Abstract
Background and purpose:
Transient receptor potential ankyrin 1 (TRPA1) channels are expressed by primary afferent neurones and activated by irritant chemicals including allyl isothiocyanate (AITC). Here we investigated whether intracolonic AITC causes afferent input to the spinal cord and whether this response is modified by mild colitis, morphine or a TRPA1 channel blocker.
Experimental approach:
One hour after intracolonic administration of AITC to female mice, afferent signalling was visualized by expression of c-Fos in laminae I–IIo of the spinal dorsal horn at sacral segment S1. Mild colitis was induced by dextran sulphate sodium (DSS) added to drinking water for 1 week.
Key results:
Relative to vehicle, AITC (2%) increased expression of c-Fos in the spinal cord. Following induction of mild colitis by DSS (2%), spinal c-Fos responses to AITC, but not vehicle, were augmented by 41%. Colonic inflammation was present (increased myeloperoxidase content and disease activity score), whereas colonic histology, locomotion, feeding and drinking remained unchanged. Morphine (10 mg·kg−1) or the TRPA1 channel blocker HC-030031 (300 mg·kg−1) inhibited the spinal c-Fos response to AITC, in control and DSS-pretreated animals, whereas the response to intracolonic capsaicin (5%) was blocked by morphine but not HC-030031.
Conclusions and implications:
Activation of colonic TRPA1 channels is signalled to the spinal cord. Mild colitis enhanced this afferent input that, as it is sensitive to morphine, is most likely of a chemonociceptive nature. As several irritant chemicals can be present in chyme, TRPA1 channels may mediate several gastrointestinal pain conditions.
Keywords: allyl isothiocyanate, capsaicin, TRPA1, TRPV1, c-Fos expression, colitis, chemonociception, chemical hyperresponsiveness, abdominal pain, morphine, HC-030031
Introduction
Visceral hypersensitivity to distension is a key feature of functional gastrointestinal disorders such as irritable bowel syndrome (IBS), which is characterized by pain and altered bowel habits in the absence of overt structural changes (Azpiroz et al., 2007; Knowles and Aziz, 2009). However, emerging evidence indicates that IBS is associated with low grade inflammation (Bercik et al., 2005; Spiller, 2007; De Giorgio and Barbara, 2008) and that the concomitant hyperalgesia is induced by inflammatory mediators (Barbara et al., 2007). Although the actual cause of abdominal pain in IBS is not known, we hypothesize that potentially noxious chemicals present in the chyme contribute to the symptoms of IBS. In a translational context it is therefore important to study the mechanisms and pharmacological characteristics of chemonociception in the gut.
In order to survey their chemical environment, primary afferent neurones express many receptors and ion channels that transduce potentially noxious stimuli into afferent nerve activity (Blackshaw et al., 2007). One of these multimodal sensors is the transient receptor potential ankyrin 1 (TRPA1) ion channel, which is preferentially expressed by C-fibre afferents and frequently colocalized with the TRP vanilloid 1 (TRPV1) ion channel (Zhang et al., 2004; Kobayashi et al., 2005; Anand et al., 2008; Brierley et al., 2009; Wrigley et al., 2009; Cattaruzza et al., 2010; channel nomenclature follows Alexander et al., 2009). TRPA1 is activated by several electrophilic compounds such as allyl isothiocyanate (AITC), which are responsible for the pungency of mustard, horseradish, wasabi, garlic and onion as well as several irritant chemicals (Jordt et al., 2004; Bautista et al., 2005; Andrèet al., 2009). Its wide spectrum of chemosensory modalities places TRPA1 in a position to monitor potentially deleterious conditions arising from the gastrointestinal presence of ammonia, oxidative insults and environmental toxins such as p-hydroxybenzoate (Bautista et al., 2006; Fujita et al., 2007, 2008; Trevisani et al., 2007, Macpherson et al., 2007a; 2007b;). Apart from direct activation by these stimuli, TRPA1 contributes to the sensitization of afferent neurones by inflammatory mediators such as bradykinin and protease-activated receptor agonists (Eid et al., 2008; Wang et al., 2008; Brierley et al., 2009; Yu et al., 2009; Yu and Ouyang, 2009; Cattaruzza et al., 2010).
The current work pursued four specific questions. Firstly, we examined whether activation of TRPA1 in the mouse colon by AITC caused afferent signalling to the spinal cord as visualized by expression of c-Fos, a marker of neuronal excitation (Munglani and Hunt, 1995). Secondly, we tested whether the AITC-induced input to the spinal cord was enhanced by mild inflammation of the mouse colon in the absence of overt structural damage. In this, we attempted to reproduce the situation of low-grade inflammation seen in IBS and to strictly avoid the artificial condition of necrotic inflammation, which in rats has been found to enhance behavioural pain responses to colorectal distension and intracolonic AITC (Yang et al., 2008). Thirdly, we asked whether the AITC-evoked afferent signalling from the colon can be inhibited by an analgesic drug such as morphine and thus is likely to reflect chemonociception. Fourthly, we explored whether the spinal c-Fos response to intracolonic AITC was inhibited by the TRPA1 blocker HC-030031 (McNamara et al., 2007; Eid et al., 2008; Taylor-Clark et al., 2008), thereby proving that the spinal input from the AITC-exposed colon is indeed mediated by TRPA1. In this way we also wanted to differentiate the spinal input caused by AITC from that caused by capsaicin-induced stimulation of TRPV1, given that TRPA1 and TRPV1 are frequently colocalized in the same afferent neurones (Zhang et al., 2004; Kobayashi et al., 2005).
Methods
Experimental animals
All animal care and experimental procedures were approved by an ethical committee at the Federal Ministry of Science and Research of the Republic of Austria and conducted according to the Directive of the European Communities Council of 24 November 1986 (86/609/EEC) and the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. The experiments were designed in such a way that the number of animals used and their suffering was minimized, which according to the recommendations of the ethical committee precluded the recording of full dose–response curves for the drugs and agents under study. The study was carried out with adult female mice of the outbred strain Him : OF1 (Division of Laboratory Animal Science and Genetics, Department of Biomedical Research, Medical University of Vienna, Himberg, Austria). The animals were housed in groups of three to five per cage under controlled temperature (set point 21°C), relative air humidity (set point 50%) and light conditions (lights on at 6:00 h, lights off at 18:00 h, maximal intensity 150 lux). One week before the start of the experiments the animals were housed individually, and at the time of the experiments they weighed 25–35 g. Food and water were available ad libitum throughout the study.
Induction of experimental colitis
Colitis was induced by adding dextran sulphate sodium (DSS; 1 or 2%, molecular weight: 36 000–50 000; MP Biochemicals, Illkirch, France) to the drinking water (tap water) for 7 days (Reber et al., 2006; Eijkelkamp et al., 2007). The control animals received normal tap water. The DSS-containing drinking water was made up fresh every day to avoid bacterial contamination. At the end of the 7 day pretreatment period, the clinical status of the animals was evaluated by recording a disease activity score (DAS).
Experimental protocols
Three different experimental protocols were used, depending on the major outcome of the experiment.
In protocol 1, the major outcome was the expression of c-Fos in the spinal cord 1 h after intracolonic administration of AITC (1 or 2%), capsaicin (5%) or their vehicle (peanut oil, 0.05 mL). One hour after intracolonic treatment the mice were killed by i.p. injection of an overdose of pentobarbital (600 mg·kg−1). The whole colon was quickly removed, its length measured, and the distal part of the colon processed for determination of its myeloperoxidase (MPO) content or for histological examination. Immediately afterwards the mice were transcardially perfused with fixative and the spinal cords removed and processed for immunocytochemistry. The experiments were carried out with control animals and animals that had been pretreated with DSS (1 or 2%), added to the drinking water, for 7 days.
Protocol 2 was used to assess the effect of DSS on the circadian pattern of locomotion, exploration, drinking and feeding in the LabMaster system (TSE Systems, Bad Homburg, Germany). These parameters were measured continuously for 14 days, with a 5 min break after 1 week for recording the weight of the animals. During the first week all animals received normal tap water, whereas in the second week DSS (2%) was added to the drinking water.
In protocol 3 the effect of intracolonic administration of AITC (2%) or its vehicle (peanut oil, 0.05 mL) on the circulating levels of corticosterone were examined. The basal levels of corticosterone were measured 2 days before the intracolonic treatment. To this end, blood (0.02–0.05 mL) was collected by incision of the tail base (Fluttert et al., 2000). The post-stimulus values of corticosterone were determined 20 min after intracolonic administration of AITC. For this purpose, the animals were deeply anaesthetized with pentobarbital (600 mg·kg−1, i.p.), the thoracic cavity was opened quickly, and heart blood was collected within 2 min following pentobarbital injection. The experiments were carried out with control animals and animals that had been pretreated with DSS (2%), added to the drinking water for 7 days.
Drug treatments
Peanut oil, AITC and capsaicin were administered into the colon in a volume of 0.05 mL. For this procedure, a feeding cannula with a rounded tip (length 38.1 mm, gauge 20) was used, the cannula being advanced 3.5 cm beyond the anus (Laird et al., 2001). The choice of the AITC concentrations used here (1–2%) was modelled according to the reports of Laird et al. (2001) and Lu and Westlund (2001), whereas the choice of the capsaicin concentration (5%) utilized in the present study was in between the concentrations tested by Laird et al. (2001) and Christoph et al. (2006). Peanut oil was chosen as vehicle because this compound had been reported to minimally affect the spinal expression of c-Fos (Lu and Westlund, 2001). Morphine was administered subcutaneously at a dose of 10 mg·kg−1 (Laird et al., 2001; Dogrul and Seyrek, 2006) and an injection volume of 2 mL·kg−1 1 h prior to the intracolonic administration of AITC or capsaicin. HC-030031 was given i.p. at 300 mg·kg−1 (McNamara et al., 2007) and an injection volume of 9 mL·kg−1, 30 min before the intracolonic administration of AITC or capsaicin.
c-Fos immunocytochemistry
Following death, the mice were transcardially perfused with 0.1 M phosphate-buffered saline (PBS) of pH 7.24 (20 mL), followed by buffered paraformaldehyde, 4%, Sigma-Aldrich, Vienna, Austria) of pH 7.24 (30 mL). The spinal cords were removed and postfixed overnight in 4% paraformaldehyde at 4°C. Then the tissues were cryoprotected for 24 h in sucrose (20%) at 4°C, divided into a thoracolumbar and a lumbosacral part, surrounded with the cryogel Tissue-TecR O.C.T.™ compound (Sakura Finetec Europe BV, Zoeterwoude, the Netherlands) and frozen in 2-methylbutane (Carl Roth GmbH, Karlsruhe, Germany) on dry ice and stored at –70°C until use. Serial coronal sections of 40 µm thickness were cut from the spinal cord with a cryostat. Only every third section of the lumbosacral spinal cord at segments L6 to S2 and every sixth section of the thoracolumbar part at segments T11 to L1 was used. Immunocytochemistry was performed with free-floating sections that first were washed once in 0.1 M PBS, then washed twice in washing buffer (WB; 0.1 M PBS with 0.03% Triton X 100), and incubated in 0.3% H2O2 for 30 min. After two further washes (each for 10 min in WB), the tissues were incubated with the primary antibody (rabbit polyclonal anti-c-Fos, 1:40 000, Santa Cruz Biotech, Santa Cruz, CA, USA) for 40 h at 4°C. The primary antibody was dissolved in 0.1 M PBS containing 0.3% Triton X 100, 1% bovine serum albumin and 2.5% goat serum. Afterwards the sections were washed twice in WB and incubated for 45 min in a solution containing the biotinylated secondary antibody (goat anti-rabbit IgG, Vectastain Elite Kit, Vector Laboratories, Burlingame, CA, USA). After two other washes in WB they were incubated for 1 h in avidin-biotin complex (Vectastain Elite Kit). The tissues were rinsed twice afterwards and developed with 3,3-diaminobenzidine substrate (Vectastain Elite Kit) intensified with nickel sulphate for 150 s. Subsequently the sections were mounted on gelatin-covered slides, air-dried and dehydrated by 100% xylol. The slides were coverslipped with Entellan (Merck, Darmstadt, Germany). To control for the specificity of the anti-c-Fos antibody signal, a c-Fos blocking peptide (Santa Cruz Biotech) was added to the primary antibody dilution.
The immunocytochemically processed spinal cord sections were examined with a light microscope (Axiophot, Zeiss, Oberkochen, Germany) coupled to a computerized image analysis system (MCID-M2, version 3.0, Rev 1.1, Imaging Research Inc., Brock University, St. Catharines, Ontario, Canada). The sections were coded such that the examiner did not know which treatment group they came from. Five sections from segments T11, T12, T13, L1, L6, S1 and S2 of each animal were analysed and all c-Fos-positive cells in the superficial layers of the dorsal horn (laminae I–IIo) counted separately on both sides of the spinal cord. The counts on the side with the higher number of c-Fos positive cells of each animal were averaged to give the number of c-Fos-positive cells of that animal. These average values from each animal were then used to calculate the mean number of c-Fos-positive cells of each experimental group.
DAS
To asses the clinical appearance of DSS-induced colitis, a DAS covering body weight change, stool consistency and presence of blood traces in the faeces was recorded. The scoring method was similar to that used by Reber et al. (2006) and Eijkelkamp et al. (2007). The body weight was measured before and 1 week after pretreatment of the animals with DSS, relative to those recorded in control animals, whereas the other parameters were evaluated at the end of the DSS pretreatment period only. A weight gain of more than 1 g, no change (±1 g), or a loss of weight of more than 1 g was scored as 0, 1 or 2, respectively. Loose, soft or normal stool consistency was rated as 2, 1 or 0, respectively. The presence of blood in the faeces was evaluated with the HEMDETECTR test (DIPROmed, Weigelsdorf, Austria), the presence or absence of blood being counted as 1 or 0, respectively. According to this rating system, the maximum DAS was 5.
Colon length
The reduction of the length of the colon was used as a parameter to assess colonic inflammation (Reber et al., 2006; Eijkelkamp et al., 2007). After death, the whole colon was excised and placed on tissue paper such that the length of the colon could be measured. Care was taken not to stretch the colon.
MPO levels in the colon
The tissue levels of MPO were used to quantify inflammation-associated infiltration of neutrophils and monocytes into the tissue (Krawisz et al., 1984). After death and measurement of the colon length, full-thickness pieces of the distal colon were excised, shock-frozen in liquid nitrogen and stored at –70°C until assay. After weighing, the frozen tissues were placed, at a ratio of 1 mg: 0.02 mL, in MPO lysis buffer. The composition of this buffer was: 200 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycine, 0.1 mM phenylmethylsulphonyl fluoride, 1 µg·mL−1 leupeptide, 28 µg·mL−1 aprotinin, pH 7.4. The samples were homogenized on ice with an Ultraturrax (IKA, Staufen, Germany) and then subjected to two centrifugation steps at 6000×g and 4°C for 15 min. The MPO (donor : H2O2 oxidoreductase, EC 1.11.1.7) content of the supernatant was measured with an enzyme-linked immunosorbent assay kit specific for the rat and mouse protein (Hycult Biotechnology, Uden, the Netherlands). The sensitivity of this assay is 1 ng·mL−1 at an intra- and inter-assay variation of around 10%.
Histological examination of the colon
For histological examination, specimens of the distal colon were fixed in a medium containing 2% paraformaldehyde, 2.5% glutaraldehyde and 0.1 M cacodylate buffer of pH 7.4 and embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany). Then 2.5 µm sections were cut and stained with a mixture of methylene blue–azure II and basic fuchsin. The sections were taken randomly from the distal colon, and histological injury was assessed in qualitative terms.
Locomotor, exploratory and ingestive behaviour
The circadian pattern of locomotion, exploration, drinking and feeding was assessed with the LabMaster system that consisted of six recording units, each unit comprising a test cage (type III, 42 cm × 26.5 cm × 15 cm, length × width × height), two external infrared frames and a cage lid fitted with two weight transducers (Edelsbrunner et al., 2009). These devices were connected to a personal computer that was used to collect and analyze the data with the LabMaster software. The system was configured such that 720 values of each test parameter were collected over a 12 h interval.
The two weight transducers were employed to quantify ingestive behaviour. To this end, a feeding bin filled with standard rodent chow (altromin 1324 FORTI; Altromin, Lage, Germany) and a drinking bottle filled with tap water were each attached to a transducer on the cage lid, and the animals were allowed to drink and feed ad libitum. Water and food intake over time was measured in mL and g, respectively. For data analysis, the amount of water and food ingested over select time intervals was normalized to the body weight of the animals.
For recording locomotion and exploration, the two external infrared frames were positioned in a horizontal manner above one another at a distance of 4.3 cm, with the lower frame being fixed 2.0 cm above the bedding floor. The bottom frame was used to record horizontal locomotion (ambulatory movements) of the mice, whereas the top frame served to record vertical movements (rearing, exploration). The measures of activity (locomotion, exploration) were derived from the light beam interruptions (counts) of the corresponding infrared frames.
Circulating corticosterone levels
The plasma levels of corticosterone were determined between 11:00 and 13:00 h 2 days before as well as 20 min after AITC (2%) or its vehicle (peanut oil, 0.05 mL) had been administered into the colon. Blood was collected into vials coated with ethylenediamine tetraacetate (Greiner, Kremsmünster, Austria) kept on ice. Following centrifugation for 20 min at 4°C and 1200×g, blood plasma was collected and stored at –20°C until assay. The plasma levels of corticosterone were determined with an enzyme immunoassay kit (Assay Designs, Ann Arbor, MI, USA). According to the manufacturer's specifications, the sensitivity of the assay is 27 pg·mL−1, and the intra- and inter-assay coefficient of variation amounts to 7.7 and 9.7%, respectively.
Statistics
Statistical evaluation of the results was performed on GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA, USA) with Student's t-test (two treatments groups), one-way or two-way analysis of variance or the Kruskal–Wallis test. The choice of post-tests for multiple comparisons depended on the number of treatment groups and the results of the normality test. To assess the normal distribution of the data, the Kolmogorov–Smirnov test was used. If the normality test was passed, Bonferroni's multiple comparison test was used as post-test, whereas Dunn's multiple comparison test was employed when the normality test failed. Outliers detected by Grubb's test were excluded from the analysis. All data are presented as means ± SEM, n referring to the number of mice in each group. Probability values of P < 0.05 and P < 0.01 were considered as significant and highly significant, respectively, whereas values of P ≤ 0.1 were interpreted as trends.
Materials
AITC, capsaicin, peanut oil and pentobarbital were obtained from Sigma-Aldrich, morphine hydrochloride from Gerot-Lannach (Lannach, Austria), and HC-030031 (2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)-N-(4-isopropylphenyl)acetamide) from ChemBridge Corporation (San Diego, CA, USA). AITC (1 or 2%) and capsaicin (5%) were dissolved in peanut oil, whereas morphine hydrochloride (5 mg·mL−1) was dissolved in saline (0.9% NaCl). HC-030031 was suspended in methyl cellulose (0.5% in saline) at a concentration of 33 mg·mL−1. Pentobarbital was dissolved in an aqueous solution containing 20% ethylene glycol and 10% ethanol at a concentration of 120 mg·mL−1.
Results
Effect of intracolonic administration of AITC and capsaicin on the spinal c-Fos response
Preliminary experiments were conducted to identify the segments of the spinal cord (L6–S2) that responded to intracolonic administration of AITC (1%) with maximal expression of c-Fos. The pertinent data showed that the c-Fos response was most prominent in the superficial layers (laminae I–IIo) of the S1 dorsal horn. Further experiments indicated that the number of c-Fos positive cells in S1 depended on the intracolonic concentration of AITC. Compared with vehicle, AITC (1%) enhanced the expression of c-Fos at S1 by a factor of 1.3 (derived from the mean number of c-Fos positive cells counted after AITC stimulation divided by the mean number counted after vehicle administration), whereas AITC (2%) increased it by a factor of 1.8 (Figures 1 and 2). The c-Fos response to 2% AITC was statistically significant. For comparison, intracolonic capsaicin (5%), an agonist at TRPV1, augmented the number of c-Fos positive cells in laminae I–IIo of the S1 dorsal horn by a factor of 1.6 (Figure 2).
Figure 1.
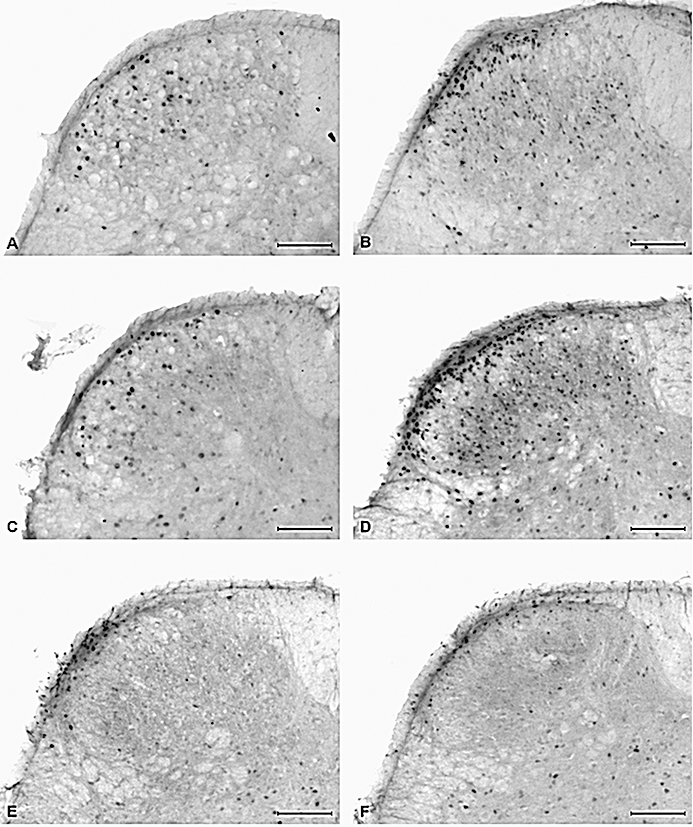
Photomicrographs showing the expression of c-Fos in laminae I–IIo of the S1 spinal dorsal horn as visualized by immunohistochemistry 1 h after intracolonic administration of vehicle (peanut oil) or allyl isothiocyanate (AITC; 2%) to control mice and mice pretreated with dextran sulphate sodium (DSS; 2%) for 1 week. (A) Intracolonic vehicle in control mice, (B) intracolonic AITC in control mice, (C) intracolonic vehicle in DSS-pretreated mice, (D) intracolonic AITC in DSS-pretreated mice, (E) intracolonic AITC in control mice injected with HC-030031, (F) intracolonic AITC in DSS-pretreated mice injected with HC-030031. Calibration bar: 100 µm.
Figure 2.
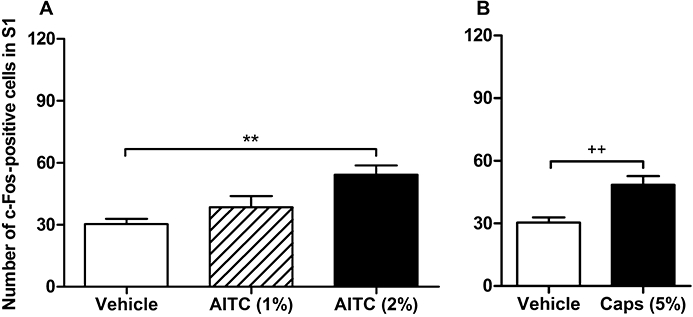
Quantitative estimates of c-Fos positive cells in laminae I–IIo of the S1 spinal dorsal horn as visualized by immunohistochemistry 1 h after intracolonic administration of (A) vehicle (peanut oil), 1% or 2% allyl isothiocyanate (AITC) and (B) vehicle or 5% capsaicin (Caps) in control mice. The values represent means + SEM, n = 5–16 (n = 16 for vehicle in panel A, combining the two vehicle groups run in parallel with the 1% AITC and the 2% AITC group, n = 5–7 for the other groups). **P < 0.01 (one-way anova followed by Bonferroni's multiple comparison test), ++P < 0.01 (Student's t-test).
Because there is evidence for an innervation of the colon by thoracolumbar afferent neurones (Traub, 2000; Robinson et al., 2004), the effect of intracolonic AITC (2%) on the expression of c-Fos in the spinal dorsal horn of segments T11–L1 was also assessed. However, the number of c-Fos positive cells in laminae I–IIo of these spinal segments did not differ between mice treated intracolonically with vehicle or AITC (Table 1).
Table 1.
Number of c-Fos positive cells in laminae I–IIo of the thoracolumbar segments T11–L2 of the spinal cord as visualized by immunohistochemistry 1 h after intracolonic administration of vehicle (peanut oil) or allyl isothiocyanate (AITC, 2%) to control mice and mice pretreated with dextran sulphate sodium (DSS; 2%) for 1 week
| Pretreatment | Treatment | T11 | T12 | T13 | L1 | L2 |
|---|---|---|---|---|---|---|
| Control | Vehicle | 8.5 ± 07 | 9.4 ± 0.5 | 9.3 ± 1.0 | 7.6 ± 0.8 | 4.8 ± 0.8 |
| Control | AITC | 6.8 ± 0.6 | 7.1 ± 1.1* | 7.8 ± 1.0 | 7.0 ± 1.2 | 4.6 ± 0.9 |
| DSS | Vehicle | 7.7 ± 0.7 | 8.4 ± 0.7 | 8.3 ± 1.7 | 9.0 ± 1.9 | 4.7 ± 1.3 |
| DSS | AITC | 5.8 ± 0.9 | 5.5 ± 0.8 | 6.4 ± 0.7 | 6.1 ± 1.3 | 7.3 ± 1.5 |
DSS was added to the drinking water. The values shown are means ± SEM of five animals in each group.
P ≤ 0.1 versus vehicle (Student's t-test).
The specificity of the c-Fos immunoreactivity in the spinal cord was demonstrated by the absence of any immunoreactive signal after preincubation of the primary antibody with the corresponding blocking peptide.
Effect of pretreatment with DSS on parameters of colonic inflammation and on circadian locomotor, exploratory and ingestive behaviour
Four parameters of colonic inflammation were evaluated: DAS, colonic MPO activity, colon length and colonic histology. The DAS of animals pretreated with 1% DSS (0.5 ± 0.3, n = 6) did not significantly differ from that of control animals (0.3 ± 0.2, n = 6). Mice pretreated with 2% DSS exhibited signs of diarrhoea and occasionally had blood in their faeces, whereas their weight gain was comparable to that of control animals (Table 2). The DAS of animals pretreated with 2% DSS (2.7 ± 0.2, n = 6) was significantly larger than that seen in control animals (P < 0.01, Dunn's multiple comparison test) and animals treated with 1% DSS (P < 0.05, Dunn's multiple comparison test).
Table 2.
Body weight of control mice and mice pretreated with dextran sulphate sodium (DSS; 1 and 2%) for 1 week
| Pretreatment | Weight at the start of pretreatment | Weight 1 week after the start of the pretreatment | Δ weight (g) |
|---|---|---|---|
| Control | 30.7 ± 0.7 g | 32.2. ± 1.0 g | 1.4 ± 0.5 g |
| DSS (1%) | 30.1 ± 0.4 g | 31.3 ± 0.9 g | 1.2 ± 0.6 g |
| DSS (2%) | 31.2. ± 1.0 g | 32.0 ± 1.2 g | 0.8 ± 0.4 g |
DSS was added to the drinking water. The values shown are means ± SEM of six animals in each group. There were no significant differences between control and DSS-pretreated mice.
The colon length, determined on the day of killing, did not differ between control mice (11.07 ± 0.26 cm, n = 14) and mice pretreated with 1% DSS (11.14 ± 0.32 cm, n = 7). In contrast, the colon of mice pretreated with 2% DSS (8.86 ± 0.32 cm, n = 7) was significantly shorter than that of control animals (P < 0.01, Bonferroni's multiple comparison test). Intracolonic treatment of control animals with 2% AITC (10.93 ± 0.53 cm, n = 7) did not alter colon length relative to vehicle treatment (11.29 ± 0.39 cm, n = 7).
The MPO content in the distal part of the colon was determined 1 week after the start of the DSS pretreatment. Compared with that in control animals, the colonic MPO content was amplified by factors of 5.4 and 16.9, respectively, in mice pretreated with 1 and 2% DSS (Figure 3A). In contrast, intracolonic administration of 2% AITC to control animals 1 h before tissue sampling did not alter the MPO content relative to intracolonic vehicle treatment (vehicle-treated mice: 0.59 ± 0.07 µg·g−1 MPO, n = 13; AITC-treated mice: 0.69 ± 0.15 µg·g−1 MPO, n = 14).
Figure 3.
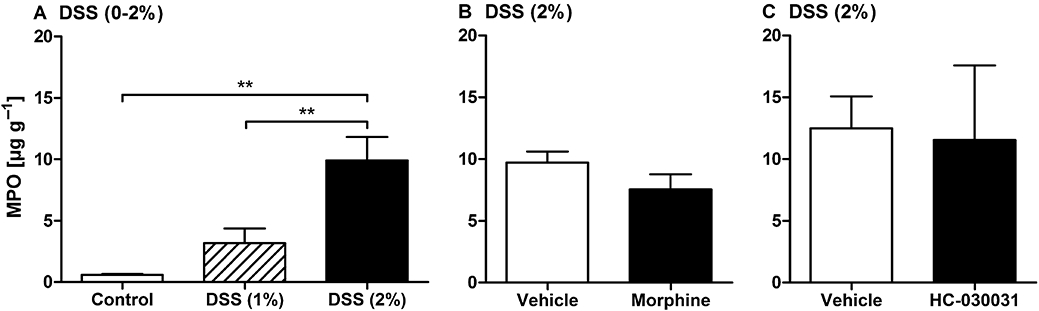
Myeloperoxidase (MPO) content of the distal colonic wall. (A) Colonic MPO content in control mice and mice pretreated with dextran sulphate sodium (DSS; 1 or 2%) for 1 week. DSS was added to the drinking water, whereas control mice drank normal tap water. (B) Colonic MPO content in mice pretreated with DSS (2%) for 1 week and treated with morphine (10 mg·kg−1 subcutaneously) or its vehicle 2 h before tissue collection. (C) Colonic MPO content in mice pretreated with DSS (2%) for 1 week and treated with HC-030031 (300 mg·kg−1) or its vehicle 1.5 h before tissue collection. The values represent means + SEM, n = 4–13 (n = 13 for control in panel A, combining the two control groups run in parallel with the 1% DSS and the 2% DSS group, n = 4 for the groups in panel C, n = 7 for the other groups). One outlier in panel A (control) and one outlier in panel C (HC-030031) were removed on the basis of Grubb's test. **P < 0.01 (one-way anova followed by the Bonferroni's multiple comparison test).
Histological examination of tissue sections revealed that pretreatment of mice with DSS (2%) failed to cause major structural changes of the colonic mucosa, relative to the mucosal architecture seen in control animals. In some specimens, accumulations of leucocytes in the lamina propria and submucosa were observed. Representative histographs of the colonic wall of a control mouse and an animal pretreated with DSS (2%) are shown in Figure 4.
Figure 4.
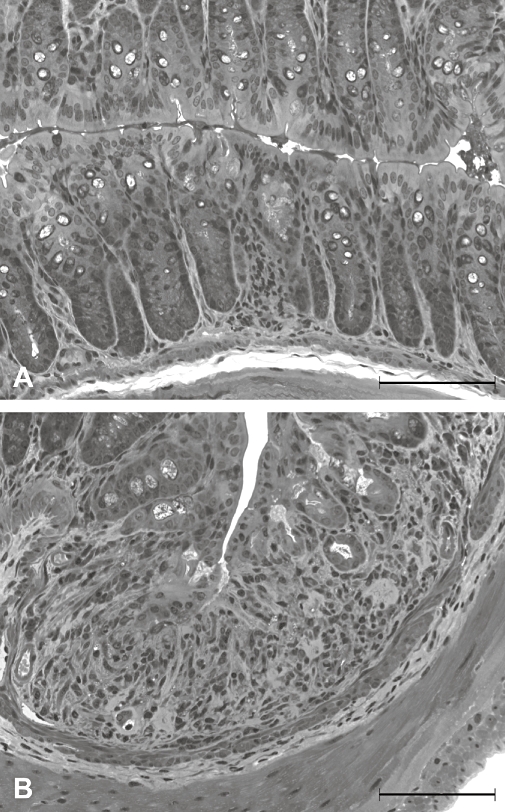
Light microscopic appearance of the colonic mucosa taken from a control mouse (A) and a mouse pretreated with dextran sulphate sodium (2%) for 1 week (B). Calibration bar: 100 µm.
The circadian pattern of locomotion, exploration, feeding and drinking was not significantly altered when, after 1 week under control conditions, the animals' drinking water contained 2% DSS for the following week (Figure 5).
Figure 5.
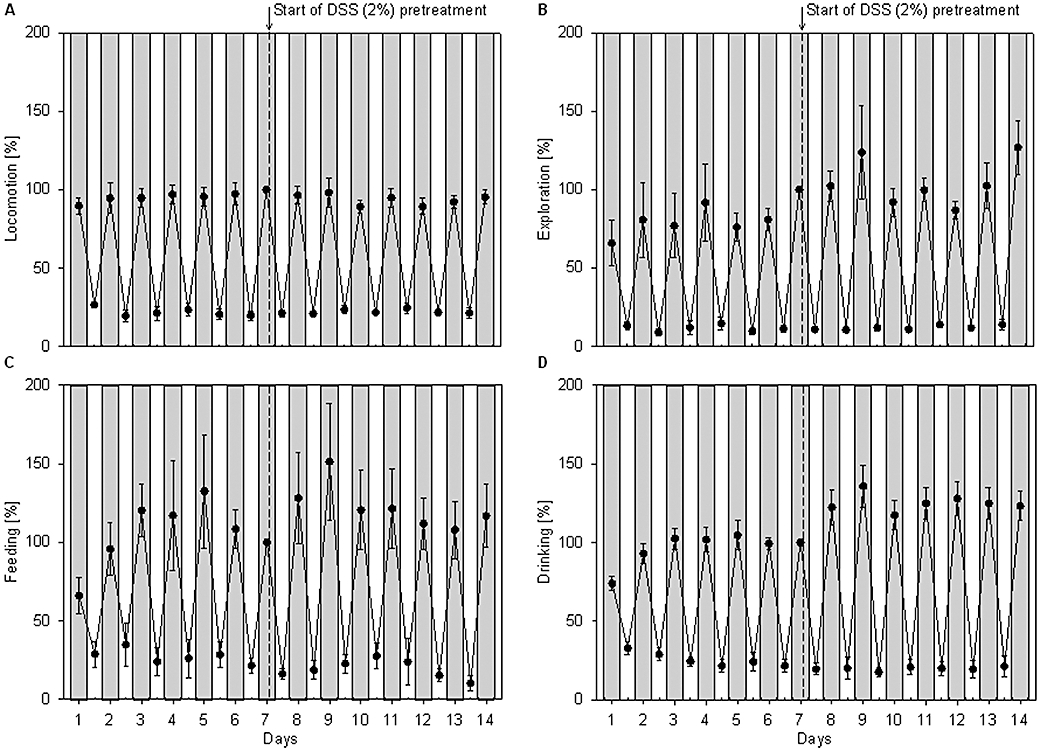
Time course of the circadian locomotor (A), exploratory (B), feeding (C) and drinking (D) activities in mice over a period of 2 weeks, before and after pretreatment with dextran sulphate sodium (DSS; 2%). During the first week, the mice drank normal tap water, whereas during the second week they drank water containing 2% DSS. The cumulative activities measured during the light (white areas) and dark (shaded areas) phases were expressed as a percentage of the activity measured during the dark phase of day 7, immediately before the pretreatment with DSS was begun. The values represent means ± SEM, n = 6. There were no significant differences between control and DSS-pretreated mice.
Effect of pretreatment with DSS on the ability of intracolonic AITC to enhance c-Fos expression in the spinal cord and to elevate circulating corticosterone levels
The expression of c-Fos measured in the spinal dorsal horn at segment S1 after intracolonic administration of vehicle did not significantly differ between control animals and animals pretreated with DSS (1 and 2%) for 1 week (Figure 6A,B,C). The spinal c-Fos response to intracolonic AITC (2%) tended to be enhanced in mice pretreated with the lower dose of DSS (1%), relative to the response measured in control animals (Figure 6A). Pretreatment with the higher dose of DSS (2%) for 1 week resulted in a significant increase in the c-Fos expression that intracolonic AITC (2%) induced in laminae I–IIo of the S1 dorsal horn, whereas the c-Fos response to intracolonic vehicle did not differ between control and DSS-pretreated animals (Figure 6B). The number of spinal neurones expressing c-Fos in response to intracolonic capsaicin (5%) was likewise significantly enhanced in mice pretreated with 2% DSS (Figure 6C).
Figure 6.
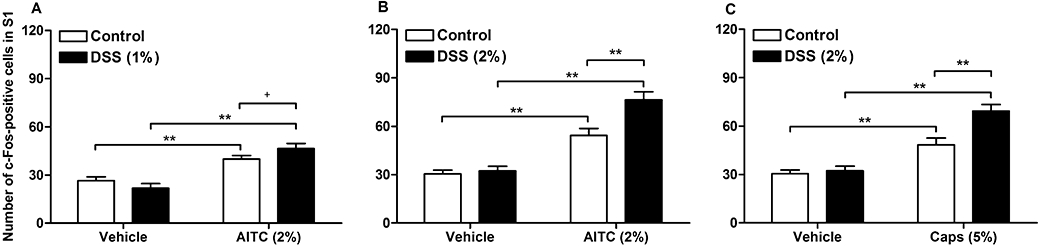
Quantitative estimates of c-Fos positive cells in laminae I–IIo of the S1 spinal dorsal horn as visualized by immunohistochemistry 1 h after intracolonic administration of (A) vehicle (peanut oil) and allyl isothiocyanate (AITC, 2%) in control mice and mice pretreated with 1% dextran sulphate sodium (DSS), (B) vehicle and AITC (2%) in control mice and mice pretreated with 2% DSS, and (C) vehicle and capsaicin (Caps, 5%) in control mice and mice pretreated with 2% DSS. DSS was added to the drinking water for 1 week, whereas control mice drank normal tap water. The values represent means + SEM, n = 5–7. +P≤ 0.1 (Student's t-test), **P < 0.01 (two-way anova followed by Bonferroni's multiple comparison test).
The baseline levels of circulating corticosterone did not significantly differ between control mice and mice pretreated with 2% DSS (Figure 7). In both groups of mice, intracolonic administration of vehicle (peanut oil) caused a nominal, but insignificant, increase in the plasma levels of corticosterone as measured 20 min post-treatment (Figure 7). In contrast, intracolonic administration of AITC (2%) resulted in a significant elevation of the corticosterone concentration in the plasma, an effect that was seen in both control animals and mice pretreated with 2% DSS (Figure 7).
Figure 7.
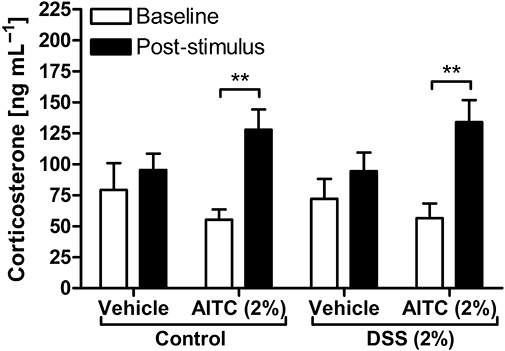
Corticosterone levels in blood plasma as measured 2 days before (baseline) and 20 min after intracolonic administration of vehicle (peanut oil) and allyl isothiocyanate (AITC, 2%) to control mice and mice pretreated for 1 week with 2% dextran sulphate sodium (DSS). The values represent means + SEM, n = 6–7. One outlier in the control group treated with AITC (baseline) was removed on the basis of Grubb's test. **P < 0.01 (two-way repeated measures anova followed by Bonferroni's multiple comparison test).
Effect of morphine and HC-030031 on the spinal c-Fos response to intracolonic AITC
Morphine (10 mg·kg−1) or its vehicle was injected subcutaneously 1 h before intracolonic administration of AITC (2%) or capsaicin (5%). The opioid receptor agonist was able to significantly inhibit the spinal c-Fos response to intracolonic AITC and capsaicin in control mice as well as in mice pretreated with 2% DSS in which the spinal c-Fos expression was significantly enhanced (Figure 8A,B). In both groups of animals, the spinal c-Fos expression was reduced by morphine to a level that was similar to that seen following intracolonic administration of peanut oil (the vehicle for AITC and capsaicin) to control animals. In contrast, the colonic MPO content of DSS-pretreated mice did not differ between animals injected with morphine and those injected with vehicle (Figure 3B).
Figure 8.
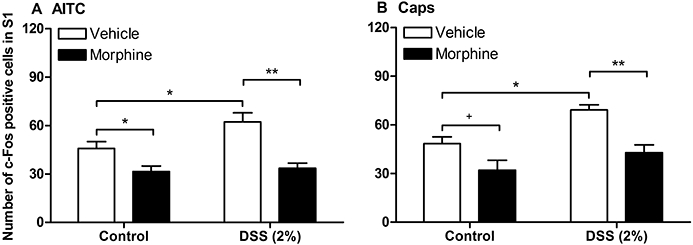
Effect of morphine on the quantitative estimates of c-Fos positive cells in laminae I–IIo of the S1 spinal dorsal horn as visualized by immunohistochemistry 1 h after intracolonic administration of (A) 2% allyl isothiocyanate (AITC) or (B) 5% capsaicin (Caps) to control mice and mice pretreated for 1 week with dextran sulphate sodium (DSS, 2%). Morphine (10 mg·kg−1) or its vehicle was injected subcutaneously 1 h before intracolonic administration of AITC or capsaicin. The values represent means + SEM, n = 5–7. +P≤ 0.1 (Student's t-test), *P < 0.05, **P < 0.01 (two-way anova followed by Bonferroni's multiple comparison test).
The TRPA1 blocker HC-030031 (300 mg·kg−1) or its vehicle was injected i.p., 30 min before intracolonic administration of AITC (2%) or capsaicin (5%). As shown in Figure 9A, HC-030031 significantly attenuated the spinal c-Fos response to intracolonic AITC in control mice as well as in mice pretreated with 2% DSS in which the number of spinal c-Fos positive neurones was significantly increased. In both groups of mice, the spinal c-Fos expression was lowered to the level seen following intracolonic administration of peanut oil. However, the colonic MPO content of DSS-pretreated mice did not differ between animals treated with HC-030031 and those treated with vehicle (Figure 3C). The specificity of HC-030031 as a TRPA1 blocker was confirmed by its failure to significantly inhibit the spinal c-Fos expression evoked by intracolonic administration of the TRPV1 agonist capsaicin (5%) in control rats, although a trend towards inhibition was noted (Figure 9B).
Figure 9.
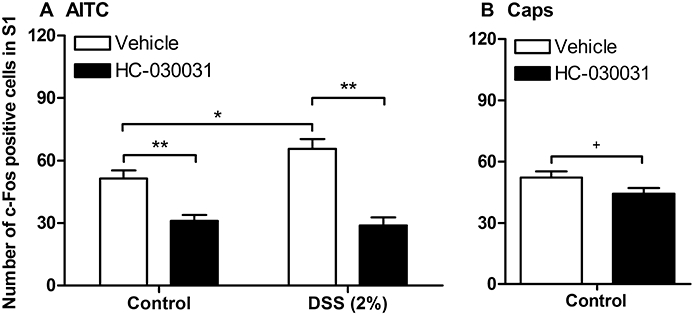
Effect of HC-030031 on the quantitative estimates of c-Fos positive cells in laminae I–IIo of the S1 spinal dorsal horn as visualized by immunohistochemistry 1 h after intracolonic administration of (A) 2% allyl isothiocyanate (AITC) or (B) 5% capsaicin (Caps) to control mice and mice pretreated for 1 week with 2% dextran sulphate sodium (DSS). HC-030031 (300 mg·kg−1) or its vehicle was injected intraperitoneally 30 min before intracolonic administration of AITC or capsaicin. The values represent means + SEM, n = 5–6. +P≤ 0.1 (Student's t-test), *P < 0.05, **P < 0.01 (two-way anova followed by Bonferroni's multiple comparison test).
Discussion
In the present study, chemical signalling from the colon to the mouse spinal cord was examined with AITC and capsaicin. AITC has long been known to stimulate nociceptive afferent neurones (Bruce, 1910), although it has only recently been elucidated that it does so by activating TRPA1 (Jordt et al., 2004; Bautista et al., 2005), whereas capsaicin excites sensory neurones via TRPV1 (Caterina and Julius, 2001; Holzer, 2008). The major findings of our present work can be summarized as follows: (i) like capsaicin, intracolonic AITC caused afferent signalling to the spinal cord (S1) as visualized by expression of c-Fos; (ii) this afferent input was enhanced by mild colitis in the absence of overt structural damage; (iii) the spinal c-Fos response to intracolonic AITC was associated with an increase in the circulating corticosterone level; (iv) the AITC- and capsaicin-induced afferent signalling was inhibited by morphine; and (v) the spinal c-Fos response to intracolonic AITC, but not capsaicin, was prevented by the TRPA1 channel blocker, HC-030031.
Afferent signalling from the mouse colon was visualized by c-Fos expression in the spinal cord, a standard method of functional neuroanatomy to delineate stimulus-evoked activation of neurones (Munglani and Hunt, 1995). The appearance of the c-Fos protein was measured 1 h post-stimulus, because c-Fos protein translation in the mouse brainstem reaches its maximum at this time (Wultsch et al., 2005). Neuroanatomical evidence indicates that the descending colon of rodents is innervated by both thoracolumbar (T8–L1) and lumbosacral (L6–S1) spinal afferent neurones Traub, 2000; Robinson et al., 2004). However, the spinal c-Fos response to intracolonic AITC was restricted to the lumbosacral level, as in segments T11–T12 no increase in the number of c-Fos positive cells was noted. As has been observed in the rat (Lu and Westlund, 2001), the c-Fos response was largest in the S1 dorsal horn. We conclude that intracolonic AITC activated only lumbosacral afferents, despite the fact that TRPA1 is expressed by both splanchnic and pelvic afferents of the mouse (Brierley et al., 2009).
The number of c-Fos positive cells induced by AITC and capsaicin was highest in the superficial layers (laminae I–IIo) of the S1 dorsal horn. The same topography of the c-Fos response to intracolonic AITC was found in the rat (Lu and Westlund, 2001), which is consistent with the prominent termination of visceral sensory neurones in lamina I and the outer part of lamina II of the spinal cord (Cervero and Connell, 1984; Neuhuber et al., 1986; Traub et al., 1992; Lanteri-Minet et al., 1993). Although visceral afferents also project to deeper laminae of the spinal cord (Cervero and Connell, 1984; Neuhuber et al., 1986; Traub et al., 1992; Lanteri-Minet et al., 1993), we failed to observe an appreciable c-Fos response in these layers. As discussed by Lu and Westlund (2001), expression of c-Fos in the deep dorsal horn may only occur in response to more intense stimulation, for example, colorectal distension (Traub et al., 1992; Lanteri-Minet et al., 1993).
AITC and capsaicin can excite sensory neurones directly by an action on their peripheral fibres and/or indirectly by altering intestinal functions that stimulate afferent nerve activity. Intracolonic administration of vehicle, AITC or capsaicin is likely to cause some distension and mechanical irritation of the colon. The part of the c-Fos response to AITC and capsaicin that exceeds that to intracolonic vehicle is thought to arise from neural mechanisms activated by TRPA1 and TRPV1, respectively. Apart from a direct excitation, afferent nerve fibres might also respond to AITC-induced stimulation of intestinal motor activity (Penuelas et al., 2007; Nozawa et al., 2009). In addition, AITC has been reported to induce colonic inflammation (Traub, 2000; Lu and Westlund, 2001; Kimball et al., 2006, 2007), yet a substantial participation of inflammation-derived mediators in the spinal c-Fos response to AITC is negated by the failure of AITC to enhance colonic MPO and reduce colonic length within 1 h post-treatment. Another way whereby AITC could indirectly stimulate afferent neurones is via release of cholecystokinin (Purhonen et al., 2008) and 5-HT (Nozawa et al., 2009) from enteroendocrine cells that express TRPA1, a possibility that awaits to be tested.
The ability of the TRPA1 blocker HC-030031 (McNamara et al., 2007; Eid et al., 2008; Taylor-Clark et al., 2008) to reduce the spinal c-Fos response to intracolonic AITC to a magnitude seen in vehicle-treated mice shows that the effect of AITC is mediated by TRPA1. This conclusion is in keeping with the finding that knockout of TRPA1 abolished AITC-evoked neuronal stimulation (Bautista et al., 2006; Kwan et al., 2006) and the effect of intracolonic AITC to enhance c-Fos expression in spinal neurones (Cattaruzza et al., 2010). The inability of HC-030031 to significantly blunt the c-Fos response to capsaicin attests to its selectivity as a TRPA1 blocker. This observation also indicates that AITC and capsaicin excite sensory neurones by activating different molecular sensors, that is, TRPA1 and TRPV1.
In addressing the question whether the spinal c-Fos response to intracolonic AITC and capsaicin reflects nociception, we found that morphine significantly reduced the number of c-Fos positive cells in the S1 dorsal horn. The behavioural pain response to intracolonic AITC is likewise blunted by morphine (Laird et al., 2001). In analogy with other studies (Hammond et al., 1992; Traub et al., 1995; Schuligoi et al., 1998; Laird et al., 2001) we therefore conclude that µ-opioid receptors control the AITC- and capsaicin-evoked input to the spinal cord and that the opioid-sensitive expression of c-Fos in the spinal cord is a molecular correlate of colonic chemonociception. In addition, we suppose that the spinal c-Fos response to noxious chemical stimulation of the colon has predictive validity for the pharmacological evaluation of analgesic drug candidates.
IBS is commonly associated with hypersensitivity to colorectal distension (Azpiroz et al., 2007; Knowles and Aziz, 2009) and low grade inflammation in the absence of any mucosal lesions (Bercik et al., 2005; Spiller, 2007; De Giorgio and Barbara, 2008). In examining the question whether there is also hypersensitivity to chemical stimuli under conditions of low grade inflammation, we used a model of mild colitis induced by addition of DSS to the drinking water for 7 days. In the animals pretreated with DSS, the DAS was increased because of diarrhoea and the presence of blood in the faeces, the colon length was reduced and the colonic submucosa infiltrated by leucocytes as revealed by histology and confirmed by an increased MPO content of the colonic wall. Although these changes are typical of colonic inflammation induced by DSS (Kimball et al., 2006; Reber et al., 2006; Eijkelkamp et al., 2007), the severity of colitis was mild, given that the architecture of the colonic mucosa remained essentially preserved and the locomotor, exploratory and ingestive behaviour as well as the weight gain of the animals stayed unaltered.
The activation of S1 spinal neurones by intracolonic AITC and capsaicin was significantly increased in mice with mild colitis. As the c-Fos response recorded after vehicle administration did not differ between control and DSS-pretreated animals (Figure 6B,C), it would appear that DSS per se did not change c-Fos expression in the spinal cord. In addition, the finding that the c-Fos response to intracolonic vehicle remained unchanged in DSS-pretreated mice indicates that the hypersensitivity to AITC and capsaicin did not result from hypersensitivity to mechanical stimuli associated with the intracolonic administration procedure. We therefore conclude that the exaggerated expression of c-Fos, which AITC and capsaicin induced in the spinal cord of DSS-pretreated mice, reflects chemical hypersensitivity. It need be emphasized that this increase in chemosensitivity occurs with mild colitis and can be studied under conditions that resemble those in functional gastrointestinal pain syndromes. Thus it is possible to avoid artificial experimental conditions such as necrotic colitis, which in rats has been reported to enhance behavioural pain responses to intracolonic AITC and colorectal distension (Yang et al., 2008).
The sensory processing of colorectal distension in healthy rats occurs in the lumbosacral spinal cord, whereas the inflammatory hypersensitivity to mechanical stimulation of the colon is in part mediated by T11–L1 thoracolumbar afferent neurones (Traub, 2000). In contrast, intracolonic AITC administration to both control and DSS-pretreated mice failed to increase the expression of c-Fos in the T11–L2 segments of the spinal cord. Thus, mild inflammation does not seem to recruit thoracolumbar afferent neurones to respond to chemical irritation of the colon, and it is tempting to speculate that different afferent pathways participate in the inflammatory hypersensitivity to chemical and mechanical stimulation of the large intestine.
The increased expression of c-Fos in the S1 spinal dorsal horn, which intracolonic AITC and capsaicin evoked in mice with mild colitis, was prevented by morphine, whereas the DSS-induced increase in colonic MPO content remained unaltered. These findings indicate that the exaggerated c-Fos response represented a correlate of chemical hyperalgesia of the colon and that the anti-hyperalgesic action of morphine resulted from an inhibitory action on nociceptive afferent pathways and did not reflect a reduction of colonic inflammation. The hyperalgesic response to AITC was mediated by TRPA1 channels because it was blocked by the TRPA1 channel blocker HC-030031, whereas the DSS-induced colitis remained unaffected by treatment with HC-030031 as judged from the colonic MPO content. It awaits to be investigated whether the colonic hypersensitivity to AITC and capsaicin reflects sensitization of TRPA1 (Wang et al., 2008; Yang et al., 2008; Brierley et al., 2009) and TRPV1 (Holzer, 2008), respectively, an increase in their expression by primary afferent neurones, or other phenotypic alterations of TRPA1- and TRPV1-positive afferents. There are reports that experimental colitis causes upregulation of TRPA1 in the colon (Kimball et al., 2007) and visceral sensory neurones (Yang et al., 2008), much as TRPV1 is sensitized and upregulated in colonic inflammation and functional gastrointestinal pain syndromes without overt inflammation (Miranda et al., 2007; Winston et al., 2007; De Schepper et al., 2008; Holzer, 2008).
Gastrointestinal pain is associated with neuroendocrine reactions (Blackburn-Munro, 2004; Knowles and Aziz, 2009), a relationship that was borne out by the increase in the plasma concentration of corticosterone following intracolonic administration of AITC, but not vehicle. Colonic chemonociception mediated by TRPA1 thus results in stimulation of the hypothalamic-pituitary-adrenal axis. As the AITC-evoked elevation of circulating corticosterone was seen both in control and DSS-pretreated mice, it appears as if the hypersensitivity to AITC in mild colitis did not translate into a hyperresponsiveness of the neuroendocrine output from the brain.
In conclusion, our data attested to an important role of TRPA1 in colonic chemo-nociception and in the chemical hyperalgesia associated with mild colitis. TRPA1 is a multimodal chemo-nocisensor (Bautista et al., 2006; Fujita et al., 2007, 2008; Trevisani et al., 2007; Macpherson et al., 2007a, 2007b; Eid et al., 2008; Wang et al., 2008; Andrèet al., 2009; Yu et al., 2009; Yu and Ouyang, 2009), and its sensory modalities place TRPA1 in a position to survey the alimentary canal for spicy compounds, pro-inflammatory mediators, environmental toxins and potentially deleterious conditions arising from the presence of alkalosis, H2S and oxidative insults. Given that several irritant chemicals can be present in the chyme, TRPA1 may be of relevance to several gastrointestinal pain conditions. In addition, TRPA1 appears to contribute to mechanical pain of the oesophagus, stomach and colon (Yang et al., 2008; Kondo et al., 2009; Yu et al., 2009; Yu and Ouyang, 2009; Cattaruzza et al., 2010).
Acknowledgments
This work was supported by the Zukunftsfonds Steiermark (grant 262) and the Austrian Science Funds (FWF grant L25-B05).
Glossary
Abbreviations:
- AITC
allyl isothiocyanate
- DAS
disease activity score
- DSS
dextran sulphate sodium
- IBS
irritable bowel syndrome
- MPO
myeloperoxidase
- PBS
phosphate-buffered saline
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid 1
- WB
washing buffer
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, et al. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett. 2008;438:221–227. doi: 10.1016/j.neulet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Andrè E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, et al. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol. 2009;158:1621–1628. doi: 10.1111/j.1476-5381.2009.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19(Suppl 1):62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Collins SM. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol Clin North Am. 2005;34:235–245. doi: 10.1016/j.gtc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G. Hypothalamo-pituitary-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Curr Pain Headache Rep. 2004;8:116–124. doi: 10.1007/s11916-004-0025-9. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce NA. Über die Beziehung der sensiblen Nervenendigungen zum Entzündungsvorgang. Arch Exp Pathol Pharmakol. 1910;63:424–433. [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner SJ, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol. 2010;298:G81–G91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Connell LA. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol. 1984;230:88–98. doi: 10.1002/cne.902300108. [DOI] [PubMed] [Google Scholar]
- Christoph T, Grünweller A, Mika J, Schäfer MK, Wade EJ, Weihe E, et al. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350:238–243. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- De Giorgio R, Barbara G. Is irritable bowel syndrome an inflammatory disorder? Curr Gastroenterol Rep. 2008;10:385–390. doi: 10.1007/s11894-008-0073-0. [DOI] [PubMed] [Google Scholar]
- De Schepper HU, De Man JG, Ruyssers NE, Deiteren A, Van Nassauw L, Timmermans JP, et al. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am J Physiol. 2008;294:G245–G253. doi: 10.1152/ajpgi.00351.2007. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Seyrek M. Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br J Pharmacol. 2006;149:498–505. doi: 10.1038/sj.bjp.0706854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelsbrunner ME, Herzog H, Holzer P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian cycle- and gender-dependent manner. Behav Brain Res. 2009;203:97–107. doi: 10.1016/j.bbr.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Kavelaars A, Elsenbruch S, Schedlowski M, Holtmann G, Heijnen CJ. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-Fos expression and behavior. Am J Physiol. 2007;293:G749–G757. doi: 10.1152/ajpgi.00114.2007. [DOI] [PubMed] [Google Scholar]
- Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim. 2000;34:372–378. doi: 10.1258/002367700780387714. [DOI] [PubMed] [Google Scholar]
- Fujita F, Moriyama T, Higashi T, Shima A, Tominaga M. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br J Pharmacol. 2007;151:153–160. doi: 10.1038/sj.bjp.0707219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, et al. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest. 2008;118:4049–4057. doi: 10.1172/JCI35957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond DL, Presley R, Gogas KR, Basbaum AI. Morphine or U-50,488 suppresses fos protein-like immunoreactivity in the spinal cord and nucleus tractus solitarii evoked by a noxious visceral stimulus in the rat. J Comp Neurol. 1992;315:244–253. doi: 10.1002/cne.903150210. [DOI] [PubMed] [Google Scholar]
- Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol. 2008;155:1145–1162. doi: 10.1038/bjp.2008.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol. 2006;291:G364–G371. doi: 10.1152/ajpgi.00407.2005. [DOI] [PubMed] [Google Scholar]
- Kimball ES, Prouty SP, Pavlick KP, Wallace NH, Schneider CR, Hornby PJ. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol Motil. 2007;19:390–400. doi: 10.1111/j.1365-2982.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- Knowles CH, Aziz Q. Basic and clinical aspects of gastrointestinal pain. Pain. 2009;141:191–209. doi: 10.1016/j.pain.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Adelta/C-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Kondo T, Obata K, Miyoshi K, Sakurai J, Tanaka J, Miwa H, et al. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut. 2009;58:1342–1352. doi: 10.1136/gut.2008.175901. [DOI] [PubMed] [Google Scholar]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Lanteri-Minet M, Isnardon P, de Pommery J, Menetrey D. Spinal and hindbrain structures involved in visceroception and visceronociception as revealed by the expression of Fos, Jun and Krox-24 proteins. Neuroscience. 1993;55:737–753. doi: 10.1016/0306-4522(93)90439-m. [DOI] [PubMed] [Google Scholar]
- Lu Y, Westlund KN. Effects of baclofen on colon inflammation-induced Fos, CGRP and SP expression in spinal cord and brainstem. Brain Res. 2001;889:118–130. doi: 10.1016/s0006-8993(00)03124-3. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007a;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007b;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munglani R, Hunt SP. Proto-oncogenes: basic concepts and stimulation induced changes in the spinal cord. Prog Brain Res. 1995;104:283–298. doi: 10.1016/s0079-6123(08)61796-3. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Sandoz PA, Fryscak T. The central projections of primary afferent neurons of greater splanchnic and intercostal nerves in the rat. A horseradish peroxidase study. Anat Embryol. 1986;174:123–144. doi: 10.1007/BF00318344. [DOI] [PubMed] [Google Scholar]
- Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuelas A, Tashima K, Tsuchiya S, Matsumoto K, Nakamura T, Horie S, et al. Contractile effect of TRPA1 receptor agonists in the isolated mouse intestine. Eur J Pharmacol. 2007;576:143–150. doi: 10.1016/j.ejphar.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Purhonen AK, Louhivuori LM, Kiehne K, Akerman KEO, Herzig KH. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008;582:229–232. doi: 10.1016/j.febslet.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology. 2006;147:4968–4976. doi: 10.1210/en.2006-0347. [DOI] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Jocic M, Heinemann A, Schöninkle E, Pabst MA, Holzer P. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology. 1998;115:649–660. doi: 10.1016/s0016-5085(98)70144-1. [DOI] [PubMed] [Google Scholar]
- Spiller RC. Irritable bowel syndrome: bacteria and inflammation – clinical relevance now. Curr Treat Options Gastroenterol. 2007;10:312–321. doi: 10.1007/s11938-007-0074-3. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Traub RJ. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport. 2000;11:2113–2116. doi: 10.1097/00001756-200007140-00011. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Pechman P, Iadarola MJ, Gebhart GF. Fos-like proteins in the lumbosacral spinal cord following noxious and non-noxious colorectal distention in the rat. Pain. 1992;49:393–403. doi: 10.1016/0304-3959(92)90247-9. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Stitt S, Gebhart GF. Attenuation of c-fos expression in the rat lumbosacral spinal cord by morphine or tramadol following noxious colorectal distention. Brain Res. 1995;701:175–182. doi: 10.1016/0006-8993(95)00990-5. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, et al. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Jeong HJ, Vaughan CW. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br J Pharmacol. 2009;157:371–380. doi: 10.1111/j.1476-5381.2009.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wultsch T, Painsipp E, Thoeringer CK, Herzog H, Sperk G, Holzer P. Endogenous neuropeptide Y depresses the afferent signaling of gastric acid challenge to the mouse brainstem via neuropeptide Y type Y2 and Y4 receptors. Neuroscience. 2005;136:1097–1107. doi: 10.1016/j.neuroscience.2005.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- Yu S, Gao G, Peterson BZ, Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am J Physiol. 2009;297:G34–G42. doi: 10.1152/ajpgi.00068.2009. [DOI] [PubMed] [Google Scholar]
- Yu S, Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am J Physiol. 2009;296:G255–G265. doi: 10.1152/ajpgi.90530.2008. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol. 2004;286:G983–G991. doi: 10.1152/ajpgi.00441.2003. [DOI] [PubMed] [Google Scholar]


