Abstract
Background and purpose:
Resveratrol, a naturally occurring polyphenolic antioxidant, has been shown to exhibit chemoprophylactic effects on cancer development. Previously, we reported that 2,3′,4,4′,5′-pentamethoxy-trans-stilbene (PMS), a methoxylated resveratrol derivative, exerted a highly potent anti-proliferative effect on human colon cancer cells as compared with its parent compound. In the present study, the chemopreventive effect of PMS was evaluated in a mouse model of colitis-associated colon carcinogenesis.
Experimental approach:
Seven-week-old Balb/c mice were injected i.p. with 10 mg·kg−1 azoxymethane (AOM). After 1 week, 3% dextran sodium sulphate (DSS) was administered in the drinking water for 7 days followed by 14 days of tap water for recovery, and this cycle was repeated twice.
Key results:
Intragastric administration of PMS (25, 50 mg·kg−1 body weight) for 16 weeks significantly reduced the multiplicity of colonic neoplasms by 15% and 35% (P < 0.01) respectively. Moreover, PMS at 50 mg·kg−1 inhibited colon cancer cell proliferation and promoted apoptosis. Such changes were accompanied by reduction of Akt (protein kinase B) phosphorylation, inactivation of β-catenin and down-regulation of inducible nitric oxide synthase. In parallel, in vitro studies also demonstrated that PMS inhibited proliferation and induced apoptosis in the murine colon adenocarcinoma cell line Colon26 with concomitant inhibition of Akt phosphorylation and inactivation of β-catenin.
Conclusions and implications:
PMS effectively suppressed colon carcinogenesis in an AOM/DSS animal model and may merit further clinical investigation as a chemoprophylactic agent against colitis-associated colon cancer in humans.
Keywords: resveratrol derivatives, colitis, colon carcinogenesis, chemoprevention
Introduction
Colorectal cancer was the third most frequent non-cutaneous malignancy and also the third leading cause of cancer-related death in USA in 2008 (Jemal et al., 2008). Despite the recent improvements in preventive strategies, screening techniques and development of chemotherapy, the median overall survival period for patients with metastatic colorectal cancer is only 24 months. Moreover, the optimal regimen for early detection, for examples, by colonoscopy and occult blood test is controversial and patient compliance with screening recommendations remains a major hurdle to overcome. Fortunately, during colorectal carcinogenesis, the transition from normal mucosa to adenoma and final carcinoma is a protracted event that offers opportunities for preventive interventions. Actually, chemoprevention has been applied successful and appears to be a promising strategy for prevention of colorectal cancer (Jänne and Mayer, 2000; Regula et al., 2006).
Interest in the pharmacological effects of phytochemicals on cancer treatment and prevention has increased dramatically over the last two decades. During the course of compound identification, resveratrol (3,4′,5-trihydroxy-trans-stilbene) and its derivatives have emerged to hold promise for their potent in vitro and in vivo anticancer bioactivities (Jang et al., 1997; Wang et al., 1999; Kim et al., 2002; Robeti et al., 2003; Murias et al., 2004; 2005; Sale et al., 2005; Simoni et al., 2006; Park et al., 2007). Among resveratrol analogues studied so far, experimental evidence increasingly suggests that methoxylated stilbenes may be a novel class of cancer chemopreventive and therapeutic agents. Compared with resveratrol, most of methoxylated resveratrol derivatives exhibit much more potent cytotoxic and pro-apoptotic activity against cancer cells (Robeti et al., 2003; Gosslau et al., 2005; Park et al., 2007; Ma et al., 2008). In vivo studies also indicate that methoxylated stilbenes display superior pharmacokinetics properties in the gastrointestinal tract of mice and result in much higher bioavailability than resveratrol (Sale et al., 2004). In addition, several methoxystilbenes have been shown to prevent colon carcinogenesis (Sale et al., 2005). Structure–activity relationship analysis further reveals that 3,5-dimethoxy and 3,4,5-trimethoxy motifs are important to the pro-apoptotic activity while the 2-methoxyl group in the stilbene skeleton confers selectivity against cancer cells by targeting cytochrome P450 CYP1B1 that is usually involved in the metabolic activation of polycyclic aromatic hydrocarbons (Kim et al., 2002; Robeti et al., 2003; Simoni et al., 2006). In our previous study, 2,3′,4,4′,5′-pentamethoxy-trans-stilbene (PMS) was found to be a potent apoptosis-inducing agent against human colon cancer cells via targeting microtubules and suppressed tumour growth in a colon cancer xenograft model (Li et al., 2009). In addition to its chemotherapeutic action, we presumed that PMS might exert chemoprophylactic effect on colon carcinogenesis. To this end, the present study was designed to investigate the chemopreventive potential of PMS in a murine model of colitis-associated colon carcinogenesis as well as its underlying mechanism of action.
Methods
Animals and diets
All animal care and experimental procedures were approved by the Committee for Use of Live Animals for the Teaching and Research of The Chinese University of Hong Kong. Male Balb/c mice (5-week-old) were fed a standard laboratory diet (Ralston Purina Co., Chicago, IL, USA) and kept in an air-conditioned room with controlled temperature (22 ± 1°C), humidity (65–70%) and day/night cycle (12 h light, 12 h dark).
Experimental procedure
To evaluate the chemopreventive effect of PMS, an azoxymethane (AOM)/dextran sodium sulphate (DSS)-induced colitis-associated colon carcinogenesis model as shown in Figure 1A was adopted (Jia et al., 2008). At week 2, 7-week-old Balb/c mice were injected with AOM (10 mg·kg−1, i.p.). After 1 week, 3% DSS (molecular weight: 36 000–50 000, International Lab, Chicago, IL, USA) was administered in the drinking water for 7 days followed by 14 days of tap water for recovery, and this cycle was repeated twice. PMS (25 or 50 mg·kg−1) or the vehicle [5%, v/v, dimethyl sulphoxide (DMSO) in olive oil] was administered using an intragastric tube at 0.1 mL per 10 g every other day throughout the experiment. The mice were killed by cervical dislocation at week 16 and the colons (from the ileocecal junction to the anal verge) were removed. After measurement of the length and weight, the colons were cut open longitudinally along the main axis, washed with phosphate-buffered saline (pH 7.4) and then macroscopically inspected. Number, size and location of presumed pre-neoplastic and neoplastic lesions (dysplasia and carcinoma) in the colons were documented based on gross examination. All identified lesions with an elevated growth pattern in the whole colon were initially classified as positive for dysplasia, and their sizes were determined by measuring the largest diameter using an ocular micrometer. After gross examination, the colons were cut into pieces at about 1 cm intervals. Half of the pieces of colon was fixed in 10% buffered formalin (pH 7.4) for 24 h for further histopathological assessment and immunohistochemical study; while the other half was kept in liquid nitrogen for determination of intestinal glutathione (GSH) and prostaglandin E2 (PGE2) level and for Western blot analysis.
Figure 1.
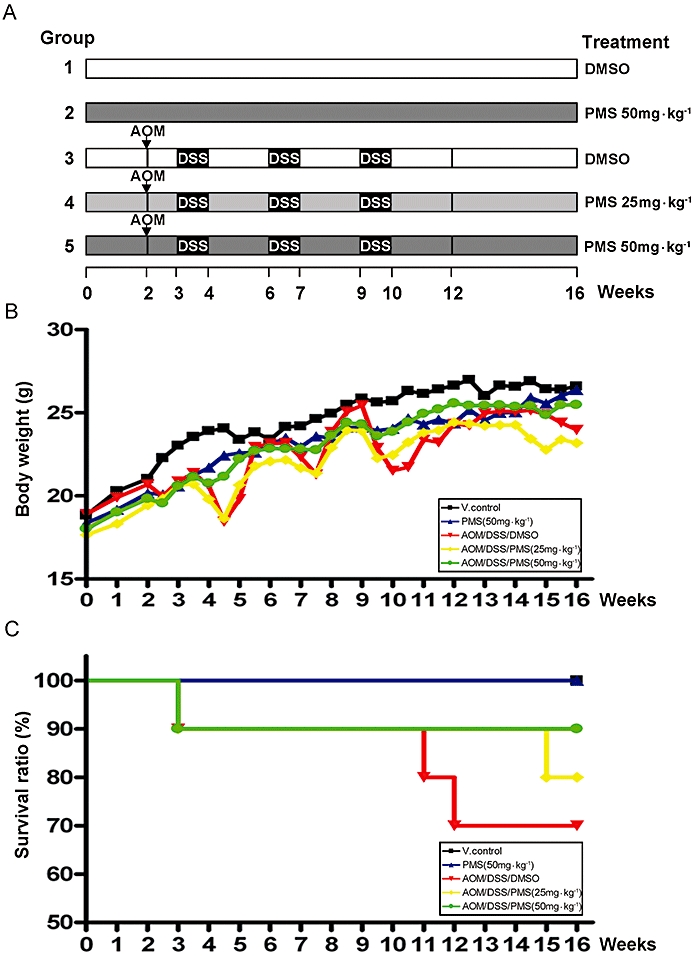
Chemopreventive activity of 2,3′,4,4′,5′-pentamethoxy-trans-stilbene (PMS) on colitis-associated colon carcinogenesis was evaluated in Balb/c mice. (A) Experimental protocol for colitis-associated colon carcinogenesis model. (B) Effect of PMS on body weight of mice. (C) Effect of PMS on survival ratio of mice. In group 3–5, 7-week-old Balb/c mice received a single i.p. injection [10 mg·kg−1 body weight for azoxymethane (AOM)], followed by three cycles exposure of dextran sodium sulphate (DSS) (3%, w/w, in drinking water) to induce colitis-associated colon carcinogenesis. PMS dissolved in 5% (v/v) dimethyl sulphoxide (DMSO)/olive oil was given to the mice in groups 2, 4 and 5 by gavage every other day for total 16 weeks. Animals in groups 1 and 3 received 5% (v/v) DMSO/olive oil. The daily drinking water intake was about 5 mL per mouse and there was no obvious change during DSS treatment. At the start of the experiment, there were ten mice in each group except group 2 (n = 6), but some of them died during the experiment especially when challenged with the third cycle of DSS treatment. Data are presented as mean (n = 6–10 mice per group). V. control, vehicle control.
Histopathological assessment of colonic mucosa
For histopathological examination, haematoxylin and eosin (H&E) staining was performed on formalin fixed, paraffin-embedded colon tissue by a routine procedure. Based on H&E staining, histological alterations such as mucosal ulceration, dysplasia and carcinoma were verified by a board-certified pathologist, unaware of the treatments, according to the criteria previously reported. Carcinoma was defined as a high-grade dysplasia of colonic mucosa that had invaded beyond the muscularis mucosa and into the submucosa.
Determination of colonocyte proliferation
Cell proliferation in the colonic mucosa was determined by staining for the proliferating cell nuclear antigen (PCNA). Formalin-fixed colon tissues were deparaffinized and rehydrated. Following incubation with 0.3% (v/v) H2O2-methanol, the sections were subjected to trypsin digestion for another 30 min and then blocked by the addition of the normal serum in 0.05 M Tris-HCl buffer (pH 7.4) for 1 h. They were then incubated overnight with anti-PCNA mouse monoclonal antibody (1:50) at 4°C. On the following day a labelled streptavidin-biotin DAKO kit (DAKO, Glostrup, Denmark) and 3,3′-diaminobenzidine were used to visualize the PCNA-positive proliferating cells in the tissue. Mayer's haematoxylin was used to counterstain the sections. The positively stained mucosal cells were counted in six to eight randomized fields (200×) with a light microscope (Olympus, Melville, NY, USA), and the average number of cells per 20 crypts was taken.
Measurement of colonocyte apoptosis
Apoptotic cells were measured using a TdT-mediated dUTP nick-end labelling (TUNEL) stain with the Dead-End kit (Promega, Madison, WI, USA) as recommended by the manufacturer. Briefly, after the colon tissue was fixed, deparaffinized and rehydrated, proteinase K (Boehringer Mannheim, Mannheim, Germany) was added to digest the cells. TdT buffer solution (140 mM sodium cacodylate, 1 mg·mL−1 BSA, 1 mM cobalt chloride in 30 mM Tris-HCl) containing 50 U per 20 µL TdT (ICN Biomedicals, Costa Mesa, CA, USA) and 50 nmol per 50 µL dUTP (Boehringer Mannheim) was added to the sections and incubated at 37°C for 90 min. The sections were then immersed in the buffer containing 300 mM sodium chloride and 30 mM sodium citrate. Apoptotic cells were labelled by the addition of peroxidase-conjugated streptavidin followed by 3,3′-diaminobenzidine. The apoptotic mucosal cells were counted in six to eight randomized fields (200×) with a light microscope, and the average was taken and expressed as the number of apoptotic cells per 20 crypts.
Determination of intestinal glutathione level
Colorimetric measurement was used to determine the intracellular GSH. Briefly, Colon tissues were homogenized in the enzyme immunoassay buffer [50 mM Tris-HCl (pH 7.5), 150 mM sodium chloride, 0.5% α-cholic acid, 0.1% SDS, 2 mM EDTA, 1% Triton X-100 and 10% glycerol] for 30 s on ice. Then the samples were centrifuged at 10 000×g at 4°C for 15 min. The resulting supernatant was separated into two parts for protein quantification by the Bradford assay and for GSH determination respectively. To determine GSH levels, trichloroacetic acid (5% w/v) was added to the supernatant for protein precipitation. The resulting mixture was centrifuged at 3000×g at 4°C for 10 min, and the supernatant was incubated with 0.01 M 5,5′-dithiobis-2-nitrobenzoic acid (DNTB) and 0.2 M phosphate buffer (pH 8.0) at room temperature for 15 min to develop yellow colour. Finally, the optical density was measured by spectrophotometer at 412 nm. GSH concentrations were calculated from standard curves and then normalized to total protein.
Detection of intestinal PGE2 level
Intestinal PGE2 levels were measured using a Correlated-EIA Prostaglandin E2 Enzyme Immunoassay Kit from Assay Designs (Ann Arbor, MI, USA) as recommended by the manufacturer. Briefly, colon tissues were homogenized in the enzyme immunoassay buffer for 30 s on ice. Then the samples were centrifuged for 15 min at 10 000×g at 4°C. The obtained supernatant was separated into two parts for protein quantification by the Bradford assay and PGE2 determination using a PGE2 immunoassay kit respectively. PGE2 levels were calculated from standard curves and then normalized to total protein.
Analysis of oncogeneic protein expression in colonic tissue by Western blot
Briefly, colon tissues were homogenized in radioimmunoprecipitation assay buffer [50 mM Tris-HCl (pH 7.5), 150 mM sodium chloride, 0.5% α-cholic acid, 0.1% SDS, 2 mM EDTA, 1% Triton X-100 and 10% glycerol] containing 1.0 mM phenylmethylsulfonyl fluoride and 1 µg·mL−1 aprotinin. After sonication for 30 s on ice and centrifuging at 12 000×g at 4°C for 20 min, the supernatant was collected and total protein concentration was determined by a standard Bradford assay reagent (Bio-Rad) using bovine serum albumin as standard. Twenty micrograms of protein samples were resolved on SDS-PAGE and transferred to Hybond C nitrocellulose membranes (Amersham Corporation, Arlington Heights, IL, USA). The membranes were probed with primary antibodies (1:1000) that dissolved in wash buffer containing 5% non-fat milk powder overnight at 4°C and incubated for 1 h with secondary antibodies conjugated with peroxidase (1:2000). Chemiluminescent signals were then developed with Lumiglo reagent (Cell Signaling Technology) and detected and quantified by the ChemiDoc XRS gel documentation system (Bio-rad, Hercules, CA, USA).
Cell culture and Western blot analysis
The murine colon adenocarcinoma cell line Colon26, which was established by giving N-nitroso-5-methyl-1,3-oxazolidine (NMO) to female BALB/c mice was obtained from the Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer, Tohoku University, Japan. It was maintained in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (Invitrogen), 100 U·mL−1 penicillin G, 100 µg·mL−1 streptomycin and maintained at 37°C, 95% humidity and 5% carbon dioxide. Briefly, 1.5 × 105 Colon26 cells per well in 2 mL medium were seeded onto six-well plates and incubated for 24 h for attachment, then treated with either PMS (10, 30 µM) or DMSO (0.1%, v/v) for 48 h. After treatment, the cells were collected and their protein was extracted for Western blot analysis.
Statistical analysis
Statistical analysis was done by using the Prism statistical package. Tukey's t-test was used to compare data between two groups. One-way anova and the Bonferroni correction were used to compare data between three and more groups. Values were expressed as means ± SEM. P < 0.05 was considered statistically significant.
Materials
2,3′,4,4′,5′-Pentamethoxy-trans-stilbene was chemically synthesized by the classic Wittig Reaction as described previously (Li et al., 2009). Antibodies to cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) and the PCNA were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All other primary antibodies were purchased from Cell Signaling Technology (Beverley, MA, USA). All other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA) unless otherwise specified.
Results
Effects of PMS and AOM/DSS on the general well-being of mice
2,3′,4,4′,5′-Pentamethoxy-trans-stilbene was well tolerated in mice, and no obvious systemic toxicity was observed during the entire period of drug treatment as indicated by the body weight, general appearance and organ histology. In agreement with previous studies (Clapper et al., 2008), body weight loss and bloody stools were observed in mice acutely exposed to DSS (Figure 1B). However, these symptoms were relieved when they received tap water during the recovery periods. Compared with the control mice, there was a significant increase in mean colon weight and shortening in mean colon length in mice receiving both AOM and DSS (Table 1). Such significant increase in colon weight to colon length ratio was a result of apparent mucosal thickening. It should be noted that such body weight fluctuation and mucosal thickening were substantially alleviated in mice receiving the higher dose of PMS (50 mg·kg−1). Based on Kaplan-Meier survival curves (Figure 1C), PMS treatment seemed also to increase the survival of mice.
Table 1.
Body weight and colon assessment in mice
| Group | Treatment (no. mice examined) | Body weight (g) |
Colon |
Neoplasms in colon |
|||
|---|---|---|---|---|---|---|---|
| Weight (g) | Length (cm) | W/L ratio | Incidence | Multiplicity | |||
| 1 | V. control (n = 10) | 26.46 ± 1.86 | 0.33 ± 0.05 | 10.24 ± 1.41 | 0.032 ± 0.004 | 0 | 0 |
| 2 | PMS 50 mg·kg−1 (n = 6) | 26.37 ± 2.02 | 0.31 ± 0.07 | 10.61 ± 1.27 | 0.029 ± 0.007 | 0 | 0 |
| 3 | AOM/DSS/DMSO (n = 7) | 23.97 ± 3.49 | 0.75 ± 0.22c | 8.41 ± 0.54a | 0.090 ± 0.026c | 100% | 20.86 ± 5.64c |
| 4 | AOM/DSS/PMS 25 mg·kg−1 (n = 8) | 23.18 ± 4.09a | 0.74 ± 0.23c | 8.63 ± 0.81a | 0.083 ± 0.024c | 100% | 17.75 ± 3.81c |
| 5 | AOM/DSS/PMS 50 mg·kg−1 (n = 9) | 25.48 ± 1.47 | 0.54 ± 0.15 | 8.73 ± 0.86a | 0.061 ± 0.016Ab | 100% | 13.56 ± 4.16Bc |
At the start of the experiment, there were ten mice in each group except group 2 (n = 6), and some of them died during treatment.
Data are shown as the mean ± SD.
P < 0.05 versus AOM/DSS/DMSO-treated group.
P < 0.01 versus AOM/DSS/DMSO-treated group.
P < 0.05 versus V. control group (vehicle control).
P < 0.01 versus V. control group.
P < 0.001 versus V. control group.
AOM, azoxymethane; DMSO, dimethyl sulphoxide; DSS, dextran sodium sulphate; PMS, 2,3′,4,4′,5′-pentamethoxy-trans-stilbene; W/L ratio (g·cm−1), colon weight per colon length.
PMS reduced the incidence and multiplicity of colonic neoplasms
Mucosal thickening in mice receiving both AOM and DSS as mentioned above seemed to be due to the burden of colonic neoplasms. Three cycles of DSS treatment in combination with AOM injection resulted in 100% incidence of colonic neoplasms, which were most frequently observed in the middle and distal colon (Figure 2A). In this study, we found that the administration of PMS (25 or 50 mg·kg−1) for 16 weeks decreased the total multiplicity of colorectal neoplasms by 15% and 35% (P < 0.01) respectively (Table 1). More noteworthy was that PMS at the higher dose (50 mg·kg−1) significantly retarded the development of large neoplasms (diameter >3 mm) by 51% (Figure 2B). Histologically, most of the lesions in the colon were consistent with tubular adenoma or adenocarcinoma after H&E staining (Figure 2C).
Figure 2.
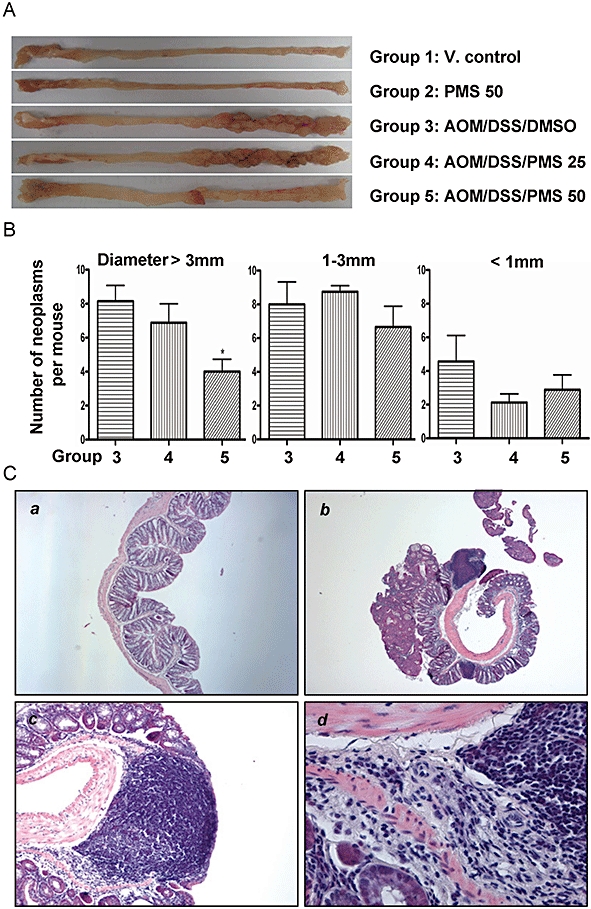
Effect of 2,3′,4,4′,5′-pentamethoxy-trans-stilbene (PMS) on the burden of colonic neoplasms. (A) Macroscopic view of colon in mice. Three cycles of dextran sodium sulphate (DSS) treatment in combination with azoxymethane (AOM) resulted in 100% incidence of colonic neoplasms that were most frequently observed in the middle and distal colon. In control group, the incidence of colonic neoplasm was zero. (B) Effect of PMS on colonic neoplasms size. PMS administration (50 mg·kg−1) for 16 weeks significantly retarded the development of the large neoplasms (diameter >3 mm). After treatment, the colons were cut open longitudinally and washed with saline. Then images were captured against a scaled ruler for tumour size measurement. Data are presented as mean ± SEM. *P < 0.05 versus AOM/DSS/dimethyl sulphoxide (DMSO)-treated group. (C) Most of colorectal neoplasms were histologically consistent with tubular adenoma or adenocarcinoma. The incidence of colonic adenocarcinoma was 100% with multiplicity of 9.15 ± 2.35 per mouse in the AOM/DSS/DMSO group. Although PMS administration (25 or 50 mg·kg−1) for 16 weeks failed to reduce the incidence of colonic adenocarcinoma, it did decrease its multiplicity by 25% and 60% respectively. Histological studies were carried out based on haematoxylin and eosin (H&E) staining as described. (Ca) Normal colon; (Cb, Cc and Cd) colon in mice receiving both AOM and three cycles of DSS. Original magnification: Ca, 40×; Cb, 40×; Cc, 100×; Cd, 400×. V. control, vehicle control.
PMS did not affect oxidative stress in the colonic tissue
Elevated oxidative stress has been implicated in the development of chronic inflammatory bowel diseases (IBD), and the available evidence suggests that oxidative cellular damage may act as a potential force during colitis-associated colon carcinogenesis. It was reported that the process of colitis-associated colon carcinogenesis was greatly alleviated by consumption of the antioxidants and aggravated by iron supplementation (Seril et al., 2003). Accordingly, we measured the intestinal GSH levels, which is a well-known indicator of oxidative stress, although it may also reflect the binding of electrophilic agents (Haridas et al., 2004). As showed in Figure 3A, chronic colitis induced by DSS markedly depleted the GSH levels in colonic tissue, which was indicative of the presence of oxidative stress. However, PMS did not significantly alter the GSH levels in mice with colitis, suggesting that antioxidation might not be the principal mechanism of the chemopreventive action of PMS.
Figure 3.
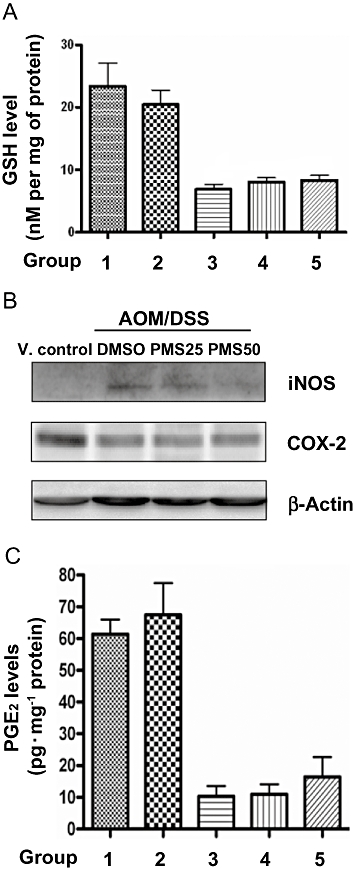
Effects of PMS on oxidative stress and inflammatory mediators in the colonic tissue. (A) PMS treatment does not affect the levels of oxidative stress markers GSH. Colonic levels of GSH were measured as described. Data are presented as mean ± SEM (n = 6–10 mice per group). (B) Effects of PMS treatment on iNOS and COX-2 protein expression. Colon tissues (10 mg per mouse) were randomly selected from the middle and distal colon. The tissues in same group (6–10 mice per group) were pooled for Western blot analysis. (C) PMS treatment did not affect the levels of inflammatory mediator PGE2. Colonic levels of PGE2 were measured as described. Data are presented as mean ± SEM. AOM, azoxymethane; COX-2, cyclooxygenase-2; DMSO, dimethyl sulphoxide; DSS, dextran sodium sulphate; GSH, reduced glutathione; iNOS, inducible nitric oxide synthase; PGE2, prostaglandin E2; PMS, 2,3′,4,4′,5′-pentamethoxy-trans-stilbene; V. control, vehicle control.
PMS reduced iNOS expression
It is now widely accepted that chronic inflammation substantially contributes to colitis-associated colon carcinogenesis. We therefore questioned whether the chemopreventive activity of PMS was mediated via its anti-inflammatory properties. The expression of iNOS is a reliable indicator of mucosal inflammation. In this regard, we found that AOM/DSS induced a dramatic up-regulation of iNOS in colonic tissues, which was reversed by the administration of PMS at either 25 or 50 mg·kg−1. Nevertheless, our data about either intestinal PGE2 level or COX-2 expression clearly indicated that there is no significant difference among the three groups receiving DSS treatment (Figure 3B and C), a finding that excluded the possibility of COX-2 targeting by PMS treatment. Surprisingly, unlike previous reports about animal models of colon cancer (Sinicrope and Gill, 2004), the down-regulation of COX-2 expression as well as PGE2 level was found in all mice suffering chronic colitis in this study. It is possible that chronic ulcerative colitis severely damages mouse colon colonic mucosa where COX-2 is located and PGE2 produced. In this connection, the down-regulation of PGE2 was also reported in a trinitrobenzenesulphonic acid (TNBS)-induced colitis mouse model (Martín et al., 2006).
PMS reduced proliferating cells and induced apoptosis in colonic tissues
Irreparable genetic lesions, out-of-control colonocyte proliferation and evasion of apoptosis represent common events at the cellular level during colon carcinogenesis (Hanahan and Weinberg, 2000; Feagins et al., 2009). Accordingly, cellular proliferation and apoptosis in colorectal mucosal crypts was evaluated using PCNA staining and TUNEL assay respectively (Figure 4). We noticed that although there was a marked increase in the number of PCNA-labelled cells in the mucosa of mice receiving both AOM and DSS, such increases in PCNA-positive cells were substantially reduced after PMS treatment. For example, compared with the AOM/DSS/DMSO group, PMS (25 or 50 mg·kg−1) significantly reduced colonocyte proliferation by 24% (P < 0.05) and 55% (P < 0.001) respectively. Meanwhile, we determined the number of apoptotic cells in colorectal mucosal crypts by TUNEL assay. Compared with the control mice, apoptosis was also increased in the colonic tissues of mice receiving both AOM and DSS, which might be a consequence of increased cell turnover. However, PMS treatment further increased the number of apoptotic colonic cells, as evidenced by the result of TUNEL assay as well as the cleavage of poly(ADP-ribose) polymerase (PARP) (Figures 4B and 5A). Compared with the AOM/DSS/DMSO group, PMS (25 or 50 mg·kg−1) significantly increased apoptotic cell death by 76% (P < 0.001) or 85% (P < 0.001) respectively.
Figure 4.
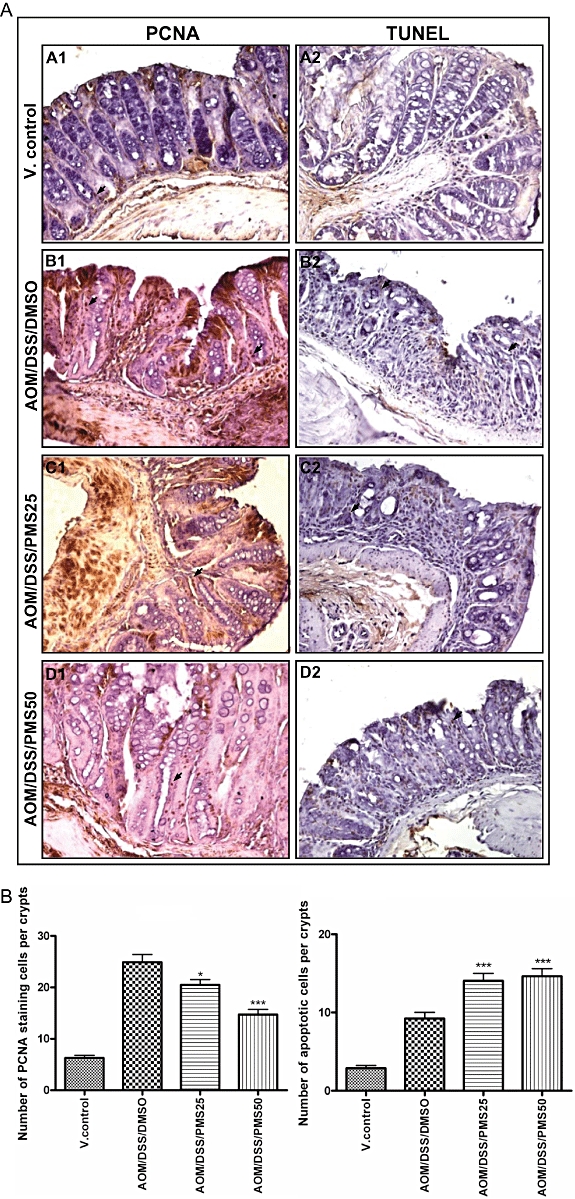
Effects of PMS on colonocyte proliferation and apoptosis. Colonic cellular proliferation and apoptosis was evaluated using PCNA staining and TUNEL assay respectively. (A) Representative section of colon samples from the normal control group (A1 and A2), colitis control group (B1 and B2) and PMS-treated groups (C1, C2, D1 and D2). Original magnification is 200× except D1 (400×). (B) Colonocyte proliferation and apoptosis index. Compared with AOM/DSS/DMSO group, PMS (25 or 50 mg·kg−1) significantly reduced colonocyte proliferation by 24% (P < 0.05) and 55% (P < 0.001); on the other hand, PMS increased apoptotic cell death by 76% (P < 0.001) and 85% (P < 0.001). Colon mucosa was stained and cell counting was performed as described. The positively stained mucosal cells were counted in six to eight randomized fields (200×) with the aid of a light microscope (Olympus, Melville, NY, USA), and the average number of cells per 20 crypts was taken. Data are presented as mean ± SEM (n = 6–10 mice per group). *P < 0.05, ***P < 0.001 versus AOM/DSS/DMSO group. AOM, azoxymethane; DMSO, dimethyl sulphoxide; DSS, dextran sodium sulphate; PCNA, proliferating cell nuclear antigen; PMS, 2,3′,4,4′,5′-pentamethoxy-trans-stilbene; TUNEL assay, TdT-mediated dUTP nick-end labelling assay; V. control, vehicle control.
Figure 5.
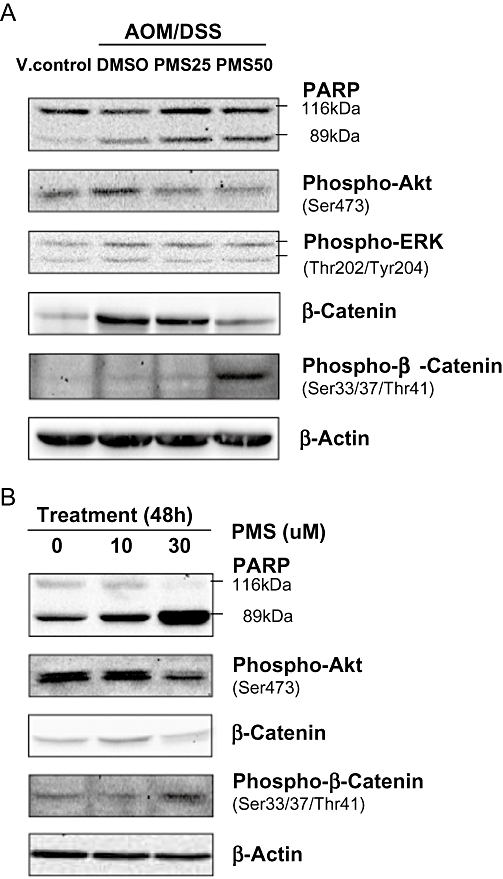
Effects of PMS on oncogenic protein expression. (A) In vivo study. The colonic tissues (10 mg per mouse) were randomly selected from the middle and distal colon. The tissues in same group (6–10 mice per group) were pooled for Western blot analysis. (B) In vitro study.The murine colon adenocarcinoma cell line Colon26 was selected for in vitro study. After PMS treatment for 48 h, Colon26 cells were lysed, and protein was extracted for Western blot analysis. Akt, protein kinase B; AOM, azoxymethane; DMSO, dimethyl sulphoxide; DSS, dextran sodium sulphate; PARP, poly(ADP-ribose) polymerase; PMS, 2,3′,4,4′,5′-pentamethoxy-trans-stilbene; V. control, vehicle control.
PMS inhibited Akt (protein kinase B) phosphorylation and promoted the inactivation of β-catenin
To elucidate the mechanism of action underlying the chemopreventive action of PMS, we examined the expression of several oncogenic proteins, which were reported to play key roles in colitis-associated colon carcinogenesis (Figure 5A). In line with findings by others, the pro-survival signalling phosphatidylinositol 3-kinase (PI3K)/Akt pathway was markedly up-regulated during colon carcinogenesis (Tanaka et al., 2003; Bertagnolli and Hamilton, 2005). It was manifested as an up-regulation of Akt phosphorylation of Ser473. Activation of the PI3K/Akt pathway has been linked to the stabilization of β-catenin, a crucial transcription factor and biomarker in the early stage of colorectal tumorigenesis. In normal tissue, the phosphorylation of β-catenin at Ser33, Ser37 and Thr41 destabilized this protein and triggered the subsequent elimination. Here we demonstrated that the suppression of Akt phosphorylation by PMS was accompanied by an increased level of phosphorylated β-catenin at Ser33, Ser37 and Thr41, suggesting that inhibition of the Akt/β-catenin axis might be responsible for the chemoprophylactic effects of PMS.
PMS inhibited Akt phosphorylation and triggered apoptosis in a murine colon adenocarcinoma cell line
To better understand the mechanism of action involved in the chemopreventive activity of PMS, we examined the effect of PMS on Colon26, a murine colon adenocarcinoma cell line that was established by treating female BALB/c mice with NMO. Similar to the results of in vivo experiments, PMS potently inhibited cell proliferation (IC50 = 29.2 ± 2.6 µM) and induced apoptosis in Colon26 as shown by the cleavage of PARP. Likewise, PMS treatment substantially down-regulated Akt phosphorylation at Ser473 and increased the level of destabilized β-catenin (Figure 5B).
Discussion
A large body of evidence has indicated that the chronic IBD, which include ulcerative colitis and Crohn's disease, increase the risk for colon cancer. Such association between IBDs and increased colorectal cancer risk has been established in colitis-associated colon carcinogenesis animal models. In this preclinical study, three cycles of DSS in combination with a single injection of AOM resulted in 100% incidence of colonic neoplasms in mice. We noticed that the induction of chronic ulcerative colitis was accompanied by the increase in oxidative stress. Both flat neoplastic lesions and polyps were also found in mice with chronic colitis. Of note, those flat neoplastic lesions seemed to be much more malignant than polypoid neoplasia as invasive cancers arose predominantly from flat lesions (Figure 2C). All of these clinicopathological features are similar to those of ulcerative colitis in humans, suggesting that the AOM/DSS model is a reliable preclinical system mimicking ulcerative colitis in human (Clapper et al., 2007; Feagins et al., 2009).
For colon cancer chemoprevention, the burden of colonic neoplasms including dysplasia and carcinoma has been used as a validated surrogate end point either in animal models or clinical settings. In this study, we also found that PMS treatment significantly reduced the mean number of neoplasms in mice exposed to AOM and DSS treatment. Based on the data presented here, we believe that PMS, a methoxylated resveratrol derivative, may be a novel compound with clinical potentials for colitis-associated colon cancer chemoprevention. Actually, some stilbene compounds (such as resveratrol and pterostilbene) have been ranked as the most promising colon cancer chemopreventive agents, and several phase I clinical trials are currently underway (Baur and Sinclair, 2006). These studies will provide a foundation for future chemoprevention trials with this agent.
Colorectal carcinogenesis is a multistep process during which epithelial cells in colon undergo the malignant transformation. At the initial stage, activation of proto-oncogenes and inactivation of tumour suppressor genes followed by increased cell proliferation or evasion of apoptosis represent a common pathway at the cellular level; while at the middle or late stage, the capability of sustained angiogenesis, tissue invasion and metastasis were required (Hanahan and Weinberg, 2000). To this end, the most promising cancer chemopreventive agents currently under investigation are likely to be ones that target the early factors and/or events leading to initial colon lesions (Bertagnolli and Hamilton, 2005). In this connection, reduction in staining for PCNA and increase of TUNEL-labelled cells were found in PMS-treated mice. And further investigation demonstrated that PMS inhibited Akt phosphorylation and induced apoptotic cell death both in vivo and in vitro. All of present findings are in line with our previous study in which PMS induced apoptosis in human colon cancer cells (HT-29 and Caco-2) with concomitant down-regulation of the pro-survival PI3K/Akt signalling. In addition, we show for the first time that PMS enhances the destabilization of β-catenin, a transcriptional factor downstream to the PI3K/Akt pathway and involved in colon carcinogenesis.
As to the effect of PMS on inflammatory mediators in colon tissues, we found that PMS inhibited the protein expression of iNOS that is markedly elevated in most colonic adenomas of human as well as mouse. This finding was in agreement with a previous chemoprevention study using pterostilbene (3,5-dimethoxy-4′-hydroxy-trans-stilbene), another methoxylated resveratrol derivative (Suh et al., 2007). Although the precise functions of iNOS in colon cancer is still unknown, growing experimental evidence suggests that iNOS may accelerate the process of colitis-associated colon carcinogenesis via nitric oxide synthesis. In addition, it is well known that nitric oxide production could act as one of the reactive nitrogen species that have been implicated in the chronic inflammation-related colon cancer (McKenzie et al., 1996).
Our present findings are in line with previous studies on the structure–activity relationship of stilbenes. Chemically, PMS belongs to a class of chemical known as stilbenes that exhibit variable antioxidant, anti-inflammatory and apoptosis-inducing properties mainly based on the substituents on the stilbene skeleton. In general, the number and position of the hydroxyl groups on the benzene rings determine their radical-scavenging activity, while methoxyl groups are mainly related to their apoptosis-inducing activity. It may partially explain why PMS did not affect the intestinal GSH level in mice with chronic colitis.
Although the findings of our present studies are promising, there are several issues that need to be addressed. For examples, for oxidative stress measurement, the GSSG/GSH ratio is a much better biomarker than GSH alone. More rigorous experiments should be conducted to determine whether the chemopreventive activity of PMS is mediated by attenuating the pro-inflammatory effect of DSS although the down-regulation of iNOS expression was observed. In this connection, a study of acute colitis animal models induced by DSS alone or earlier time points in this study should be included to determine the effect of PMS on myeloperoxidase (MPO) activity, malondialdehyde (MDA) levels and the expression of pro-inflammatory cytokines [e.g. interleukin (IL)-1, IL-6, and tumour necrosis factor-α] (Martín et al., 2006). Considering most methoxylated stilbenes exerted their anticancer activity after metabolic activation (Sale et al., 2004), data about the pharmacokinetics properties of PMS in vivo are greatly in need not only for elucidation of its mechanism of action but also for further rational design in drug development. Another issue need to be addressed is that the findings in this study may be only potentially applicable to colitis-associated colon carcinogenesis as the pathogenic mechanism and genetic changes are quite different from those of sporadic colorectal cancer development (Feagins et al., 2009).
In summary, in this study we demonstrated for the first time that PMS, a methoxylated derivative of resveratrol, effectively suppressed colitis-related colon carcinogenesis in an AOM/DSS animal model. Our data also clearly indicated that the observed chemopreventive effect of PMS on colon carcinogenesis may, at least in part, be explained by suppression of cell proliferation, promotion of apoptosis as well as inactivation of β-catenin and down-regulation of iNOS. The present work together with our previous studies has shed light on the potential application of PMS in colon cancer chemoprevention.
Acknowledgments
This study was supported by HKU seed fund for basic research. HL is supported by a postgraduate studentship from The University of Hong Kong.
Glossary
Abbreviations:
- Akt
protein kinase B
- AOM
azoxymethane
- COX-2
cyclooxygenase-2
- DMSO
dimethyl sulphoxide
- DNTB
5,5′-dithiobis-2-nitrobenzoic acid
- DSS
dextran sodium sulphate
- GSH
reduced glutathione
- IBD
inflammatory bowel disease
- IL-1
interleukin-1
- IL-6
interleukin-6
- iNOS
inducible nitric oxide synthase
- MDA
malondialdehyde
- MPO
myeloperoxidase
- NMO
N-nitroso-5-methyl-1,3-oxazolidine
- PARP
poly(ADP-ribose) polymerase
- PCNA
proliferating cell nuclear antigen
- PGE2
prostaglandin E2
- PI3K
phosphatidylinositol 3-kinase
- TNBS
trinitrobenzenesulphonic acid
- TUNEL assay
TdT-mediated dUTP nick-end labelling assay
Statement of conflicts of interest
The authors state no conflict of interest.
References
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM, Hamilton SR. Chemoprevention of colorectal cancer. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention: Strategies for Cancer Chemoprevention. Totowa, NJ: Humana Press; 2005. pp. 267–286. [Google Scholar]
- Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- Clapper ML, Gary MA, Coudry RA, Litwin S, Chang WCL, Devarajan K, et al. 5-Aminosalicylic acid inhibits colitis-associated colorectal dysplasias in the mouse model of azoxymethane/dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2008;14:1341–1347. doi: 10.1002/ibd.20489. [DOI] [PubMed] [Google Scholar]
- Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nature. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- Gosslau A, Chen M, Ho CT, Chen KY. A methoxy derivative of resveratrol analogue selectively induced activation of mitochondrial apoptotic pathway in transformed fibroblasts. Br J Cancer. 2005;92:513–521. doi: 10.1038/sj.bjc.6602300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Haridas V, Hanausek M, Nishimura G, Soehnge H, Gaikwad A, Narog M, et al. Triterpenoid electrophiles (avicins) activate the innate stress response by redox regulation of a gene battery. J Clin Invest. 2004;113:65–73. doi: 10.1172/JCI18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jänne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:1960–1968. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, et al. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ko H, Park JE, Jung S, Lee SK, Chun YJ. Design, synthesis, and discovery of novel trans-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. J Med Chem. 2002;45:160–164. doi: 10.1021/jm010298j. [DOI] [PubMed] [Google Scholar]
- Li H, Wu WK, Zheng Z, Che CT, Yu L, Li ZJ, et al. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, is a potent inducer of apoptosis in colon cancer cells via targeting microtubules. Biochem Pharmacol. 2009;78:1224–1232. doi: 10.1016/j.bcp.2009.06.109. [DOI] [PubMed] [Google Scholar]
- Ma Z, Molavi O, Haddadi A, Lai R, Gossage RA, Lavasanifar A. Resveratrol analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) mediates anti-tumor effects via mechanism different from that of resveratrol. Cancer Chemother Pharmacol. 2008;63:27–35. doi: 10.1007/s00280-008-0704-z. [DOI] [PubMed] [Google Scholar]
- McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín AR, Villegas I, Sánchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol. 2006;147:873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Handler N, Erker T, Pleban K, Ecker G, Saiko P, et al. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: synthesis and structure-activity relationship. Bioorg Med Chem. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Murias M, Jager W, Handler N, Erker T, Horvath Z, Szekeres T, et al. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure-activity relationship. Biochem Pharmacol. 2005;69:903–912. doi: 10.1016/j.bcp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Park H, Aiyar SE, Fan P, Wang J, Yue W, Okouneva T, et al. Effects of tetramethoxystilbene on hormone-resistant breast cancer cells: biological and biochemical mechanisms of action. Cancer Res. 2007;67:5717–5726. doi: 10.1158/0008-5472.CAN-07-0056. [DOI] [PubMed] [Google Scholar]
- Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- Robeti M, Pizziranti D, Simoni D, Rondanin R, Baruchello R, Bonora C, et al. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J Med Chem. 2003;46:3546–3554. doi: 10.1021/jm030785u. [DOI] [PubMed] [Google Scholar]
- Sale S, Verschoyle RD, Boocock D, Jones DJL, Wilsher N, Rupareliak C, et al. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br J Cancer. 2004;90:736–744. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale S, Tunstall RG, Potter GA, Steward WP, Gescher AJ. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene(DMU212) on adenoma development in the ApcMin+ mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- Simoni D, Romagnoli R, Baruchello R, Rondanin R, Rizzi M, Pavani MG, et al. Novel combretastatin analogues endowed with antitumor activity. J Med Chem. 2006;49:3143–3152. doi: 10.1021/jm0510732. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metast Rev. 2004;23:63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- Suh N, Paul S, Hao X, Simi B, Xiao H, Rimando AM, et al. Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin Cancer Res. 2007;13:350–355. doi: 10.1158/1078-0432.CCR-06-1528. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Jin Y, Ho CT. Evaluation of resveratrol derivatives as potential antioxidants and identification of a reaction product of resveratrol and 2,2-dipphenyl-1-picryhydrazyl radical. J Agric Food Chem. 1999;47:3974–3977. doi: 10.1021/jf990382w. [DOI] [PubMed] [Google Scholar]


