Abstract
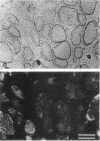
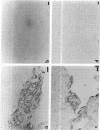
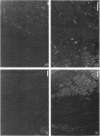
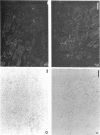
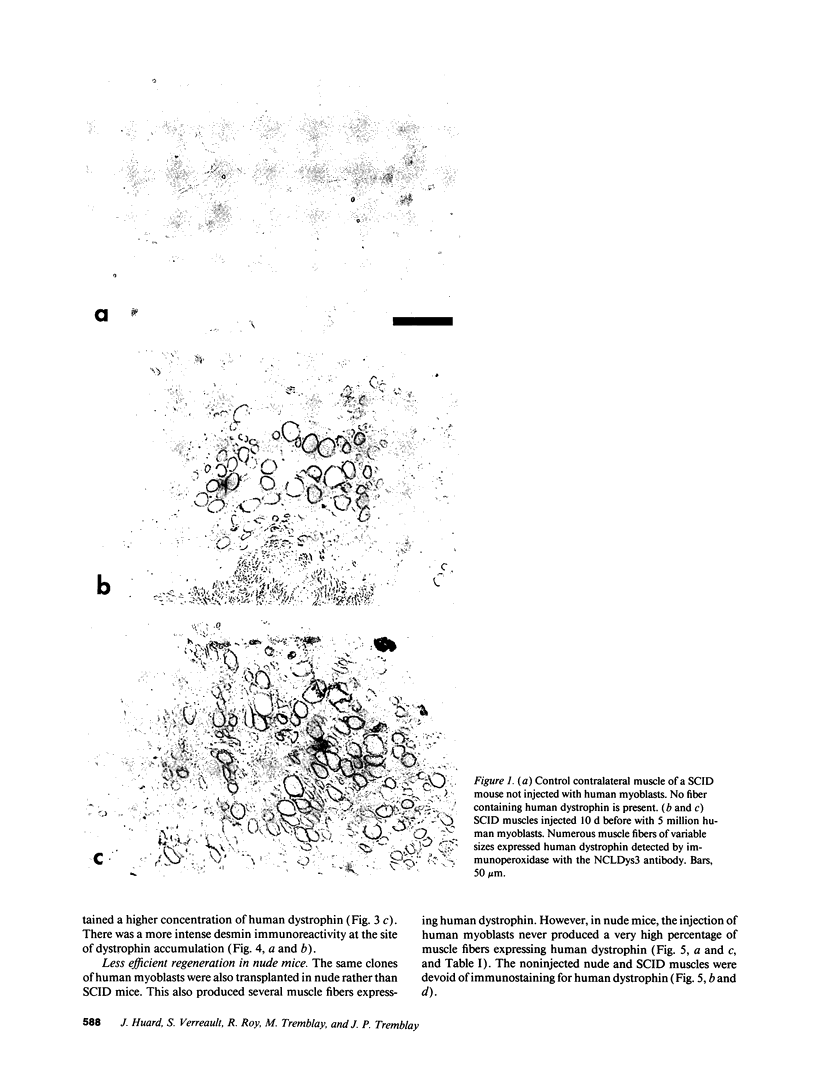
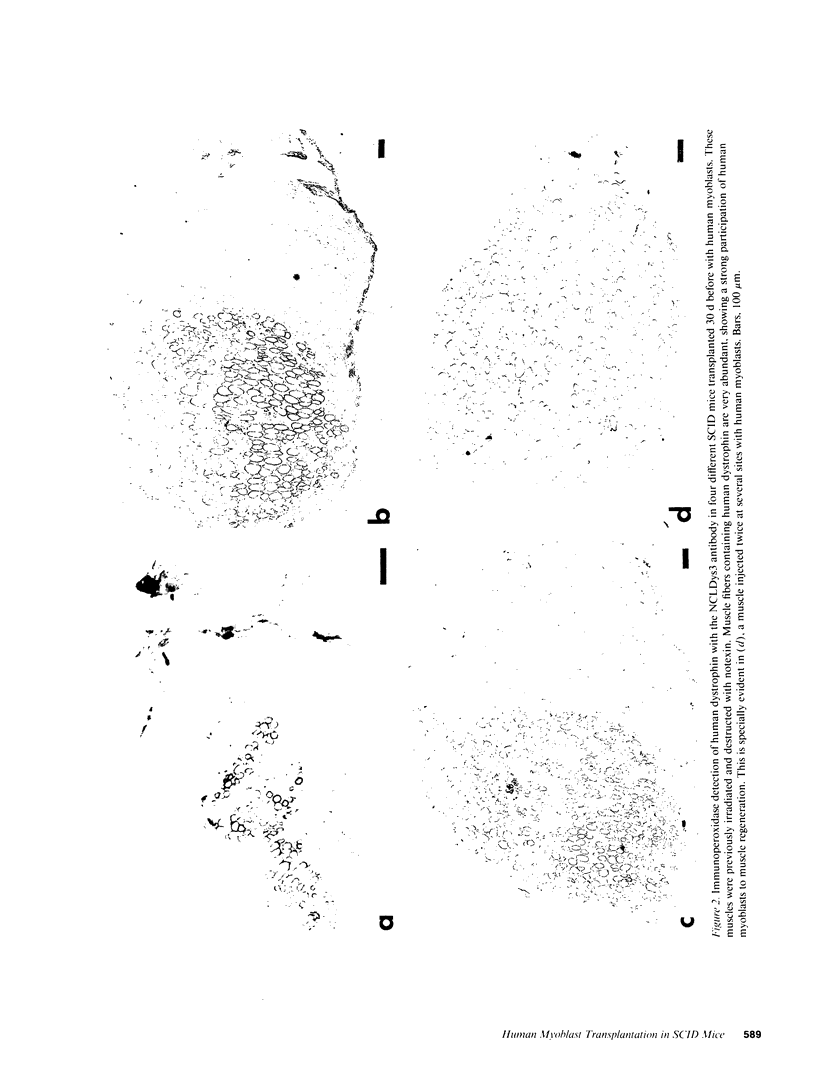
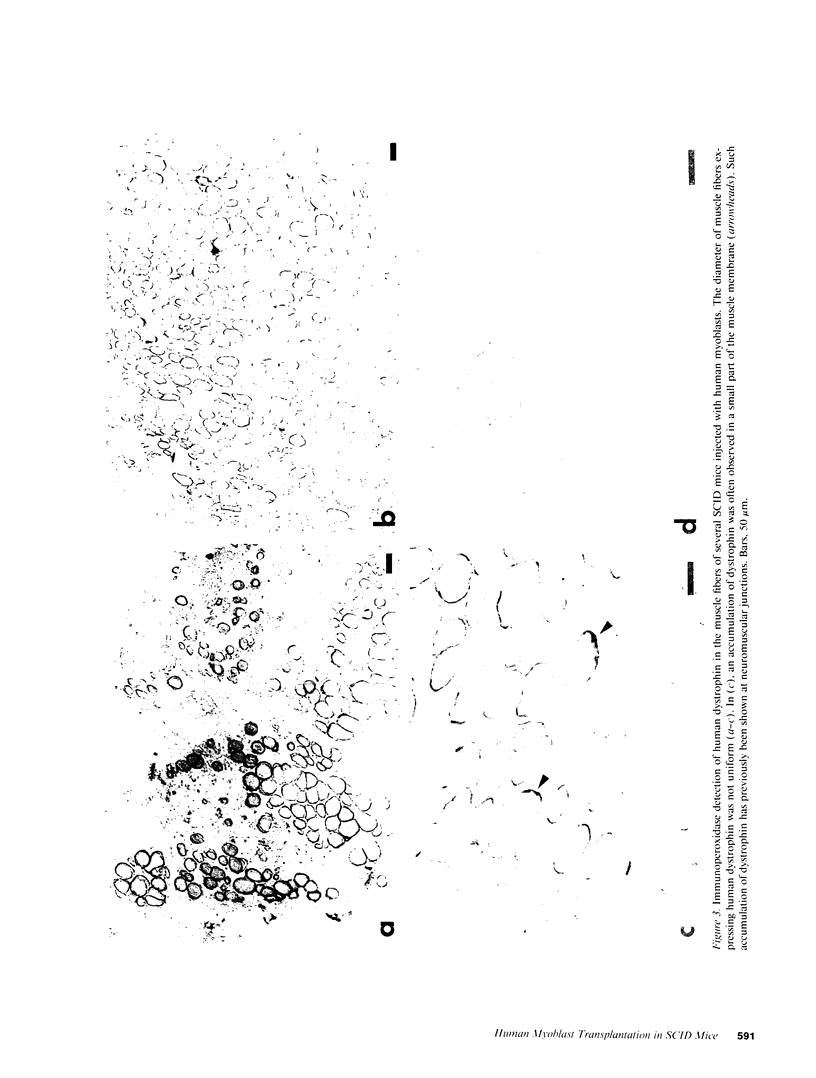
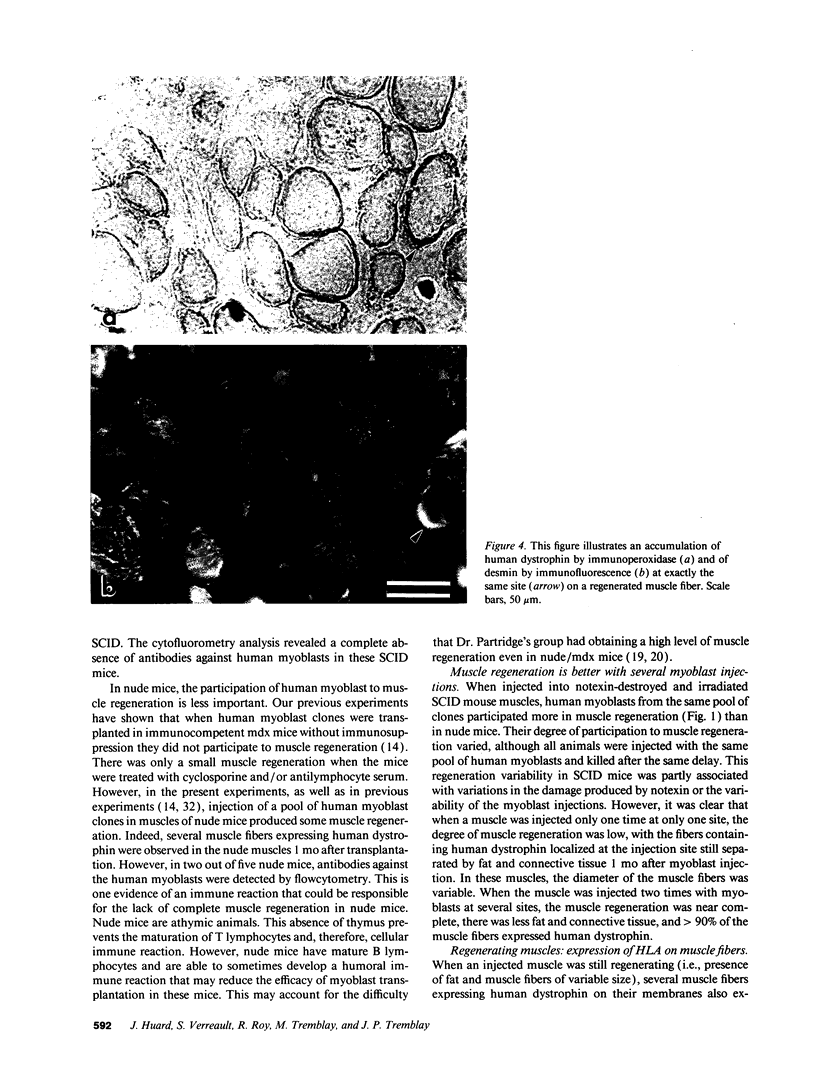
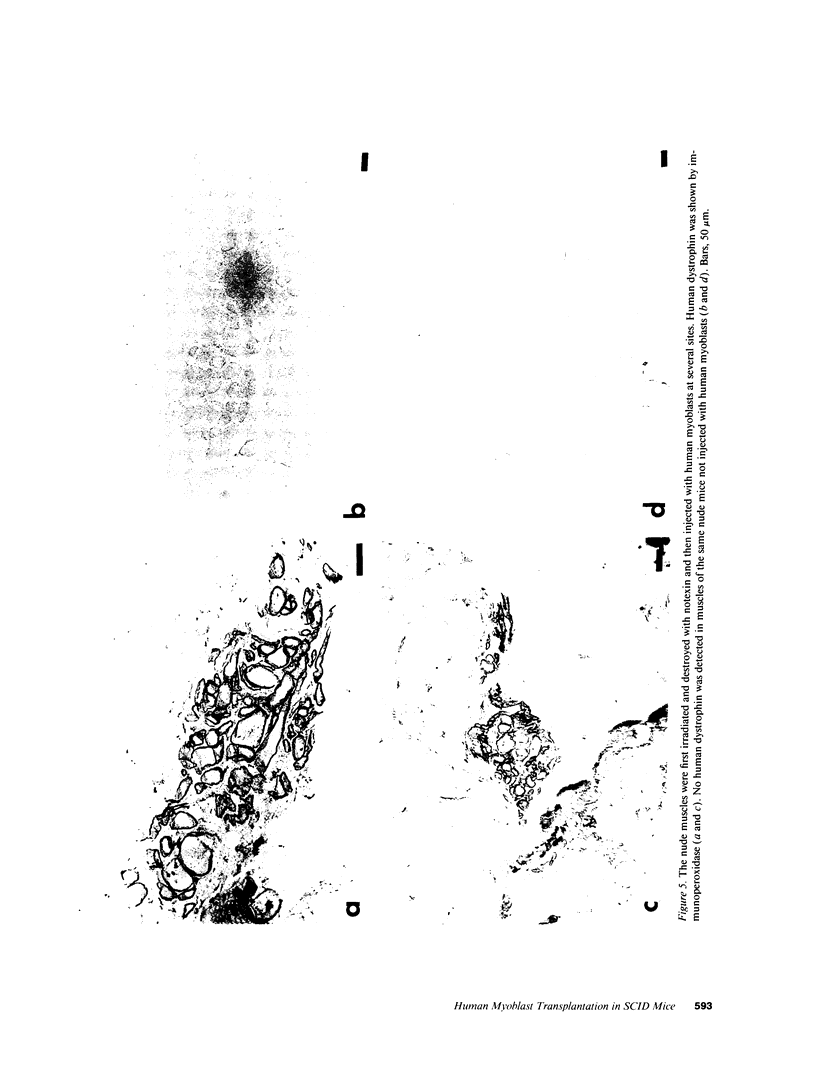
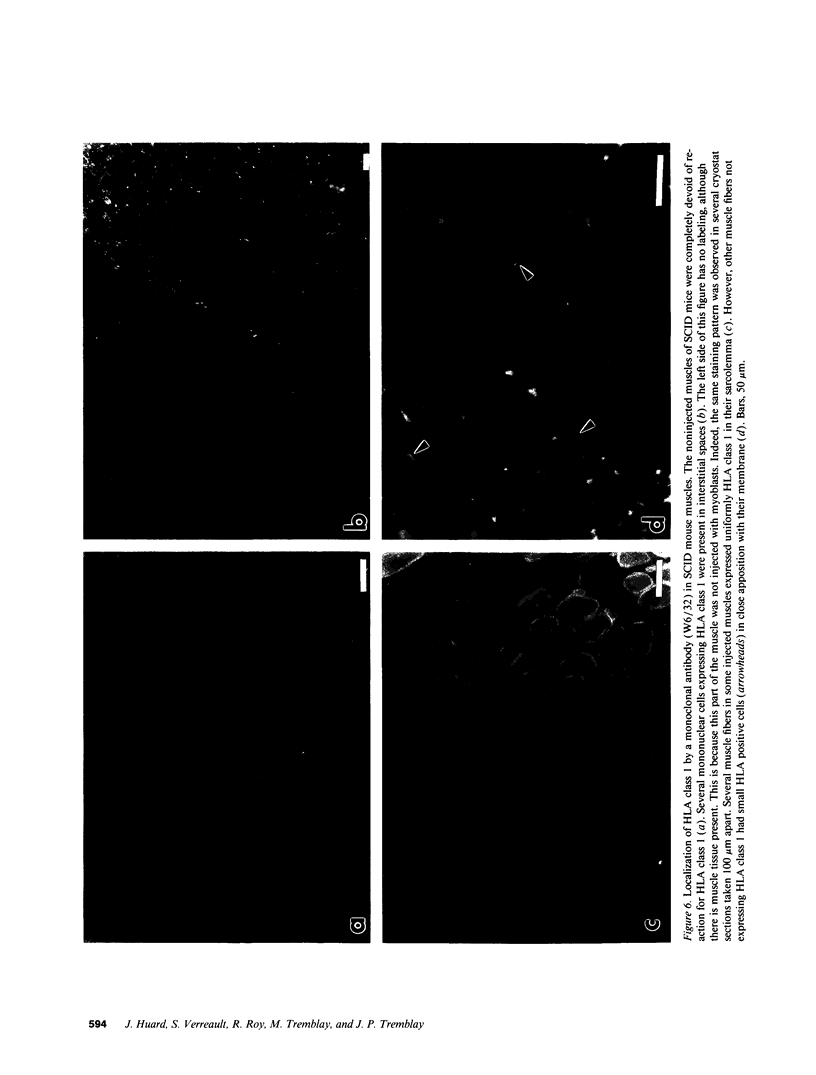
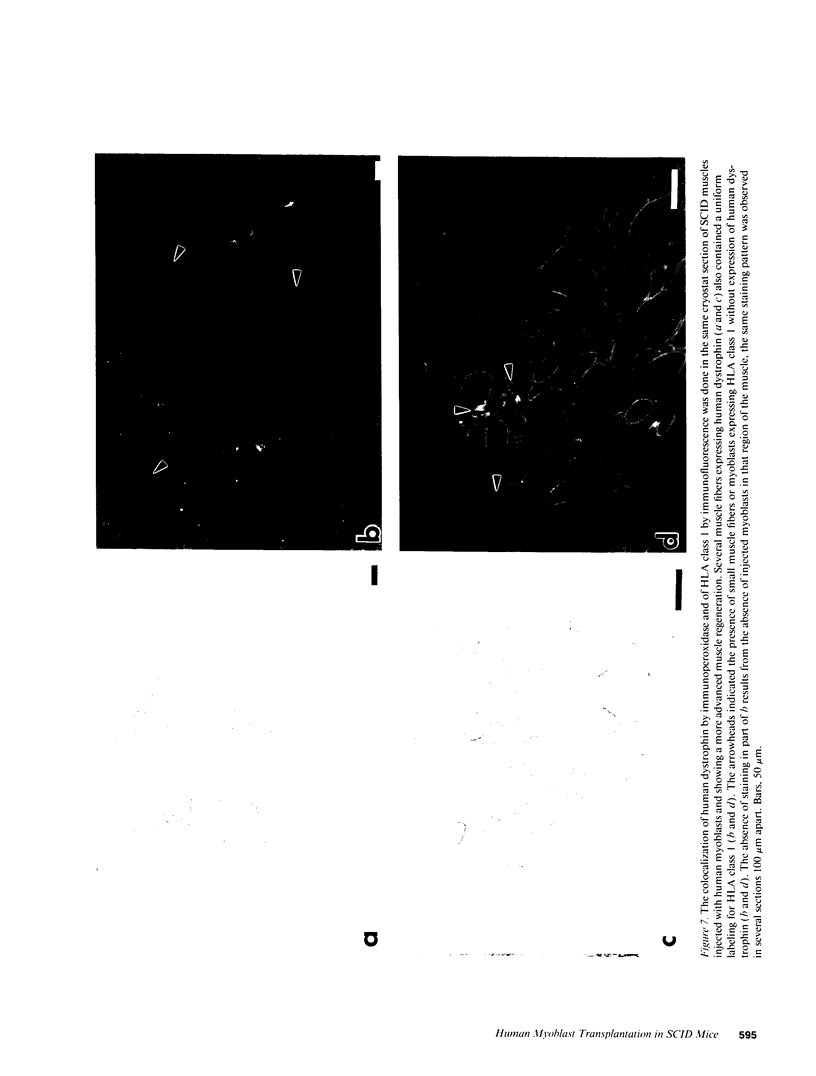
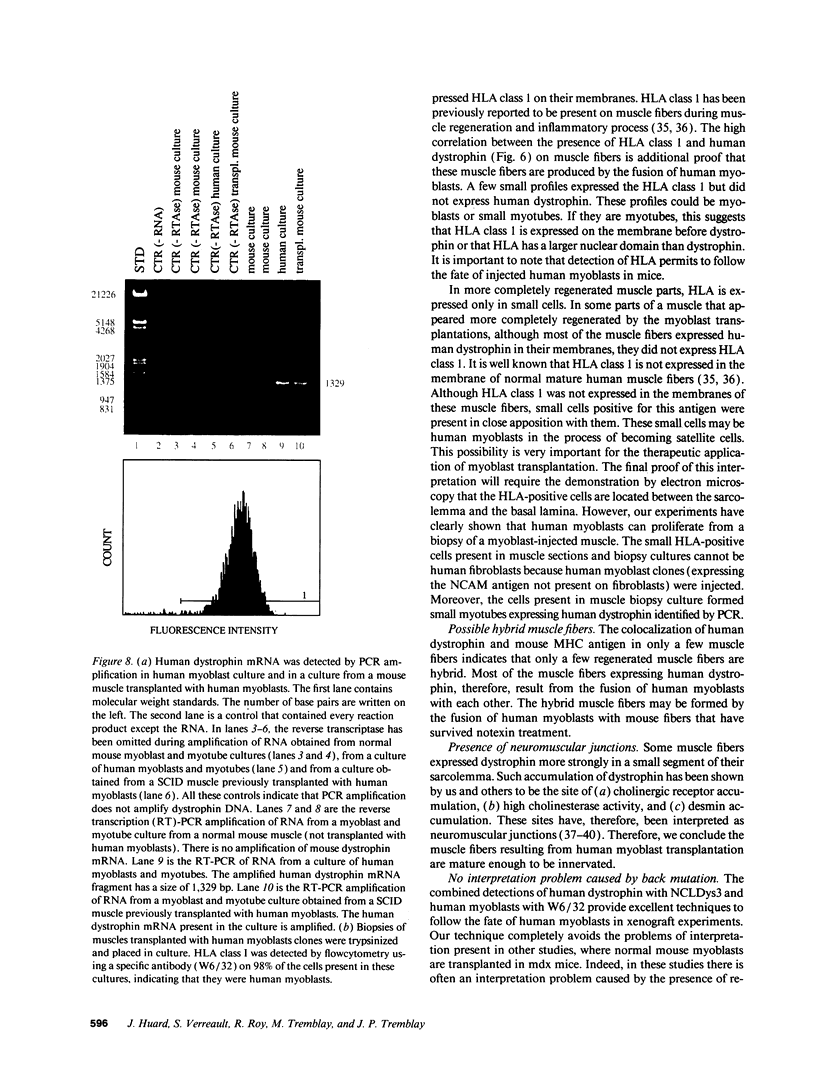
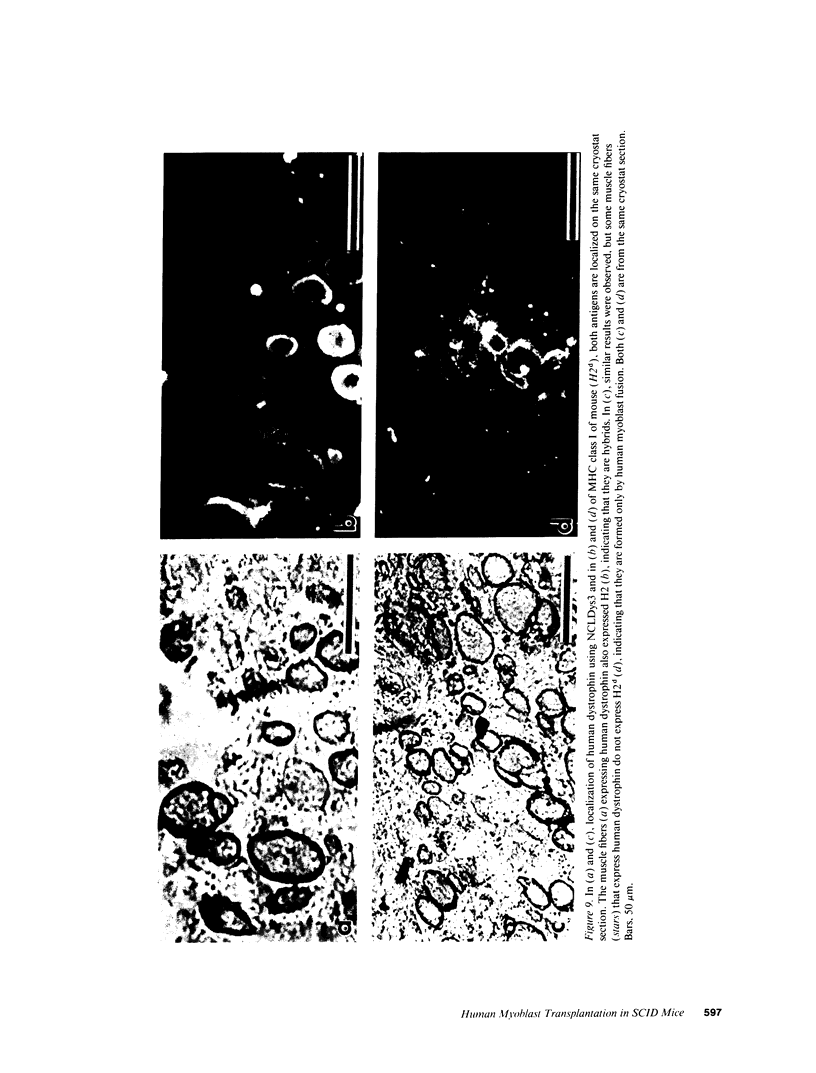
SCID mouse tibialis anterior muscles were first irradiated to prevent regeneration by host myoblasts and injected with notexin to damage the muscle fibers and trigger regeneration. The muscles were then injected with roughly 5 million human myoblasts. 1 mo later, 16-33% of the normal number of muscle fibers were present in the injected muscle, because of incomplete regeneration. However, > 90% of these muscle fibers contained human dystrophin. Some newly formed muscle fibers had an accumulation of human dystrophin and desmin on a part of their membrane. Such accumulations have been demonstrated at neuromuscular junctions before suggesting that the new muscle fibers are innervated and functional. The same pool of clones of human myoblasts produced only < or = 4% of muscle fibers containing human dystrophin when injected in nude mice muscles. Several of the human myoblasts did not fuse and remained in interstitial space or tightly associated with muscle fibers suggesting that some of them have formed satellite cells. Moreover, cultures of 98% pure human myoblasts were obtained from transplanted SCID muscles. In some mice where the muscle regeneration was not complete, the muscle fibers containing human dystrophin also expressed uniformly HLA class 1, confirming that the fibers are of human origin. The presence of hybrid muscle fibers containing human dystrophin and mouse MHC was also demonstrated following transplantation. These results establish that in absence of an immune reaction, transplanted human myoblasts participate to the muscle regeneration with a high degree of efficacy even if the animals were killed only 1 mo after the transplantation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Beam K. G. Duchenne muscular dystrophy. Localizing the gene product. Nature. 1988 Jun 30;333(6176):798–799. doi: 10.1038/333798a0. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Emslie-Smith A. M., Arahata K., Engel A. G. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol. 1989 Mar;20(3):224–231. doi: 10.1016/0046-8177(89)90128-7. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Pavlath G. K., Lanctot A. M., Sharma K. R., Miller R. G., Steinman L., Blau H. M. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992 Apr 2;356(6368):435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Ham R. G., St Clair J. A., Webster C., Blau H. M. Improved media for normal human muscle satellite cells: serum-free clonal growth and enhanced growth with low serum. In Vitro Cell Dev Biol. 1988 Aug;24(8):833–844. doi: 10.1007/BF02623656. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Johnson M. A. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin Exp Pharmacol Physiol. 1978 Nov-Dec;5(6):587–600. doi: 10.1111/j.1440-1681.1978.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Hudecki M. S., Rosenberg P. A., Pollina C. M., Kunkel L. M. Cell and fiber-type distribution of dystrophin. Neuron. 1988 Jul;1(5):411–420. doi: 10.1016/0896-6273(88)90191-2. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Knudson C. M., Campbell K. P., Kunkel L. M. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987 Dec 24;330(6150):754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Morgan J. E., Watkins S. C., Partridge T. A. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990 Oct;99(1):9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- Huard J., Bouchard J. P., Roy R., Malouin F., Dansereau G., Labrecque C., Albert N., Richards C. L., Lemieux B., Tremblay J. P. Human myoblast transplantation: preliminary results of 4 cases. Muscle Nerve. 1992 May;15(5):550–560. doi: 10.1002/mus.880150504. [DOI] [PubMed] [Google Scholar]
- Huard J., Fortier L. P., Dansereau G., Labrecque C., Tremblay J. P. A light and electron microscopic study of dystrophin localization at the mouse neuromuscular junction. Synapse. 1992 Feb;10(2):83–93. doi: 10.1002/syn.890100202. [DOI] [PubMed] [Google Scholar]
- Huard J., Roy R., Bouchard J. P., Malouin F., Richards C. L., Tremblay J. P. Human myoblast transplantation between immunohistocompatible donors and recipients produces immune reactions. Transplant Proc. 1992 Dec;24(6):3049–3051. [PubMed] [Google Scholar]
- Huard J., Tremblay G., Verreault S., Labrecque C., Tremblay J. P. Utilization of an antibody specific for human dystrophin to follow myoblast transplantation in nude mice. Cell Transplant. 1993 Mar-Apr;2(2):113–118. doi: 10.1177/096368979300200204. [DOI] [PubMed] [Google Scholar]
- Karpati G., Pouliot Y., Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol. 1988 Jan;23(1):64–72. doi: 10.1002/ana.410230111. [DOI] [PubMed] [Google Scholar]
- Karpati G., Pouliot Y., Zubrzycka-Gaarn E., Carpenter S., Ray P. N., Worton R. G., Holland P. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989 Jul;135(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Law P. K., Goodwin T. G., Fang Q., Duggirala V., Larkin C., Florendo J. A., Kirby D. S., Deering M. B., Li H. J., Chen M. Feasibility, safety, and efficacy of myoblast transfer therapy on Duchenne muscular dystrophy boys. Cell Transplant. 1992;1(2-3):235–244. doi: 10.1177/0963689792001002-305. [DOI] [PubMed] [Google Scholar]
- Law P. K., Goodwin T. G., Li H. J. Histoincompatible myoblast injection improves muscle structure and function of dystrophic mice. Transplant Proc. 1988 Jun;20(3 Suppl 3):1114–1119. [PubMed] [Google Scholar]
- Menke A., Jockusch H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature. 1991 Jan 3;349(6304):69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- Miike T., Miyatake M., Zhao J., Yoshioka K., Uchino M. Immunohistochemical dystrophin reaction in synaptic regions. Brain Dev. 1989;11(5):344–346. doi: 10.1016/s0387-7604(89)80067-1. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Bonilla E., Martucci G., Moraes C. T., Hays A. P., Dimauro S. Immunocytochemical study of dystrophin in muscle cultures from patients with Duchenne muscular dystrophy and unaffected control patients. Am J Pathol. 1988 Sep;132(3):410–416. [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Hoffman E. P., Partridge T. A. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol. 1990 Dec;111(6 Pt 1):2437–2449. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. E. Practical aspects of myoblast implantation: investigations on two inherited myopathies in animals. Adv Exp Med Biol. 1990;280:89–96. doi: 10.1007/978-1-4684-5865-7_11. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Watt D. J., Sloper J. C., Partridge T. A. Partial correction of an inherited biochemical defect of skeletal muscle by grafts of normal muscle precursor cells. J Neurol Sci. 1988 Sep;86(2-3):137–147. doi: 10.1016/0022-510x(88)90093-7. [DOI] [PubMed] [Google Scholar]
- Partridge T. A. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve. 1991 Mar;14(3):197–212. doi: 10.1002/mus.880140302. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Ray P. N., Belfall B., Duff C., Logan C., Kean V., Thompson M. W., Sylvester J. E., Gorski J. L., Schmickel R. D., Worton R. G. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature. 1985 Dec 19;318(6047):672–675. doi: 10.1038/318672a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. G., Barby T. F., Manners E., Bobrow M., Bentley D. R. Direct detection of dystrophin gene rearrangements by analysis of dystrophin mRNA in peripheral blood lymphocytes. Am J Hum Genet. 1991 Aug;49(2):298–310. [PMC free article] [PubMed] [Google Scholar]
- Salviati G., Betto R., Ceoldo S., Biasia E., Bonilla E., Miranda A. F., Dimauro S. Cell fractionation studies indicate that dystrophin is a protein of surface membranes of skeletal muscle. Biochem J. 1989 Mar 15;258(3):837–841. doi: 10.1042/bj2580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A., Huard J., Labrecque C., Tremblay J. P. Use of fluorescent latex microspheres (FLMs) to follow the fate of transplanted myoblasts. J Histochem Cytochem. 1993 Oct;41(10):1579–1582. doi: 10.1177/41.10.8245416. [DOI] [PubMed] [Google Scholar]
- Tremblay J. P., Malouin F., Roy R., Huard J., Bouchard J. P., Satoh A., Richards C. L. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993 Mar-Apr;2(2):99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- Tremblay J. P., Roy B., Goulet M. Human myoblast transplantation: a simple assay for tumorigenicity. Neuromuscul Disord. 1991;1(5):341–343. doi: 10.1016/0960-8966(91)90120-h. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Wernig A., Irintchev A., Härtling A., Stephan G., Zimmermann K., Starzinski-Powitz A. Formation of new muscle fibres and tumours after injection of cultured myogenic cells. J Neurocytol. 1991 Dec;20(12):982–997. doi: 10.1007/BF01187916. [DOI] [PubMed] [Google Scholar]
- Yasin R., Van Beers G., Nurse K. C., Al-Ani S., Landon D. N., Thompson E. J. A quantitative technique for growing human adult skeletal muscle in culture starting from mononucleated cells. J Neurol Sci. 1977 Jul;32(3):347–360. doi: 10.1016/0022-510x(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]