 | Change From Baseline at Week 24 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TwHF Group* | Sulfasalazine Group* |
P Value | TwHF Group* | Sulfasalazine Group* |
P Value | TwHF Group* | Sulfasalazine Group* |
P Value | |
| Mean QTc (SE), ms† | |||||||||
| QTc Bazett | 408.9 ± 3.1 | 412.5 ± 3.3 | 0.43 | 10.2 ± 2.7‡ | 2.4 ± 2.5 | 0.045 | 7.0 ± 3.0§ | 2.3 ± 3.2 | 0.27 |
| QTc Fridericia | 399.1 ± 3.1 | 400.0 ± 2.6 | 0.82 | 10.5 ± 2.4‡ | −1.6 ± 2.0 | <0.001 | 7.1 ± 2.5‖ | 0.1 ± 2.5 | 0.051 |
| Completed Therapy¶ | P Value | Completed Therapy¶ | P Value | Completed Therapy¶ | P Value | ||||
| Mean QTc (SE), ms ‖ | |||||||||
| QTc Bazett | 409.5 ± 4.6 | 414.7 ± 4.4 | 0.44 | 12.7 ± 3.5‡ | −1.6 ± 4.0 | 0.001 | 6.6 ± 4.2 | −0.8 ± 4.9 | 0.26 |
| QTc Fridericia | 399.3 ± 4.5 | 400.5 ± 3.8 | 0.845 | 12.9 ± 3.2‡ | −2.4 ± 3.6 | 0.003 | 7.6 ± 3.4‖ | 0.4 ± 4.0 | 0.178 |
| Baseline | Change at Week 2 or Week 24 | ||||||||
|
TwHF Group (n = 60) |
Sulfasalazine Group (n = 61) |
P Value |
TwHF Group (n = 60) |
Sulfasalazine Group (n = 61) |
P Value | ||||
| Patients with ECG abnormalities, n | |||||||||
| At baseline** | 14 | 13 | 0.83 | – | – | NA | |||
| Changes during the study†† | – | – | NA | 4 | 5 | 1.00 | |||
| Changes during the study, but no abnormalities at baseline | – | – | NA | 3 | 2 | 0.68 | |||
ECG = electrocardiography; NA = not available; QTc = corrected QT interval; TwHF = Tripterygium wilfordii Hook F.
Based on 60 TwHF recipients and 61 sulfasalazine recipients.
A last-observation-carried-forward approach to data presentation and analysis was used.
Statistically significant change from baseline to week 2 or 24 within the same treatment group (P < 0.001).
Statistically significant change from baseline to week 2 or 24 within the same treatment group (P < 0.05).
Statistically significant change from baseline to week 2 or 24 within the same treatment group (P < 0.01).
Based on 36 TwHF recipients and 25 sulfasalazine recipients.
In the TwHF group, baseline abnormalities included atrial premature complexes (n = 1), bundle-branch block (n = 2), flat T waves (n = 4), ST-segment depression in a patient with left ventricular hypertrophy (n = 1), ectopic supraventricular rhythm (n = 1), anterior hemiblock (n = 4), and atrioventricular block (n = 1). In the sulfasalazine group, baseline abnormalities included inverted T waves (n = 3), anterior hemiblock (n = 3), bundle-branch block (n = 1), depressed ST segment (n = 2), atrial fibrillation (n = 1), sinus tachycardia (n = 1), and atrioventricular block (n = 2).
Changes in ECG consisted of bundle-branch block, inverted T waves, evidence of septal infarction, and flat T waves in the TwHF group and consisted of sinus tachycardia, atrial fibrillation, inverted T waves (n = 2), and anterior hemiblock in the sulfasalazine group.
DISCUSSION
Our results indicate that patients with active rheumatoid arthritis can be effectively treated with a standardized extract of the roots of TwHF, a medicinal plant that has been widely used in Chinese traditional medicine (6–8). During the 6-month study, treatment with TwHF extract resulted in rapid improvement in clinical signs and symptoms of rheumatoid arthritis, including joint pain, joint swelling, and measures of overall well-being, and in markers of inflammation, such as CRP, ESR, and the pro-inflammatory cytokine interleukin-6. Compared with sulfasalazine, 2 g/d (an approved standard therapy for rheumatoid arthritis), TwHF led to statistically significantly greater improvement in terms of patients achieving ACR 20, ACR 50, and ACR 70 responses and to moderate to good improvement in DAS 28. The improvement in clinical and laboratory markers translated into clinically and statistically significant improvement in patient function as measured by the HAQ disability score.
Because rates of noncompletion were relatively high, especially in the sulfasalazine group, we performed a sub-analysis on patients who completed the trial to assure that the effects of TwHF were not overestimated. We observed a significantly greater benefit of TwHF compared with sulfasalazine when we analyzed only patients who completed the study. Many other analyses, including a modified worst-case scenario analysis, also confirmed that the unequal withdrawal rate did not bias the study results in favor of TwHF. The sensitivity analyses, including patients who completed the study; as-treated patients; worst-case scenario; and a mixed-models repeated measures approach, which provides a sophisticated method of handling the problem of missing data explicitly, also yielded treatment results strongly in favor of TwHF, with estimated treatment effects often larger than those seen in these other approaches.
The oral administration of TwHF, 3 times per day, required a study design with another daily oral agent, such as sulfasalazine. Like TwHF, sulfasalazine is associated with gastrointestinal side effects at treatment initiation and, therefore, blinding was maintained during our study. Furthermore, because methotrexate is the most commonly used disease-modifying antirheumatic drug in the United States, using this drug as the active comparator would have made it likely that recruited participants in whom this therapy had failed might be randomly assigned to receive the same treatment again in the study. Sulfasalazine has been used as an active drug comparator in other studies (27–29) and has been reported to be similar to other oral disease-modifying drugs.
The rapid improvement in HAQ disability measure in our trial may be because TwHF not only has potent anti-inflammatory and immunomodulatory effects but also inhibits the transcription of cyclooxygenase-2 (20), which may result in the reduced production of prostaglandin E2 at inflammatory sites and therefore have a direct analgesic effect. This analgesic effect may have contributed to the early and significant improvement in pain and HAQ scores that we observed in patients who received TwHF.
Although our sample size was relatively small and the study was not powered to detect group differences in radiographic joint damage scores, the TwHF group trended to slower progression of radiographic joint damage than the sulfasalazine group. Because sulfasalazine has been shown to limit radiographic progression (30), our result indicates that TwHF limits radiographic progression at least as well. Because our patient population was similar to patients in other recent studies in terms of joint damage and disease activity (31–35), our results are encouraging but need to be verified in a larger cohort.
The withdrawal rates in our study were higher than the attrition rates of 19% to 28.5% in other rheumatoid arthritis efficacy trials that compared monotherapies, including sulfasalazine (36). This difference may reflect a lower current threshold in the United States for rheumatoid arthritis study participants and physicians to exit an investigational study if a rapid clinical benefit is not observed or if adverse events occur. However, similar to our study, a review of placebo-controlled clinical trials in patients with rheumatoid arthritis (27) reported overall attrition rates of 25% to 50% with sulfasalazine monotherapy. Furthermore, a second meta-analysis of 71 trials and 88 observational studies reported that with long-term use, only 22% of patients with rheumatoid arthritis continued sulfasalazine monotherapy compared with 36% receiving methotrexate (37). Therefore, the withdrawal rate with sulfasalazine in our study may be as expected.
Gastrointestinal symptoms were the most frequently reported adverse events, occurring early in the course of treatment and leading to similar numbers of drug discontinuation in the TwHF and sulfasalazine groups. The gastrointestinal side effects subsided in more patients who continued receiving TwHF (59%) than in patients who continued receiving sulfasalazine (49%). However, a lower drug dose at initiation of therapy or a gradual dose increase to full levels may improve tolerability, and counseling patients on the improvement of the gastrointestinal symptoms with continuation of therapy may further improve drug adherence. Despite these limitations, the effect of TwHF in terms of HAQ improvement and the persistence of this benefit, even when we analyzed only patients who completed the study (data not shown), stress the magnitude of the benefit of this treatment.
Toxicities reported with the use of various nonstandardized preparations of TwHF are difficult to compare with the adverse events that occurred in our study, because peeling the roots and using a standardized extraction with ethanol followed by ethyl acetate partitioning (10, 38) seems to result in better tolerability and less toxicity. Adverse hematologic events included reversible neutropenia in 1 patient and thrombocytopenia in 2 patients who received TwHF. Reversible amenorrhea, which has also been reported in other studies (39, 40), occurred in 3 patients receiving TwHF; this effect may make this drug more attractive in the treatment of postmenopausal women.
Anti-inflammatory therapies with anti–tumor necrosis factor and anti–interleukin-6 activity have been associated with elevated serum levels of total cholesterol (31, 41). Further studies need to evaluate whether the increase in HDL and LDL cholesterol levels seen with administration of TwHF is mediated through the reduction in interleukin-6 levels or through unknown mechanisms and, more important, whether the effect on prostaglandins, the increase in HDL and LDL cholesterol levels, and the decrease in CRP and interleukin-6 levels will result in an increase or a decrease in atherosclerotic risk. Prolongation of the corrected QT interval on ECG may warrant monitoring when other drugs with similar effects are used in combination (42).
Animal data (43) suggested modification of the pituitary axis when TwHF is administered to rats, and initial in vitro results (13) suggested that triptolide may exert some of its anti-inflammatory properties by binding to the glucocorticoid receptor. Nonetheless, we observed no weight gain, increase in glucose intolerance, or alterations in adrenocorticotropic hormone and cortisol in our patients, suggesting limited or no effect on the hypothalamic–pituitary–adrenal axis. Triptolide, the major mediator of the anti-inflammatory effect of the extract, has been reported (44) to bind to the calcium channel, PC-2, mediating calcium release in kidney cells, which arrested the growth of kidney cysts (45). Additional investigation is needed to determine whether all the clinical effects of this extract in the different tissues can be explained by these mechanisms. However, several diterpenoids have been described to have potent anti-inflammatory and analgesic properties and may serve as useful models of new drug development (13).
In summary, our study demonstrates that treatment with a standardized extract from the peeled roots of the Chinese herbal remedy TwHF administered over 24 weeks may be both effective and safe in treating patients with active rheumatoid arthritis. The rapid improvement in function and pain and the profound effect on inflammation may make this extract an attractive and affordable alternative to currently available agents. The long-term effects and toxicities and the potential combination of TwHF with other antirheumatic therapies need to be addressed in further studies.
Acknowledgment
The authors thank Dr. Richard Trout for help with the statistical analysis; Albert Nitche of Phytomedics, for his help with the logistical assistance with this study; Dr. Douglas Rosing for his help with the ECG analysis; Dr. Sack, Dr. Alan K. Matsumoto, Dr. Justus Fiechtner, and Dr. Lourie for contributing patients; and Elizabeth Joyal and Cedric McClinton for their help with the organization of patient material.
Grant Support: By the Intramural Research Program of the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and Phytomedics.
Footnotes
ClinicalTrials.gov registration number: NCT00062465.
Potential Financial Conflicts of Interest: Consultancies: P.E. Lipsky (Phytomedics). Honoraria: Y. Sherrer (Abbott, Amgen, Bristol-Myers Squibb, Genentech, Wyeth). Grants received: N.J. Olsen (Phytomedics). Patents received: N.J. Olsen (2 patents related to the use of TwHF). Other: Y. Sherrer (Abbott, Amgen, Biogen Idec, Genentech, Genmab, Medarex, Roche).
Reproducible Research Statement: Study protocol: Available from Dr. Goldbach-Mansky (e-mail, goldbacr@mail.nih.gov). Statistical code: Available from Dr. Wesley (e-mail, bwesley@cc.nih.gov). Data set: Available to qualified investigators by contacting Dr. Goldbach-Mansky (e-mail, goldbacr@mail.nih.gov).
Current author addresses and author contributions are available at www.annals.org.
Author Contributions: Conception and design: R. Goldbach-Mansky, F. Pucino, X. Tao, R. Wesley, P.E. Lipsky.
Analysis and interpretation of the data: R. Goldbach-Mansky, G. Csako, R. Costello, T.H. Pham, D. van der Heijde, P.E. Lipsky.
Drafting of the article: R. Goldbach-Mansky, P.E. Lipsky.
Critical revision of the article for important intellectual content: R. Goldbach-Mansky, R. Fleischmann, N. Olsen, J. Silverfield, P. Kempf, A. Kivitz, Y. Sherrer, F. Pucino, G. Csako, R. Costello, D. van der Heijde, R. Wesley, P.E. Lipsky.
Final approval of the article: R. Goldbach-Mansky, R. Fleischmann, N. Olsen, J. Silverfield, P. Kempf, A. Kivitz, Y. Sherrer, F. Pucino, G. Csako, D. van der Heijde, R. Wesley, P.E. Lipsky.
Provision of study materials or patients: R. Goldbach-Mansky, M. Wilson, R. Fleischmann, N. Olsen, J. Silverfield, P. Kempf, A. Kivitz, Y. Sherrer, D. van der Heijde, X. Tao.
Statistical expertise: R. Wesley.
Administrative, technical, or logistic support: R. Goldbach-Mansky, M. Wilson, T.H. Pham, C. Snyder.
Collection and assembly of data: R. Goldbach-Mansky, M. Wilson, F. Pucino, G. Csako, R. Costello, T.H. Pham, C. Snyder, D. van Heijde.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [PMID: 12748655] [DOI] [PubMed] [Google Scholar]
- 2.Scott DL, Symmons DP, Coulton BL, Popert AJ. Long-term outcome of treating rheumatoid arthritis: results after 20 years. Lancet. 1987;1:1108–1111. doi: 10.1016/s0140-6736(87)91672-2. [PMID: 2883443] [DOI] [PubMed] [Google Scholar]
- 3.O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–2602. doi: 10.1056/NEJMra040226. [PMID: 15201416] [DOI] [PubMed] [Google Scholar]
- 4.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [PMID: 17570481] [DOI] [PubMed] [Google Scholar]
- 5.Yen JH. Treatment of early rheumatoid arthritis in developing countries. Biologics or disease-modifying anti-rheumatic drugs? Biomed Pharmacother. 2006;60:688–692. doi: 10.1016/j.biopha.2006.09.008. [PMID: 17049202] [DOI] [PubMed] [Google Scholar]
- 6.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [PMID: 12568630] [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Hachida M, Enosawa S, Li XK, Suzuki S, Koyanagi H. Immunosuppressive effect of triptolide in vitro. Transplant Proc. 1999;31:2056–2057. doi: 10.1016/s0041-1345(99)00262-6. [PMID: 10455969] [DOI] [PubMed] [Google Scholar]
- 8.Kupchan SM, Court WA, Dailey RG, Jr, Gilmore CJ, Bryan RF. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94:7194–7195. doi: 10.1021/ja00775a078. [PMID: 5072337] [DOI] [PubMed] [Google Scholar]
- 9.Ji SM, Wang QW, Chen JS, Sha GZ, Liu ZH, Li LS. Clinical trial of Tripterygium wilfordii Hook F. in human kidney transplantation in China. Transplant Proc. 2006;38:1274–1279. doi: 10.1016/j.transproceed.2006.03.017. [PMID: 16797280] [DOI] [PubMed] [Google Scholar]
- 10.Tao X, Lipsky PE. The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum Dis Clin North Am. 2000;26:29–50. viii. doi: 10.1016/s0889-857x(05)70118-6. [PMID: 10680192] [DOI] [PubMed] [Google Scholar]
- 11.Ren J, Tao Q, Wang X, Wang Z, Li J. Efficacy of T2 in active Crohn’s disease: a prospective study report. Dig Dis Sci. 2007;52:1790–1797. doi: 10.1007/s10620-007-9747-y. [PMID: 17410440] [DOI] [PubMed] [Google Scholar]
- 12.Zheng JR, Fang JL, Gu KX, Xu LF, Gao JW, Guo HZ, et al. [Screening of active anti-inflammatory-immunosuppressive and antifertile compositions from Tripterygium wilfordii. I. Screening of 8 components from total glucosides of Tripterygium wilfordii (TII)] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1987;9:317–322. [PMID: 2968853] [PubMed] [Google Scholar]
- 13.Brinker AM, Ma J, Lipsky PE, Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae) Phytochemistry. 2007;68:732–766. doi: 10.1016/j.phytochem.2006.11.029. [PMID: 17250858] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao X, Cai JJ, Lipsky PE. The identity of immunosuppressive components of the ethyl acetate extract and chloroform methanol extract (T2) of Tripterygium wilfordii Hook. F. J Pharmacol Exp Ther. 1995;272:1305–1312. [PMID: 7891348] [PubMed] [Google Scholar]
- 15.Tao X, Cush JJ, Garret M, Lipsky PE. A phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii hook F in rheumatoid arthritis. J Rheumatol. 2001;28:2160–2167. [PMID: 11669150] [PubMed] [Google Scholar]
- 16.Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2002;46:1735–1743. doi: 10.1002/art.10411. [PMID: 12124856] [DOI] [PubMed] [Google Scholar]
- 17.Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274:13451–13455. doi: 10.1074/jbc.274.19.13451. [PMID: 10224110] [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Liu ZH, Chen ZH, Yang JW, Li LS. Triptolide: a potent inhibitor of NF-kappa B in T-lymphocytes. Acta Pharmacol Sin. 2000;21:782–786. [PMID: 11501157] [PubMed] [Google Scholar]
- 19.Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [PMID: 10224109] [DOI] [PubMed] [Google Scholar]
- 20.Tao X, Schulze-Koops H, Ma L, Cai J, Mao Y, Lipsky PE. Effects of Tripterygium wilfordii hook F extracts on induction of cyclooxygenase 2 activity and prostaglandin E2 production. Arthritis Rheum. 1998;41:130–138. doi: 10.1002/1529-0131(199801)41:1<130::AID-ART16>3.0.CO;2-4. [PMID: 9433878] [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Ma L, Tao X, Lipsky PE. Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum. 2004;50:2995–2303. doi: 10.1002/art.20459. [PMID: 15457469] [DOI] [PubMed] [Google Scholar]
- 22.Li K, Yuan Y, Dai X, Qao X. [Determination of triptolide in extract from leigongteng (Tripterygium wilfordii Hook. F.) by RP-HPLC] Se Pu. 1998;16:356–357. [PMID: 11367766] [PubMed] [Google Scholar]
- 23.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–735. doi: 10.1002/art.1780380602. [PMID: 7779114] [DOI] [PubMed] [Google Scholar]
- 24.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–263. [PMID: 10648051] [PubMed] [Google Scholar]
- 25.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. 2nd ed. Oxford: Oxford Univ Pr; 2002. [Google Scholar]
- 26.Redelmeier DA, Lorig K. Assessing the clinical importance of symptomatic improvements. An illustration in rheumatology. Arch Intern Med. 1993;153:1337–1342. [PMID: 8507124] [PubMed] [Google Scholar]
- 27.Suarez-Almazor ME, Belseck E, Shea B, Wells G, Tugwell P. Sulfasalazine for rheumatoid arthritis. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000958. CD000958 [PMID: 10796400] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smolen JS, Kalden JR, Scott DL, Rozman B, Kvien TK, Larsen A, et al. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999;353:259–266. doi: 10.1016/s0140-6736(98)09403-3. [PMID: 9929017] [DOI] [PubMed] [Google Scholar]
- 29.Proudman SM, Conaghan PG, Richardson C, Griffiths B, Green MJ, McGonagle D, et al. Treatment of poor-prognosis early rheumatoid arthritis. A randomized study of treatment with methotrexate, cyclosporin A, and intraarticular corticosteroids compared with sulfasalazine alone. Arthritis Rheum. 2000;43:1809–1819. doi: 10.1002/1529-0131(200008)43:8<1809::AID-ANR17>3.0.CO;2-D. [PMID: 10943871] [DOI] [PubMed] [Google Scholar]
- 30.Sharp JT, Strand V, Leung H, Hurley F, Loew-Friedrich I. Treatment with leflunomide slows radiographic progression of rheumatoid arthritis: results from three randomized controlled trials of leflunomide in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis Rheum. 2000;43:495–505. doi: 10.1002/1529-0131(200003)43:3<495::AID-ANR4>3.0.CO;2-U. [PMID: 10728741] [DOI] [PubMed] [Google Scholar]
- 31.Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–1167. doi: 10.1136/ard.2006.068064. [PMID: 17485422] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, Cannon GW, Schiff M, Weaver A, Fox R, Olsen N, et al. Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Arthritis Rheum. 2001;44:1984–1992. doi: 10.1002/1529-0131(200109)44:9<1984::AID-ART346>3.0.CO;2-B. [PMID: 11592358] [DOI] [PubMed] [Google Scholar]
- 33.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [PMID:16947627] [DOI] [PubMed] [Google Scholar]
- 34.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [PMID: 16785475] [DOI] [PubMed] [Google Scholar]
- 35.van der Heijde D, Klareskog L, Rodriguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J, et al. TEMPO Study Investigators. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–1074. doi: 10.1002/art.21655. [PMID: 16572441] [DOI] [PubMed] [Google Scholar]
- 36.Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med. 2008;148:124–134. doi: 10.7326/0003-4819-148-2-200801150-00192. [PMID: 18025440] [DOI] [PubMed] [Google Scholar]
- 37.Maetzel A, Wong A, Strand V, Tugwell P, Wells G, Bombardier C. Meta-analysis of treatment termination rates among rheumatoid arthritis patients receiving disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2000;39:975–981. doi: 10.1093/rheumatology/39.9.975. [PMID: 10986302] [DOI] [PubMed] [Google Scholar]
- 38.Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2002;46:1735–1743. doi: 10.1002/art.10411. [PMID: 12124856] [DOI] [PubMed] [Google Scholar]
- 39.Gu CX. [An experimental study on delayed calcification of bovine pericardial valve using Triton X-100] Zhonghua Xin Xue Guan Bing Za Zhi. 1989;17:236–237. [PMID: 2627881] [PubMed] [Google Scholar]
- 40.Gu J, Zhu C, Wang W, Wang L. The effects of Lei Gong Teng on reproductive hormones. J Tradit Chin Med. 2001;21:50–51. [PMID: 11360541] [PubMed] [Google Scholar]
- 41.Seriolo B, Paolino S, Sulli A, Fasciolo D, Cutolo M. Effects of anti-TNF-alpha treatment on lipid profile in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:414–419. doi: 10.1196/annals.1351.039. [PMID: 16855168] [DOI] [PubMed] [Google Scholar]
- 42.Arizona Center for Education and Research on Therapeutics. [22 June 2009];QT Drug Lists. Accessed at on www.torsades.org.
- 43.Chen L, Wang H, Zhao Z, Zhang Y, Huang G. Effects of the extract of a Chinese herb Tripterygium wilfordii hook f on rat pituitary gland. Am J Chin Med. 2005;33:945–955. doi: 10.1142/S0192415X05003521. [PMID: 16355451] [DOI] [PubMed] [Google Scholar]
- 44.Bai JP, Shi YL. Inhibition of Ca(2+) channels in mouse spermatogenic cells by male antifertility compounds from Tripterygium wilfordii Hook. f. Contraception. 2002;65:441–445. doi: 10.1016/s0010-7824(02)00312-8. [PMID: 12127645] [DOI] [PubMed] [Google Scholar]
- 45.Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104:4389–4394. doi: 10.1073/pnas.0700499104. [PMID: 17360534] [DOI] [PMC free article] [PubMed] [Google Scholar]