Abstract
Recent studies suggest that endothelial cells are a critical component of the normal hematopoietic microenvironment. Therefore, we sought to determine whether primary endothelial cells have the capacity to repair damaged hematopoietic stem cells. Highly purified populations of primary CD31+ microvascular endothelial cells isolated from the brain or lung did not express the pan hematopoietic marker CD45, hematopoietic lineage markers, or the progenitor marker c-kit and did not give rise hematopoietic cells in vitro or in vivo. Remarkably, the transplantation of small numbers of these microvascular endothelial cells consistently restored hematopoiesis following bone marrow lethal doses of irradiation. Analysis of the peripheral blood of rescued recipients demonstrated that both short term and long term multilineage hematopoietic reconstitution was exclusively of host origin. Secondary transplantation studies revealed that microvascular endothelial cell-mediated hematopoietic regeneration also occurs at the level of the hematopoietic stem cell. These findings suggest a potential therapeutic role for microvascular endothelial cells in the self-renewal and repair of adult hematopoietic stem cells.
Keywords: hematopoietic stem cells, endothelial cells, radioprotection, lethal irradiation
Introduction
Hematopoietic stem cells (HSCs) and hematopoietic progenitors reside in close proximity to sinusoidal endothelium in the bone marrow and other hematopoietic organs such as the spleen (reviewed by Kiel and Morrison (Kiel and Morrison, 2008)). It has been increasingly appreciated that endothelial cells (ECs) comprise an important functional component of the HSC/hematopoietic progenitor niche (Colmone and Sipkins, 2008). The ability of endothelial cells to support HSCs in vitro (Brandt et al., 1999; Chute et al., 2005; Chute et al., 2002; Li et al., 2004) as well as their expression of numerous cytokines with HSC-supportive activity (Li et al., 2004) suggests an active role of endothelium in normal hematopoiesis.
We previously showed that transplanted intact adult blood vessels have the capability to restore host hematopoiesis following lethal irradiation (Montfort et al., 2002). Our findings demonstrated the presence of a population of cells within normal adult vascular tissue that has the capacity to protect host hematopoietic stem cells from radiation induced death. To further investigate the cellular source of this radioprotective activity we evaluated mature, adult microvascular ECs. Moreover, we wished to determine whether these protective cells have cell autonomous hematopoietic potential and/or exert their effects on host hematopoiesis in a non-cell autonomous manner.
Using a lethal irradiation model, we now demonstrate that a single infusion of a low dose of microvascular ECs isolated from adult brain or lung protect lethally irradiated recipients from bone marrow failure. Peripheral blood engraftment analysis in rescued recipients shows that hematopoiesis is exclusively host derived and serial transplantation studies demonstrate that true HSCs are rescued in these EC protected recipients. Our findings provide strong evidence that microvascular endothelial cells have the capacity to rescue mice from lethal irradiation through restoring host derived hematopoiesis.
Materials and Methods
Mice
8-12 week old Thy1.1 /5.2 Hyb or C57Bl/6 mice were used as donors and age-matched C57Bl/6 (Ly5.1) mice were used as recipients. Mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in an SPF animal care facility at Oregon Health & Science University (Portland, OR). Recipient mice were kept on acidified water (pH 2.2) prior to transplantation. All procedures were approved by the Institutional Animal Care and Use Committee of the Oregon Health & Science University.
Donor tissue preparation and surface marker expression analysis
Microvascular endothelial cells were isolated using a modification of published protocols (Abbott et al., 1992; Unger et al., 2002). Specifically, brains and lungs were collected from donor mice, washed with modified Hank’s Balanced Salt Solution (HBSS; with 5% FCS and 10mM HEPES buffer), digested with 0.5% collagenase in Buffer A (DMEM with 1% Penicillin/Streptomyocin, 10mM HEPES and 3% BSA) at 37°C for 1 hour, and then passed through 70 μm filter to remove tissue debris, and the resulting cell pellets were then washed in modified HBSS. Lipids were removed from the brain cell preparation by resuspending the cell pellet in Buffer A containing 25% BSA and centrifuging at 2500 rpm for 20 minutes. Brain and lung cell preparations were stained with CD31/PECAM-1 (MEC13.3; BD Pharmingen, San Diego, CA) and CD31 positive cells were sorted using a Vantage Cell Sorter (Becton Dickinson, San Jose CA). Co-expression analysis was performed with Ly6A/E (Sca-1), CD34, CD105, c-kit, and VE cadherin antibodies (BD Pharmingen).
Radioprotection Assay
Recipient mice were irradiated twice with 575-600 cGy 3 hours apart using a J. L. Shepherd Co. Cesium irradiator. Sorted CD31+ cells from donor brain and lung were diluted in 200 μl of modified HBSS at doses of 3×104, 1×104 and 1×103. Following the second dose of irradiation, donor cells were injected into the retroorbital plexus of recipients anesthetized with isoflurane. Irradiated control mice received 200 μl modified HBSS only. Recipient mice that had been maintained acidified water were switched to non-acidified water containing antibiotics (106 unit/liter Polymyxin B sulfate and 1.1g/liter neomycin sulfate) and monitored daily over 60 days. For secondary transplantation, bone marrow was obtained from brain CD31+ cell radioprotected recipients eight months after the primary transplantation. Primary recipients transplanted with bone marrow received 1×106 donor cells with 2 ×104 host cells, while secondary recipients received 2 × 106 unfractionated BM cells.
Assessment of hematopoietic reconstitution
Peripheral blood was obtained from primary or secondary recipients by retro-orbital puncture. Aliquots of 200 μl were analyzed for complete blood counts and platelet counts (Antech Diagnostics, Portland, OR). For the determination of donor-derived hematopoiesis, peripheral blood was collected and nucleated cells were prepared by sedimenting erythrocytes in 2% Dextran (T-500) followed by hypotonic lysis. Cell pellets were washed and incubated with anti-CD45.1-FITC and anti-CD45.2-PE in combination with lineage specific markers for T-cells (CD3-APC), B-cells ( B220-APC) or myelomonocytic cells (Mac-1-APC and Gr-1-APC) (BD Pharmingen). The co-expression of these cell surface antigens was determined by using a FACscan II and dead cells were excluded using scatter gates and propidium iodide. Up to 50 thousand events were analyzed to provide a sensitivity of 0.5%.
Hemoglobin analysis
Hemoglobin analysis was performed on peripheral blood isolated as described previously (Baumann et al., 2004). Donor Hbbd CD31+ cells were transplanted into Hbbs recipients. Approximately 70 μl of peripheral blood was collected from each recipient mouse, centrifuged and the pellets were lysed with 1X cystamine solution. Hemoglobin lysates were applied to a cellulose acetate plate (Helena Laboratories, Beaumont, TX) and electrophoresed at 300 volts for 30 minutes. Following electrophoresis, plates were stained with Ponceau S for 20 minutes, rinsed in deionized water, and destained in 2 changes of 7% glacial acetic acid prior to imaging.
Methylcellulose Assay
Complete methylcellulose medium with recombinant cytokines (Methocult M3434, Stem Cell Technologies Inc, Vancouver, Canada) was used for colony forming assays. CD31+ cells from brain and lung were sorted and plated in triplicate at a concentration of 5×103/plate - 1.5×104/plate in a 1:10 (v/v) ratio of methylcellulose. As a positive control 1.5 ×104mononuclear whole bone marrow cells/plate were cultured in triplicate. Cells were incubated at 37°C in a humidified incubator with 5% CO2 in air and colonies were counted on day 14.
RT-PCR Analysis
Total RNA was isolated from 2000-5000 sorted CD31 positive cells from brain and lung using Qiagen one-step RT-PCR kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The same number of unfractionated bone marrow cells was used as a positive control. PCR reactions were performed at 94° C, 1 min, 58° C, 1 min, and 72° C, 1 min, for a total of 30 cycles. Amplicons were electrophoresed on a 1% agarose gel, and then stained by ethidium bromide. RNA isolation and RT-PCR were performed a minimum of two times. The primers used and the corresponding amplicon sizes are listed in Table 1.
Table 1.
Primers used for RT-PCR analysis
| Gene | Forward Primer | Reverse Primer | size (bp) |
|---|---|---|---|
| CD31 | AGGGGACCAGCTGCACATTAGG | AGGCCGCTTCTCTTGACCACTT | 452 |
| flk1 | TGAGCCAAGTGTTAAGTGTGG | GAGCAAGCTGCATCATTTCC | 292 |
| tie2 | GGATGGCAATCGAATCACTG | TCTGCTCTAGGCTGCTTCTT | 371 |
| vWF | CTC AGA GCT TCG GCG CAT CAC CAG | GAC AAA CAC CAC ATCCAG AAC CAT | 495 |
| c-kit | TGT CTC TCC AGT TTC CCT GC | TTC AGG GAC TCA TGG GCT CA | 765 |
| pu.1 | AACCACTTCACAGAGCTGCA | CAAGCCATCAGCTTCTCCAT | 260 |
| gata1 | ATG CCT GTA ATC CCA GCA CT | TCA TGG TGG TAG CTG GTA GC | 581 |
| gata2 | GACTATGGCAGCAGTCTCTTCC | GGTGGTTGTCGTCTGACAATT | 296 |
| mb-1 | GCC AGG GGG TCT AGA AGC | TCA CTT GGC ACC CAG TAC AA | 308 |
| hprt | CACAGGACTAGAACACCTGC | GCTGGTGAAAAGGACCTCT | 249 |
Abbreviations: bp: base pairs, vWF: von Willebrand Factor
Results
CD31 expressing microvascular endothelial cells from the brain and lung provide protection against lethal irradiation
We previously demonstrated that transplanted whole adult blood vessels can restore host hematopoiesis following lethal irradiation without significantly contributing to circulating the hematopoietic cells (Montfort et al., 2002). As our studies revealed that signals generated in the irradiated mice caused significant proliferation of the endothelial cells within the transplanted vascular grafts, we speculated that endothelial cells might mediate the radioprotective activity of the vascular grafts.
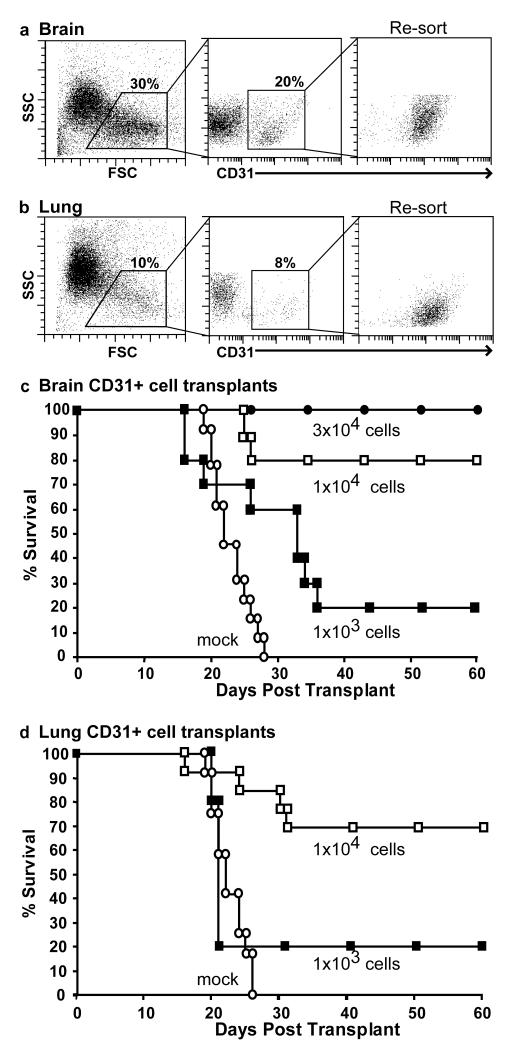
Microvascular endothelial cells (ECs) were isolated from brain and lung tissue using a well established approach (Abbott et al., 1992; Unger et al., 2002). Briefly, following collagenase treatment, enriched EC preparations were labeled with antibodies to CD31 and Sca-1 and then sorted to homogeneity by flow cytometry. As shown in Figure 1, CD31+ cells comprised ~10- 20% of the total mononuclear cell preparations derived from these tissues. To test the potential of microvascular endothelial cell to restore hematopoiesis, highly purified brain or lung-derived CD31+ cells were transplanted into lethally irradiated recipients (1200cGy) at doses of 1×103, 1×104 or 3×104 cells per mouse. As a control, lethally irradiated mice were injected with media only. Transplanted and control recipients were monitored daily for survival. Combined results from several independent experiments revealed that purified CD31+ microvascular cells from both brain (Figure 1c) and lung (Figure 1d) provide similar degrees of protection to lethally irradiated recipients. Specifically, transplantation of 3×104 CD31+ cells from brain rescued 100% of recipients, whereas 1×104 CD31+ cells from either brain or lung rescue 80% of recipients from lethal irradiation, and a dose of 1×103 CD31+ cells isolated from either brain or lung saves 20% of recipients. Importantly, 100% of control mice, included in all experiments, died from hematopoietic failure. These results clearly demonstrate that a single, low dose of purified CD31+ cells is sufficient to rescue mice from bone marrow lethal doses of irradiation.
Figure 1. Purified populations of CD31+ microvascular endothelial cells rescue lethally irradiated recipients.
CD31+ cells were sorted from adult mouse brain (a) and lung (b). Re-analysis of the twice sorted populations confirmed their high degree of purity. Survival of lethally irradiated recipients transplanted with CD31+ cells from brain (c) or lung (d) were were monitored daily for survival. CD31+ cells rescued lethally irradiated hosts in a dose-dependent manner. In (c), (●) 3×104 cells, n=4; (□) 1×104 cells, n=10; (■) 1×103, cells n=10; (○) mock, n=13. In (d), (□)1×104 cells, n=10; (■)1×103, n=10; (○) mock, n=14. Combined results from 2 or 3 independent experiments for each experimental group. Mock is media only.
CD31+ microvascular cells express endothelial cell markers and are devoid of most differentiated hematopoietic cell markers
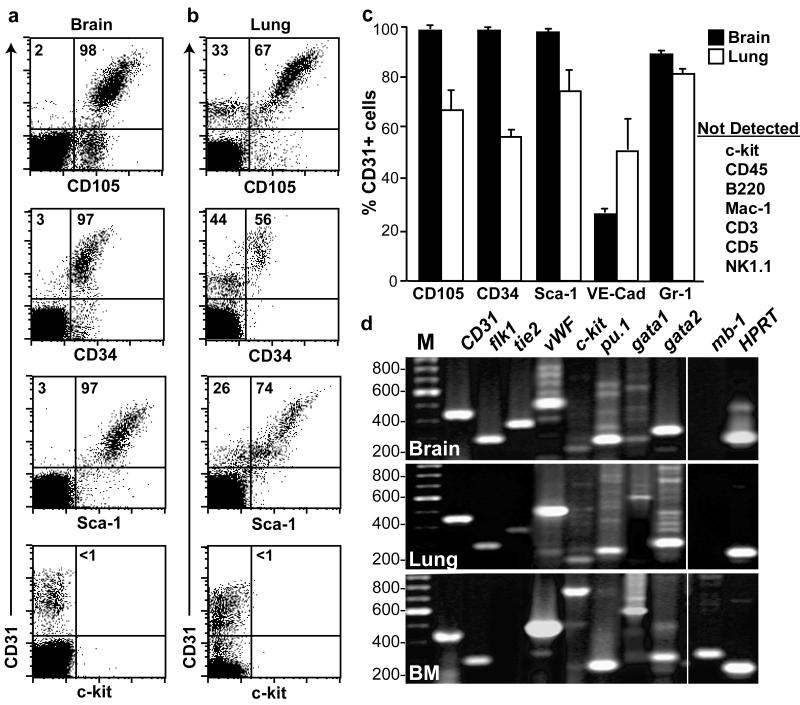
CD31+ cells isolated from brain and lung were evaluated for expression of endothelial, HSC, and hematopoietic lineage markers by flow cytometry (Figure 2a-c) and RT-PCR (Figure 2d). Unfractionated bone marrow was used as a positive control for this analysis (Figure 3d, bottom panel). As expected, most CD31+ cells showed cell surface expression of EC markers such as CD105 (endoglin) and CD144 (VE-cadherin) (Figure 2a-c), and expressed EC specific transcripts including flk1, tie2, and vWF (Figure 2d). Consistent with previous reports, the vast majority of CD31+ cells isolated from brain and lung also expressed cell surface markers common to both ECs and HSCs, including CD34 and Sca-1 (Ieronimakis et al., 2008; Kotton et al., 2003; Luna et al., 2004; van de Rijn et al., 1989). Similarly, expression of gata2 mRNA, which is also common to both HSCs and ECs (Akashi et al., 2000; Dorfman et al., 1992; Lee et al., 1991; Tsai and Orkin, 1997) was detected in the sorted CD31+ microvascular EC population. Importantly, expression of the HSC/hematopoietic progenitor marker c-kit was not detected by flow cytometry or by RT-PCR. Furthermore, most hematopoietic lineage-specific markers including CD45, Mac-1, B220, CD3, CD5 and NK1.1 and mb-1 were also not detected in the CD31+ cells. Interestingly, however, the transcription factors PU.1 and gata1 and the granulocytic marker Gr-1 were expressed in the CD31+ cells. Taken together, these data indicate that the phenotype of CD31+ cells from lung and brain are consistent with microvascular ECs and not hematopoietic stem or progenitor cells.
Figure 2. CD31+ microvascular cells express endothelial cell markers but not most hematopoietic markers.
Characterization of hematopoietic and endothelial cell surface marker expression on CD31+ cells isolated from brain (a) and lung (b) by flow cytometry. (c) Mean frequency of brain and lung derived CD31 cells expressing cell surface markers. Error bars are SEM. Whereas the majority of CD31+ cells in both brain and lung co-express CD105, CD34 and Sca-1 and Gr-1 on the cell surface (a & b), c-kit and other hematopoietic markers were not detected. (d) RT-PCR analysis of sorted CD31+ cells from brain (top panel) and lung (middle panel) compared to unfractionated bone marrow (lower panel). DNA size (in base pairs) is indicated on the left side of the gel. M: DNA ladder.
Long-term hematopoiesis in rescued recipients is exclusively of host origin
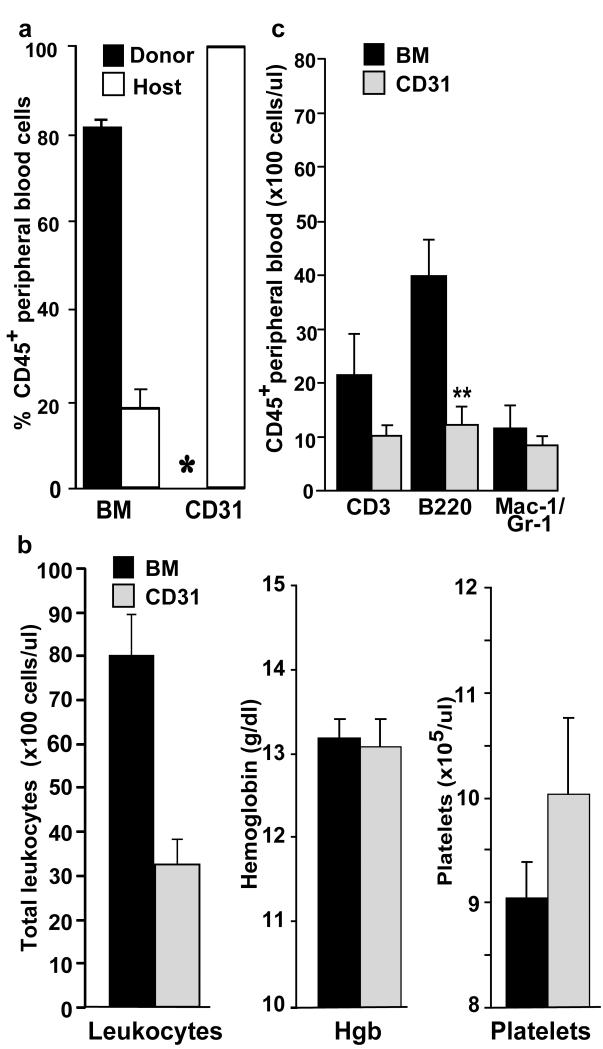
To address the possibility that brain and lung derived CD31+ microvascular cells may possess long-term, in vivo hematopoietic repopulating activity, we investigated the potential contribution of CD31+ cells donor-derived cells to the hematopoietic systems of rescued mice. As a positive control for donor derived hematopoiesis, 1×106 whole bone marrow (BM) cells were infused into irradiated hosts. Peripheral blood was harvested at various time points after transplantation, and the frequency of donor derived blood cells (CD45.2) and host derived blood cells (CD45.1) was evaluated by flow cytometry. No donor derived leukocytes were detected in the blood of recipient mice up to 8 months after CD31+ cell transplantation (Figure 3a, sensitivity 0.5%). In contrast, in the peripheral blood of control recipients that received bone marrow, a mean of 85% of the hematopoietic cells were donor-derived. Thus, CD31+ cells do not possess any measurable long term hematopoietic repopulating activity.
Figure 3. CD31+ cells do not directly contribute to multilineage hematopoiesis in rescued hosts.
Peripheral nucleated blood cell analysis of bone marrow (BM) recipients (n=3) and CD31+ cell recipients (n=7), 8 months after transplantation. (a) Levels of donor and host engraftment revealed that all peripheral blood cells in the CD31+ cell recipients were of host origin. * indicates below level of detection (0.5%). (b) CD31+ cell recipients had fewer total circulating nucleated cells (left panel) while the analysis of hemoglobin levels (middle panel, Hgb) and platelets (right panel) revealed no differences compared to BM. (c) Multilineage reconstitution analysis of radioprotected hosts. Populations of total peripheral blood leukocytes cells from BM recipients and host cells from brain CD31+ cell recipients were assayed by flow cytometry for T-cell (CD3), B-cell (B220), or myelomonocytic cell (Mac-1/Gr-1) marker expression. Only the absolute number of B-cells was decreased in CD31+ cell recipients (**P<0.004). In all panels, error bars show standard error of the mean. P value was determined using an unpaired, two-tailed Students t-test.
To determine the extent of multilineage hematopoiesis in both CD31+ and bone marrow recipients, cohorts of mice transplanted with either cell type were analyzed at 8 months. Evaluation of the peripheral blood in CD31+ cell rescued recipients, revealed a moderate reduction in total leukocytes, normal hemoglobin levels and normal platelet counts, comparable to bone marrow transplant recipients (Figure 3b). Analysis of hematopoietic lineages showed a similar frequency of myelomonocytic cells along with a reduction in the number of both B and T lymphocytes (Figure 3c). Taken together these results demonstrate that CD31+ cell rescued recipients maintain stable, long term multilineage hematopoiesis.
CD31+ microvascular cells derived from the brain and lung do not possess short term hematopoietic activity
We and others have previously demonstrated that populations of bone marrow-derived hematopoietic progenitors are capable of rescuing irradiated hosts by providing short term hematopoiesis until host stem/progenitor cells (Baumann et al., 2004; Na et al. 2002). These findings raise the possibility that transplanted CD31+ cells rescue lethally irradiated hosts by acting as a source of short term hematopoietic progenitors. To address this possibility, we first assayed the in vitro hematopoietic colony-forming activity of CD31+ endothelial cells. Sorted CD31+ cells were seeded into methylcellulose and unfractionated bone marrow cells were used as a positive control. Bone marrow cells gave rise to an average of 19 ± 1.1 total hematopoietic colonies per 5000 input cells, whereas sorted CD31 positive cells from both the brain and lung did not give rise to any detectable colonies (Table 2). Thus, CD31+ cells lack significant in vitro hematopoietic colony forming capacity.
Table 2.
In vitro colony forming activity of sorted CD31+ cells
| Input cells |
# of experiments |
CFU per 5×103 cells (±SEM) |
|---|---|---|
| Unfractionated BM | 3 | 19.0 (±1.1) |
| Brain CD31+ ECs | 3 | 0 |
| Lung CD31+ ECs | 2 | 0 |
Abbreviations: BM: bone marrow, ECs: endothelial cells, CFU: colony forming units, SEM: standard error of the mean.
Severe anemia is a major cause of death following bone marrow irradiation injury. We have previously identified a short-lived, CD31 expressing erythroid lineage progenitor cell population in the bone marrow that also provides radioprotection (Baumann et al., 2004). To address the possibility that donor CD31+ endothelial cells have the erythroid lineage progenitor potential, mice congeneic at the hemoglobin locus (Hbb) were utilized. During the recovery phase following CD31+ cell transplant, analysis of the irradiated recipients (Hbbs), revealed no contribution from donor derived hemoglobin (Hbbd , Figure 4). Together with the colony forming data, these results indicate that CD31+ endothelial cells do not demonstrate any significant short term hematopoietic progenitor activity.
Figure 4. CD31+ microvascular endothelial cells do not exhibit erythroid progenitor activity.
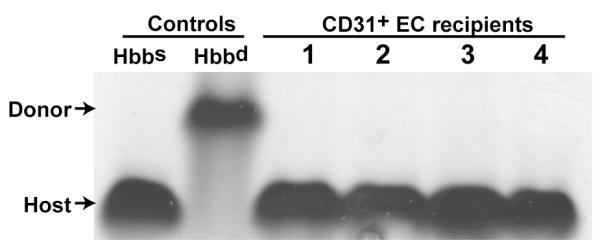
CD31+ cells isolated from mice congeneic at the hemoglobin locus were used to evaluate their potential to give rise to the erythroid lineage following transplant into irradiated recipients. Specifically, donor Hbbd CD31+ cells were transplanted into Hbbs recipients. Two weeks after transplantation, blood samples were obtained and total hemoglobin was evaluated by protein electrophoresis. Lanes 1-4 show the absence of detectable donor hemoglobin Hbbd in 4 of 4 recipient mice.
Host-derived HSC are regenerated after CD31+ cell transplantation
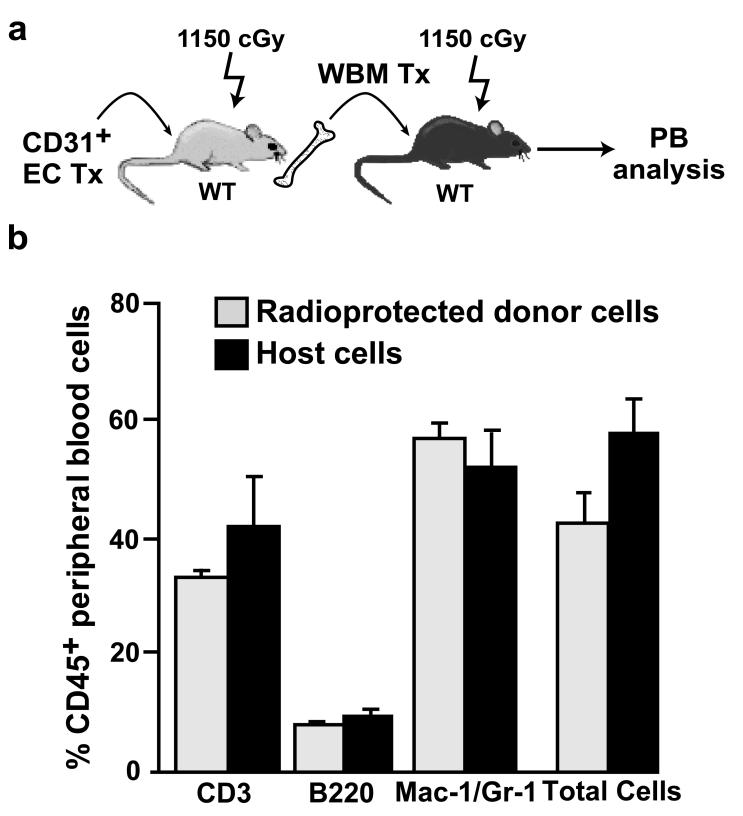
Serial transplantation was used to determine whether a population of true, self-renewing host HSC had been regenerated in lethally irradiated CD31+ EC recipients. Eight months after CD31 cell infusion, bone marrow was harvested from rescued primary recipients and 2×106 unfractionated cells were injected into CD45 congeneic, lethally irradiated secondary recipients (Figure 5a). Analysis of the peripheral blood of these secondary recipients revealed robust, multilineage hematopoietic reconstitution by the donor cells (Figure 5b). These serial transplantation findings indicate that irradiated host HSCs are regenerated following the infusion of CD31+ microvascular cells.
Figure 5. CD31+ microvascular endothelial cells restore host HSCs with self renewal activity.
(a) Schematic of serial transplantation strategy. Eight months after transplantation with brain derived CD31+ cells, the BM from radioprotected hosts (CD45.2) was harvested and 2×106 cells were transplanted into lethally, irradiated secondary recipients (CD45.1, n=5). (b) Multilineage reconstitution of peripheral blood (PB) from each secondary recipient was analyzed 6-8 weeks after transplantation. No significant differences in the extent of multilineage hematopoietic reconstitution was observed in cells derived from radioprotected hosts. Error bars are SEM.
Discussion
Our results demonstrate that highly purified populations of primary CD31+ microvascular endothelial cells can restore hematopoiesis following bone marrow lethal doses of radiation. The peripheral blood of rescued recipients shows that both short term and long term multilineage hematopoietic reconstitution are exclusively of host origin. Direct evidence for the protection of the self-renewing the hematopoietic stem cell is provided by secondary transplantation studies. Taken together, these findings demonstrate an important role for microvascular endothelial cells in the repair of irradiated hematopoietic stem cells.
Consistent with our findings, Chute and coworkers recently demonstrated a radioprotective effect of cultured endothelial cells (Chute et al., 2007). However, there are several important differences in the experimental approaches utilized. In addition to using cultured cell preparations, repetitive administration of 1×105 mouse brain endothelial cells (i.e., a total dosage of 5×105 cells) mediated the survival of only 57% of lethally irradiated hosts. By contrast, we demonstrate that only a single dose of 3×104 primary brain endothelial cells is fully radioprotective. Moreover, we show that different sources of primary endothelium, such as lung CD31+ cells, have comparable radioprotective activity when transplanted at the same dose as brain ECs. Our findings suggest that this activity may be a general property of adult endothelium. Presently, it is unclear why cultured endothelium is less efficient than freshly isolated endothelium in restoring host hematopoiesis. One possibility is that since cultured brain ECs are derived as outgrowths from isolated vessels (Chute et al., 2007; Chute et al., 2002), they are a more heterogeneous population of cells than those found in the CD31+ sorted populations we employed for our studies. Alternatively, the culture conditions employed may simply reduce the HSC repair capacity of the endothelial cells.
The mechanism by which transplanted endothelial cells rescue host hematopoiesis and support host HSC recovery is currently unknown. Given that during embryonic development, endothelial cells differentiate into hematopoietic cells (Chen et al., 2009; Eilken et al., 2009; Lancrin et al., 2009; Zovein et al., 2008), it is possible that adult CD31+ cells may also possess hemogenic potential in certain experimental settings. Short lived populations of myeloerythroid progenitors, including the common myeloid progenitor (CMP) and the megakaryocyte/erythrocyte restricted progenitors (MEP), rescue mice from lethal irradiation and restore host HSC activity (Na et al., 2002; Nakorn et al., 2003). However, the data from these studies do not support the presence of CMP and MEP populations in these CD31+ cell populations. First, neither we nor Chute and colleagues (Chute et al., 2007) found evidence of the transplanted endothelial cells adopting hematopoietic fates in vivo as donor cell derived hematopoiesis was not detected. This is not a question of sensitivity as we were readily able to detect the progeny of small numbers of CMPs in a previous study (Bailey et al., 2006). In addition, whereas both CMPs and MEPs express c-kit (Akashi et al., 2000; T.N. et al., 2002), c-kit was not detectable on the cell surface or at the mRNA level in the sorted CD31+ cells. As we have previously identified a CD31+,c-kit+, Sca-1− population of bone marrow cells that have transient erythroid lineage generating potential (Baumann et al., 2004), congenic CD31+ cell recipients were evaluated for the presence of donor derived hemoglobin (Hbbd into Hbbs, Figure 4). No early wave of donor derived erythroid engraftment was detected, excluding a role for CD31+ cell derived erythroid progenitor in mediating radioprotection. In addition, hematopoietic progenitor cell activity was not detected in CFU assays of sorted CD31+ donor cells and these cells did not express the panhematopoietic marker CD45 (Figure S1). This assay is sensitive enough to detect single progenitor cells; therefore, the lack of CFU activity rules out the presence of even a small number of myeloerythroid progenitors in the CD31+ EC preparations. Based on all of these findings, we conclude that CD31+ cells mediate host hematopoietic recovery in a non-cell autonomous manner. However, it is worth noting that the infused CD31+ EC may also be providing some other form of indirect hematopoietic support. Potential mechanisms could include improving vascular stability and minimizing bleeding from thrombocytopenia, leading to enhanced hematopoietic recovery and survival.
In addition to inducing hematopoietic failure, myeloablative treatments such as ionizing irradiation and chemotherapy significantly damage endothelium in the bone marrow sinusoids (Kopp et al., 2005; Li et al., 2008; Narayan et al., 1994; Salter et al., 2009; Slayton et al., 2007). Moreover, repair of BM sinusoids appears to be a critical step in restoring normal hematopoiesis (Avecilla et al., 2004; Kopp et al., 2005; Salter et al., 2009). Intriguingly, neither we (Goldman & Fleming, unpublished observations) nor others (Chute et al., 2007) have found any evidence of CD31+ cell engraftment within the hematopoietic or endothelial compartments in the BM of lethally irradiated recipient mice. Similar to mature ECs, transplanted endothelial progenitor cells do not integrate into BM vasculature in measurable numbers yet still they still protect lethally irradiated hosts (Salter et al., 2009). The non-cell autonomous rescue of hematopoietic cells and BM sinusoidal endothelium by transplanted ECs and EPCs is very likely mediated through the actions of secreted and/or cell surface expressed cytokines. To date, a number of cytokines have been identified that provide some degree of hematopoietic radioprotection to lethally irradiated mice (Singh and Yadav, 2005), including interleukin-1, granulocyte colony-stimulating factor and c-kit ligand/stem cell factor, all of which are normally expressed by ECs (Li et al., 2000). Interestingly, the administration of individual cytokines is not nearly as effective in rescuing lethally irradiated mice as combinations of cytokines (Streeter et al., 2003). These findings suggest that more than one EC derived cytokine mediates the EC mediated regeneration of hematopoiesis. In the future, it will be important to determine if the repair of HSC is due to EC mediated attenuation of DNA damage, the enhancement of DNA repair mechanisms or a combination of both mechanisms.
Conclusions
These studies demonstrate that transplantation of small numbers of highly purified CD31+ microvascular endothelial cells can restore hematopoiesis following bone marrow lethal doses of radiation. Importantly, in these transplanted recipients, host hematopoietic stem cell activity was restored by the endothelial cells in a non-cell autonomous manner. Taken together, these findings highlight a biologically important and potentially therapeutic role for microvascular endothelial cells in the repair of irradiated hematopoietic stem cells.
Supplementary Material
Acknowledgements
We thank Mandy Boyd of the OHSU Flow Cytometry Core for assistance with cell sorting. This work was supported by grants from the NIH (R01 HL077818 and R01 HL069133) to WHF.
Abbreviations
- (HSCs)
hematopoietic stem cells
- (ECs)
endothelial cells
- (BM)
bone marrow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992;103(Pt 1):23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CI, Bailey AS, Li W, Ferkowicz MJ, Yoder MC, Fleming WH. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood. 2004;104:1010–1016. doi: 10.1182/blood-2004-03-0989. [DOI] [PubMed] [Google Scholar]
- Brandt JE, Bartholomew AM, Fortman JD, Nelson MC, Bruno E, Chen LM, Turian JV, Davis TA, Chute JP, Hoffman R. Ex vivo expansion of autologous bone marrow CD34(+) cells with porcine microvascular endothelial cells results in a graft capable of rescuing lethally irradiated baboons. Blood. 1999;94:106–113. [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute JP, Muramoto GG, Fung J, Oxford C. Soluble factors elaborated by human brain endothelial cells induce the concomitant expansion of purified human BM CD34+CD38-cells and SCID-repopulating cells. Blood. 2005;105:576–583. doi: 10.1182/blood-2004-04-1467. [DOI] [PubMed] [Google Scholar]
- Chute JP, Muramoto GG, Salter AB, Meadows SK, Rickman DW, Chen B, Himburg HA, Chao NJ. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute JP, Saini AA, Chute DJ, Wells MR, Clark WB, Harlan DM, Park J, Stull MK, Civin C, Davis TA. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- Colmone A, Sipkins DA. Beyond angiogenesis: the role of endothelium in the bone marrow vascular niche. Transl Res. 2008;151:1–9. doi: 10.1016/j.trsl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Dorfman DM, Wilson DB, Bruns GA, Orkin SH. Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. J Biol Chem. 1992;267:1279–1285. [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Ieronimakis N, Balasundaram G, Reyes M. Direct isolation, culture and transplant of mouse skeletal muscle derived endothelial cells with angiogenic potential. PLoS ONE. 2008;3:e0001753. doi: 10.1371/journal.pone.0001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Shmelkov SV, Ramos CA, Zhang F, Rafii S. Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005;106:505–513. doi: 10.1182/blood-2004-11-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton DN, Summer RS, Sun X, Ma BY, Fine A. Stem cell antigen-1 expression in the pulmonary vascular endothelium. Am J Physiol Lung Cell Mol Physiol. 2003;284:L990–996. doi: 10.1152/ajplung.00415.2002. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ME, Temizer DH, Clifford JA, Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991;266:16188–16192. [PubMed] [Google Scholar]
- Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp Hematol. 2004;32:1226–1237. doi: 10.1016/j.exphem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Li WM, Huang WQ, Huang YH, Jiang DZ, Wang QR. Positive and negative hematopoietic cytokines produced by bone marrow endothelial cells. Cytokine. 2000;12:1017–1023. doi: 10.1006/cyto.1999.0678. [DOI] [PubMed] [Google Scholar]
- Li XM, Hu Z, Jorgenson ML, Wingard JR, Slayton WB. Bone marrow sinusoidal endothelial cells undergo nonapoptotic cell death and are replaced by proliferating sinusoidal cells in situ to maintain the vascular niche following lethal irradiation. Exp Hematol. 2008;36:1143–1156. doi: 10.1016/j.exphem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Luna G, Paez J, Cardier JE. Expression of the hematopoietic stem cell antigen Sca-1 (LY-6A/E) in liver sinusoidal endothelial cells: possible function of Sca-1 in endothelial cells. Stem Cells Dev. 2004;13:528–535. doi: 10.1089/scd.2004.13.528. [DOI] [PubMed] [Google Scholar]
- Montfort MJ, Olivares CR, Mulcahy JM, Fleming WH. Adult blood vessels restore host hematopoiesis following lethal irradiation. ExpHematol. 2002;30:950–956. doi: 10.1016/s0301-472x(02)00813-5. [DOI] [PubMed] [Google Scholar]
- Na NT, Traver D, Weissman IL, Akashi K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. JClinInvest. 2002;109:1579–1585. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakorn TN, Miyamoto T, Weissman IL. Characterization of mouse clonogenic megakaryocyte progenitors. Proc Natl Acad Sci U S A. 2003;100:205–210. doi: 10.1073/pnas.262655099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan K, Juneja S, Garcia C. Effects of 5-fluorouracil or total-body irradiation on murine bone marrow microvasculature. Exp Hematol. 1994;22:142–148. [PubMed] [Google Scholar]
- Salter AB, Meadows SK, Muramoto GG, Himburg H, Doan P, Daher P, Russell L, Chen B, Chao NJ, Chute JP. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Yadav VS. Role of cytokines and growth factors in radioprotection. Exp Mol Pathol. 2005;78:156–169. doi: 10.1016/j.yexmp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Slayton WB, Li XM, Butler J, Guthrie SM, Jorgensen ML, Wingard JR, Scott EW. The role of the donor in the repair of the marrow vascular niche following hematopoietic stem cell transplant. Stem Cells. 2007;25:2945–2955. doi: 10.1634/stemcells.2007-0158. [DOI] [PubMed] [Google Scholar]
- Streeter PR, Dudley LZ, Fleming WH. Activation of the G-CSF and Flt-3 receptors protects hematopoietic stem cells from lethal irradiation. Exp Hematol. 2003;31:1119–1125. [PubMed] [Google Scholar]
- T.N. N, Traver D, Weissman IL, Akashi K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J Clin Invest. 2002;109:1579–1585. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–3643. [PubMed] [Google Scholar]
- Unger RE, Oltrogge JB, von Briesen H, Engelhardt B, Woelki U, Schlote W, Lorenz R, Bratzke H, Kirkpatrick CJ. Isolation and molecular characterization of brain microvascular endothelial cells from human brain tumors. In Vitro Cell Dev Biol Anim. 2002;38:273–281. doi: 10.1290/1071-2690(2002)038<0273:IAMCOB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989;86:4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.