Abstract
Activation of the renin–angiotensin–aldosterone system (RAAS) results in vasoconstriction, muscular (vascular and cardiac) hypertrophy and fibrosis. Established arterial stiffness and cardiac dysfunction are key factors contributing to subsequent cardiovascular and renal complications. Blockade of RAAS has been shown to be beneficial in patients with hypertension, acute myocardial infarction, chronic systolic heart failure, stroke and diabetic renal disease. An aggressive approach for more extensive RAAS blockade with combination of two commonly used RAAS blockers [ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs)] yielded conflicting results in different patient populations. Combination therapy is also associated with more side effects, in particular hypotension, hyperkalaemia and renal impairment. Recently published ONTARGET study showed ACEI/ARB combination therapy was associated with more adverse effects without any increase in benefit. The Canadian Hypertension Education Program responded with a new warning: ‘Do not use ACEI and ARB in combination’. However, the European Society of Cardiology in their updated heart failure treatment guidelines still recommended ACEI/ARB combo as a viable option. This apparent inconsistency among guidelines generates debate as to which approach of RAAS inhibition is the best. The current paper reviews the latest evidence of isolated ACEI or ARB use and their combination in cardiovascular diseases, and makes recommendations for their prescriptions in specific patient populations.
Keywords: renin–angiotensin–aldosterone system, angiotensin converting enzyme inhibitors, angiotensin II type 1 receptor blockers, hypertension, myocardial infarction, heart failure, stroke, diabetic nephropathy
Introduction
The renin–angiotensin–aldosterone system (RAAS) is a complex system that plays an important role in maintaining haemodynamic stability in the human body through regulation of arterial blood pressure, water and electrolyte balance (Skeggs et al., 1976). However, pathological activation of the RAAS results in excessive vasoconstriction, abnormal muscular (vascular and cardiac) hypertrophy and fibrosis. Established arterial stiffness and cardiac dysfunction are key factors contributing to subsequent cardiovascular and renal complications (Topouchian et al., 2007). Blockade of RAAS has been shown to be beneficial in patients with hypertension, acute myocardial infarction (AMI), chronic systolic heart failure, stroke and diabetic nephropathy (DN). ACE inhibitor (ACEI) and angiotensin receptor blocker (ARB) are two major RAAS inhibitors commonly used in clinical practice. Recently, an aggressive approach of more extensive RAAS blockade with their combination (ACEI/ARB combo) has been addressed in a number of large randomized trials. In this paper, the latest evidence concerning the use of these two agents in cardiovascular diseases will be discussed.
RAAS: historical perspective
The RAAS has been discovered for more than a century. In 1898, Tigerstedt and Bergman first demonstrated that an extract from the renal cortex of rabbits (later named renin) increased blood pressure when it was injected intravenously to recipient rabbits (Tigerstedt and Bergman, 1898). However, the findings of Tigerstedt could not be reproduced in other studies, and the discovery of renin was once disputed and ignored. It took another 40 years for scientists to realize that renin functioned as an enzyme on a protein substrate to produce a peptide that mediated the vasopressor effect of renin (Braun-Menendez et al., 1940; Page and Helmer, 1940). The protein substrate was later named angiotensinogen (Skeggs et al., 1956; Kageyama et al., 1984), and the peptide known as angiotensin (Braun-Menendez and Page, 1958). Further work by Skeggs et al. demonstrated that angiotensin existed in two distinct forms: angiotensin I (A-I) and angiotensin II (A-II) (Skeggs et al., 1957a), where A-I was cleaved by ACE to generate the biologically active A-II (Skeggs et al., 1957b). The relationship between A-II and aldosterone was hypothesized by Gross (1958) and subsequently confirmed by Davis (1959). These precious works led to ongoing research and increasing understanding of the RAAS (Hedner et al., 1998).
Current concept of the RAAS
The classical RAAS hormonal cascade begins with production of renin (Figure 1). Renin, an aspartyl protease produced by the juxtaglomerular cells of the kidney, regulates the initial and rate-limiting step of the RAAS by converting angiotensinogen to A-I (Hackenthal et al., 1990; Persson et al., 2004). Angiotensinogen is an alpha-2-globulin mainly produced by the liver (Ménard et al., 1983; Deschepper, 1994; Hall, 2003). A-I is a biologically inactive decapeptide, which requires further activation by ACE, a dipeptidyl carboxypeptidase, to form the biologically active octapeptide A-II (Ng and Vane, 1967; Corvol et al., 1995). ACE is a membrane-bound zinc metalloprotease, mainly produced by the lungs (Corvol et al., 1995). A-II acts on the adrenal cortex and causes the release of aldosterone (Quinn and Williams, 1988). The net effects of the activation of the RAAS include vasocontriction, sodium and water retention, increased arterial blood pressure and increased myocardial contractility, which in combination increase the effective circulating volume. An increase in perfusion of the juxtaglomerular apparatus inhibits the release of renin through a negative feedback mechanism. Apart from the classical endocrine pathway in the circulation, there is increasing evidence that the renin–angiotensin system (RAS) functions at tissue level in a paracrine or autocrine manner (Paul et al., 2006). Generally speaking, it is thought that the tissue or local RAS works with the classical circulating RAAS in a complementary manner.
Figure 1.
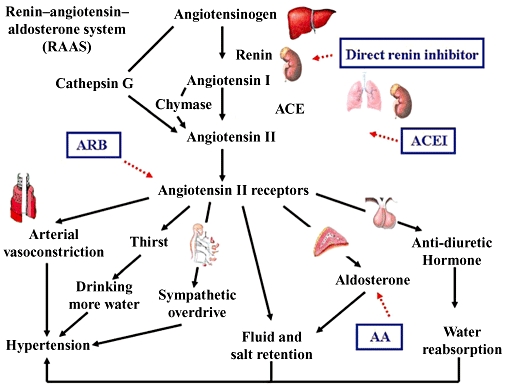
RAAS. Renin, produced by the juxtaglomerular cells of the kidney converts angiotensinogen to A-I. Angiotensinogen is an alpha-2-globulin mainly produced by the liver. A-I is biologically inactive and is activated by ACE, mainly produced by the lungs to form A-II. A-II acts on A-II receptors. The angiotensin type 1 (AT1) receptor governs most physiological effects. The net effects of activation of the RAAS include vasocontriction, increased arterial blood pressure, increased myocardial contractility, sodium and water retention which subsequently increases the effective circulating volume. Renin–angiotensin–aldosterone blockade can be achieved by direct renin inhibitor, ACEI, ARB and AA. A-II can also be produced by alternative pathways by enzymes like chymase and cathepsin G, which form the basis of ‘A-II escape’. This is also the rationale for using dual blockade of the system by ACEI and ARB.
Pathophysiological role of RAAS in cardiovascular disorders
Dysregulation of the RAAS has been implicated in the pathophysiology of various cardiovascular disorders including hypertension, AMI, congestive heart failure (CHF) and stroke, as well as renal disorders especially DN.
RAAS and hypertension
RAAS is involved in certain forms of secondary hypertension, including renin-secreting neoplasms, renovascular hypertension (e.g. renal artery stenosis), malignant hypertension, pheochromocytoma and primary hyperaldosteronism. In patients with primary (essential) hypertension, the plasma renin activity (PRA) can be high, normal or low (Bühler et al., 1984). ‘Low-renin’ hypertension is more commonly seen in the eldery, diabetic patients, and those with chronic renal parenchymal disease. Studies have shown that the PRA may not necessarily reflect tissue activities (such as the vascular endothelium, kidneys, brain and the adrenal glands) (Redgrave et al., 1985; Johnston, 1992). This was evidenced by experimental models that in transgenic rats into which a mouse renin gene was inserted to produce angiotensin-mediated hypertension, the PRA, plasma A-II level and renal renin content were all below normal, while adrenal renin content, vascular A-II formation and plasma level of prorenin (the precursor of active renin) were all markedly elevated (Bachmann et al., 1992). These findings suggest a possible link between primary hypertension and abnormal activation of local RAS.
RAAS and AMI
Activation of the RAAS begins shortly after AMI. Traditionally, such activation has been considered as a compensatory and adaptive response to maintain blood pressure and systemic perfusion. However, sustained activation of the RAAS has been shown to be associated with a poor prognosis (Vaney et al., 1984; Remes, 1994; Isnard et al., 2000). A-II, being positively inotropic, increases myocardial oxygen demand, but causes vasoconstriction of the coronary vasculatures at the same time. This further exacerbates oxygen imbalance and myocardial ischaemia after myocardial infarction, and may result in irreversible myocardial damage (Perondi et al., 1992). A-II also has direct toxic effect on myocytes and stimulates myocyte hypertrophy, growth of vascular smooth muscle cells and fibroblasts (Lonn et al., 1994). Moreover, loss of myocytes triggers abnormal deposition of fibrillar collagen in the heart (Weber and Brilla, 1991). All these factors lead to progressive ventricular dysfunction after myocardial infarction.
RAAS and systolic heart failure
Packer (1992) first proposed that neurohormonal mechanisms played a central role in the progression of systolic heart failure, where activation of the sympathetic nervous system and RAAS had a direct deleterious effect on the heart that was independent of the haemodynamic disturbance produced by these endogenous systems. The degree of neurohormonal activation in patients with heart failure has been shown to be related to the severity of left ventricular dysfunction (Benedict et al., 1994). The major neurohormonal systems involved are the sympathetic nervous system, RAAS and anti-diuretic hormone (Francis et al., 1984; Dzau, 1987). Other vasoactive substances including endothelin, atrial natriuretic peptide and nitric oxide are also involved. A-II is an important mediator of cardiac remodelling. It stimulates fibroblasts to produce collagen, causes hypertrophy of cardiac myocytes (Sadoshima and Izumo, 1993) and promotes cardiac fibrosis (Kawano et al., 2000). There is now increasing evidence that the local cardiac (Raman et al., 1995; Dostal and Baker, 1999) and renal (Schunkert et al., 1992) activation of renin and angiotensin is involved in the neurohormonal adaptation. Secondary hyperaldosteronism is commonly seen in patients with chronic heart failure, and aldosterone per se may also induce cardiac fibrosis (Lijnen and Petrov, 2000). The net result will be pathological and maladaptive cardiac remodelling, which runs in a viscious cycle and causes progressive decline in the cardiac function.
RAAS and atherosclerosis
Hypertension is a major risk factor for both haemorrhagic and ischaemic stroke (especially small vessel disease). Atherosclerosis is another important factor in the pathophysiology of ischaemic stroke. There is increasing evidence that RAAS, in particular A-II, is closely related to atherosclerosis (Weiss et al., 2001). A-II promotes generation of oxidative stress in the vasculatures, which appears to be a key mediator of endothelial dysfunction, endothelial cell apoptosis and lipoprotein peroxidation (Dimmeler and Zeiher, 2000). A-II also induces cellular adhesion molecules, and chemotactic and pro-inflammatory cytokines, all of which participate in the induction of an inflammatory response in the vessel wall (Phillips and Kagiyama, 2002). In addition, A-II triggers responses in vascular smooth muscle cells that lead to proliferation, migration and a phenotypic modulation, resulting in production of growth factors and extracellular matrix. While all these effects contribute to neointima formation and development of atherosclerotic lesions, A-II may also be involved in acute complications of atherosclerosis by promoting plaque rupture and a hyperthrombotic state (Schmidt-Ott et al., 2000).
DN
DN is a major complication of both type 1 (insulin-dependent diabetes mellitus) and type 2 (non-insulin-dependent diabetes mellitus) diabetes. It is characterized by microalbuminuria in the early stage, followed by overt proteinuria and progressive, irreversible decline in glomerular filtration rate (GFR). Albuminuria is a risk factor for cardiovascular events in individuals with or without DM (Gerstein et al., 2001). Dysregulation of RAAS plays a pivitol role in the pathogenesis of DN. Pathological hallmarks of DN include expansion of mesangial cells, accumulation of extracellular matrix protein, thickening of glomerular and tubular basement membranes, tubulointerstitial fibrosis, glomerulosclerosis and renal endothelial dysfunction (Schrijvers et al., 2004; Kanwar et al., 2008). Hyperglycaemia is associated with increased production of A-II in glomerular mesangial cells (Singh et al., 2003). A-II increases the expression of transforming growth factor, which stimulates the mesangial matrix synthesis (Kagami et al., 1994; Davis et al., 2008). It also decreases mesangial matrix degradation through promoting synthesis of type 1 plasminogen activator inhibitor (PAI-1) (Wilson et al., 1997) and inhibiting activity of mesangial cell collagenase (Singh et al., 1999). Other mechanisms of renal injury include production of reactive oxygen species (ROS) (Giacchetti et al., 2005) and renal fibrosis by up-regulating the expression of Rho A and activating Rho/Rho kinase pathway (Ruiz-Ortega et al., 2006). These structural changes lead to microalbuminuria, followed by macroalbuminuria and finally chronic renal failure.
RAAS: an important therapeutic target
Four groups of RAAS blockers (Figure 1) have been developed, namely direct renin inhibitor (DRI), ACEI, ARB and aldosterone antagonist (AA). Aliskiren was the first DRI and was approved by the United States Food and Drug Administration in 2007 for the treatment of primary hypertension. Remikiren is another DRI currently under development. ACEI inhibits ACE and can be divided into three groups based on their chemical structures, namely dicarboxylate containing (e.g. benazepril, enalapril, lisinopril, perindopril, ramipril, quinapril), sulphydryl containing (e.g. captopril, zofenopril) and phosphate containing (fosinopril). ARBs block the activation of A-II type 1 receptors. Examples include candesartan, eprosartan, irbesartan, losartan, olmesartan and telmisartan. AA blocks the action of aldosterone on mineralocorticoid receptors. Spironolactone was the first member of the class. Other examples include eplerenone and canrenone. In this review, only the role of ACEI and ARB, and their combination in hypertension, myocardial infarction, heart failure, stroke and DN will be discussed.
Hypertension
Anti-hypertensive therapy has been shown to reduce the risk of stroke (35–40%), myocardial infarction (20–25%) and heart failure (>50%) (Neal et al., 2000). Despite the negative result of the Captopril Prevention Project (Hansson et al., 1999b), subsequent large trials involving other ACEIs (e.g. ramipril, perindopril, lisinopril, enalapril) like STOP-2 (Hansson et al., 1999a), HOPE (Yusuf et al., 2000) and ACCOMPLISH (Jamerson et al., 2008) showed that anti-hypertensive treatment with ACEI improved clinical outcomes (Table 1). Previous meta-analysis raised the concern that controlling hypertension (with thiazide or beta-blocker as first line treatment, resulting in a mean reduction of systolic blood pressure by 15.0 mm Hg and diastolic blood pressure by 6.1 mm Hg) in the very old may increase the risk of death (Gueyffier et al., 1999). In the HYVET-Pilot study which involved hypertensive patients older than 80, use of ACEI (lisinopril and enalapril) reduced the risk of stroke by 40%, but was associated with a trend of increased risk of total deaths (RR 1.14, 95% confidence interval [CI] 0.65–2.02) and cardiac deaths (RR 1.40, 95% CI 0.50–3.92) (Bulpitt et al., 2003). The later HYVET study, in which indapamide was used as the first line therapy and perindopril as add-on therapy, however, showed improvement in cardiovascular outcomes with no increase in adverse events (Beckett et al., 2008). In this study, 23.9% and 49.5% of patients were receiving perindopril 2 mg and perindopril 4 mg in addition to indapamide at 2 years.
Table 1.
Effect of ACEI/ARB blockade on hypertension
| Trial acronym (year of publication) | Population | Patient no. | Comparators | Mean follow-up duration | Major results |
|---|---|---|---|---|---|
| ACEI | |||||
| CAPPP (1999) Hansson et al., 1999b | Aged 25–66 years | 10 985 | Captopril (50 mg daily) versus diuretics or beta-blockers | 6.1 years | No difference in major adverse cardiovascular end points. |
| STOP-2 (1999) Hansson et al., 1999a | Aged 70–84 years | 6614 | Beta-blocker or diuretic versus ACEI or calcium channel blockers | 54 months | No difference in major adverse cardiovascular end points. |
| HOPE (2000) Yusuf et al., 2000 | Aged ≥ 55 years with vascular disease or diabetes plus one other cardiovascular risk factor | 9297 | Ramipril (10 mg daily) versus placebo | 5.4 years | Ramipril associated with 26, 20, 32 and 33% reduction in cardiovascular death, myocardial infarction, stroke and heart failure, respectively (all P < 0.01) |
| HYVET-Pilot (2003) Bulpitt et al., 2003 | Aged ≥ 80 years | 1283 | Diuretics versus ACEI versus no treatment | 13 months | ACEI was associated with a 53% reduction in stroke and 43% reduction in stroke mortality (P < 0.01) |
| HYVET (2008) Beckett et al., 2008 | Aged ≥ 80 years | 3845 | Indapamide (sustained release 1.5 mg daily) ± perindopril (2 or 4 mg daily) versus placebo | 1.8 years | ACEI was associated with 21% reduction in all cause mortality (P = 0.02) and 64% reduction in heart failure (P < 0.001) |
| ACCOMPLISH (2008) Jamerson et al., 2008 | High cardiovascular risk | 11 506 | Benazepril (20 mg) + amlodipine (5 mg daily) versus benazepril (20 mg) + hydrochlorothiazide (12.5 mg daily) | 36 months | Benazepril/amlodipine was associated 20% reduction in cardiovascular end points (P < 0.001) |
| ARB | |||||
| LIFE (2002) Dahlöf et al., 2002 | Aged 55–80 years | 9193 | Losartan (mean dose 82 mg) versus atenolol (mean dose 79 mg) | 4.8 years | Losartan was associated with 13 and 25% reduction in composite end points (P = 0.02) and stroke (P = 0.001) respectively |
| VALUE (2004) Julius et al., 2004 | Aged ≥ 50 years | 15 245 | Valsartan (80 mg daily) versus amlodipine (5 mg daily) | 4.2 years | No difference in composite primary end points. Amlodipine group had fewer myocardial infarction than valsartan group (11.4 vs. 9.6%, P = 0.02) |
ARBs have been compared with other classes of anti-hypertensive drugs in large clinical trials. In the LIFE study, losartan and atenolol achieved similar blood pressure reduction in patients with essential hypertension and left ventricular hypertrophy (Dahlöf et al., 2002). Losartan was superior to atenolol in decreasing risk of cardiovascular death, stroke or MI and new-onset diabetes. In the VALUE study, valsartan was comparable to amlodipine in terms of reducing cardiac mortality and morbidity in hypertensive patients at high cardiovascular risk (Julius et al., 2004). Although the amlodipine group had a significantly lower incidence of myocardial infarction than the valsartan group, it could be explained by the fact that the amlodipine group attained a lower blood pressure. Similar to the LIFE study, patients receiving valsartan had a lower incidence of new-onset diabetes.
AMI
Treatment with ACEI is beneficial following AMI (Table 2). Seven major prospective randomized trials have evaluated the use of ACEI following AMI. These trials could be divided into: (i) those in which ACEI were given to all AMI patients in a randomized fashion (ISIS-4, GISSI-3 and CONSENSUS II); and (ii) those that required evidence of asymptomatic or symptomatic left ventricular dysfunction before randomization [SAVE, TRACE, Acute Infarction Ramipril Efficacy (AIRE) and SMILE]. In the ISIS-4 (ISIS-4 Collaborative Group, 1995) and GISSI-3 (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico, 1994; 1996;) studies, captopril and lisinopril as compared to placebo resulted in a 7% (at 5 weeks) and 12% (12% at 6 weeks; 6.2% at 6 months) reduction in mortality, respectively, beyond that achieved by thrombolytic therapy. The negative result shown by the CONSENSUS II study (Swedberg et al., 1992) was likely explained by the higher frequency of hypotension caused by intravenous enalaprilat given in the first 24 h after AMI. These data supported the use of oral ACEI in the early phase of AMI if hypotension could be avoided. In patients with AMI and left ventricular systolic dysfunction, captopril and trandolapril versus placebo resulted in 32% and 25–30% reduction in mortality in SAVE (Rutherford et al., 1994) and TRACE trials (Kober et al., 1995; Torp-Pedersen and Kober, 1995). Sustained clinical benefits were observed at 10- to 12-year follow-up (Buch et al., 2005). In patients with clinically evident CHF, ramipril reduced mortality and heart failure progression as compared to placebo (The AIRE Study Investigators, 1993; Cleland et al., 1997). The AIRE results suggested halting heart failure progression in AMI patients could improve survival by reducing the risks of circulatory failure and sudden death, and the benefit was sustained for many years (Hall et al., 1997). The SMILE study showed that in patients with anterior AMI without thrombolysis, zofenopril reduced mortality and incidence of severe heart failure (Ambrosioni et al., 1995) when the drug was started within 24 h after the onset of AMI. Several meta-analyses of ACEI trials have consistently demonstrated a favourable effect on survival after AMI (ACE Inhibitor Myocardial Infarction Collaborative Group, 1998; Flather et al., 2000; Latini et al., 2000; Rodrigues et al., 2003). Studies regarding ACEI in low-risk patients with stable coronary heart disease were more conflicting. The PEACE (Braunwald et al., 2004), QUIET (Pitt et al., 2001) and CAMELOT (MacMahon et al., 2000) all showed negative results, whereas the large-scale EUROPA (Fox and The European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease Investigators, 2003) study demonstrated beneficial effect of perindopril in low-risk patients with stable coronary heart disease and no apparent heart failure, in particular those with history of myocardial revascularization (Bertrand et al., 2009). Meta-analysis of pooled data showed that use of ACEI was associated with a reduction in cardiovascular mortality (RR 0.83, 95% CI 0.72–0.96, P = 0.01), non-fatal MI (RR 0.84, 95% CI 0.75–0.94, P = 0.003), all-cause mortality (RR 0.87, 95% CI 0.81–0.94, P = 0.0003) and revascularization rates (RR 0.93, 95% CI 0.87–1.00, P = 0.04) (Al-Mallah et al., 2006).
Table 2.
Effect of ACEI/ARB on AMI
| Trial acronym (year of publication) | Population | Patient no. | Comparators | Mean follow-up duration | Major results |
|---|---|---|---|---|---|
| ACEI | |||||
| ISIS-4 (1995) ISIS-4 Collaborative Group, 1995 | AMI | 58 050 | Captopril (6.25 mg daily up to 50 mg twice daily) versus placebo | 15 months | Captopril was associated with 7% reduction in mortality at 5 weeks (P = 0.02). |
| GISSI-3 (1994, 1996) Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico, 1994; 1996 | AMI | 19 394 | Lisinopril (10 mg daily) versus placebo | 6 weeks, 6 months | Lisinopril started within 24 h of AMI was associated with 12 and 6% reduction in mortality at 6 weeks and 6 months, respectively (P = 0.03) |
| CONSENSUS II (1992) Swedberg et al., 1992 | AMI | 6090 | Enalapril (intravenous enalaprilat 1 mg followed by enalapril 20 mg daily) versus placebo | 6 months | Enalapril started within 24 h of AMI did not improve survival at 6 months |
| SAVE (1994) Rutherford et al., 1994 | AMI and LVEF ≤ 40% | 2231 | Captopril (12.5–50 mg thrice daily) versus placebo | 42 months | Captopril was associated with 32% reduction in mortality (P = 0.029). |
| TRACE (1995, 1999, 2005) Kober et al., 1995; Torp-Pedersen and Kober, 1995; Buch et al., 2005 | AMI and LVEF ≤ 35% | 1749 | Tradolapril (4 mg daily) versus placebo | Up to 10–12 years | Tradolapril was associated with 25–30% reductions in mortality, sudden death and heart failure progression at 2–4 year follow-up (all P < 0.05) |
| AIRE (1993, 1997) The AIRE Study Investigators, 1993; Cleland et al., 1997 | AMI with heart failure | 2006 | Ramipril (5 mg twice daily) versus placebo | 15 months | Ramipril reduced risk of sudden cardiac death (P = 0.011) and heart failure progression by 30 and 23%, respectively (P = 0.017) |
| SMILE (1995) Ambrosioni et al., 1995 | AMI | 1556 | Zofenopril (15 mg daily) | 1 year | Zofenopril reduced mortality or heart failure by 34% (P = 0.018) and 29% (P = 0.011) at 6 weeks and 1 year, respectively |
| ARB | |||||
| VALIANT (2003) Pfeffer et al., 2003 | AMI and heart failure | 14 793 | Valsartan (20–160 mg twice daily) versus captopril (6.25–50 mg thrice daily) versus both (valsartan 20–80 mg twice daily + captopril 6.25–50 mg thrice daily) | 24.7 months | Valsartan was as effective as captopril. Combination therapy caused more adverse events without improvement in survival |
| OPTIMAAL (2002) Dickstein et al., 2002 | AMI with heart failure | 5477 | Losartan (50 mg daily) versus captopril (50 mg thrice daily) | 2.7 years | Losartan was better tolerated than captopril despite having similar efficacy |
ARBs also save lives in AMI patients as shown in two major randomized control studies. In OPTIMAAL study, AMI patients with CHF randomized to losartan or captopril had similar outcomes after a mean follow-up of 2.7 years (Dickstein et al., 2002). However, losartan was better tolerated than captopril with fewer patients withdrawing from treatment (17% vs. 23%, RR 0.70, 95% CI 0.62–0.79, P < 0.0001). Similarly, valsartan had been shown to be equally effective as captopril in reducing mortality in AMI patients in the VALIANT study (Pfeffer et al., 2003).
Chronic systolic heart failure
Previous meta-analysis showed that ACEI therapy increased survival, reduced heart failure-related hospitalizations and improved symptoms in patients with left ventricular dysfunction or heart failure (Flather et al., 2000). Three out of the five trials included for analysis enrolled patients within a week after AMI. The CONSENSUS (The CONSENSUS Trial Study Group, 1987) and SOLVD (The SOLVD Investigators, 1991) studies showed that enalapril reduced mortality by up to 40% in patients with symptomatic severe systolic heart failure (Table 3). In asymptomatic patients with left ventricular dysfunction, enalapril could reduce the incidence of heart failure, rate of related hospitalizations (The SOLVD Investigators, 1992) and a statistically insignificant trend towards reduced mortality due to cardiovascular causes. Compared with hydralazine and isosorbide dinitrate combination, enalapril was superior in terms of reducing mortality as shown in the V-HeFT II study (Cohn et al., 1991), although subsequent analysis showed that the mortality benefit was only seen in white patients with hypertension and higher PRA (Carson et al., 1999). In the FEST study, fosinopril increased exercise tolerance and reduced the frequency of clinical events indicative of worsening heart failure in patients with mild to moderately severe heart failure, despite no significant improvement in overall mortality (Erhardt et al., 1995).
Table 3.
Effect of ACEI/ARB on heart failure
| Trial acronym (year of publication) | Population | Patient no. | Comparators | Mean follow-up duration | Major results |
|---|---|---|---|---|---|
| ACEI | |||||
| CONSENSUS (1987) The CONSENSUS Trial Study Group, 1987 | NYHA class IV | 253 | Enalapril (2.5–40 mg daily) versus placebo | 188 days | Enlapril was associated with 40% (P = 0.002) and 31% (P = 0.001) reduction in mortality at 6 months and 1 year respectively |
| SOLVD (1991) The SOLVD Investigators, 1991 | LVEF ≤ 35% Symptomatic | 2569 | Enalapril (2.5–20 mg daily) versus placebo | 41.4 months | Enalapril reduced mortality by 16% (P = 0.004) and combined end point of death or heart failure hospitalization by 26% (P < 0.0001) |
| SOLVD (1992) The SOLVD Investigators, 1992 | LVEF ≤ 35% Asymptomatic | 4228 | Enalapril (2.5–20 mg daily) versus placebo | 47.4 months | Enalapril reduced the risk of death and heart failure by 29% (P < 0.001) |
| V-HeFT II (1991) Cohn et al., 1991 | NYHA class II–III | 806 | Enalapril (10 mg daily) versus hydralazine (150 mg daily)/isorsobide dinitrate (80 mg twice daily) | 2.5 years | Enalapril reduced 2-year mortality by 28% (P = 0.02) |
| FEST (1995) Erhardt et al., 1995 | NYHA class II–III | 308 | Fosinopril (10–40 mg daily) versus placebo | 12 weeks | Fosinopril increased exercise tolerance and reduced worsening of heart failure (8 vs. 20%; P = 0.002) without change in mortality. |
| ARB | |||||
| ARCH-J (2003) Matsumori and ARCH-J Study Investigators, 2003 | ACEI intolerant | 305 | Candesartan (8 mg daily) versus placebo | 6 months | Candesartan reduced progression of heart failure by 67% (P < 0.001) and cardiovascular events by 53% (P < 0.01) |
| CHARM-alternative (2003) Granger et al., 2003 | ACEI intolerant; LVEF ≤ 40% | 2028 | Candesartan (4–16 mg daily) versus placebo | 33.7 months | Candesartan reduced the risk of cardiovascular deaths or heart failure admission by 23% (P < 0.001) |
| ELITE I (1997) Pitt et al., 1997 | ACEI naïve; LVEF ≤ 40%, | 722 | Losartan (50 mg daily) versus captopril (50 mg thrice daily) | 48 weeks | Losartan reduced all-cause mortality by 46% (P = 0.04) |
| ELITE II (2000) Pitt et al., 2000 | ACEI naïve, NYHA class II–IV; LVEF ≤ 40% | 3152 | Losartan (50 mg daily) versus captopril (50 mg thrice daily) | 555 days | Losartan and captopril had similar efficacy |
| SPICE (2000) Granger et al., 2000 | ACEI intolerant; LVEF ≤ 35% | 270 | Candesartan versus placebo | 12 weeks | No difference in cardiovascular end points |
| HEAAL (2009) Konstam et al., 2009 | ACEI intolerant, NYHA class II–IV; LVEF ≤ 40% | 3846 | Losartan 50 mg once daily versus 150 mg once daily | 4.7 years | Higher dose losartan was associated with reduced rate of death or heart-failure-related admissions (43 vs. 46%, P = 0.03) |
The role of ARB in heart failure has been evaluated as primary therapy compared with ACEI (ELITE-I and ELITE-II) or placebo [Assessment of Response to Candesartan in Heart Failure in Japan (ARCH-J)], as an alternative in patients intolerant of ACEIs (CHARM-alternative and SPICE) and as add-on therapy in patients already treated with an ACEI (which will be discussed later). The ELITE-I study showed losartan provided a smilar benefit when compared with captopril, and had a similar risk of renal dysfunction (Pitt et al., 1997). There was a trend towards better clinical outcome in terms of death and/or hospital admission for heart failure in the losartan group (9.4% in losartan group vs. 13.2% in captopril group) (P = 0.075), primarily due to a decrease in all-cause mortality (4.8% vs. 8.7%; P = 0.035). However, such mortality benefit was not seen in the ELITE-II study, which showed a similar all-cause mortality (11.7% vs. 10.4% average annual mortality rate), sudden death or resuscitated cardiac arrests (9.0% vs. 7.3%) between losartan group and captopril group (Pitt et al., 2000). The authors commented that the superiority of losartan to captopril in reducing mortality in the ELITE-I study should be taken as a chance finding as the observations were based on a small number of deaths. The ELITE-II study had four times as many patients and 10 times more events (Pitt et al., 2000). Subsequent analysis of the ELITE-II study suggested that losartan and captopril did not differ in terms of heart failure-related outcomes, NYHA class and quality of life (Konstam et al., 2005). The ARCH-J study was prematurely terminated when candesartan was shown to reduce progression of CHF by 66.7% (P < 0.001) and incidence of cardiovascular events by 53% (P < 0.01) when compared with placebo (Matsumori and ARCH-J Study Investigators, 2003). In the CHARM-alternative study, candesartan was shown to reduce cardiovascular deaths or hospital admissions for heart failure by 30% (P < 0.0001) in patients intolerant to ACEI (Granger et al., 2003). Although the SPICE trial failed to demonstrate any benefit in terms of mortality and morbidity, this may be accountable by the relatively small sample size, low event rates and short follow-up period (Granger et al., 2000). One meta-analysis showed that ARB reduced all-cause mortality and heart failure hospitalizations as compared with placebo, and had similar efficacy when compared with ACEI in patients with chronic heart failure (Lee et al., 2004). The recent HEAAL study suggested that in patients with heart failure (NYHA class II-IV), LVEF 40% or less, and intolerant to ACEI, losartan 150 mg daily was superior to 50 mg daily in reducing the mortality or admissions for heart failure, suggesting that increased doses of an ARB would be needed to achieve the maximal benefit (Konstam et al., 2009). While the HEAAL study certainly added information that losartan at 150 mg daily seemed to be more effective and generally tolerated, it did not provide any information about whether a high-dose ARB is better than ACEI monotherapy. Neither did it offer insights about whether maximizing the dose of one RAAS blocker would be better than the use of combination therapy (e.g. comparing high-dose ARB with low-dose ARB plus low-dose ACEI).
Stroke
RAAS blockers could lower stroke risk by mechanisms other than blood pressure-lowering effect. The benefit of ACEI in stroke was supported by two large trials (PROGRESS and HOPE) (Table 4). In the PROGRESS study, which was a randomized, double-blind, placebo-controlled trial of 6105 individuals with history of cerebrovascular disease, patients were randomly assigned to receive perindopril with or without addition of indapamide or placebo (PROGRESS Collaborative Group, 2001). After a mean follow up of 4 years, perindopril alone reduced the incidence of recurrent stroke by 28%, while the combination with indapamide reduced stroke risk by 43%. Benefits of treatment were consistent across important patient subgroups, including those with and without hypertension, and for both ischaemic and haemorrhagic strokes. Similarly, ramipril reduced the relative risk of any stroke by 32% (Yusuf et al., 2000) and the risk of fatal stroke by 61% (Bosch et al., 2002) in the HOPE study.
Table 4.
Effect of ACEI/ARB blockade on stroke
| Trial acronym (year of publication) | Population | Patient no. | Comparators | Mean follow-up duration | Major results |
|---|---|---|---|---|---|
| ACEI | |||||
| PROGRESS (2001) PROGRESS Collaborative Group, 2001 | History of stroke or transient ischaemic attack | 6105 | Perindopril (4 mg daily) ± indapamide (2 or 2.5 mg daily) versus placebo | 4.2 years | Perindopril ± indapamide reduced the risk of stroke by 28% (P < 0.0001) and major adverse cardiovascular events by 26% (P < 0.01) |
| HOPE (2000) Yusuf et al., 2000 | High vascular-risk; aged ≥ 55 years | 9297 | Ramipril (10 mg daily) versus placebo | 5.4 years | Ramipril reduced the risk of any stroke by 32% (P < 0.001) |
| ARB | |||||
| ACCESS (2003) Schrader et al., 2003 | Hypertensive; history of ischaemic stroke | 339 | Candesartan (4–16 mg daily) versus placebo | 12 months | Candesartan reduced the risk of mortality by 60% (P = 0.07) and the number of vascular events by 47.6% (P = 0,026) |
| SCOPE (2003) Lithell et al., 2003 | Hypertensive, aged 70–89 years; MMSE score ≥ 24 | 4964 | Candesartan (8–16 mg daily) versus placebo | 3.7 years | Candesartan reduced the risk of non-fatal stroke by 27.8% (P = 0.04) and all stroke by 23.6% (P = 0.056) |
| LIFE (2002) Dahlöf et al., 2002 | Hypertensive; aged 55–80 years; LVH ascertained by electrocardiography | 9193 | Losartan (mean dose 82 mg) versus atenolol (mean dose 79 mg) | 4.8 years | Losartan reduced the risk of fatal or non-fatal stroke by 25% (P = 0.001) |
| MOSES (2005) Schrader et al., 2005 | Hypertensive; high vascular risk; previous stroke | 1405 | Eprosartan (600 mg daily) versus nitrendipine (10 mg daily) | 2.5 years | Eprosartan reduced the risk of combined cardiovascular events by 21% (P = 0.014) and cerebrovascular events by 25% (P = 0.03) |
| PRoFESS (2008) Yusuf et al., 2008a | History of ischaemic stroke | 20 332 | Telmisartan (80 mg daily) versus placebo | 2.5 years | Telmisartan did not significantly lower the rate of stroke (8.7 vs. 9.2%) or major cardiovascular events (13.5 vs. 14.4%) |
| TRANSCEND (2008) Yusuf et al., 2008b | ACEI intolerant; established cardiovascular diseases or diabetes with end-organ damage | 5926 | Telmisartan (80 mg daily) versus placebo | 56 months | Telmisartan did not reduce the risk of stroke (hazard ratio 0.83, P = 0.136) |
Four ARBs (candesartan, losartan, telmisartan, eprosartan) have been tested in large clinical trials, both as primary prevention (LIFE, SCOPE, TRANSCEND and ONTARGET) and secondary prevention (ACCESS, MOSES and PRoFESS) in the treatment of stroke. In the LIFE study, losartan was shown to be superior to atenolol in terms of stroke prevention, where losartan reduced the risk of fatal or non-fatal stroke by 25% (P = 0.001) (Dahlöf et al., 2002). Similarly, candesartan reduced the risk of non-fatal stroke by 27.8% (P = 0.04), and all stroke by 23.6% (P = 0.056) in the SCOPE study (Lithell et al., 2003). In the TRANSCEND study, despite a 13% reduction in the risk of the secondary composite outcome of cardiovascular death, myocardial infarction or stroke by telmisartan (P = 0.048), the reduction of stroke risk in isolation was statistically insignificant (3.8% vs. 4.6%; P = 0.136) (Yusuf et al., 2008b). The stroke subgroup of ONTARGET study also showed that telmisartan treatment only produced a statistically insignificant trend towards reduced risk of recurrent stroke compared with ramipril (HR 0.91; P = 0.85) (Yusuf et al., 2008c). Studies on benefit of ARB in terms of secondary prevention of stroke generated inconsistent results as well. In the ACCESS study, candesartan reduced the number of vascular events by more than 50% (P = 0.026) when compared with placebo in patients with ischaemic stroke and hypertension. There was a trend towards improved 12 month mortality in the candesartan group (2.9% vs. 7.2%; P = 0.07). (Schrader et al., 2003). In the MOSES study, eprosartan was superior to nitrendipine by reducing the risk of combined cardiovascular events by 21% (P = 0.014) and cerebrovascular events by 25% (P = 0.03) despite a similar degree of blood pressure reduction (Schrader et al., 2005). However, the PRoFESS trial, which was the largest stroke trial, failed to demonstrate such benefit. The PRoFESS study was designed to compare the effects of telmisartan against placebo, in addition to standard stroke prevention therapy including other anti-hypertensive drugs on the further reduction of recurrent stroke. In this study, 20 332 patients with ischaemic stroke were randomized to telmisartan versus placebo and to two anti-platelets (aspirin and dipyridamole) in a 2 × 2 factorial design. After a mean follow-up of 2.5 years, telmisartan showed an insignificant lower rate of recurrent stroke (HR 0.95, 95% CI 0.86–1.04, P = 0.23) (Yusuf et al., 2008a). The findings of the PRoFESS study have raised the question of whether ARBs offer additional benefits independent of their effects on blood pressure, as suggested in both the HOPE and LIFE studies. Afterall, ARB remains an appropriate alternative in patients who are intolerant to ACEI, but whether ARB should be used as the first line agent in stroke prevention requires further clarification.
DN
ACEI and ARB have been shown to be effective in delaying disease progression in both type 1 and type 2 diabetic patients with microalbuminuria or established DN, although there is no evidence that they are effective in the primary prevention of DN (Table 5). Early randomized trials showed that in normotensive patients with type 1 diabetes and persistent microalbuminuria, captopril significantly reduced the risk of progression to clinical proteinuria (Viberti et al., 1994; The Microalbuminuria Captopril Study Group, 1996). In the captopril study, which involved patients with overt proteinuria and mild renal impairment (creatinin ≤ 220 µmol·L−1), captopril reduced the risk of combined end point of mortality, dialysis and transplatation by 50% (Lewis et al., 1993). Further analysis of the captopril study showed that captopril induced remission of nephrotic-range proteinuria in 16.7% patients, compared with only 1.5% with placebo (Hebert et al., 1994). The beneficial effect of captopril is consistent among both hypertensive and normotensive subjects (Kasiske et al., 1993). Long-term remission of nephrotic syndrome and preservation of renal function have also been described (Wilmer et al., 1999). Remission of nephrotic range proteinuria has been associated with a 28% risk reduction in terms of end-stage renal diseaes (dialysis or transplantation) or death (Hovind et al., 2004).
Table 5.
Effect of ACEI/ARB blockade on diabetic (DM) nephropathy
| Trial acronym (year of publication) | Population | Patient no. | Comparators | Mean follow-up duration (years) | Major results |
|---|---|---|---|---|---|
| ACEI | |||||
| Viberti et al., 1994 | Normotensive, insulin-dependent DM with microalbuminuria | 92 | Captopril (50 mg twice daily) versus placebo | 2 | Captopril was associated with significant reduction in albumin excretion compared to placebo (P < 0.01) |
| The Microalbuminuria Captopril Study Group, 1996 | Normotensive insulin-dependent DM with microalbuminuria | 253 | Captopril (50 mg twice daily) versus placebo | 2 | Progression to overt albuminuria over 24 months was significantly reduced by captopril by 69% (P = 0.004) |
| Captopril Study, 1993 Lewis et al., 1993 | Insulin-dependent DM with overt proteinuria and creatinine ≤ 2.5 mg·L−1 (220 µmol·L−1) | 409 | Captopril (25 mg thrice daily) versus placebo | 3 | Captopril reduced the risk of doubling of the serum creatinine by 48% (P = 0.007) |
| Hebert et al., 1994 | DM with nephritic-range proteinuria | 108 | Captopril (25 mg thrice daily) versus placebo | 3 | Captopril was associated with higher remission of nephrotic-range proteinuria compared to placebo (6.7 vs. 1.5%; P = 0.005). |
| Ravid et al., 1994 | Normotensive, type II DM with microalbuminuria and normal renal function | 94 | Enalapril 10 mg daily versus placebo | 5 | Enalapril prevented decline of kidney function (13% decline in the placebo group and remained stable in the enalapril group; P < 0.05) |
| MICRO-HOPE (2000) Heart Outcomes Prevention Evaluation Study Investigators, 2000 | DM patients in the HOPE study | 3577 | Ramipril 10 mg daily versus placebo | 4.5 | Ramipril lowered the risk of the combined primary outcome by 25% (P = 0.0004), myocardial infarction by 22% (P = 0.01), stroke by 33% (P = 0.0074), cardiovascular death by 37% (P = 0.0001), total mortality by 24% (P = 0.004), revascularisation by 17% (P = 0.031) and overt nephropathy by 24% (P = 0.036) |
| ARB | |||||
| IDNT (2001) Lewis et al., 2001 | Hypertensive, type II DM with nephropathy | 1715 | Irbesartan (300 mg daily) versus amlodipine (10 m daily) versus placebo | 2.6 | Irbesartan was associated 23% (P = 0.02) and 20% (P = 0.006) reduction in combined end points (doubling of the plasma creatinine, development of end-stage renal disease or death from any cause) compared with amlodipine and placebo, respectively |
| Parving et al., 2001a | Hypertensive, type II DM with microalbuminuria | 590 | Irbesartan (150 or 300 mg daily) versus placebo | 2 | Irbesartan 300 mg daily reduced the risk of DM nephropathy compared with placebo (HR 0.30, 95% CI 0.14–0.61, P < 0.001) while 150 mg daily was associated with a statistically insignificant risk reduction of 39% (P = 0.081) |
| RENAAL (2001) Brenner et al., 2001 | Type II DM with nephropathy | 1517 | Losartan (50–100 mg daily) versus placebo | 3.4 | Losartan reduced doubling of the plasma creatinine by 25% (P = 0.006) and end-stage renal disease by 28% (P = 0.002) |
| DETAIL (2004) Barnett et al., 2004 | Type II DM with nephropathy | 250 | Telmisartan (80 mg daily) versus enalapril (20 mg daily) | 5 | Telmisartan and enalapril were associated with similar decline in GFR |
A similar benefit has been observed in patients with type 2 diabetes. In one early study, enalapril significantly decreased albuminuria and prevented decline in kidney function in type 2 diabetics with microalbuminuria and normal renal function (Ravid et al., 1994). In the MICRO-HOPE study, which was a sub-study of the diabetic population in the HOPE study, ramipril reduced the risk of developing overt nephropathy by 24% (P = 0.027) (Heart Outcomes Prevention Evaluation Study Investigators, 2000). The ADVANCE study showed a 9% relative risk reduction in a major macrovascular or microvascular event with perindopril/indapamide combination (Patel et al., 2007). In terms of renal outcome, perindopril/indapamide significantly reduced the rate of new-onset microalbuminuria and in the combined end point of new-onset or worsening microalbuminuria or proteinuria. Such benefit was seen even among those with initial BP < 120/70 mm Hg (de Galan et al., 2009). However, it is impossible to predict the degree to which the renal benefits in ADVANCE study were due to the ACEI or to the lower blood pressure.
Several major trials have demonstrated a clear benefit in terms of renoprotection with ARB in patients with nephropathy due to type 2 diabetes (IDNT, RENAAL and DETAIL). In the IDNT study, 1715 hypertensive patients with nephropathy due to type 2 diabetes were randomly assigned to irbesartan, amlodipine or placebo (Lewis et al., 2001). At 2.6 years, irbesartan was associated with a risk of the combined end point (doubling of the plasma creatinine, development of end-stage renal disease or death from any cause) that was 23 and 20% lower than with amlodipine and placebo respectively. These benefits were independent of the differences in the magnitude of blood pressure reduction among the groups (Berl et al., 2005; Pohl et al., 2005). In another randomized trial which involved 590 hypertensive patients with type 2 diabetes and microalbuminuria, irbesartan at a dose of 300 mg daily reduced the risk of overt DN by 70% when compared with placebo (P < 0.001) (Parving et al., 2001b). In the RENAAL study, 1513 patients with type 2 diabetes and nephropathy were randomly assigned to losartan or placebo, both in addition to conventional anti-hypertensive therapy (excluding ACEI). At 3.4 years, losartan reduced the incidence of a doubling of the plasma creatinine by 25% (P = 0.006) and end-stage renal disease by 28% (P = 0.002). The composite end point of doubling of the base-line serum creatinine concentration, end-stage renal disease or death was reduced by 16% in the losartan group (P = 0.02). These benefits were again not associated with differences in blood pressure levels between the groups (Brenner et al., 2001). The DETAIL study demonstrated similar efficicacy of telmisartan and enalapril in patients with early nephropathy (Barnett et al., 2004).
Combination therapy of ACEI and ARB
After the publication of several large clinical trials involving combination therapy of ACEI and ARB (ACEI/ARB combo), the Canadian Hypertension Education Program (CHEP) responded with a bold new warning: ‘Do not use ACEI and ARB in combination’. However, the European Society of Cardiology (ESC) in their updated heart failure treatment guidelines still recommended ACEI/ARB combo as a viable option. The rationale of giving ACEI/ARB combo is large based on a phenomenon called ‘A-II escape’. There is evidence that standard doses of ACEI only offer a partial blockade of ACE (Ennezat et al., 2000). One proposed explanation was that enzymes such as chymase, cathepsin G and chymostatin-sensitive angiotensin-generating enzyme can form A-II from angiotensinogen and other peptide substrates (Balcells et al., 1997). Because this mode of A-II generation is independent of ACE, it can proceed regardless of the presence of an ACEI. By targeting both the ACE and the angiotensin receptors, the goal of therapy is to provide a more complete blockade of the effect of A-II produced by the alternative pathway. Certain ARBs (especially telmisartan) have also been shown to be selective peroxisome proliferator-activated receptor modulators (Schupp et al., 2005) implicating an effect on the metabolism, proliferation and inflammation of cardiovascular cells (Brown and Plutzky, 2007). In the following section, the role of ACEI and ARB combination therapy in the aforementioned cardiovascular diseases and DN will be discussed (Table 6).
Table 6.
Effect of ACEI/ARB combination in cardiovascular diseases
| Trial acronym (year of publication) | Population | Patient no. | Comparators | Mean follow-up duration | Major efficacy end points | Major adverse events (in particular hypotension, renal impairment and hyperkalaemia) |
|---|---|---|---|---|---|---|
| Hypertension | ||||||
| ONTARGET (2008) Yusuf et al., 2008c | Established atherosclerotic diseases or DM with end-organ damage | 25 620 | Ramipril (10 mg daily) versus telmisartan (80 mg daily) versus combination | 56 months | Combination therapy was associated with trend in greater reduction in mean blood pressure but no difference in primary outcome (death from cardiovascular causes, myocardial infarction, stroke or hospitalization for heart failure) | Combination therapy was associated with greater discontinuation of study medication due to: (i) hypotensive symptoms (ramipril 1.7% vs. telmisartan 2.7% vs. combination 4.8%, P < 0.001); (ii) renal impairment (ramipril 0.7% vs. talmisartan 0.8% vs. combination 1.1%. P < 0.001); and (iii) hyperkalaemia (not mentioned) |
| Myocardial infarction | ||||||
| VALIANT (2003) Pfeffer et al., 2003 | AMI with heart failure and/or left ventricular systolic dysfunction | 14 793 | Valsartan (20–160 mg twice daily) versus captopril (6.25–50 mg thrice daily) versus both (valsartan 20–80 mg twice daily + captopril 6.25–50 mg thrice daily) | 24.7 months | Combination therapy did not improve mortality | Adverse events resulting in dose reduction: (i) hypotension (valsartan 15.1%* vs. captopril 11.9% vs. combination 18.2%*); (ii) renal causes (valsartan 4.9%* vs. captopril 3.0% vs. combination 4.8%*); and (iii) hyperkalemia (valsartan 1.3% vs. captopril 0.9% vs. combination 1.2%) *P < 0.05 |
| ONTARGET (2008) Yusuf et al., 2008c | Established atherosclerotic diseases or DM with end-organ damage | 25 620 | Ramipril (10 mg daily) versus telmisartan (80 mg daily) versus combination | 56 months | The incidence of cardiovascular events were similar among the groups (ramipril group 16.5% vs. telmisartan group 16.7% [P = 0.83] versus combination group 16.3% [P = 0.38]) | Combination therapy was associated with trend in greater reduction in mean blood pressure, but no difference in primary outcome (death from cardiovascular causes, myocardial infarction, stroke or hospitalization for heart failure) |
| Heart failure | ||||||
| Val-HeFT (2001) Cohn et al., 2001 | NYHA class II–IV; receiving standard therapy | 5010 | Valsartan (160 mg twice daily) + ACEI | 23 months | Combination therapy reduced the incidence of the combined endpoints by 13.2% (P = 0.009) driven by reduction in heart failure hospitalization (13.8% with valsartan vs. 18.2% with placebo, P < 0.001) | Adverse events leading to discontinuation of study medication: (i) hypotension (valsartan 1.3% vs. placebo 0.8%, P = 0.124); (ii) renal impairment (valsartan 1.1% vs. placebo 0.2%, P < 0.001); mean change of serum potassium level (0.12 mmol·L−1with candesartan vs. 0.07 decrease with placebo, P < 0.001) |
| CHARM-added (2003) McMurray et al., 2003 | NYHA class II–IV; being treated with ACEI | 2548 | Candesartan (32 mg daily) + ACEI or placebo | 41 months | Candesartan + ACEI was associated with lower composite end points, defined as cardiovascular death or unplanned admission to hospital for the management of worsening congestive heart failure (38 vs. 42%, P = 0.011) | Adverse events leading to drug discontinuation: (i) (i) hypotension (candesartan 4·5% vs. placebo 3·1%, P = 0·079); (ii) increase in creatinine (candesartan 7·8% vs. placebo 4·1%, P = 0·0001); and (iii) hyperkalaemia (candesartan 3·4% vs. placebo 0·7%, P < 0.0001) |
| Stroke | ||||||
| ONTARGET (2008) Yusuf et al., 2008c | Established atherosclerotic diseases or DM with end-organ damage | 25 620 | Ramipril (10 mg daily) versus telmisartan (80 mg daily) versus combination | 56 months | Combination therapy did not reduce the risk of stroke or transient ischaemic attacks | As above |
| Diabetic nephropathy | ||||||
| Jennings et al., 2007 | DM nephropathy | 315 | ACEI/ARB | Not applicable | ACEI/ARB combination was associated with reduced proteinuria, but also worsening of renal renal function | ACEI/ARB combination was associated with a mean decrease in GFR of 3.87 mL/min (P = 0.03). Serum potassium was increased by a mean of 0.2 mmol/l (95% CI 0.08–0.32; P < 0.01) with combination therapy |
| ONTARGET (2008) Yusuf et al., 2008c | Established atherosclerotic diseases or DM with end-organ damage | 25 620 | Ramipril (10 mg daily) versus telmisartan (80 mg daily) versus combination | 56 months | Ramipril/telmisartan combination reduced proteinuria more than monotherapy, but was associated with worsened major renal outcomes | As above |
ACEI/ARB combo and hypertension
Doulton et al. (2005) analysed the blood pressure effect of ACEI/ARB combo in different populations (uncomplicated essential or isolated systolic hypertension, chronic renal failure, type 1 and type 2 diabetes) in a meta-analysis which included 14 trials. Overall, ACEI/ARB combo reduced 24 h ambulatory blood pressure by 4.7/3.0 mm Hg compared with ACEI monotherapy, and 3.8/2.9 mm Hg compared with ARB monotherapy. Proteinuria was also reduced by 30 and 39% when compared with ACEI monotherapy and ARB monotherapy respectively. However, one drawback of this meta-analysis was that the majority of included studies used submaximal doses or once-daily dosing of shorter-acting ACEIs and had a short duration of follow-up (4–8 weeks). Hence, the long-term effect of adding ARB to chronic ACEI therapy could not be concluded. The study with the longest duration of follow-up (2.9 years) included in this meta-analysis was the COOPERATE study, which used the longest acting ACEI trandolapril and showed no additional reduction in trough blood pressure with combination therapy (trandolapril plus losartan) compared with monotherapy (Nakao et al., 2003). However, the COOPERATE study was recently retracted due to problems with authenticity of the data. As a result, most of the evidence concerning the effect of ACEI/ARB combo on hypertension came from the ONTARGET study (Yusuf et al., 2008c). The ONTARGET study was the world's largest morbidity and mortality trial involving ARB and ACEI/ARB combination so far which compared ramipril, telmisartan and their combination in patients with vascular disease or high-risk diabetes. The principle questions to be addressed were: (i) whether an ARB, specifically telmisartan, is as effective as ACEI, specifically ramipril, in high-risk patients (as shown in the HOPE study); (ii) whether combination therapy of ramipril and telmisartan can further improve clinical outcomes; and (iii) whether such combination was associated with more adverse side effects. A total of 25 620 patients were randomized to ramipril 10 mg daily, telmisartan 80 mg dialy or combination of both. Both telmisartan and ramipril reduced blood pressure to a similar extent (about 6 mm Hg reduction for systolic and 5 mm Hg for diastolic blood pressure). Mean blood pressure reductions were slightly greater, although statistically insignificant with ramipril/telmisartan combination compared with ramipril alone (8.4/6.0 vs. 6.0/4.6 mm Hg) (Elliott, 2009). However, there was no significant difference in the incidence of the primary outcome (death from cardiovascular causes, myocardial infarction, stroke or hospitalization for heart failure). The JNC-7 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) (Chobanian et al., 2003) and 2007 guidelines for the management of arterial hypertension from the European Society of Hypertension and ESC (Mancia et al., 2007), both of which were published before the ONTARGET study, did not have specific mentioning on the role of ACEI/ARB combination in the management of hypertension. However, the latest 2009 CHEP recommendations specifically stated that regarding treatment for adults with hypertension without compelling indications for specific agents, ‘the combination of ACEI and ARB is not recommended (grade A)’, and therapy using the combination of an ACEI and an ARB should only be considered in selected and closely monitored patients with advanced heart failure or proteinuric nephropathy (Campbell et al., 2009).
ACEI/ARB combo and AMI
The role of ACEI/ARB combination in patients with myocardial infarction was addressed in the VALIANT study (Pfeffer et al., 2003). A total of 14 703 patients with less than 10 day history of myocardial infarction and heart failure were randomized into three groups: captopril 50 mg daily (n = 4909), valsartan 160 mg twice daily (n = 4909) or combination of two drugs (n = 4885). After a median follow-up of 24.7 months, the mortality rate was 19.5% in the captopril group, 19.9% in the valsartan group and 19.3% in the combination group. The rate of the secondary end point of death from cardiovascular causes, recurrent myocardial infarction or hospitalizations for heart failure was also similar in the three groups. However, patients receiving combination therapy had a significantly higher rate of adverse effects, in particular hypotension and renal impairment. The incidence of hyperkalaemia was similar among the three groups. Combining valsartan with captopril in patients with myocardial infarction and heart failure increased the rate of adverse events without improving survival. The ONTARGET study population shared a high cardiovascular risk (Yusuf et al., 2008c). In the three treatment arms, about 75% had a clinicaly history of coronary artery disease and about 50% had previous myocardial infarction. Besides, in each of the three groups, more than 20% of patients had coronary artery bypass grafting and about 30% had previous percutaneous coronary intervention. All patients received similar medical therapies in terms of anti-platelets, statins and anti-hypertensives in the form of beta-blockers, diuretics or calcium channel blockers. In this high-risk population, the combination of ramipril and telmisartan did not reduce the risk of death from cardiovascular causes, myocardial infarction or stroke when compared with ramipril alone. All the current evidence does not support ACEI/ARB combination therapy in the setting of AMI. For patients with coronary artery disease, the CHEP recommendation suggested ‘the combination of an ACEI and ARB is not recommended in patients without co-existing systolic heart failure’ (Campbell et al., 2009).
ACEI/ARB combo and chronic systolic heart failure
Studies showed that ACEI/ARB combination therapy dampened neurohormonal and haemodynamic disturbance, reduced cardiac remodelling and improved left ventricular dysfunction in patients with chronic systolic heart failure (Baruch et al., 1999; McKelvie et al., 1999; Murdoch et al., 2001). It may also improve symptoms, exercise capacity and quality of life (Kum et al., 2008). In the Val-HeFT study, 5010 patients with heart failure of NYHA class II–IV and receiving standard therapy were randomly assigned to receive 160 mg of valsartan or placebo twice daily (Cohn et al., 2001). At the time of randomization, 93% of the patients were being treated with ACEIs. Overall mortality was similar in the two groups. The incidence of the combined end point of mortality and morbidity (defined as the incidence of cardiac arrest with resuscitation, hospitalizations for heart failure or receipt of intravenous inotropic or vasodilator therapy for at least 4 h) was 13.2% lower with valsartan than with placebo (P = 0.009). Treatment with valsartan also resulted in significant improvements in NYHA class, left ventricular ejection fraction and quality of life as compared with placebo. In the subgroup analysis, valsartan had an adverse effect on mortality and was associated with a trend towards an increase in the combined end point of mortality and morbidity among those who were receiving both ACEI and beta-blocker at base line. However, further analysis of subjects in Val-HeFT receiving ACEI, but not beta-blocker at baseline, showed that mortality was not affected by valsartan, but morbidity end points were significantly reduced (Krum et al., 2004). Quality of life was significantly improved; ejection fraction was significantly increased; left ventricular diameter was significantly reduced; and plasma B-type natriuretic peptide, norepinephrine and aldosterone levels were significantly reduced with valsartan compared to placebo. In the CHARM-Added study, 2548 patients with NYHA class II–IV heart failure and being treated with ACEI were randomized to candesartan or placebo. The primary outcome (composite of cardiovascular death or hospital admission for heart failure) was significantly lower in the candesartan group (38% vs. 42% in placebo group, HR 0.85, 95% CI 0.75–0.96, P = 0.011). The benefits of candesartan were similar in patients receiving baseline beta-blocker treatment (McMurray et al., 2003). Incorporating the positive findings of the Val-HeFT and CHARM-Added studies, the latest AHA/ACC guidelines suggest that addition of an ARB may be considered in persistently symptomatic patients with reduced LVEF who are already being treated with conventional therapy (Hunt et al., 2009). This was supported by the latest guidelines by the Canadian Cardiovascular Society (Howlett et al., 2009) and ESC (Dickstein et al., 2008). It should be noted that the Val-HeFT investigators have subsequently pointed out that in those patients on optimal or maximally tolerated doses of ACEI, there was no benefit of adding valsartan. A recent meta-analysis also suggested that overall combination therapy did not reduce mortality in patients with heart failure, although it may reduce hospitalizations for heart failure (Phillips et al., 2007). It also led to more adverse effects (especially hypotension and hyperkalaemia), and did not change overall hospitalization rates. ACEI/ARB combination therapy in heart failure should be individualized. Some specific patients, for instance, those with good renal function or younger patients might still benefit from this combination. A close monitoring of renal function and serum potassium level is mandatory.
ACEI/ARB combo and stroke
Majority of the evidence came from the ONTARGET study. Although subgroup analysis showed that telmisartan showed a trend towards reducing recurrent stroke versus ramipril (HR 0.91, 95% CI 0.79–1.05), ACEI/ARB combination therapy was not associated with any additional benefit in this group of high-risk patients (Yusuf et al., 2008c). The CHEP 2009 recommendations advised against ACEI/ARB combination for patients with stroke (Campbell et al., 2009).
ACEI/ARB combo and DN
Although several early studies suggested that ACEI/ARB combination provided additive benefit in DN, most of these studies were small in size and had a short duration of follow-up. In one meta-analysis which included 10 trials, 156 patients received ACEI/ARB combination therapy and 159 received ACEI only (Jennings et al., 2007). The duration of follow-up for most studies was between 8 and 12 weeks. ACEI/ARB combination was shown to reduce proteinuria, at the expense of statistically and clinically significant reduction in GFR and increase in serum creatinine. The authors suggested that this decrease could be secondary to the observed reductions in both systolic and diastolic blood pressure, which could have resulted in diminished renal perfusion. The duration of the included studies was relatively short, and hence such decrease in GFR could also have been a transient reduction. However, it was also stated that ‘a decrease of nearly 4 mL·min−1 in GFR after only 2–3 months of dual therapy is somewhat concerning and should be considered in assessing the risk/benefit of this treatment strategy’. Analysis of the renal outcome of the ONTARGET study showed that the primary renal outcome (composite of dialysis, doubling of serum creatinine and death) was similar for telmisartan (13.4%) and ramipril (13.5%), but was increased with combination therapy (14.5%, P = 0.037) (Mann et al., 2008). The secondary renal outcome (dialysis or doubling of serum creatinine) was also more frequent with combination therapy (HR 1.24, 95% CI 1.01–1.51, P = 0.038). Although combination therapy was associated with reduced albuminuria, it caused the greatest decline in the estimated GFR. These findings suggested that ACEI/ARB combination reduced proteinuria to a greater extent than monotherapy with ACEI, but overall it worsened major renal outcomes. The Combination Angiotensin Receptor Blocker and Angiotensin-converting Enzyme Inhibitor for Treatment of Diabetic Nephropathy (VA NEPHRON-D) study is an ongoing, randomized, double-blind, multicentre clinical trial to assess the effect of combination losartan and lisinopril, compared with losartan alone, on the progression of kidney disease in 1850 patients with diabetes and overt proteinuria. The result will certainly provide more information on this particular area (Fried et al., 2009). The KDOQI guideline in 2004 (Kidney Disease Outcomes Quality Initiative, 2004) suggested ‘ACEIs and ARBs can be used in combination to lower blood pressure or reduce proteinuria’, but this recommendation was based on early small studies. The CHEP 2009 recommendation advised against combination of an ACEI and ARB for patients with non-proteinuric chronic kidney disease or in patients with diabetes and normal urinary albumin levels (Campbell et al., 2009). Overall, although evidence from previous short-term studies indicates that combined therapy with ACEI/ARB reduced proteinuria, there was no evidence of a beneficial effect of ACEI/ARB on progression of DN, and combination therapy resulted in a clinically significant decrease in GFR in some studies (Dalla Vestra et al., 2009).
Safety profile of ACEI/ARB combination
Adverse effects observed with ACEI/ARB have been relatively mild. Most commonly reported were hypotension, dizziness, increased serum creatinine and hyperkalaemia. Careful monitoring of renal function and serum potassium level is necessary. In one meta-analysis which involved 17 337 patients, ACEI/ARB combination was associated with significantly higher rates of medication discontinuation because of adverse effects. There were also significant increases in worsening renal function, hyperkalaemia and symptomatic hypotension (Phillips et al., 2007). The ONTARGET study also showed a worse clinical outcome with combination therapy, but patients with heart failure were excluded. Given the potential benefit of ACEI/ARB combo in patients with systolic heart failure, such combination remains a reasonable option, provided a close monitoring of renal function and potassium level during the course of treatment. There has been no concensus in terms of how to monitor patients on ACEI/ARB combination therapy. The ESC 2008 guideline only mentioned how to use ACEI or ARB monotherapy in heart failure (Dickstein et al., 2008). In real clinical practice, many patients with advanced heart failure are of advanced age and have brittle haemodynamic status. Addition of ARB to pre-existing chronic ACEI therapy should be started at a low dose and titrated up slowly on an individual basis in order to prevent excessive hypotension. Baseline renal function should be obtained before adding ARB to ACEI and preferrably, renal function should be checked after 1 week of treatment. Early onset renal impairment (e.g. increase in serum creatinin > 30% of baseline) or hyperkalaemia (serum potassium > 5.5 mmol·L−1) should alert the clinician to seriously reconsider the risks and benefits of combination therapy. It seems reasonable to recheck renal function at 1 week after any up-titration of treatment. Preferrably, patients on maintenance therapy should have at least a monthly monitoring of renal function. The dosage of ACEI and ARB should also be individualized based on tolerability.
Other types of combined RAAS blockade
There was some evidence that combination therapy with the DRI aliskiren and an ACEI or ARB provided additional blood pressure reductions compared with monotherapy in patients with mild-to-moderate hypertension, and reduced surrogate markers of organ damage in patients with heart failure or DN (Düsing and Sellers, 2009). Such combination appeared to be safe and generally well tolerated. However, longer-term trials are required to establish whether more complete RAAS blockade with aliskiren-based therapy translates into improved clinical outcomes. Aldosterone blockade with spironolactone, eplerenone or canrenone was shown to improve all-cause mortality by 20% in patients with heart failure and post-MI (Ezekowitz and McAlister, 2009). The AHA/ACC guidelines suggested addition of AAs in selected patients with moderately severe to severe symptoms of heart failure (NYHA III or IV) and reduced LVEF (Hunt et al., 2009). The ESC guidelines recommended that low-dose AA should be considered for patients with LVEF less than 35% and severe symptoms of heart failure (NYHA III B or IV) (Dickstein et al., 2008). In patients with chronic kidney disease who are already on ACEI and ARB, addition of AAs may further reduce proteinuria (Navaneethan et al., 2009). However, such combination was associated with increased risk of hyperkalaemia. Most studies were small and had relatively short duration of follow-up. Therefore, long-term effects on renal outcomes, mortality and safety are still largely unknown.
Conclusion
There is ample evidence that RAAS plays an important role in the pathophysiology of many cardiovascular diseases. RAAS blockade by ACEI or ARB has greatly improved clinical outcomes in a wide range of patients. ACEI intolerance is common, majority of which being attributed to cough. ARB has evolved to become an effective alternative to ACEI and may even be used as first line treatment in selected cases. The aim of ACEI/ARB combination therapy is to overcome the phenomenon of ‘angiotensin escape’ and provides more complete blockade of the RAAS. However, despite a theoretical advantage, ‘ACEI/ARB combo’ has not been shown to provide additional benefits in most patients, with the exception of systolic heart failure and possibly overt proteinuria due to DN (Table 7). It should not be routinely prescribed and if indicated, a close monitoring of renal function and potassium level will be warranted. Further studies are warranted to look for the optimal strategy of RAAS blockade.
Table 7.
Expert guidelines regarding ACEI/ARB combination
| CHEP recommendations 2009 | Therapy using the combination of an ACE inhibitor and an ARB should only be considered in selected and closely monitored people with advanced heart failure or proteinuric nephropathy. |
| Canadian Cardiovascular Society Consensus 2009 | ARBs should be added to an ACE inhibitor for patients with persistent HF symptoms despite optimal treatment with other recommended drugs. |
| ACC/AHA guideline 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults | The addition of an ARB may be considered in persistently symptomatic patients with reduced LVEF who are already being treated with conventional therapy. |
| ESC Guidelines for Diagnosis and Treatment for Acute or Chronic Heart Failure 2008 | Unless contraindicated or not tolerated, ARB is recommended in patients with HF and LVEF < 40% who remain symptomatic despite optimal treatment with ACEI or beta blocker. |
| K/DOQI Clinical Practice Guidelines on Hypertension and Anti-hypertensive Agents in Chronic Kidney Disease 2004 | ACE inhibitors and ARBs can be used in combination to lower blood pressure or reduce proteinuria. |
Glossary
Abbreviations:
- AA
aldosterone antagonist
- ACE
angiotensin converting enzyme
- ACEI
angiotensin converting enzyme inhibitor
- AMI
acute myocardial infarction
- A-I
angiotensin I
- A-II
angiotensin II
- ARB
angiotensin receptor blocker
- CHF
congestive heart failure
- CI
confidence interval
- DM
diabetes mellitus
- DN
diabetic nephropathy
- DRI
direct renin inhibitor
- HR
hazard ratio
- LVEF
left ventricular ejection fraction
- PRA
plasma renin activity
- RAAS
renin–angiotensin–aldosterone system
- RAS
renin–angiotensin system
Conflict of interest
The paper has not been published and is not under consideration for publication elsewhere. All authors have read and approved the paper, and have no real or perceived conflicts of interests.
References
- ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. Circulation. 1998;97:2202–2212. doi: 10.1161/01.cir.97.22.2202. [DOI] [PubMed] [Google Scholar]
- Al-Mallah MH, Tleyjeh IM, Abdel-Latif AA, Weaver WD. Angiotensin-converting enzyme inhibitors in coronary artery disease and preserved left ventricular systolic function: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2006;47:1576–1583. doi: 10.1016/j.jacc.2005.11.073. [DOI] [PubMed] [Google Scholar]
- Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-term Evaluation (SMILE) study investigators. N Engl J Med. 1995;332:80–85. doi: 10.1056/NEJM199501123320203. [DOI] [PubMed] [Google Scholar]
- Bachmann S, Peters J, Engler E, Ganten D, Mullins J. Transgenic rats carrying the mouse renin gene – morphological characterization of a low-renin hypertension model. Kidney Int. 1992;41:24–36. doi: 10.1038/ki.1992.4. [DOI] [PubMed] [Google Scholar]
- Balcells E, Meng QC, Johnson WH, Jr, Oparil S, Dell'Italia LJ. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol. 1997;273:H1769–H1774. doi: 10.1152/ajpheart.1997.273.4.H1769. [DOI] [PubMed] [Google Scholar]
- Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- Baruch L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN. Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Vasodilator Heart Failure Trial (V-HeFT) study group. Circulation. 1999;99:2658–2664. doi: 10.1161/01.cir.99.20.2658. [DOI] [PubMed] [Google Scholar]
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- Benedict CR, Johnstone DE, Weiner DH, Bourassa MG, Bittner V, Kay R, et al. Relation of neurohumoral activation to clinical variables and degree of ventricular dysfunction: a report from the Registry of Studies of Left Ventricular Dysfunction. SOLVD investigators. J Am Coll Cardiol. 1994;23:1410–1420. doi: 10.1016/0735-1097(94)90385-9. [DOI] [PubMed] [Google Scholar]
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16:2170–2179. doi: 10.1681/ASN.2004090763. [DOI] [PubMed] [Google Scholar]
- Bertrand ME, Fox KM, Remme WJ, Ferrari R, Simoons ML. Angiotensin-converting enzyme inhibition with perindopril in patients with prior myocardial infarction and/or revascularization: a subgroup analysis of the EUROPA trial. Arch Cardiovasc Dis. 2009;102:89–96. doi: 10.1016/j.acvd.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Bosch J, Yusuf S, Pogue J, Sleight P, Lonn E, Rangoonwala B, et al. Heart outcomes prevention evaluation. Use of ramipril in preventing stroke: double blind randomised trial. BMJ. 2002;324:699–702. doi: 10.1136/bmj.324.7339.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Menendez E, Page IH. Suggested revision of nomenclature – angiotensin. Science. 1958;127:242. doi: 10.1126/science.127.3292.242-a. [DOI] [PubMed] [Google Scholar]
- Braun-Menendez E, Fasciolo JC, Leloir LF, Muñoz JM. The substance causing renal hypertension. J Physiol. 1940;98:283–298. doi: 10.1113/jphysiol.1940.sp003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, et al. PEACE Trial investigators. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- Buch P, Rasmussen S, Abildstrom SZ, Køber L, Carlsen J, Torp-Pedersen C, et al. The long-term impact of the angiotensin-converting enzyme inhibitor trandolapril on mortality and hospital admissions in patients with left ventricular dysfunction after a myocardial infarction: follow-up to 12 years. Eur Heart J. 2005;26:145–152. doi: 10.1093/eurheartj/ehi021. [DOI] [PubMed] [Google Scholar]
- Bühler FR, Bolli P, Kiowski W, Erne P, Hulthén UL, Block LH. Renin profiling to select antihypertensive baseline drugs. Renin inhibitors for high-renin and calcium entry blockers for low-renin patients. Am J Med. 1984;77:36–42. [PubMed] [Google Scholar]
- Bulpitt CJ, Beckett NS, Cooke J, Dumitrascu DL, Gil-Extremera B, Nachev C, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens. 2003;21:2409–2417. doi: 10.1097/00004872-200312000-00030. [DOI] [PubMed] [Google Scholar]
- Campbell NR, Khan NA, Hill MD, Tremblay G, Lebel M, Kaczorowski J, et al. 2009 Canadian Hypertension Education Program recommendations: the scientific summary – an annual update. Can J Cardiol. 2009;25:271–277. doi: 10.1016/s0828-282x(09)70490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. J Card Fail. 1999;5:178–187. doi: 10.1016/s1071-9164(99)90001-5. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Erhardt L, Murray G, Hall AS, Ball SG. Effect of ramipril on morbidity and mode of death among survivors of acute myocardial infarction with clinical evidence of heart failure. A report from the AIRE Study investigators. Eur Heart J. 1997;18:41–51. [PubMed] [Google Scholar]
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. Valsartan Heart Failure Trial Investigators. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- Corvol P, Michaud A, Soubrier F, Williams TA. Recent advances in knowledge of the structure and function of the angiotensin I converting enzyme. J Hypertens Suppl. 1995;13:S3–S10. doi: 10.1097/00004872-199509003-00002. [DOI] [PubMed] [Google Scholar]
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction in Hypertension Study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Dalla Vestra M, Simioni N, Masiero A. Renal effects of dual renin–angiotensin–aldosterone system blockade in patients with diabetic nephropathy. Int Urol Nephrol. 2009;41:119–126. doi: 10.1007/s11255-008-9490-0. [DOI] [PubMed] [Google Scholar]
- Davis JO. Mechanisms regulating the secretion and metabolism of aldosterone in experimental secondary hyperaldosteronism. Recent Prog Horm Res. 1959;17:293–352. [PubMed] [Google Scholar]
- Davis LK, Rodgers BD, Kelley KM. Angiotensin II- and glucose-stimulated extracellular matrix production: mediation by the insulin-like growth factor (IGF) axis in a murine mesangial cell line. Endocrine. 2008;33:32–39. doi: 10.1007/s12020-008-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschepper CF. Angiotensinogen: hormonal regulation and relative importance in the generation of angiotensin II. Kidney Int. 1994;46:1561–1563. doi: 10.1038/ki.1994.446. [DOI] [PubMed] [Google Scholar]
- Dickstein K, Kjekshus J, OPTIMAAL Steering Committee of the OPTIMAAL Study Group Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal trial in myocardial infarction with angiotensin II antagonist losartan. Lancet. 2002;360:752–760. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;19:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Reactive oxygen species and vascular cell apoptosis in response to angiotensin II and pro-atherosclerotic factors. Regul Pept. 2000;90:19–25. doi: 10.1016/s0167-0115(00)00105-1. [DOI] [PubMed] [Google Scholar]
- Dostal DE, Baker KM. The cardiac renin–angiotensin system: conceptual, or a regulator of cardiac function? Circ Res. 1999;85:643–650. doi: 10.1161/01.res.85.7.643. [DOI] [PubMed] [Google Scholar]
- Doulton TW, He FJ, MacGregor GA. Systematic review of combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade in hypertension. Hypertension. 2005;45:880–886. doi: 10.1161/01.HYP.0000161880.59963.da. [DOI] [PubMed] [Google Scholar]
- Düsing R, Sellers F. ACE inhibitors, angiotensin receptor blockers and direct renin inhibitors in combination: a review of their role after the ONTARGET trial. Curr Med Res Opin. 2009;25:2287–2301. doi: 10.1185/03007990903152045. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. Renal and circulatory mechanisms in congestive heart failure. Kidney Int. 1987;31:1402. doi: 10.1038/ki.1987.156. [DOI] [PubMed] [Google Scholar]
- Elliott HL. Focus on the ONTARGET results. J Hypertens. 2009;27:S8–S10. doi: 10.1097/01.hjh.0000354512.14086.2a. [DOI] [PubMed] [Google Scholar]
- Ennezat PV, Berlowitz M, Sonnenblick EH, Le Jemtel TH. Therapeutic implications of escape from angiotensin-converting enzyme inhibition in patients with chronic heart failure. Curr Cardiol Rep. 2000;2:258–262. doi: 10.1007/s11886-000-0077-3. [DOI] [PubMed] [Google Scholar]
- Erhardt L, MacLean A, Ilgenfritz J, Gelperin K, Blumenthal M. Fosinopril attenuates clinical deterioration and improves exercise tolerance in patients with heart failure. Fosinopril Efficacy/Safety Trial (FEST) study group. Eur Heart J. 1995;16:1892–1899. doi: 10.1093/oxfordjournals.eurheartj.a060844. [DOI] [PubMed] [Google Scholar]
- Ezekowitz JA, McAlister FA. Aldosterone blockade and left ventricular dysfunction: a systematic review of randomized clinical trials. Eur Heart J. 2009;30:469–477. doi: 10.1093/eurheartj/ehn543. [DOI] [PubMed] [Google Scholar]
- Flather MD, Yusuf S, Kober L, Pfeffer M, Hall A, Murray G, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355:1575–1581. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. The European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease Investigators. [DOI] [PubMed] [Google Scholar]
- Francis GS, Goldsmith SR, Levine TB, Olivari MT, Cohn JN. The neurohumoral axis in congestive heart failure. Ann Intern Med. 1984;101:370–377. doi: 10.7326/0003-4819-101-3-370. [DOI] [PubMed] [Google Scholar]
- Fried LF, Duckworth W, Zhang JH, O'Connor T, Brophy M, Emanuele N, et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D) Clin J Am Soc Nephrol. 2009;4:361–368. doi: 10.2215/CJN.03350708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, et al. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol. 2009;20:883–892. doi: 10.1681/ASN.2008070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin–angiotensin–aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab. 2005;16:120–126. doi: 10.1016/j.tem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Granger CB, Ertl G, Kuch J, Maggioni AP, McMurray J, Rouleau JL, et al. Randomized trial of candesartan cilexetil in the treatment of patients with congestive heart failure and a history of intolerance to angiotensin-converting enzyme inhibitors. Am Heart J. 2000;139:609–617. doi: 10.1016/s0002-8703(00)90037-1. [DOI] [PubMed] [Google Scholar]
- Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- Gross F. Renin und hypertensin, physiologische oder pathologische Wirkstoffe? Klin Wochenschr. 1958;36:693–706. doi: 10.1007/BF01493136. [DOI] [PubMed] [Google Scholar]
- Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. Effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. Six-month effects of early treatment with lisinopril and transdermal glyceryl trinitrate singly and together withdrawn six weeks after acute myocardial infarction: the GISSI-3 trial. J Am Coll Cardiol. 1996;27:337–344. [PubMed] [Google Scholar]
- Gueyffier F, Bulpitt C, Boissel JP, Schron E, Ekbom T, Fagard R, et al. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. INDANA group. Lancet. 1999;353:793–796. doi: 10.1016/s0140-6736(98)08127-6. [DOI] [PubMed] [Google Scholar]
- Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- Hall JE. Historical perspective of the renin–angiotensin system. Mol Biotechnol. 2003;24:27–39. doi: 10.1385/MB:24:1:27. [DOI] [PubMed] [Google Scholar]
- Hall AS, Murray GD, Ball SG. Follow-up study of patients randomly allocated ramipril or placebo for heart failure after acute myocardial infarction: AIRE Extension (AIREX) Study. Acute infarction ramipril efficacy. Lancet. 1997;349:1493–1497. doi: 10.1016/s0140-6736(97)04442-5. [DOI] [PubMed] [Google Scholar]
- Hansson L, Lindholm LH, Ekbom T, Dahlöf B, Lanke J, Scherstén B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 Study. Lancet. 1999a;354:1751–1756. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999b;353:611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- Hebert LA, Bain RP, Verme D, Cattran D, Whittier FC, Tolchin N, et al. Remission of nephrotic range proteinuria in type I diabetes. Collaborative Study Group. Kidney Int. 1994;46:1688–1693. doi: 10.1038/ki.1994.469. [DOI] [PubMed] [Google Scholar]
- Hedner T, Hansson L, Himmelmann A. The renin–angiotensin system – a century of progress. Blood Press. 1998;7:68–70. doi: 10.1080/080370598437411. [DOI] [PubMed] [Google Scholar]
- Hovind P, Tarnow L, Rossing P, Carstensen B, Parving HH. Improved survival in patients obtaining remission of nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2004;66:1180–1186. doi: 10.1111/j.1523-1755.2004.00870.x. [DOI] [PubMed] [Google Scholar]
- Howlett JG, McKelvie RS, Arnold JM, Costigan J, Dorian P, Ducharme A, et al. Canadian Cardiovascular Society Consensus Conference guidelines on heart failure, update 2009: diagnosis and management of right-sided heart failure, myocarditis, device therapy and recent important clinical trials. Can J Cardiol. 2009;25:85–105. doi: 10.1016/s0828-282x(09)70477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- Isnard R, Pousset F, Trochu J, Chafirovskaïa O, Carayon A, Golmard J, et al. Prognostic value of neurohormonal activation and cardiopulmonary exercise testing in patients with chronic heart failure. Am J Cardiol. 2000;86:417–421. doi: 10.1016/s0002-9149(00)00957-7. [DOI] [PubMed] [Google Scholar]
- Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- Jennings DL, Kalus JS, Coleman CI, Manierski C, Yee J. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486–493. doi: 10.1111/j.1464-5491.2007.02097.x. [DOI] [PubMed] [Google Scholar]
- Johnston CI. Franz Volhard Lecture. Renin–angiotensin system: a dual tissue and hormonal system for cardiovascular control. J Hypertens Suppl. 1992;10:S13–S26. [PubMed] [Google Scholar]
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohkubo H, Nakanishi S. Primary structure of human preangiotensinogen deduced from the cloned cDNA sequence. Biochemistry. 1984;23:3603–3609. doi: 10.1021/bi00311a006. [DOI] [PubMed] [Google Scholar]
- Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med. 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- Kasiske BL, Kalil RS, Ma JZ, Liao M, Keane WF. Effect of antihypertensive therapy on the kidney in patients with diabetes: a meta-regression analysis. Ann Intern Med. 1993;118:129–138. doi: 10.7326/0003-4819-118-2-199301150-00009. [DOI] [PubMed] [Google Scholar]
- Kawano H, Do YS, Kawano Y, Starnes V, Barr M, Law RE, et al. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation. 2000;101:1130–1137. doi: 10.1161/01.cir.101.10.1130. [DOI] [PubMed] [Google Scholar]
- Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–290. [PubMed] [Google Scholar]
- Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- Konstam MA, Neaton JD, Poole-Wilson PA, Pitt B, Segal R, Sharma D, et al. Comparison of losartan and captopril on heart failure-related outcomes and symptoms from the losartan heart failure survival study (ELITE II) Am Heart J. 2005;150:123–131. doi: 10.1016/j.ahj.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- Krum H, Carson P, Farsang C, Maggioni AP, Glazer RD, Aknay N, et al. Effect of valsartan added to background ACE inhibitor therapy in patients with heart failure: results from Val-HeFT. Eur J Heart Fail. 2004;6:937–945. doi: 10.1016/j.ejheart.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kum LC, Yip GW, Lee PW, Lam YY, Wu EB, Chan AK, et al. Comparison of angiotensin-converting enzyme inhibitor alone and in combination with irbesartan for the treatment of heart failure. Int J Cardiol. 2008;125:16–21. doi: 10.1016/j.ijcard.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Latini R, Tognoni G, Maggioni AP, Baigent C, Braunwald E, Chen ZM, et al. Clinical effects of early angiotensin-converting enzyme inhibitor treatment for acute myocardial infarction are similar in the presence and absence of aspirin: systematic overview of individual data from 96,712 randomized patients. Angiotensin-converting Enzyme Inhibitor Myocardial Infarction Collaborative Group. J Am Coll Cardiol. 2000;35:1801–1807. doi: 10.1016/s0735-1097(00)00638-0. [DOI] [PubMed] [Google Scholar]
- Lee VC, Rhew DC, Dylan M, Badamgarav E, Braunstein GD, Weingarten SR. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann Intern Med. 2004;141:693–704. doi: 10.7326/0003-4819-141-9-200411020-00011. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- Lijnen P, Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- Lonn EM, Yusuf S, Jha P, Montague TJ, Teo KK, Benedict CR, et al. Emerging role of angiotensin-converting enzyme inhibitors in cardiac and vascular protection. Circulation. 1994;90:2056–2069. doi: 10.1161/01.cir.90.4.2056. [DOI] [PubMed] [Google Scholar]
- McKelvie RS, Yusuf S, Pericak D, Avezum A, Burns RJ, Probstfield J, et al. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study investigators. Circulation. 1999;100:1056–1064. doi: 10.1161/01.cir.100.10.1056. [DOI] [PubMed] [Google Scholar]
- MacMahon S, Sharpe N, Gamble G, Clague A, Mhurchu CN, Clark T, et al. Randomized, placebo-controlled trial of the angiotensin-converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease. PART-2 Collaborative Research Group. Prevention of atherosclerosis with ramipril. J Am Coll Cardiol. 2000;36:438–443. doi: 10.1016/s0735-1097(00)00736-1. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- Matsumori A. Efficacy and safety of oral candesartan cilexetil in patients with congestive heart failure. Eur J Heart Fail. 2003;5:669–677. doi: 10.1016/s1388-9842(03)00162-4. Assessment of Response to Candesartan in Heart Failure in Japan (ARCH-J) Study Investigators. [DOI] [PubMed] [Google Scholar]
- Ménard J, Bouhnik J, Clauser E, Richoux JP, Corvol P. Biochemistry and regulation of angiotensinogen. Clin Exp Hypertens A. 1983;5:1005–1019. doi: 10.3109/10641968309048838. [DOI] [PubMed] [Google Scholar]
- Murdoch DR, McDonagh TA, Farmer R, Morton JJ, McMurray JJ, Dargie HJ. ADEPT: addition of the AT1 receptor antagonist eprosartan to ACE inhibitor therapy in chronic heart failure trial: hemodynamic and neurohormonal effects. Am Heart J. 2001;141:800–807. doi: 10.1067/mhj.2001.114802. [DOI] [PubMed] [Google Scholar]
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361:117–124. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009;8:CD007004. doi: 10.1002/14651858.CD007004.pub2. [DOI] [PubMed] [Google Scholar]
- Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet. 2000;356:1955–1964. doi: 10.1016/s0140-6736(00)03307-9. Blood Pressure Lowering Treatment Trialists' Collaboration. [DOI] [PubMed] [Google Scholar]
- Ng KK, Vane JR. Conversion of angiotensin I to angiotensin II. Nature. 1967;216:762–766. doi: 10.1038/216762a0. [DOI] [PubMed] [Google Scholar]
- Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–254. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. J Exp Med. 1940;71:29–42. doi: 10.1084/jem.71.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parving HH, Hommel E, Jensen BR, Hansen HP. Long-term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int. 2001a;60:228–234. doi: 10.1046/j.1523-1755.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001b;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin–angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Perondi R, Saino A, Tio RA, Pomidossi G, Gregorini L, Alessio P, et al. ACE inhibition attenuates sympathetic coronary vasoconstriction in patients with coronary artery disease. Circulation. 1992;85:2004–2013. doi: 10.1161/01.cir.85.6.2004. [DOI] [PubMed] [Google Scholar]
- Persson PB, Skalweit A, Thiele BJ. Controlling the release and production of renin. Acta Physiol Scand. 2004;181:375–381. doi: 10.1111/j.1365-201X.2004.01308.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Kagiyama S. Angiotensin II as a pro-inflammatory mediator. Curr Opin Investig Drugs. 2002;3:569–577. [PubMed] [Google Scholar]
- Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med. 2007;167:1930–1936. doi: 10.1001/archinte.167.18.1930. [DOI] [PubMed] [Google Scholar]
- Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE) Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial-the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355:1582–1587. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- Pitt B, O'Neill B, Feldman R, Ferrari R, Schwartz L, Mudra H, et al. QUIET Study Group. The QUinapril Ischemic Event Trial (QUIET): evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol. 2001;87:1058–1063. doi: 10.1016/s0002-9149(01)01461-8. [DOI] [PubMed] [Google Scholar]
- Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027–2037. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- Quinn SJ, Williams GH. Regulation of aldosterone secretion. Annu Rev Physiol. 1988;50:409–426. doi: 10.1146/annurev.ph.50.030188.002205. [DOI] [PubMed] [Google Scholar]
- Raman VK, Lee YA, Lindpaintner K. The cardiac renin–angiotensin–aldosterone system and hypertensive cardiac hypertrophy. Am J Cardiol. 1995;76:18D–23D. doi: 10.1016/s0002-9149(99)80487-1. [DOI] [PubMed] [Google Scholar]
- Ravid M, Savin H, Jutrin I, Bental T, Lang R, Lishner M. Long-term effect of ACE inhibition on development of nephropathy in diabetes mellitus type II. Kidney Int Suppl. 1994;45:S161–S164. [PubMed] [Google Scholar]
- Redgrave J, Rabinowe S, Hollenberg NK, Williams GH. Correction of abnormal renal blood flow response to angiotensin II by converting enzyme inhibition in essential hypertensives. J Clin Invest. 1985;75:1285–1290. doi: 10.1172/JCI111828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remes J. Neuroendocrine activation after myocardial infarction. Br Heart J. 1994;72:S65–S69. doi: 10.1136/hrt.72.3_suppl.s65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues EJ, Eisenberg MJ, Pilote L. Effects of early and late administration of angiotensin-converting enzyme inhibitors on mortality after myocardial infarction. Am J Med. 2003;115:473–479. doi: 10.1016/s0002-9343(03)00435-2. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sánchez-López E, Carvajal G, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21:16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- Rutherford JD, Pfeffer MA, Moye LA, Davis BR, Flaker GC, Kowey PR, et al. Effects of captopril on ischemic events after myocardial infarction. Results of the Survival and Ventricular Enlargement trial. SAVE investigators. Circulation. 1994;90:1731–1738. doi: 10.1161/01.cir.90.4.1731. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Kagiyama S, Phillips MI. The multiple actions of angiotensin II in atherosclerosis. Regul Pept. 2000;93:65–77. doi: 10.1016/s0167-0115(00)00178-6. [DOI] [PubMed] [Google Scholar]
- Schrader J, Lüders S, Kulschewski A, Berger J, Zidek W, Treib J, et al. The ACCESS study: evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke. 2003;7:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- Schrader J, Lüders S, Kulschewski A, Hammersen F, Plate K, Berger J, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;6:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25:971–1010. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- Schunkert H, Ingelfinger JR, Hirsch AT, Tang SS, Litwin SE, Talsness CE, et al. Evidence for tissue-specific activation of renal angiotensinogen mRNA expression in chronic stable experimental heart failure. J Clin Invest. 1992;90:1523–1529. doi: 10.1172/JCI116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp M, Clemenz M, Gineste R, Witt H, Janke J, Helleboid S, et al. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes. 2005;54:3442–3452. doi: 10.2337/diabetes.54.12.3442. [DOI] [PubMed] [Google Scholar]
- Singh R, Alavi N, Singh AK, Leehey DJ. Role of angiotensin II in glucose-induced inhibition of mesangial matrix degradation. Diabetes. 1999;48:2066–2073. doi: 10.2337/diabetes.48.10.2066. [DOI] [PubMed] [Google Scholar]
- Singh R, Singh AK, Alavi N, Leehey DJ. Mechanism of increased angiotensin II levels in glomerular mesangial cells cultured in high glucose. J Am Soc Nephrol. 2003;14:873–880. doi: 10.1097/01.asn.0000060804.40201.6e. [DOI] [PubMed] [Google Scholar]
- Skeggs LT, Jr, Lentz KE, Kahn JR, Shumway NP, Woods KR. The amino acid sequence of hypertensin. II. J Exp Med. 1956;104:193–197. doi: 10.1084/jem.104.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs LT, Jr, Kahn JR, Lentz K, Shumway NP. The existence of two forms of hypertensin. J Exp Med. 1957a;99:275–282. doi: 10.1084/jem.99.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs LT, Jr, Kahn JR, Lentz K, Shumway NP. The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med. 1957b;106:439–453. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs LT, Dorer FE, Kahn JR, Lentz KE, Levine M. The biochemistry of the renin–angiotensin system and its role in hypertension. Am J Med. 1976;60:737–748. doi: 10.1016/0002-9343(76)90888-3. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Held P, Kjekshus J, Rasmussen K, Ryden L, Wedel H. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II) N Engl J Med. 1992;327:678–684. doi: 10.1056/NEJM199209033271002. [DOI] [PubMed] [Google Scholar]
- The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet. 1993;342:821–828. [PubMed] [Google Scholar]
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- The Microalbuminuria Captopril Study Group. Captopril reduces the risk of nephropathy in IDDM patients with microalbuminuria. Diabetologia. 1996;39:587–593. doi: 10.1007/BF00403306. [DOI] [PubMed] [Google Scholar]
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- Tigerstedt R, Bergman P. Niere und Kreislauf. Skand Arch Physiol. 1898;8:223–271. [Google Scholar]
- Topouchian J, El Feghali R, Pannier B, Wang S, Zhao F, Smetana K, et al. Arterial stiffness and pharmacological interventions – the TRanscend arterial stiffNess Substudy (TRANS study) Vasc Health Risk Manag. 2007;3:381–387. [PMC free article] [PubMed] [Google Scholar]
- Torp-Pedersen C, Kober L. Effect of ACE inhibitor trandolapril on life expectancy of patients with reduced left-ventricular function after acute myocardial infarction. TRACE Study Group. Trandolapril cardiac evaluation. Lancet. 1995;354:9–12. doi: 10.1016/s0140-6736(98)09374-x. [DOI] [PubMed] [Google Scholar]
- Vaney C, Waeber B, Turini G, Margalith D, Brunner HR, Perret C. Renin and the complications of acute myocardial infarction. Chest. 1984;86:40–43. doi: 10.1378/chest.86.1.40. [DOI] [PubMed] [Google Scholar]
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA. 1994;271:275–279. [PubMed] [Google Scholar]
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin–angiotensin–aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- Weiss D, Sorescu D, Taylor WR. Angiotensin II and atherosclerosis. Am J Cardiol. 2001;87:25C–32C. doi: 10.1016/s0002-9149(01)01539-9. [DOI] [PubMed] [Google Scholar]
- Wilmer WA, Hebert LA, Lewis EJ, Rohde RD, Whittier F, Cattran D, et al. Remission of nephrotic syndrome in type 1 diabetes: long-term follow-up of patients in the Captopril Study. Am J Kidney Dis. 1999;34:308–314. doi: 10.1016/s0272-6386(99)70360-4. [DOI] [PubMed] [Google Scholar]
- Wilson HM, Haites NE, Booth NA. Effect of angiotensin II on plasminogen activator inhibitor-1 production by cultured human mesangial cells. Nephron. 1997;77:197–204. doi: 10.1159/000190273. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008a;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008b;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008c;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]


