Abstract
Objective
To examine the association of childhood headache disorders with markers of risk for cardiovascular and cerebrovascular disease.
Design
Information was collected on severe or recurrent headache or migraine in childhood or adolescence and on biomarkers predictive of vascular disease.
Setting
The National Health and Nutrition Survey, a nationally representative health survey.
Participants
Children or adolescents aged 4 to 19 years (n=11 770) who took part in the National Health and Nutrition Survey in 1999 through 2004.
Main Exposure
Headache.
Main Outcome Measures
Body mass index; levels of C-reactive protein, homocysteine, serum and red blood cell folate, vitamin B12, methylmalonic acid, total cholesterol, high-density lipoprotein cholesterol, non–high-density lipoprotein cholesterol, triglycerides, and uric acid; and platelet count.
Results
Mean values for body mass index, C-reactive protein, and homocysteine were higher in children with than without headaches, and more children with headaches were in the highest quintile of risk for these factors. Serum and red blood cell folate levels were lower in children with headache. More children with headache were in the highest quintile of risk for 3 or more of these factors.
Conclusions
Several important risk factors for long-term vascular morbidity cluster in children and adolescents with severe or recurrent headache or migraine. Further study and screening of children with headaches may permit improved preventive management.
Known markers of risk for cardiovascular and cerebrovascular disease include dyslipidemia,1,2 obesity,1 and elevated blood levels of C-reactive protein (CRP)3,4 and homocysteine.5,6 Adults with migraine or other headache disorders have elevated levels of these factors.7–10 Adult migraineurs with aura are at increased risk of cardiovascular disease and stroke.11,12 Hyperhomocysteinemia and genetic variants relating to homocysteine are risk factors for stroke in children.13
In adults, analysis of the association of these factors with headache disorders and other outcomes is complicated by the frequent presence of such additional risk factors as smoking, hypertension, and diabetes mellitus. In children, these and other potential confounders are much less common. There are reports from small clinical samples that pediatric migraine is associated with dyslipidemia14 and hyperhomocysteinemia.15 In addition, in a representative national sample of children, severe or recurrent headache was associated with higher levels of adiposity as measured by body mass index (BMI).16 We found no report on CRP levels in children with migraine.
Are children with headache disorders at increased risk of vascular disease? To our knowledge, no study has addressed this question. Since elevated levels of risk tend to be maintained over many years in adults17 and some of the risk factors can be modified, we investigated the association and interaction of these factors in the National Health Examination and Nutrition Survey (NHANES).
The NHANES provides unique information on the health of US children; it includes comprehensive information on diseases and services, extensive data on social and demographic correlates of health, laboratory and clinical assessments of a wide range of potential indicators of health status, and environmental exposures that may contribute to child health. The sample was representative of noninstitutionalized US children and adults. The large sample size permitted pooling of the multiple replication waves, enhancing the reliability of the estimates and increasing statistical power to evaluate the stability of findings across specific subgroups of the population.18
Recurrent or severe headaches in children are associated with recurrent headaches in later life but headache type as assigned in children is not a reliable predictor of the presence or type of headache disorder at later ages.19,20 Migraine in children differs in some respects from migraine in adults,21 and there has been considerable discussion regarding the validity of the diagnostic criteria for migraine in children.22–27 The clinical significance of recurrent headaches in childhood and their association with comorbidities and biologic correlates suggest that, despite difficulties in their subclassification in NHANES and more broadly, pediatric headache is an important disorder.
We examined the association between headaches and vascular or inflammatory biomarkers including CRP, homocysteine, folate, lipid, and uric acid levels and platelet counts and their interrelationships in children. Other well-established correlates of headaches and/or vascular biomarkers including age, sex, ethnicity, poverty income ratio (PIR) (proxy for social class), and BMI were also included. Asthma was also considered in this risk profile because our previous work in both adults28 and children16 showed a strong link between asthma and headache disorders. We found that many of these biomarkers clustered more closely in children with than without severe or recurrent headaches or migraine.
METHODS
SAMPLE
Data were drawn from the NHANES (https://www.cdc.gov/nchs/nhanes.htm), which collected questionnaire and medical examination and laboratory measurements data from a representative sample of the US civilian residents from 1999 through 2004. The design was cross-sectional with each of the 6 annual-survey years pooled into 1 analytical data set. Twelve subjects were excluded because they lacked information on headache. This produced an analytical sample of 11 770 children and adolescents aged 4 to 19 years. The NHANES uses a complex multistage probability sampling design. Variance corrections were performed by using SUDAAN version 9 (RTI International, Research Triangle Park, North Carolina) to account for stratification and clustering. Data were weighted to adjust for oversampling of low-income persons, adolescents 12 to 19 years of age, and African American and Mexican American individuals. Detailed NHANES survey operations manuals are available on the NHANES Web site (http://www.cdc.gov/nchs/nhanes.htm).
MEASURES
Headache status was determined by this yes/no question: “During the past 12 months, have you had frequent or severe headaches including migraine?” The term headaches is used to refer to frequent or severe headaches or migraine in the remainder of this report. Sex, age, and race/ethnicity were obtained by self-report. Race/ethnicity was defined as non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, and other race including multiple races. Socioeconomic status was assessed by the PIR. The PIR, based on family size, is the ratio of family income to the family’s poverty threshold level, determined by the US Census Bureau. The National Center for Health Statistics calculated respondents’ PIR values using self-reported family income data. The PIR was categorized into 3 groups in the analysis: less than 1, 1 to 2, and greater than or equal to 3. The PIR values less than 1 were deemed to be below the poverty threshold.
The NHANES data on laboratory measures from blood, urine, and swabs were collected from all respondents except those who did not meet age criteria or had excluded medical conditions such as hemophilia, chemotherapy within the last 4 weeks, or conditions preventing a clean blood draw. The measures examined herein include BMI (calculated as weight in kilograms divided by height in meters squared), triglycerides (12-hour fasting subsample), total cholesterol, high-density lipoprotein (HDL) cholesterol, non-HDL cholesterol (created variable, total cholesterol minus HDL cholesterol), low-density lipoprotein cholesterol (12-hour fasting subsample), CRP, homocysteine, methylmalonic acid, serum folate, vitamin B12, red blood cell folate, uric acid (12- to 19-year-old subsample), and platelet count. With the exception of homocysteine and methylmalonic acid, each measure is presented in non-SI units. Risk quintiles were defined to include individuals with measured values in the lowest quintile (HDL cholesterol, serum folate, red blood cell folate, and vitamin B12) or the highest quintile (remaining measures). They were first calculated in the control population for the entire sample and then within 2 defined age groups: 4 to 11 years of age and 12 years and older.
DATA ANALYSES
χ2 Tests were used to compare the prevalence of headache within the sociodemographic groups as well as the sociodemographic breakdown within the 2 headache groups. Mean values of the biological measures were calculated for the entire sample as well as by sex, and t test analysis was used to compare groups. Mean values were also calculated within the 4-level age group and compared by linear regression analysis. Logistic regression models were used to estimate the association between headache and the risk quintile separately in 2 age groups in both unadjusted models and models adjusted for sex, race/ethnicity, and PIR.
The clustering of biological risk factors by headache group was determined by creating a sum variable that measured the number of risk quintile measurements for the following variables: BMI and levels of CRP, homocysteine, serum folate, HDL cholesterol, and non-HDL cholesterol. Values ranged from 0 (respondent not categorized into the risk quintile for any of these variables) to 6 (respondents measured in the risk quintile for all variables). An additional sum variable was created that included a self-reported diagnosis of asthma (range, 0–7). For all of the analyses, a 2-sided test with P values less than .05 was considered statistically significant. We used a Bonferroni correlation as a simple and conservative correction for multiple comparisons.
Finally, to examine relationships among the measured variables, we performed a factor analysis. Given the binary nature of some of the variables, we first examined tetrachoric correlations, then applied the factor analysis (PROC FACTOR) in SAS (SAS Institute Inc, Cary, North Carolina) on its output correlation matrix with promax rotation.
RESULTS
Among 11 770 persons 4 to 19 years of age surveyed in NHANES in 1999 through 2004, there were 2295 (19.5%) for whom severe or recurrent headaches were reported. The prevalence of headache rose with increasing, age. Before puberty, rates of headache were comparable in boys and girls, but after the age of 12 years, girls with headache outnumbered boys.16 The highest headache prevalence, 27.4%, was observed in girls aged 16 to 18 years. Headaches were reported more frequently in African American individuals and in persons with low income as measured by the PIR.16
Mean BMI was higher among children with headache, the difference in BMI between children with and without headaches being highly significant statistically in boys, girls, and the total (Table 1). Mean BMI rose with age but remained higher in children with than without headache in each age group. There was an excess of children with headache in the highest quintile of BMI values (as derived from the nonheadache population; see “Methods” section). Body mass index in the highest quintile was 35% more frequent in children for whom recurrent or severe headaches were reported (Table 2, odds ratio adjusted for age, race, and PIR).
Table 1.
Mean Values of Biological Measures by Headache Statusa
| Value | Mean (SE) |
|
|---|---|---|
| No Headache | Headache | |
| BMI | 20.19 (0.10) | 22.71 (0.22)b |
| C-reactive protein, mg/L | 1.5 (0.1) | 1.9 (0.1)c |
| Homocysteine, μmol/L | 5.35 (0.04) | 5.92 (0.07)b |
| Serum folate, ng/mL | 16.23 (0.19) | 13.81 (0.34)b |
| RBC folate, ng/mL | 268.30 (2.41) | 250.37 (3.17)b |
| Vitamin B12, pg/mL | 67.24 (1.27) | 58.21 (0.96)b |
| Methylmalonic acid, μmol/L | 0.131 (0.00) | 0.126 (0.00)d |
| Total cholesterol, mg/dL | 163.67 (0.64) | 165.27 (1.02) |
| HDL cholesterol, mg/dL | 51.28 (0.23) | 50.45 (0.38) |
| Non-HDL cholesterol, mg/dL | 112.39 (0.63) | 114.82 (1.05) |
| LDL cholesterol, mg/dL | 93.32 (0.83) | 95.05 (1.41) |
| Triglycerides, mg/dL | 89.76 (1.82) | 95.91 (2.73) |
| Uric acid, mg/dL | 5.15 (0.03) | 5.07 (0.05) |
| Platelet count, ×103/μL | 300.81 (1.62) | 297.87 (2.81) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL, high-density lipoprotein; LDL, low-density lipoprotein; RBC, red blood cell.
SI conversion factors: To convert C-reactive protein to nanomoles per liter, multiply by 9.524; homocysteine to milligrams per liter, divide by 7.397; serum folate to nanomoles per liter, multiply by 2.266; vitamin B12 to picomoles per liter, multiply by 0.7378; total cholesterol, HDL cholesterol, and LDL cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113; uric acid to micromoles per liter, multiply by 59.485; platelet count to ×109/L, multiply by 1.
Bonferroni correction–applied cutoff at 0.05/13 (P <.004). This would eliminate the statistically significant results for methylmalonic acid (P=.049) and C-reactive protein (P=.005).
Significant at P <.001.
Significant at P <.01.
Significant at P <.05.
Table 2.
Logistic Regression of Headache Status on the Odds of Being in the “Risk” Quintile for Selected Biological Factors
| Value | OR (95% CI) | AORa (95% CI) | AORb (95% CI) |
|---|---|---|---|
| BMI | 2.00 (1.71–2.34) | 1.35 (1.15–1.58) | … |
| C-reactive proteinc | 1.51 (1.24–1.83) | 1.30 (1.06–1.60) | 1.23 (1.00–1.51) |
| Homocysteinec | 1.87 (1.60–2.18) | 1.33 (1.11–1.59) | 1.32 (1.10–1.58) |
| Serum folated | 1.96 (1.66–2.30) | 1.36 (1.13–1.63) | 1.34 (1.12–1.60) |
| RBC folated | 1.69 (1.49–1.93) | 1.20 (1.04–1.39) | 1.23 (1.06–1.43) |
| Total cholesterolc | 1.25 (1.08–1.44) | 1.19 (1.01–1.39) | 1.15 (0.97–1.36) |
| HDL cholesterold | 1.33 (1.13–1.58) | 1.27 (1.08–1.51) | 1.21 (1.01–1.44) |
| Non-HDL cholesterolc | 1.31 (1.11–1.56) | 1.22 (1.02–1.46) | 1.15 (0.94–1.41) |
Abbreviations: AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; OR, odds ratio; RBC, red blood cell.
Adjusted for sex, race, and poverty income ratio.
Adjusted for sex, race, poverty income ratio, and BMI.
The “risk” quintile is the highest quintile.
The “risk” quintile is the lowest quintile.
Children with headaches had higher mean CRP values than children without headache (Table 1), and girls had higher mean CRP values than boys, within headache status groups (data not shown). There was no significant difference in CRP values between boys with and without headaches. More children with headache were in the top quintile for CRP value (Table 2, second odds ratio also adjusted for BMI).
Mean values for homocysteine were higher in children with headache in boys, girls, and the total. Boys with and without headache had higher mean homocysteine levels than girls in the same groups (data not shown). Mean values of homocysteine rose over the ages studied in both the nonheadache and headache groups. More children aged 4 to 11 years with headache were in the highest risk quintile, while the difference between the nonheadache and headache groups was smaller in magnitude and not formally significant in children 12 years or older. Serum and red blood cell folate levels were lower in children with headache, without difference by sex. More children with headache were in the highest risk quintile (ie, the lowest folate levels). Vitamin B12 mean values were lower in children with headaches. Mean values of lipids and quintiles of risk were not consistently different by headache status, although HDL cholesterol level was lower in boys with than without headache. There was no clear association of headache status with low platelet count or uric acid level.
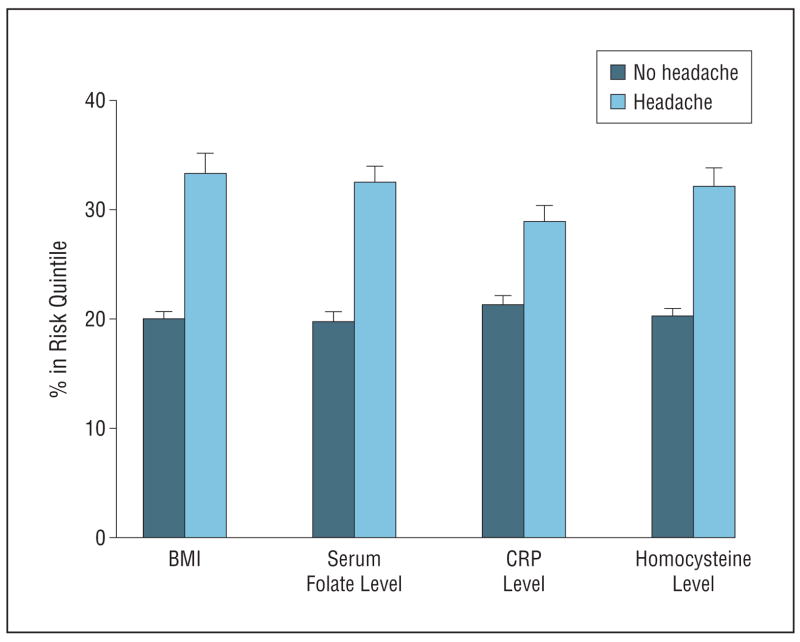
More children with severe or recurrent headache were in the highest risk quintile for BMI and levels of CRP, homocysteine, and folate (Figure 1) and more were in the riskiest quintile for 3 or more factors measured.
Figure 1.
Percentage with body mass index (BMI), serum folate level, C-reactive protein (CRP) level, and homocysteine level in risk quintiles by headache status.
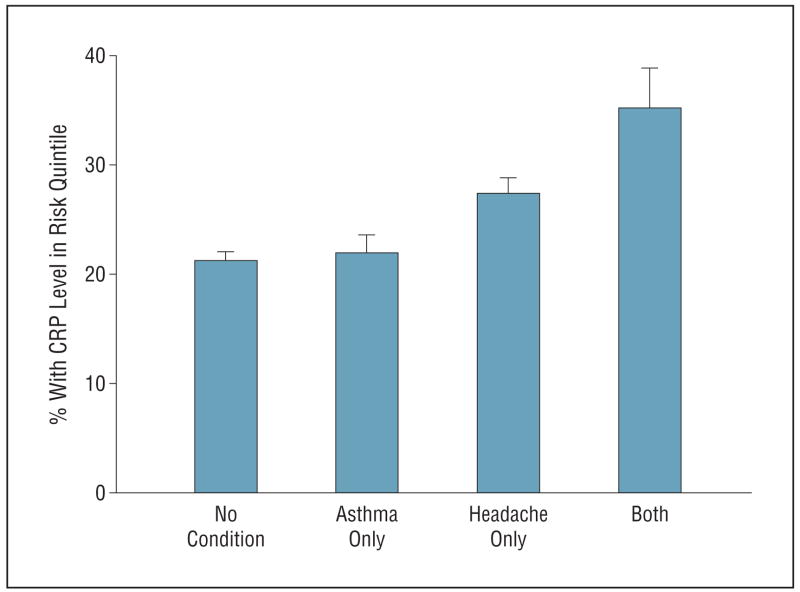
With asthma added to the group of clustered characteristics, almost twice as many young children with headache (31.7% vs 17.9%) had 3 or more of these characteristics. Thus, the factors measured in this study tended to cluster in children with headache. Children with headaches were more often in the highest quintile of risk with respect to CRP level than children with neither asthma nor headaches or with asthma alone. Children with both asthma and headaches were still more often in the highest quintile of risk with regard to CRP level (Figure 2). Factor analysis suggested 2 factors, one primarily represented by homocysteine and serum folate levels, the other by CRP level and asthma.
Figure 2.
Percentage with C-reactive protein (CRP) level in risk (highest) quintile by asthma and headache status.
In summary, a number of examined characteristics, including clinical and biochemical factors, were associated with risk for later cardiovascular and cerebrovascular morbidity and tended to cluster in children and adolescents with headaches.
In this large and representative American sample, children and adolescents with severe or recurrent headache or migraine had higher mean BMI, higher levels of CRP and homocysteine, and lower levels of serum and red blood cell folate. More children with headache were in the quintile of highest risk for each of these biomarkers, and more were in the highest quintile of risk for 3 or more. Our results suggest that there may be 2 subgroups of children with headaches: one characterized by elevated levels of homocysteine and lower serum folate levels and the other by relatively high CRP levels and asthma. These observations suggest that different mechanisms may contribute to vascular changes in these 2 groups, and these subgroups may index potential endophenotypes that could be examined in future genetic research.
Elevated CRP level is a marker of inflammation, its concentration in blood correlating with levels of inflammatory cytokines, and is a biomarker of risk for cardiovascular disease and stroke.29 The results of the study presented herein are consistent with a previous one showing increased CRP levels in young adults with migraine.9 There is a strong relationship of CRP levels and BMI in children and adolescents, such that a 1-SD increase in BMI associates with a 52% increase in CRP concentration.30 Indeed, adiposity was the major determinant of CRP levels in children.31 Persons with an elevated CRP level at initial measurement tend to continue to have elevated levels of CRP in subsequent years.32 Abnormalities in homocysteine levels are also associated with vascular risk.
In the NHANES survey years examined in this study, values for homocysteine, folate, methylmalonic acid, and vitamin B12 were all measured after the initiation of fortification of foodstuffs with folate.33
Many of the clinical or demographic indicators explored in this study are related to one another. In analyzing the association of the several factors with headache, we have tried to take the known relationships into account in multivariate analysis but cannot rule out unrecognized confounding.
While NHANES has important advantages for the study of disorders of health and their correlates, this data source has significant limitations. Information available in NHANES on headache status does not distinguish between migraine and its variants, including aura, and other frequent or severe headache types. In fact, as indicated, headaches in childhood are not easy to classify. Diagnostic categories of headache in young children are not stable over time, the proportion with a diagnosis of migraine at pubertal age being similar among those diagnosed at age 6 years with migraine or with tension-type headache.20 The basic distinction between tension-type headache and migraine has been questioned.34,35
Severe or recurrent headache or migraine, as identified in NHANES, marks a group of young people who have characteristics associated with cardiovascular risk. Identifying early indicators of vascular disease may enhance our ability to define targets for prevention of these conditions. While cardiovascular and cerebrovascular disease usually do not become symptomatic until midlife or later, changes in arteries that are detectable in childhood are thought to be part of its pathogenesis. Chronic low-grade inflammation, as measured by CRP level, appears to play a role in the development of such vascular pathology.36,37 Elevated levels of homocysteine may also contribute.
Recent randomized trials indicate that treatment with a statin to lower CRP levels reduces the risk of stroke and other vascular events.38 Statin use in children with hyperlipidemia is apparently effective and safe.39,40 Dietary improvement can lower homocysteine levels and decrease BMI. Wärnberg and Marcos stress
the possibility of using markers of low-grade inflammation for screening high-risk young subjects during the long presymptomatic phase … while most damage is likely to be reversible.41(pp13–14)
Risk of asymptomatic vascular disease can be identified early, and severity rises with increasing number of risk factors.42 We report that biomarkers of risk for vascular disease appear to cluster in children and adolescents with severe or recurrent headaches or migraine. Such young people may be an appropriate target for further study and for screening, follow-up, and efforts to prevent long-term vascular pathology and resulting cardiovascular disease and stroke.
Acknowledgments
Funding/Support: This study was supported by the National Institute of Mental Health and National Institute of Neurological Disorders and Stroke intramural research programs.
Footnotes
Author Contributions: Study concept and design: Nelson, Richardson, Lateef, Khoromi, and Merikangas. Acquisition of data: Nelson and Merikangas. Analysis and interpretation of data: Nelson, Richardson, He, Khoromi, and Merikangas. Drafting of the manuscript: Nelson, He, Khoromi, and Merikangas. Critical revision of the manuscript for important intellectual content: Richardson, He, Lateef, and Merikangas. Statistical analysis: Richardson, He, and Merikangas. Study supervision: Nelson, Lateef, and Merikangas.
Financial Disclosure: None reported.
References
- 1.Wilson PW, Bozeman SR, Burton TM, Hoaglin DC, Ben-Joseph R, Pashos CL. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. Circulation. 2008;118(2):124–130. doi: 10.1161/CIRCULATIONAHA.108.772962. [DOI] [PubMed] [Google Scholar]
- 2.Sanossian N, Tarlov NE. HDL-C and LDL-C: their role in stroke pathogenesis and implications for treatment. Curr Treat Options Cardiovasc Med. 2008;10(3):195–206. doi: 10.1007/s11936-008-0021-1. [DOI] [PubMed] [Google Scholar]
- 3.Abi-Saleh B, Iskandar SB, Elgharib N, Cohen MV. C-reactive protein: the harbinger of cardiovascular diseases. South Med J. 2008;101(5):525–533. doi: 10.1097/SMJ.0b013e31816c0195. [DOI] [PubMed] [Google Scholar]
- 4.Makita S, Nakamura M, Satoh K, et al. Serum C-reactive protein levels can be used to predict future ischemic stroke and mortality in Japanese men from the general population. Atherosclerosis. 2009;204(1):234–238. doi: 10.1016/j.atherosclerosis.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Zee RY, Mora S, Cheng S, et al. Homocysteine, 5,10-methylenetetrahydrofolate reductase 677C>T polymorphism, nutrient intake, and incident cardiovascular disease in 24,968 initially healthy women. Clin Chem. 2007;53(5):845–851. doi: 10.1373/clinchem.2006.083881. [DOI] [PubMed] [Google Scholar]
- 6.Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. Factors associated with the steep increase in late-midlife stroke occurrence among US men. J Stroke Cerebrovasc Dis. 2008;17(4):165–168. doi: 10.1016/j.jstrokecerebrovasdis.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monastero R, Pipia C, Cefalu AB, et al. Association between plasma lipid levels and migraine in subjects aged > or =50 years: preliminary data from the Zabut Aging Project. Neurol Sci. 2008;29(suppl 1):S179–S181. doi: 10.1007/s10072-008-0919-0. [DOI] [PubMed] [Google Scholar]
- 8.Keith SW, Wang C, Fontaine KR, Cowan CD, Allison DB. BMI and headache among women: results from 11 epidemiologic datasets. Obesity (Silver Spring) 2008;16(2):377–383. doi: 10.1038/oby.2007.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanmolkot FH, de Hoon JN. Increased C-reactive protein in young adult patients with migraine. Cephalalgia. 2007;27(7):843–846. doi: 10.1111/j.1468-2982.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- 10.Moschiano F, D’Amico D, Usai S, et al. Homocysteine plasma levels in patients with migraine with aura. Neurol Sci. 2008;29(suppl 1):S173–S175. doi: 10.1007/s10072-008-0917-2. [DOI] [PubMed] [Google Scholar]
- 11.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301(24):2563–2570. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72(21):1864–1871. doi: 10.1212/WNL.0b013e3181a71220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rook JL, Nugent DJ, Young G. Pediatric stroke and methylenetetrahydrofolate reductase polymorphisms: an examination of C677T and A1298C mutations. J Pediatr Hematol Oncol. 2005;27(11):590–593. doi: 10.1097/01.mph.0000188119.33452.fd. [DOI] [PubMed] [Google Scholar]
- 14.Glueck CJ, Bates SR. Migraine in children: association with primary and familial dyslipoproteinemias. Pediatrics. 1986;77(3):316–321. [PubMed] [Google Scholar]
- 15.Bottini F, Celle ME, Calevo MG, et al. Metabolic and genetic risk factors for migraine in children. Cephalalgia. 2006;26(6):731–737. doi: 10.1111/j.1468-2982.2006.01107.x. [DOI] [PubMed] [Google Scholar]
- 16.Lateef TM, Merikangas KR, He J, et al. Headache in a national sample of American children: prevalence and comorbidity. J Child Neurol. 2009;24(5):536–543. doi: 10.1177/0883073808327831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wannamethee SG, Shaper AG, Whincup PH, Walker M. Role of risk factors for major coronary heart disease events with increasing length of follow up. Heart. 1999;81(4):374–379. doi: 10.1136/hrt.81.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merikangas K, He J, Brody D, Fisher PW, Bourdon K, Koretz D. Prevalence and correlates of mental disorders and services in US children: results from NHANES 2001–2004 [published online December 14, 2009] Pediatrics. doi: 10.1542/peds.2008–2598. [DOI] [Google Scholar]
- 19.Brna P, Dooley J, Gordon K, Dewan T. The prognosis of childhood headache: a 20-year follow-up. Arch Pediatr Adolesc Med. 2005;159(12):1157–1160. doi: 10.1001/archpedi.159.12.1157. [DOI] [PubMed] [Google Scholar]
- 20.Virtanen R, Aromaa M, Rautava P, et al. Changing headache from preschool age to puberty: a controlled study. Cephalalgia. 2007;27(4):294–303. doi: 10.1111/j.1468-2982.2007.01277.x. [DOI] [PubMed] [Google Scholar]
- 21.Lewis DW. Pediatric migraine. Neurol Clin. 2009;27(2):481–501. doi: 10.1016/j.ncl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Wöber-Bingöl C, Wöber C, Wagner-Ennsgraber C, et al. IHS criteria for migraine and tension-type headache in children and adolescents. Headache. 1996;36 (4):231–238. doi: 10.1046/j.1526-4610.1996.3604231.x. [DOI] [PubMed] [Google Scholar]
- 23.Gallai V, Sarchielli P, Carboni F, Benedetti P, Mastropaolo C, Puca F Juvenile Headache Collaborative Study Group. Applicability of the 1988 IHS criteria to headache patients under the age of 18 years attending 21 Italian headache clinics. Headache. 1995;35(3):146–153. doi: 10.1111/j.1526-4610.1995.hed3503146.x. [DOI] [PubMed] [Google Scholar]
- 24.Maytal J, Young M, Shechter A, Lipton RB. Pediatric migraine and the International Headache Society (IHS) criteria. Neurology. 1997;48(3):602–607. doi: 10.1212/wnl.48.3.602. [DOI] [PubMed] [Google Scholar]
- 25.Gherpelli JL, Nagae Poetscher LM, Souza AM, et al. Migraine in childhood and adolescence: a critical study of the diagnostic criteria and of the influence of age on clinical findings. Cephalalgia. 1998;18(6):333–341. doi: 10.1046/j.1468-2982.1998.1806333.x. [DOI] [PubMed] [Google Scholar]
- 26.Hämäläinen ML, Hoppu K, Santavuori PR. Effect of age on the fulfilment of the IHS criteria for migraine in children at a headache clinic. Cephalalgia. 1995;15(5):404–409. doi: 10.1046/j.1468-2982.1995.1505404.x. [DOI] [PubMed] [Google Scholar]
- 27.Battistella PA, Fiumana E, Binelli M, et al. Primary headaches in preschool children: clinical study and follow-up in 163 patients. Cephalalgia. 2006;26(2):162–171. doi: 10.1111/j.1468-2982.2005.01008.x. [DOI] [PubMed] [Google Scholar]
- 28.Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773–780. doi: 10.1097/PSY.0b013e31817f9e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampoli AM, Tousoulis D, Antoniades C, Siasos G, Stefanadis C. Biomarkers of premature atherosclerosis. Trends Mol Med. 2009;15(7):323–332. doi: 10.1016/j.molmed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Lambert M, Delvin EE, Paradis G, O’Loughlin J, Hanley JA, Levy E. C-reactive protein and features of the metabolic syndrome in a population-based sample of children and adolescents. Clin Chem. 2004;50(10):1762–1768. doi: 10.1373/clinchem.2004.036418. [DOI] [PubMed] [Google Scholar]
- 31.Cook DG, Mendall MA, Whincup PH, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149(1):139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 32.Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem. 2009;55(2):305–312. doi: 10.1373/clinchem.2008.120642. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am J Clin Nutr. 2007;86(3):718–727. doi: 10.1093/ajcn/86.3.718. [DOI] [PubMed] [Google Scholar]
- 34.Vargas BB. Tension-type headache and migraine: two points on a continuum? Curr Pain Headache Rep. 2008;12(6):433–436. doi: 10.1007/s11916-008-0073-7. [DOI] [PubMed] [Google Scholar]
- 35.Cady RK. The convergence hypothesis. Headache. 2007;47(suppl 1):S44–S51. doi: 10.1111/j.1526-4610.2007.00676.x. [DOI] [PubMed] [Google Scholar]
- 36.Kapiotis S, Holzer G, Schaller G, et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol. 2006;26(11):2541–2546. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- 37.Järvisalo MJ, Harmoinen A, Hakanen M, et al. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22(8):1323–1328. doi: 10.1161/01.atv.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Danielson E, Fonseca FAH, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 39.Avis HJ, Vissers MN, Wijburg FA, Kastelein JJ, Hutten BA. The use of lipid-lowering drug therapy in children and adolescents. Curr Opin Investig Drugs. 2009;10(3):224–231. [PubMed] [Google Scholar]
- 40.O’Gorman CS, Higgins MF, O’Neill MB. Systematic review and meta-analysis of statins for heterozygous familial hypercholesterolemia in children: evaluation of cholesterol changes and side effects. Pediatr Cardiol. 2009;30(4):482–489. doi: 10.1007/s00246-008-9364-3. [DOI] [PubMed] [Google Scholar]
- 41.Wärnberg J, Marcos A. Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr Opin Lipidol. 2008;19(1):11–15. doi: 10.1097/MOL.0b013e3282f4096b. [DOI] [PubMed] [Google Scholar]
- 42.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]