Abstract
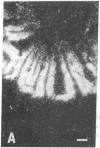
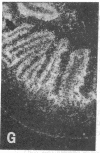
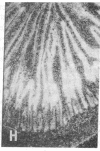
The effect of insulinopenic diabetes on the expression of glucose transporters in the small intestine was investigated. Enterocytes were sequentially isolated from jejunum and ileum of normal fed rats, streptozotocin-diabetic rats, and diabetic rats treated with insulin. Facilitative glucose transporter (GLUT) 2, GLUT5, and sodium-dependent glucose transporter 1 protein content was increased from 1.5- to 6-fold in enterocytes isolated from diabetic animals in both jejunum and ileum. Insulin was able to reverse the increase in transporter protein expression seen after induction of diabetes. There was a four- to eightfold increase in the amount of enterocyte glucose transporter mRNA after diabetes with greater changes in sodium-dependent glucose transporter 1 and GLUT2 than in GLUT5 levels. In situ hybridization showed that after the induction of diabetes there was new hybridization in lower villus and crypt enterocytes that was reversed by insulin treatment. Thus, the increase in total hexose transport caused by diabetes is due to a premature expression of hexose transporters by enterocytes along the crypt-villus axis, causing a cumulative increase in enterocyte transporter protein during maturation. These changes are likely to represent an adaptive response by the organism to increase nutrient absorption in a perceived state of tissue starvation. These adaptive changes may lead to exacerbation of hyperglycemia in uncontrolled diabetes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burant C. F., Bell G. I. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992 Oct 27;31(42):10414–10420. doi: 10.1021/bi00157a032. [DOI] [PubMed] [Google Scholar]
- Burcelin R., Eddouks M., Kande J., Assan R., Girard J. Evidence that GLUT-2 mRNA and protein concentrations are decreased by hyperinsulinaemia and increased by hyperglycaemia in liver of diabetic rats. Biochem J. 1992 Dec 1;288(Pt 2):675–679. doi: 10.1042/bj2880675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. I., Maenz D. D. Rapid regulation of D-glucose transport in basolateral membrane of rat jejunum. Am J Physiol. 1989 May;256(5 Pt 1):G878–G883. doi: 10.1152/ajpgi.1989.256.5.G878. [DOI] [PubMed] [Google Scholar]
- Colville C. A., Seatter M. J., Jess T. J., Gould G. W., Thomas H. M. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993 Mar 15;290(Pt 3):701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Chowrimootoo G. Streptozotocin diabetes and sugar transport by rat ileal enterocytes: evidence for adaptation caused by an increased luminal nutrient load. Biochim Biophys Acta. 1992 Jun 11;1107(1):86–92. doi: 10.1016/0005-2736(92)90332-g. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Ebrahim H. Y., Swaine D. J. Diabetes mellitus and sugar transport across the brush-border and basolateral membranes of rat jejunal enterocytes. J Physiol. 1990 May;424:13–25. doi: 10.1113/jphysiol.1990.sp018052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorak R. N., Chang E. B., Madara J. L., Field M. Intestinal adaptation to diabetes. Altered Na-dependent nutrient absorption in streptozocin-treated chronically diabetic rats. J Clin Invest. 1987 Jun;79(6):1571–1578. doi: 10.1172/JCI112991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Hoffman L. R., Chang E. B. Altered regulation of regional sucrase-isomaltase expression in diabetic rat intestine. Am J Physiol. 1992 Jun;262(6 Pt 1):G983–G989. doi: 10.1152/ajpgi.1992.262.6.G983. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Rossetti L., Lodish H. F., Charron M. J. Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. J Clin Invest. 1991 Jun;87(6):2197–2206. doi: 10.1172/JCI115254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T., Burant C. F., Fukumoto H., Gould G. W., Fan Y. S., Eddy R. L., Byers M. G., Shows T. B., Seino S., Bell G. I. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990 Aug 5;265(22):13276–13282. [PubMed] [Google Scholar]
- Miller D. L., Schedl H. P. Effects of experimental diabetes on intestinal strontium absorption in the rat. Proc Soc Exp Biol Med. 1976 Sep;152(4):589–592. doi: 10.3181/00379727-152-39446. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hase K., Taketani Y., Minami H., Oka T., Nakabou Y., Hagihira H. Diabetes and glucose transporter gene expression in rat small intestine. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1110–1117. doi: 10.1016/0006-291x(91)92053-m. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Akanuma Y., Takaku F. Increased liver glucose-transporter protein and mRNA in streptozocin-induced diabetic rats. Diabetes. 1990 Apr;39(4):441–446. doi: 10.2337/diab.39.4.441. [DOI] [PubMed] [Google Scholar]
- Philpott D. J., Butzner J. D., Meddings J. B. Regulation of intestinal glucose transport. Can J Physiol Pharmacol. 1992 Sep;70(9):1201–1207. doi: 10.1139/y92-167. [DOI] [PubMed] [Google Scholar]
- Schedl H. P., Wilson H. D. Effects of diabetes on intestinal growth in the rat. J Exp Zool. 1971 Apr;176(4):487–495. doi: 10.1002/jez.1401760410. [DOI] [PubMed] [Google Scholar]
- Solberg D. H., Diamond J. M. Comparison of different dietary sugars as inducers of intestinal sugar transporters. Am J Physiol. 1987 Apr;252(4 Pt 1):G574–G584. doi: 10.1152/ajpgi.1987.252.4.G574. [DOI] [PubMed] [Google Scholar]
- Thorens B., Cheng Z. Q., Brown D., Lodish H. F. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- Thorens B., Flier J. S., Lodish H. F., Kahn B. B. Differential regulation of two glucose transporters in rat liver by fasting and refeeding and by diabetes and insulin treatment. Diabetes. 1990 Jun;39(6):712–719. doi: 10.2337/diab.39.6.712. [DOI] [PubMed] [Google Scholar]
- Toloza E. M., Diamond J. Ontogenetic development of nutrient transporters in rat intestine. Am J Physiol. 1992 Nov;263(5 Pt 1):G593–G604. doi: 10.1152/ajpgi.1992.263.5.G593. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Wright E. M., Turk E., Zabel B., Mundlos S., Dyer J. Molecular genetics of intestinal glucose transport. J Clin Invest. 1991 Nov;88(5):1435–1440. doi: 10.1172/JCI115451. [DOI] [PMC free article] [PubMed] [Google Scholar]