Abstract
Objective
To describe the course of patients with juvenile dermatomyositis (JDM) treated effectively without systemic corticosteroids.
Study design
A retrospective study of 38 patients with JDM treated at a tertiary care children’s hospital identified 8 patients who had never received corticosteroids. Disease presentation and course, pharmacologic and ancillary treatments were recorded.
Results
Patients in the no corticosteroid group were followed for a median of 2.8 years (range 2.1 – 9.5 years). Treatment was primarily with intravenous immunoglobulin (IVIG) (75%) and methotrexate (MTX) (50%), with favorable response in all. No serious treatment complications were observed; headaches were reported by 3 patients receiving IVIG. Two patients had a myositis flare after discontinuing all medications for more than one year; complete resolution of symptoms was observed after either 1 or 2 further doses of IVIG. Two patients developed calcinosis (at 1 and 9 years of disease); however, no patient developed joint contractures, muscle atrophy, lipodystrophy, or functional limitations.
Conclusion
Systemic corticosteroids can be avoided in a select group of patients with JDM. Alternative agents such as MTX and IVIG may be prescribed to effectively treat JDM and prevent complications.
Keywords: pediatric, prednisone, steroid-sparing, intravenous immune globulin, methotrexate, glucocorticoids
Juvenile dermatomyositis (JDM) is a chronic childhood multisystem autoimmune disease characterized by rash and proximal muscle weakness. Although standardized treatment protocols for JDM do not exist, high-dose systemic corticosteroid therapy slowly tapered over two or more years has been the conventional approach.1, 2 Corticosteroids can have disabling side effects, including immunosuppression, growth delay and growth failure, hyperglycemia, hypertension, avascular necrosis of bone, cataracts, weight gain, cutaneous striae, acne, and osteoporosis. Many of these side effects are attributable to the dose and duration of systemic therapy.
To minimize the adverse effects of long-term systemic corticosteroids, pediatric rheumatologists have sought alternative treatments. Prospective randomized controlled trials have not been conducted, although studies have demonstrated the utility of methotrexate (MTX) as an effective steroid-sparing agent in JDM.3–5 Other drugs including cyclosporine A and hydroxychloroquine are prescribed for refractory disease.6–10 A small randomized trial of intravenous immunoglobulin (IVIG) in treatment-resistant adult dermatomyositis demonstrated significant disease improvement.11 IVIG likely has multiple immunomodulatory actions in dermatomyositis,12 although its precise mechanism of action is unknown.13 In JDM, IVIG is effective as a steroid-sparing agent, 14–20 however; because of its expense, it has often been reserved for resistant disease.21
The objective of this study was to describe the disease course of 8 patients with JDM who were effectively treated without systemic corticosteroids, primarily utilizing IVIG as a steroid-sparing agent.
STUDY DESIGN
A retrospective chart review of all patients diagnosed with probable or definite JDM22, 23 and treated at the Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center (CUMC) between January 1991 and June 2006 was conducted. Records of patients identified as “never having received corticosteroids” were thoroughly reviewed, and further data were extracted. Patient demographics, disease presentation and course, and pharmacologic and ancillary treatments were reviewed.
Although no definitions of JDM disease severity are universally accepted, no patient in this study had severe disease at diagnosis. At this center, patients with vasculitic skin lesions, dysphonia, palatal weakness, gastrointestinal involvement, pulmonary or cardiac disease, or muscle weakness of grade 3/5 or worse were identified as “severe” and received corticosteroid therapy at the time of diagnosis. Most patients without severe disease also received corticosteroids at time of JDM diagnosis. The degree of elevation of muscle enzymes [creatine phosphokinase (CPK), aldolase, lactose dehydrogenase (LDH), aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] and the presence of arthritis were not considered in the definition of disease severity at presentation.
Clinical outcome measures abstracted from chart review included strength on manual muscle testing (MMT), progression or resolution of rash, and findings on ophthalmoscopic nailfold capillaroscopy. Notation was made of growth measures, joint contractures, calcinosis, and disease exacerbations. Laboratory testing for muscle enzymes and inflammatory markers was also recorded.
Statistical analysis was performed using Stata 10.0 (College Station, Texas). Student t-tests were performed for continuous data, and Chi-squared analysis and Fisher exact test were performed for analysis of categorical data. Most data, however, were of a qualitative nature, and descriptive statistics were used. The study was approved by the Institutional Review Board at CUMC.
RESULTS
Between January 1991 and June 2006, 38 patients with JDM were identified. Of these, 8 (21%) were treated without systemic corticosteroids and included in this study. One further patient was never treated with corticosteroid; however, this patient was excluded because she had clinically amyopathic dermatomyositis24 with typical cutaneous findings, but no myositis over three years of follow-up. The corticosteroid group (30 patients) all received steroids at the time of JDM diagnosis; no patient initially treated without corticosteroid subsequently required the addition of steroids because of worsening disease or failure to improve.
Patient characteristics at diagnosis are shown in Table I; the two groups were similar, although the corticosteroid treated group had significantly greater von Willebrand factor antigen (vWF Ag) level at diagnosis (100% vs. 184%, p=.03). All patients were followed for a minimum of two years, with the end of study visit recorded as either June 2006 or last follow-up visit if earlier than this date.
Table 1.
Patient Characteristics at Diagnosis
| Treatment Received | p value | |||
|---|---|---|---|---|
| No Corticosteroid (n=8) |
Corticosteroid (n=30) |
|||
| Sex (#, %) | 6 F (75) | 22 F (73) | 1.0 | |
|
Ethnicity (#, %) |
Caucasian | 6 (75) | 14 (47) | 0.90 |
| Black | 1 (12.5) | 7 (23) | ||
| Asian | 0 | 1 (3) | ||
| Hispanic | 1 (12.5) | 6 (20) | ||
| Other | 0 | 2 (7) | ||
|
Age (median years, range) |
8.0 (4.3 – 12.3) | 7.9 (1.9 – 17.4) | 0.9 | |
|
Duration of Symptoms (median weeks, range) |
8 (4 – 52) | 8 (2 – 52) | 0.75 | |
|
Muscle Enzymes (median, range) (U/l) |
CPK (N<238) |
177 (69 – 2932) | 1022 (32 – 83440) | 0.42 |
|
aldolase (N<7.0) |
10.5 (6.7 – 26) | 15.8 (6.5 – 106.9) (n=20) |
0.14 | |
|
AST (N<38) |
35 (23 – 217) | 83 (16 – 1110) (n=24) |
0.47 | |
|
ALT (N<41) |
27 (11 – 220) | 59 (14 – 540) (n=24) |
0.33 | |
|
LDH (N<221) |
290 (240 – 415) (n=4) |
438 (180 – 3741) (n=23) |
0.34 | |
|
vWF Ag (median, range) (N 50 – 150%) |
100 (22 – 166) (n=6) |
184 (52 – 420) (n=20) |
0.03 | |
CPK = creatine phosphokinase, AST = aspartate aminotransferase, ALT = alanine aminotransferase, LDH = lactate dehydrogenase, vWF Ag= von Willebrand factor antigen. For comparison of laboratory test values, number of subjects with test results available for each group is noted if different than total number in group.
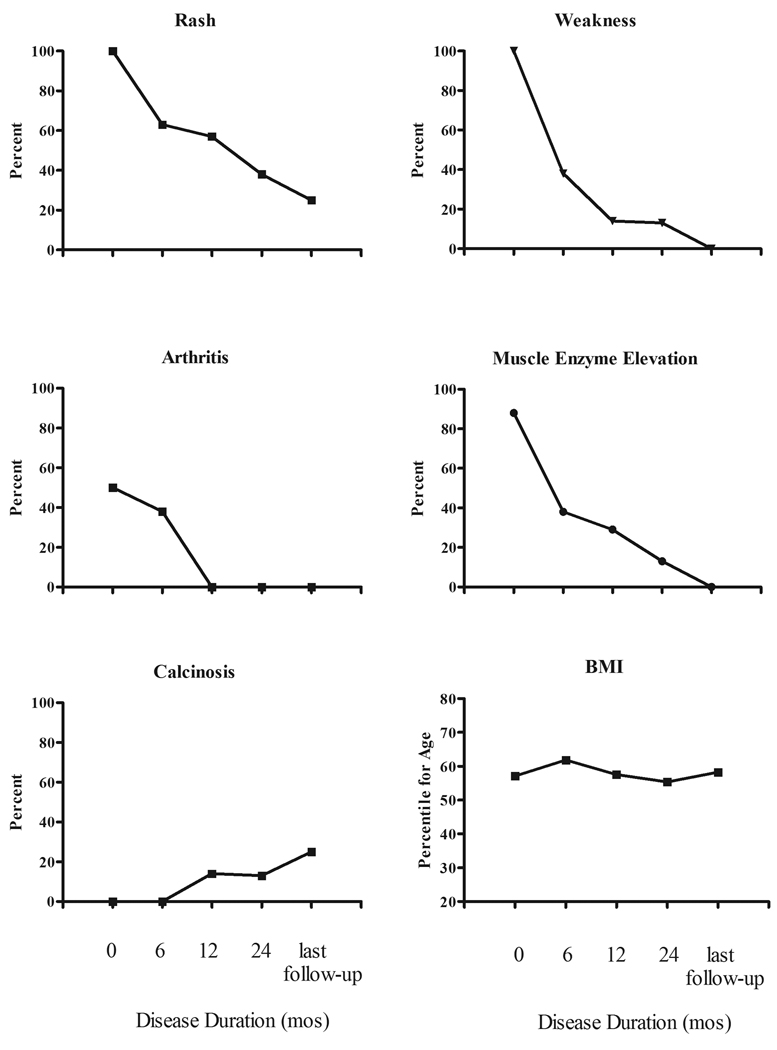
Disease characteristics at the time of JDM diagnosis for the group treated without corticosteroid are detailed in Table II. Erythrocyte sedimentation rate (ESR) was normal in all eight patients; antinuclear antibodies (ANA) were present in 4 (50%) patients; and double-stranded DNA antibodies (dsDNA) and antibodies to extractable nuclear antigens (ENA) were negative in all patients. Median body mass index (BMI) was 16.7 (range 15.3 – 18.1, equivalent to 37th – 90th percentile corrected for age). Four (50%) patients had arthritis (two to eight joints affected). No patient had signs of severe disease, and none had calcinosis at diagnosis. Change in disease characteristics over the course of study are illustrated in the Figure.
Table 2.
Disease Characteristics at Diagnosis
| Pt # | Age (y) | Symptoms | Duration (weeks) | MMT | Rash | Muscle Enzymes | MRI | Other (muscle biopsy, EMG, NCS) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UE | LE | Neck | Abd | CPK | ALD | AST | ALT | LDH | |||||||
| 1 | 4.3 | Rash, myalgias | 20 | 4+ | 4+ | 4 | 4 | Gottron's papules, nailfold abnormalities | ↑ | ↑ | N | N | ↑ | N | ND |
| 2 | 5.2 | Fatigue, fever, rash, arthralgias, falling, weakness, “waddling” gait | 8 | 4+ | 4 | 4 | 4 | Gottron's papules, heliotrope nailfold abnormalities | ↑ | ↑ | N | N | ND | + | EMG + |
| 3 | 6.2 | Fatigue, rash, leg pain, trouble walking up stairs | 8 | 5 | 4 | 5 | 4 | Gottron's papules, malar rash, nailfold abnormalities | N | ↑ | N | N | ND | + | ND |
| 4 | 7.3 | Rash | 4 | 4+ | 4+ | 4+ | 4+ | Gottron’s papules, mild heliotrope, malar rash, bilateral ear lesions | N | ↑ | ↑ | N | ND | N | ND |
| 5 | 8.8 | Leg pain | 52 | 4+ | 4+ | 5 | 4+ | Gottron's papules, nailfold abnormalities | N | N | N | N | ND | + | ND |
| 6 | 11.0 | Fatigue, rash, arthralgias, myalgias, weakness, rash | 16 | 4+ | 5 | 4+ | 5 | Gottron’s papules, nailfold abnormalities | N | ↑ | N | N | ↑ | N | ND |
| 7 | 11.1 | Fatigue, rash, abdominal pain, difficulty walking and climbing stairs | 4 | 4+ | 4+ | 4 | 5 | Gottron's papules, mild heliotrope, nailfold abnormalities, | ↑ | ↑ | ↑ | ↑ | ↑ | + | ND |
| 8 | 12.3 | Fatigue, arthralgias, difficulty with ADLs | 2 | 4 | 4 | 4 | 4 | Gottron’s papules, mild heliotrope, malar rash, shawl sign | ↑ | ↑ | ↑ | ↑ | ↑ | + | ND |
MMT = manual muscle testing, UE = upper extremities, LE = lower extremities, Abd = abdominal musculature, CPK = creatine phosphokinase, ALD = aldolase, AST = aspartate aminotransferase, ALT = alanine aminotransferase, LDH = lactate dehydrogenase, MRI = magnetic resonance imaging of proximal thighs, EMG = electromyogram, NCS = nerve conduction study, N = normal, ND = not done, ADLs = activities of daily living
Figure.
Clinical characteristics shown as percentage of subjects with clinical characteristic noted at each visit. BMI (body mass index) is shown as mean percentile for age of all subjects at each time point.
All eight patients received medical therapy as detailed in Table III. Prescribed medical treatments at presentation included IVIG, MTX (oral or subcutaneous) and oral hydroxychloroquine. All patients received physical and occupational therapy if required.
Table 3.
Treatment Course
| Patient | IVIG dose | Other Treatment | Medication Duration | Complications |
|---|---|---|---|---|
| 1 | 2 g/kg every 4 weeks | None | IVIG every 10 weeks at 4 years | None |
| 2 | 2 g/kg every 8 weeks for 3 doses; 2 doses at relapse after 2.5 years | None | Total 5 doses of IVIG | Superficial nodular calcinosis |
| 3 | 2 g/kg every 4 weeks for 2 doses; 1 dose at relapse at 1 year | None | Total 3 doses of IVIG | None |
| 4 | 2 g/kg every 6 weeks | Hydroxychloroquine | Total 5 doses of IVIG Discontinued hydroxychloroquine by 1 year | Headaches post IVIG |
| Methylphenidate hydrochloride and clonidine (for ADHD) | ||||
| 5 | None | Sulfasalazine at presentation for 4 months (for arthritis) | MTX and etanercept for arthritis at 2.1 years | Tumoral calcinosis |
| NSAID | ||||
| MTX started 4 months after presentation | ||||
| Etanercept added at 6 months | ||||
| 6 | None | NSAID | Discontinued all by 8 months | None |
| MTX | ||||
| 7 | 2 g/kg every 4 weeks | MTX | Discontinued IVIG by 1 year, MTX by 2 years | Headaches post IVIG |
| 8 | 2 g/kg every 2 weeks for 3 doses, then every 4 weeks | MTX | Discontinued IVIG by 18 months, MTX by 2.5 years | Headaches post IVIG |
| Thyroxine (for hypothyroidism) |
IVIG = intravenous immunoglobulin; ADHD = attention deficit hyperactivity disorder; MTX = methotrexate; NSAID = non-steroidal anti-inflammatory drug
At presentation, patient #4 had isolated dermatologic findings and was prescribed only hydroxychloroquine; IVIG was added when he met criteria for probable JDM four months later. In total, 4 patients (50%) received MTX, and IVIG was prescribed for 6 (75%) patients. Patient #5 was initially treated for symmetric polyarthritis, and while taking MTX and etanercept, developed Gottron’s papules and mild myositis, followed by the development of tumoral calcinosis.
Six of the eight patients (75%) received intravenous immunoglobulin (IVIG) for treatment of myositis. The standard dose of IVIG was 2 grams per kilogram per dose. Dosing was every 4 weeks for 3 patients, with alternative schedules for the other 3 patients (Table III). For comparison, in the corticosteroid treated group, 19/30 (63%) were prescribed IVIG at the onset of their disease and a further 5 (17%) were given IVIG later in their disease course for treatment of resistant disease, for a total of 24/30 (85%) patients having ever received IVIG.
Patient 8, a 12 year-old white girl, presented with a two week history of increasing weakness, fatigue, low-grade fever, and polyarthritis. She was unable to climb stairs, and had difficulty with personal grooming. She had mild heliotrope rash and Gottron’s papules. She was unable to arise from a low stool unassisted; MMT was graded as 4/5 in proximal upper and lower extremities bilaterally, as well as neck and abdominal muscles. Her medical history was significant for bilateral femoral fractures and humeral fractures with osteoporosis secondary to Graves’ disease. She had received radioactive iodine ablation, and was taking thyroxine at time of diagnosis of JDM. All muscle enzymes at diagnosis were elevated: CPK 582 U/L (normal <238), aldolase 16.7 U/L (normal <8.8), AST 164 U/L (normal <38), ALT 167 U/L (normal <41), LDH 415 U/L (normal <221), and MRI was consistent with bilateral proximal thigh myositis. A medical regimen avoiding corticosteroid was preferable to avoid further bone complications. The patient had notable improvement of her weakness within days of the first IVIG dose; MMT at 10 days was 4+/5 in lower extremities and abdomen, 4/5 in upper extremities and neck. At 26 days following the first dose of IVIG, MMT was 4+/5 in all muscle groups except for biceps (4/5). The patient was able to wash her hair unassisted and was able to resume competitive bowling within several weeks. Muscle enzymes 10 days following IVIG were CPK 264, aldolase 11.1, AST 127, ALT 148, and at 26 days following diagnosis CPK was 60, aldolase 7.3, AST 62, ALT 88, LDH 220. All muscle enzymes were normal at two months following diagnosis.
During the time period of this study, there were no serious adverse reactions to IVIG, MTX, or other immunosuppressive therapies. No patient required hospitalization for infection, no liver toxicity was noted, and only headache was reported by three (50%) of the patients who received IVIG. At this center, the usual practice is to administer IVIG at home by a visiting nurse service to avoid school absences and disruptions to the family routine.
Mean duration of follow-up was 3.7 years (median 2.8 years, range 2.1 – 9.5 years). Two patients were still receiving treatment – one was prescribed IVIG every 10 weeks, and the second patient was prescribed weekly MTX and etanercept, primarily for arthritis. Six patients had discontinued all therapy − 5 had received IVIG, and 3 had received MTX.
Over the duration of this study, 2 patients had disease flares with increased proximal muscle weakness, cutaneous findings, and muscle enzyme elevations while off all medications, at 1 year and 2.5 years respectively. Re-treatment with 1 and 2 doses of IVIG resulted in a second complete remission of symptoms in both patients.
Although cutaneous involvement improved with treatment in all 8 patients, 2 patients were noted to have rash at the most recent follow-up (“mild Gottron’s”) at disease durations of 2.6 and 4.0 years respectively. All six patients with nailfold capillary abnormalities at diagnosis (telangiectasia, dropout, hemorrhage) had complete resolution at their last visit.
Two patients developed calcinosis – one after 1 year of follow-up, the second after 9 years of follow-up. The first patient developed tumoral calcinosis in and around both elbow joints; the second patient developed superficial nodular calcinosis over a metacarpophalangeal (MCP) joint in his “pitching hand” after 7 asymptomatic years off medication.
All 8 patients maintained normal growth velocities with normal body mass index (BMI) noted at follow-up appointments (Figure). No bone fractures were observed. In this study, 21% of all pediatric patients treated for JDM over 15 years at one center were successfully treated without systemic corticosteroids. By avoiding prolonged high-dose corticosteroids, patients were able to maintain normal height velocities and BMIs, and avert other steroid side effects. At present, standard of care treatment for JDM includes high-dose daily oral corticosteroids,1, 2 although many pediatric rheumatologists utilize intravenous intermittent high-dose “pulse” methylprednisolone to avoid the side effects of this regimen. Previous studies report successful treatment with intravenous corticosteroid alone.25, 26 However, there have been no recent reports examining the successful treatment of JDM without corticosteroids.
To determine which patients with JDM would respond favorably to treatment regimens that do not include systemic corticosteroids, categorizing disease severity may be useful. At this center, any patient who presents with signs of severe disease receives corticosteroid therapy in addition to IVIG and MTX at diagnosis. Patients with less severe weakness (3+/5 by MMT) also receive IVIG and MTX, and almost all will also receive corticosteroid therapy at diagnosis. These patients are often unable to get out of bed, or off of a chair without assistance, and have difficulty with other activities of daily living. Patients presenting with milder muscle weakness (4 or 4+/5) receive IVIG with or without MTX as first line therapy. In those patients not prescribed corticosteroid, should myositis fail to improve (or worsen) within days to weeks following the initial IVIG infusion, corticosteroids would be added to the therapeutic regimen. Interestingly, in this series, no patient who initiated treatment without corticosteroid later required the addition of steroids. Additionally, although prolonged duration of symptoms prior to initiation of treatment is known to be a risk factor for disease severity at diagnosis,27 in this series, both the corticosteroid and no corticosteroid groups had a median 8 week symptom duration prior to diagnosis.
The understanding of the natural history of JDM has evolved over the past several years. Pediatric rheumatologists recognize that JDM generally runs either a monocyclic course (2 to 3 years average) or a chronic continuous course that can last 7 to 10 years or even longer.28 Ultimately, the disease will permanently remit, but the duration of disease cannot be predicted at its onset. Short-term goals of treatment are directed at improving strength and function. Treatment is often continued for 2 years or longer to prevent long- term complications, including dystrophic soft tissue calcifications and joint and muscle contractures. In this cohort, 2 of 8 patients (25%) developed calcinosis at 12 months and 9 years of follow-up, respectively, consistent with an incidence of 30% in the recent literature.29 No patient in this series developed joint or muscle contractures, muscle atrophy, or lipodystrophy, and all patients reported excellent function and full ability to complete activities of daily living at their last reported visit. Growth and BMI were normal for all 8 patients, successfully avoiding the growth delay and weight gain frequently observed with high-dose corticosteroid therapy.
In the United States, IVIG is readily available, and can be obtained for use for treatment of myositis. Since publication of the adult trial of IVIG for dermatomyositis,11 IVIG has been an integral part of the initial treatment regimen for all new patients with JDM at this center. Serious adverse effects were not observed in this patient cohort, and although the frequency of mild side effects was lower than recently reported in a large cohort,30 patients received IVIG infusions at home, so mild side effects may have been underreported. In this series, 2 of 8 patients treated without corticosteroids did not receive IVIG – both of these patients initially presented predominantly with polyarthritis, with later development of JDM rash and myositis. Both patients were successfully treated with other steroid- sparing medications (i.e., MTX, etanercept).
The current study is limited by its retrospective nature, small number of patients, and lack of routine use of standard measures such as the Childhood Myositis Assessment Scale31 (CMAS) to gauge disease activity. However, it appears that systemic corticosteroids can be avoided completely in a select group of patients with JDM. Alternative agents such as MTX and IVIG may be prescribed to effectively treat the disease and prevent complications.
Although several features of severe disease are recognized as poor prognostic indicators, and long duration of disease prior to initiation of treatment is a recognized risk factor for developing calcinosis,1, 27 there is currently no accurate way to predict a mild disease course. In this series, patients who did not receive corticosteroid had normal to minimally elevated vWF antigen levels, suggesting that endothelial activity may be useful for determining severity of disease and required intensity of treatment. Creation of a scoring system of disease severity (at presentation) should be considered to further aid in treatment decisions. This score could include a CMAS assessment and other functional indices; however, it would require validation. The recent activity core set for the evaluation of response to therapy in JDM32 may be a useful starting point for the development of a severity score.
ACKNOWLEDGMENT
The authors thank Dr. Yumna Jafri for her assistance with chart review.
ABBREVIATIONS
- ALT
alanine aminotransferase
- ANA
anti-nuclear antibodies
- AST
aspartate aminotransferase
- BMI
body mass index
- CMAS
Childhood Myositis Assessment Scale
- CPK
creatine phosphokinase
- CUMC
Columbia University Medical Center
- dsDNA
double-stranded DNA
- ENA
extractable nuclear antigens
- IVIG
intravenous immunoglobulin
- JDM
juvenile dermatomyositis
- LDH
lactose dehydrogenase
- MCP
metacarpophalangeal
- MMT
manual muscle testing
- MTX
methotrexate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no financial or other relationships that might lead to a conflict of interest.
Contributor Information
Deborah M. Levy, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center.
C. April Bingham, Milton S. Hershey Medical Center, Penn State College of Medicine.
Philip J. Kahn, New York University School of Medicine.
Andrew H. Eichenfield, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center.
Lisa F. Imundo, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center.
REFERENCES
- 1.Bowyer SL, Blane CE, Sullivan DB, Cassidy JT. Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr. 1983;103:882–888. doi: 10.1016/s0022-3476(83)80706-9. [DOI] [PubMed] [Google Scholar]
- 2.Reed AM, Lopez M. Juvenile dermatomyositis: recognition and treatment. Paediatr Drugs. 2002;4:315–321. doi: 10.2165/00128072-200204050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Miller LC, Sisson BA, Tucker LB, DeNardo BA, Schaller JG. Methotrexate treatment of recalcitrant childhood dermatomyositis. Arthritis Rheum. 1992;35:1143–1149. doi: 10.1002/art.1780351006. [DOI] [PubMed] [Google Scholar]
- 4.Ramanan AV, Campbell-Webster N, Ota S, et al. The effectiveness of treating juvenile dermatomyositis with methotrexate and aggressively tapered corticosteroids. Arthritis Rheum. 2005;52:3570–3578. doi: 10.1002/art.21378. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mayouf S, Al-Mazyed A, Bahabri S. Efficacy of early treatment of severe juvenile dermatomyositis with intravenous methylprednisolone and methotrexate. Clin Rheumatol. 2000;19:138–141. doi: 10.1007/s100670050032. [DOI] [PubMed] [Google Scholar]
- 6.Zeller V, Cohen P, Prieur AM, Guillevin L. Cyclosporin a therapy in refractory juvenile dermatomyositis. Experience and longterm followup of 6 cases. J Rheumatol. 1996;23:1424–1427. [PubMed] [Google Scholar]
- 7.Reiff A, Rawlings DJ, Shaham B, et al. Preliminary evidence for cyclosporin A as an alternative in the treatment of recalcitrant juvenile rheumatoid arthritis and juvenile dermatomyositis. J Rheumatol. 1997;24:2436–2443. [PubMed] [Google Scholar]
- 8.Olson NY, Lindsley CB. Adjunctive use of hydroxychloroquine in childhood dermatomyositis. J Rheumatol. 1989;16:1545–1547. [PubMed] [Google Scholar]
- 9.Woo TY, Callen JP, Voorhees JJ, Bickers DR, Hanno R, Hawkins C. Cutaneous lesions of dermatomyositis are improved by hydroxychloroquine. J Am Acad Dermatol. 1984;10:592–600. doi: 10.1016/s0190-9622(84)80263-7. [DOI] [PubMed] [Google Scholar]
- 10.Heckmatt J, Hasson N, Saunders C, et al. Cyclosporin in juvenile dermatomyositis. Lancet. 1989;1:1063–1066. doi: 10.1016/s0140-6736(89)92456-2. [DOI] [PubMed] [Google Scholar]
- 11.Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329:1993–2000. doi: 10.1056/NEJM199312303292704. [DOI] [PubMed] [Google Scholar]
- 12.Dalakas MC. Intravenous immunoglobulin in autoimmune neuromuscular diseases. Jama. 2004;291:2367–2375. doi: 10.1001/jama.291.19.2367. [DOI] [PubMed] [Google Scholar]
- 13.Barbasso Helmers S, Dastmalchi M, Alexanderson H, et al. Limited effects of high-dose intravenous immunoglobulin (IVIg) treatment on molecular expression in muscle tissue of patients with inflammatory myopathies. Ann Rheum Dis. 2007 doi: 10.1136/ard.2006.058644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang BA, Laxer RM, Murphy G, Silverman ED, Roifman CM. Treatment of dermatomyositis with intravenous gammaglobulin. Am J Med. 1991;91:169–172. doi: 10.1016/0002-9343(91)90010-u. [DOI] [PubMed] [Google Scholar]
- 15.Breems DA, de Haas PW, Visscher F, Sabbe LJ, Busch HF, van Doorn PA. Intravenously-administered immunoglobulins as first-choice agent in juvenile dermatomyositis. Ned Tijdschr Geneeskd. 1993;137:1979–1982. [PubMed] [Google Scholar]
- 16.Tsai MJ, Lai CC, Lin SC, Chiang BL, Chou CC, Hsieh KH. Intravenous immunoglobulin therapy in juvenile dermatomyositis. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1997;38:111–115. [PubMed] [Google Scholar]
- 17.Galeazzi M, Bellucci AM, Girardelli CR, Bono R, De Pita O, Puddu P. Efficacy of intravenous immunoglobulin therapy in a case of juvenile dermatomyositis. Clin Rheumatol. 1996;15:215–216. doi: 10.1007/BF02230347. [DOI] [PubMed] [Google Scholar]
- 18.Kokori H, Fotoulaki M, Giannakopoulou C, Hatzidaki E, Tantros S, Sbyrakis S. Intravenous immunoglobulin treatment in a girl with juvenile dermatomyositis. Pediatr Int. 1999;41:696–697. [PubMed] [Google Scholar]
- 19.Roifman CM. Use of intravenous immune globulin in the therapy of children with rheumatological diseases. J Clin Immunol. 1995;15:42S–51S. doi: 10.1007/BF01540893. [DOI] [PubMed] [Google Scholar]
- 20.Sansome A, Dubowitz V. Intravenous immunoglobulin in juvenile dermatomyositis--four year review of nine cases. Arch Dis Child. 1995;72:25–28. doi: 10.1136/adc.72.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Mayouf SM, Laxer RM, Schneider R, Silverman ED, Feldman BM. Intravenous immunoglobulin therapy for juvenile dermatomyositis: efficacy and safety. J Rheumatol. 2000;27:2498–2503. [PubMed] [Google Scholar]
- 22.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 23.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 24.Plamondon S, Dent PB. Juvenile amyopathic dermatomyositis: results of a case finding descriptive survey. J Rheumatol. 2000;27:2031–2034. [PubMed] [Google Scholar]
- 25.Laxer RM, Stein LD, Petty RE. Intravenous pulse methylprednisolone treatment of juvenile dermatomyositis. Arthritis Rheum. 1987;30:328–334. doi: 10.1002/art.1780300312. [DOI] [PubMed] [Google Scholar]
- 26.Eisenstein DM, Paller AS, Pachman LM. Juvenile dermatomyositis presenting with rash alone. Pediatrics. 1997;100:391–392. doi: 10.1542/peds.100.3.391. [DOI] [PubMed] [Google Scholar]
- 27.Pachman LM, Abbott K, Sinacore JM, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148:247–253. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Huber AM, Lang B, LeBlanc CM, et al. Medium- and long-term functional outcomes in a multicenter cohort of children with juvenile dermatomyositis. Arthritis Rheum. 2000;43:541–549. doi: 10.1002/1529-0131(200003)43:3<541::AID-ANR9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 29.Ramanan AV, Feldman BM. Clinical features and outcomes of juvenile dermatomyositis and other childhood onset myositis syndromes. Rheum Dis Clin North Am. 2002;28:833–857. doi: 10.1016/s0889-857x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- 30.Manlhiot C, Tyrrell PN, Liang L, Atkinson AR, Lau W, Feldman BM. Safety of intravenous immunoglobulin in the treatment of juvenile dermatomyositis: adverse reactions are associated with immunoglobulin A content. Pediatrics. 2008;121:e626–e630. doi: 10.1542/peds.2007-1218. [DOI] [PubMed] [Google Scholar]
- 31.Huber AM, Feldman BM, Rennebohm RM, et al. Validation and clinical significance of the Childhood Myositis Assessment Scale for assessment of muscle function in the juvenile idiopathic inflammatory myopathies. Arthritis Rheum. 2004;50:1595–1603. doi: 10.1002/art.20179. [DOI] [PubMed] [Google Scholar]
- 32.Ruperto N, Ravelli A, Pistorio A, et al. The provisional Paediatric Rheumatology International Trials Organisation/American College of Rheumatology/European League Against Rheumatism Disease activity core set for the evaluation of response to therapy in juvenile dermatomyositis: a prospective validation study. Arthritis Rheum. 2008;59:4–13. doi: 10.1002/art.23248. [DOI] [PubMed] [Google Scholar]