Abstract
Background and purpose:
Small (SKCa or KCa2) and intermediate (IKCa or KCa3.1) conductance calcium-activated potassium channels are involved in regulation of vascular tone and blood pressure. The present study investigated whether NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) and CyPPA (cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine), which are selective openers of SKCa and IKCa channels and of SKCa2 and SKCa3 channels, respectively, enhance endothelium-dependent vasodilatation in porcine retinal arterioles.
Experimental approach:
In porcine retinal arterioles, SKCa3 and IKCa protein localization was examined by immunolabelling. Endothelial cell calcium was measured by fluorescence imaging. For functional studies, arterioles with internal diameters of 116 ± 2 µm (n = 276) were mounted in microvascular myographs for isometric tension recordings.
Key results:
SKCa3 and IKCa protein was localized in the endothelium. Bradykinin, but not NS309 or CyPPA increased endothelial cell calcium. Pre-incubation with NS309 or CyPPA enhanced bradykinin relaxation without changing endothelial cell calcium. This enhanced relaxation was abolished by blocking SKCa channels with apamin. In the presence of NS309 or CyPPA, mainly inhibition of NO synthase with asymmetric dimethylarginine, but also inhibition of cyclooxygenase with indomethacin, reduced bradykinin relaxation. Bradykinin relaxation was completely abolished by NO synthase and cyclooxygenase inhibition together with a NO scavenger, oxyhaemoglobin.
Conclusions and implications:
In porcine retinal arterioles, bradykinin increases endothelial cell calcium leading to activation of SKCa and IKCa channels. Without altering endothelial cell calcium, NS309 and CyPPA open SKCa channels that enhance NO-mediated bradykinin relaxations. These results imply that opening SKCa channels improves endothelium-dependent relaxation and makes this channel a potential target for treatments aimed at restoring retinal blood flow.
Keywords: bradykinin, CyPPA, H2S, HOE-140, IKCa, NO, NS309, prostaglandins, retinal arterioles, SKCa, SNP, vasodilatation
Introduction
Disturbance in the blood supply to the retina is a common finding in retinal diseases leading to blindness. A disturbed blood supply affects the delivery of oxygen and metabolic substrates necessary for the maintenance of retinal structure and function. The vascular endothelium modulates the blood flow by smooth muscle relaxation through activation of the vasodilating pathways involving endothelial nitric oxide (NO) synthase, cyclooxygenase, and the calcium-activated potassium channels of small (SKCa or KCa2) and intermediate (IKCa or KCa3.1) conductance (Feletou and Vanhoutte, 2006; channel nomenclature follows Alexander et al., 2009). Activation of SKCa and IKCa channels is generally acknowledged as a required component for the activation of the relaxation mediated by endothelium-dependent hyperpolarizing factor (EDHF) (Burnham et al., 2002; Bychkov et al., 2002; Eichler et al., 2003), but SKCa and IKCa channel activation has also been associated with activation of NO synthase (Stankevicius et al., 2006; Sheng and Braun, 2007; Brahler et al., 2009; Sheng et al., 2009). Supporting these findings, in vivo studies in mice show that doxycycline-induced suppression of the SKCa3 channel (SKCa3T/T + Dox) (Taylor et al., 2003), knockout of the IKCa channel (IKCa−/−) (Si et al., 2006) and deficit of both SKCa3T/T+ Dox and IKCa−/− (Brahler et al., 2009) leads to elevated blood pressure. In addition, opening SKCa and IKCa channels decreases myogenic tone, increases acetylcholine-induced relaxation in rat cremaster arterioles (Sheng et al., 2009), and restores the attenuated EDHF-type relaxation in mesenteric small arteries from Zucker diabetic fatty (ZDF) rats (Brondum et al., 2009). Moreover, opening of IKCa channels decreases mean arterial blood pressure in angiotensin II-induced hypertensive mice (Sankaranarayanan et al., 2009). These results suggest that SKCa and/or IKCa channels are very important in the regulation of endothelium-dependent vasodilatation, and hence in controlling blood pressure and organ blood flow.
Patients with retinal diseases, such as glaucoma and diabetes, have a reduced ocular blood flow that in part is caused by endothelial dysfunction and a reduced release of vasodilating factors (Schmetterer et al., 1997; Delles et al., 2004; Resch et al., 2009). We have previously found that in retinal arterioles, the endothelium-dependent relaxation induced by the endogenous agonist, bradykinin, is mainly mediated by NO (Jeppesen et al., 2002; Dalsgaard et al., 2009), and that blocking SKCa and IKCa channels reduces this relaxation (Dalsgaard et al., 2009). Thus, opening of SKCa and/or IKCa channels may enhance endothelium-dependent relaxation and increase retinal blood flow, which may be beneficial for these patients.
The present study hypothesized that opening of endothelial cell SKCa and IKCa channels would enhance endothelium-dependent relaxation in retinal arterioles. To address this hypothesis, localization of SKCa3 and IKCa protein was investigated in porcine retina. Moreover, porcine retinal arterioles were isolated and bradykinin-induced relaxation was evaluated in the presence of NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) (Strobaek et al., 2004), an opener of SKCa and IKCa channels, and CyPPA (cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine), an opener of SKCa2 and SKCa3 channels (Hougaard et al., 2007). To address the mechanisms involved in the enhanced relaxation, we measured the effect on endothelial cell calcium of bradykinin, NS309, and CyPPA. We also determined whether NS309 and CyPPA directly affect the bradykinin receptor and whether increased formation of NO, prostaglandins or an EDHF-type response are involved in the enhanced bradykinin relaxation in the presence of NS309 and CyPPA. As we found no evidence for involvement of EDHF-type relaxation in the enhanced bradykinin relaxation, we investigated the role of applying different preconstrictors on bradykinin relaxation, and whether hydrogen sulphide (H2S) contributes to bradykinin relaxation.
Methods
Tissue preparation
Eyes from pigs of approximately 6 months of age and a weight of 85–90 kg were obtained from a local abattoir. From each animal, one eye was removed immediately after the pig had been exposed to carbon dioxide (CO2) and killed by exsanguination. The eyes were transferred to a container with cold (4°C) physiological saline solution (PSS) [composition (mM): 4.8 KCl, 1.14 MgSO4, 118 NaCl, 25 NaHCO3, 5 HEPES, 5.5 glucose and 1.6 CaCl2] and transported to the laboratory, where they were dissected and the retinas isolated as previously described (Jeppesen et al., 2002). In brief, the eyes were bisected by a frontal section, the vitreous was removed and the retina was detached from the underlying pigment epithelium. An arteriolar segment located approximately 1–2 mm from the optic disk with a length of ∼ 2 mm was dissected from the retina.
Histology of arteriolar segments and immunohistochemistry of SKCa3 and IKCa protein
For histological and immunohistochemical processing, arteriolar segments with surrounding retinal tissue were fixed in cold (4°C) 4% paraformaldehyde, pH 7.0 for 1 h and placed in 8% agar prior to embedding in paraffin. Longitudinal sections of 3 µm were cut on a microtome, placed on glass slides, heated at 80°C for 1 h, and deparaffinized in xylene and decreasing concentrations of ethanol. For histology, sections were stained with haematoxylin and 0.1% w/v eosin, and dehydrated in increasing concentrations of ethanol. For evaluation of SKCa3 and IKCa protein distribution, sections were incubated with 0.3% H2O2 for 20 min, rinsed with phosphate buffered saline (PBS: 1.5 M NaCl, 0.5 M NaH2PO4), transferred to TEG-buffer (10 mM Tris, 0.5 mM EGTA, pH 9.0), heated in the microwave for 2 × 7 min and rinsed with PBS. Thereafter, sections were transferred to 0.25% Triton X solution for 15 min and rinsed with PBS afterwards. Sections were incubated with 10% fetal calf serum in PBS with 1% bovine serum albumin (BSA) for 30 min in order to block non-specific antibody binding. Incubation with the primary antibodies for SKCa3 protein (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and IKCa protein (1:400, Alomone, Jerusalem, Israel) in PBS containing 1% BSA was performed overnight in a humidified chamber at 4°C. Negative controls for each sample section were without primary antibody. Sections were rinsed with PBS and incubated for 1 h at room temperature with secondary antibody, goat anti-rabbit IgG coupled to Alexa 488 (1:1000, Molecular Probes Inc., Eugene, OR, USA) with PBS containing 1% BSA for 1 h followed by rinsing with PBS. Finally, antifade solution (Bio-Rad Laboratories Ltd., Hemel Hempstead, UK) was added and a cover slip was mounted. Preparations were analysed on an inverted confocal microscopy (LSM 510 Meta, Carl Zeiss Inc, Oberkochen, Germany) equipped with an oil immersion objective (C-Aprochromat 63 × NA = 0.75) excited at a wavelength of 488 nm using the Zeiss LSM Image Browser software program (Zeiss Inc, Oberkochen, Germany).
Calcium measurements
Mounting, loading and normalization
Arteriolar segments were mounted on two stainless steel wires 20 µm in diameter in a confocal myograph (model 120CW, Danish Myo Technology, Aarhus, Denmark) and loaded with a buffer consisting of the visible light excitable calcium indicator; Oregon Green BAPTA 1-AM (7.24 µM), the nonoinic surfactants; Cremophor EL [0.066% (w/v)], and Pluronic F-127 (10.66 µM), and dimethylsulphoxide [DMSO, 0.32% (w/v)] at room temperature (21°C). The loading buffer was introduced to the luminal side of the segments and loading time was 45 min. After loading, each segment was allowed to equilibrate for 30 min at 37°C in PSS. The solutions were equilibrated with bioair of the following composition: 5% CO2, 21% O2 and 74% N2. Subsequently, they were normalized in PSS without CaCl2 (PSS0.0) to an internal circumference corresponding to 94% of the tone at a transmural pressure of 70 mmHg (Hessellund et al., 2003). After normalization, PSS0.0 was replaced with PSS, and the segments were allowed to equilibrate for another 30 min. Image acquisition of endothelial cell calcium was performed with an inverted confocal microscope (model LSM 510 Exciter, Carl Zeiss Inc, Oberkochen, Germany) equipped with a 63 × NA = 0.75 long working distance objective excited at a wavelength of 488 nm using the Zeiss LSM Image Browser software program (Zeiss Inc, Oberkochen, Germany). A fluorescent signal in the first cell layer towards the luminal side, the longitudinally orientation of this cell layer in the arteriolar segment, and an increased fluorescence upon addition of bradykinin was used to confirm successful loading of endothelial cells. In contrast, abluminal loading of smooth muscle cells showed perpendicular orientation in the segment, an increase in calcium upon addition of the thromboxane analogue, U46619 (9,11-dideoxy-11a,9a-epoxymethano-prostaglandin F2a) and a decrease in signal upon addition of bradykinin (data not shown).
Experimental protocol
After equilibration, the calcium signal in endothelial cells was recorded before and after addition of bradykinin (0.1, 1, 3 and 10 nM), NS309 (1 µM), NS309 (1 µM) + bradykinin (3 nM), CyPPA (6 µM) or CyPPA (6 µM) + bradykinin (3 nM). Bradykinin (3 nM), NS309 (1 µM) and CyPPA (6 µM) were the concentrations leading to approximately 50% relaxation in U46619 (0.1 µM)-contracted segments.
Functional studies
Mounting and normalization
An isolated arteriolar segment was transferred to a chamber of a dual wire myograph system (model 410A, Danish Myo Technology, Aarhus, Denmark) for isometric tension recording using the Chart5 software programme (ADInstruments Ltd., Oxfordshire, UK). Each arteriole was mounted in the wire myograph using two tungsten wires 25 µm in diameter and was allowed to equilibrate for 30 min at 37°C after which it was normalized as described above. A total of 276 arteriolar segments were used for the functional studies, where the internal diameter of the arterioles averaged 116 ± 2 µm.
Control of viability
After equilibration, smooth muscle cell viability was tested by addition of PSS, where equimolar concentration of NaCl was replaced with KCl giving a concentration of 60 mM K+ (KPSS), and U46619 (0.1 µM) to test contraction induced by depolarization and by activation of the thromboxane A2 receptor, followed by exposure to U46619 (0.1 µM) alone. Endothelium-dependent relaxation was tested by addition of bradykinin (0.03 µM) in U46619 (0.1 µM)-contracted arterioles. Arterioles were discarded, if U46619 contraction was less than 0.25 Nm−1, or if bradykinin relaxation was less than 50% (Dalsgaard et al., 2009).
Experimental protocol
After control of viability, segments were contracted with U46619 (0.1 µM) and concentration–response curves were obtained by cumulative additions of bradykinin (0.01 nM–300 nM) alone or in the presence of NS309 (1 µM), CyPPA (6 µM) or a NO donor, sodium nitroprusside (SNP, 1 µM). Moreover, concentration–response curves for NS309 (0.1–10 µM), CyPPA (1–100 µM), NO solution (0.01–3 µM), hydrogen sulphide (H2S, 0.01–10 mM) and sodium nitroprusside (SNP, 0.01–100 µM) were obtained. To investigate the contribution of different enzymes and channels in the concentration–response experiments, 30 min prior to contraction, segments were incubated with the NO synthase inhibitor, asymmetric dimethylarginine (ADMA, 300 µM) (Stankevicius et al., 2006), the cyclooxygenase inhibitor, indomethacin (3 µM) (Stankevicius et al., 2006), the NO-scavenger, oxyhaemoglobin (25 µM) (Simonsen et al., 1999), the IKCa channel blocker, charybdotoxin (ChTx, 0.1 µM) (Alexander et al., 2009), the SKCa channel blocker, apamin (0.5 µM) (Alexander et al., 2009), the bradykinin receptor B2 antagonist, icatibant acetate (HOE-140, 0.1, 1, 10 and 100 nM) (Tirapelli et al., 2007), the cystathionine γ-lyase inhibitor, D,l-propargylglycine (PPG, 5 mM) (Zhao et al., 2003; Yang et al., 2008) or a combination of these inhibitors or blockers. In a series of experiments, the arterial segments were contracted with KPSS (125 mM) or endothelin-1 (0.03 µM) instead of U46619 (0.1 µM). In another series of experiments, the endothelium was mechanically removed by introducing tungsten wires 25 µm in diameter into the lumen of the mounted segment. Removal of the endothelium was considered successful, if U46619 (0.1 µM) induced a contraction of more than 0.25 Nm−1, and bradykinin (0.03 µM) subsequently induced a relaxation of less than 10%.
Data analysis
For calcium measurements, 5–10 endothelial cells were selected on each isolated arterial segment and marked with a region of interest. For each region of interest, the Oregon Green fluorescent signal was determined after each treatment, minus the Oregon Green fluorescent signal before treatment.
In the functional studies, the tension in the peak response after each addition of bradykinin or NO solution, and the stable tension after each addition of NS309, CyPPA, H2S or SNP were expressed as percentage of the active tension, where the active tone was defined as the level of contraction after addition of U46619, minus the level of contraction after the normalization procedure. For bradykinin curves obtained in the presence of NS309, CyPPA or SNP, the active tone was defined as the level of contraction after addition of U46619 and NS309, CyPPA or SNP, minus the level of contraction after the normalization procedure.
Myogenic tone was calculated as the level of contraction 30 min after addition of blocker/inhibitor minus the level of contraction after the normalization procedure for bradykinin concentration–response curves. EC50 values were calculated by nonlinear regression for a variable slope, where minimum and maximum relaxation was set to 0% and 100%, respectively, using the GraphPad Prism 5.02 program (GraphPad software Inc., La Jolla, CA, USA) for bradykinin, NO solution, H2S, NS309 and CyPPA concentration–response curves. Maximum relaxation was determined for bradykinin, NO solution, H2S, NS309 and CyPPA concentration–response curves.
Statistical analysis
All data are presented as mean ± SEM with a significance level of P < 0.05, where n represents the number of segments from individual animals.
A Wilcoxon matched pair test was used to test for differences in the intracellular concentration of calcium in endothelial cells. A two-way anova was used to test for differences in concentration–response curves and a Student's t-test was used to test for differences in myogenic tone, U46619 contraction, EC50 and maximum relaxation. All data were analysed using GraphPad Prism 5.02 software (GraphPad software Inc., La Jolla, CA, USA).
Materials
For histological and immunohistochemical preparations, xylene, Meyer's haematoxylin and eosin were purchased at Bie & Berntsen, Herlev, Denmark. Fetal calf serum was purchased at Biochrom, Berlin, Germany. Triton X and BSA were purchased at Sigma Aldrich, St. Louis, MO, USA.
For calcium measurements, DMSO, Chremophore EL and Pluronic F-127 were purchased from Sigma Aldrich, St. Louis, MO, USA, while Oregon Green Bapta 1-AM was obtained from Invitrogen, Costa Mesa, CA, USA.
For the functional studies, U46619 (9,11-dideoxy-11a,9a-epoxymethano-prostaglandin F2a), endothelin-1, HOE-140 (icatibant acetate), PPG (D,l-propargylglycine), ADMA (asymmetric dimethylarginine), indomethacin, haemoglobin (bovine), bradykinin, SNP (sodium nitroprusside) and NaSH (sodium hydrosulfide hydrate) were purchased from Sigma Aldrich, St. Louis, MO, USA. Apamin and charybdotoxin were purchased from Latoxan, Valence, France. NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) and CyPPA (cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine), were kindly donated by Dr Søren-Peter Olesen, Neurosearch A/S, Ballerup, Denmark.
U46619 was dissolved in ethanol, NS309 and CyPPA in DMSO (99%), apamin and bradykinin in distilled water, charybdotoxin and indomethacin in PSS0.0. Apamin, bradykinin and charybdotoxin were prepared in 2% albumin-coated Eppendorf tubes.
Oxyhaemoglobin was prepared from commercial haemoglobin by addition of 10 mM sodium dithionite (Na2S2O4, Sigma Aldrich, St. Louis, MO, USA). The reducing agent converting methaemoglobin to oxyhaemoglobin was removed by 2 h of dialysis against 100 volumes of PSS0.0 equilibrated with N2 at 4°C. The purity of the oxyhaemoglobin solution was determined spectrophotometrically and gave a final concentration of 1 mM (Simonsen et al., 1995).
Nitric oxide solution was prepared from deoxygenated (argon-bubbled) distilled water, contained in glass vials with a septum closure. The argon gas was led through a 10 mM pyrogallol (1,2,3-trihydroxybenzene, Sigma Aldrich, St. Louis, MO, USA) solution to remove traces of oxygen. Pure NO gas, bubbled through sodium hydroxide (NaOH, 10 mM) to remove higher nitrogen oxides, was then bubbled through the deoxygenated water for 10 min at room temperature (20°C), giving a concentration of 1.9 mM, which was then diluted to 0.19 and 0.019 mM. The dissolved NO gas in solution was withdrawn from the stock solution using gas-tight Hamilton microlitre syringes (Simonsen et al., 1999). H2S was prepared by dissolving NaSH in PSS0.0 and was titrated to pH ∼7.4 with HCl shortly before experimentation (Olson et al., 2008).
Results
Localization of SKCa3 and IKCa protein
In arteriolar segments fixed with surrounding retinal tissue, SKCa3 and IKCa immunoreactions were observed in the vascular endothelium, but not in the vascular smooth muscle layer (Figure 1). Moreover, in the retina, SKCa3 immunoreaction was observed in a few single cells, whereas IKCa immunoreaction was observed in the majority of all retinal cells (data not shown).
Figure 1.
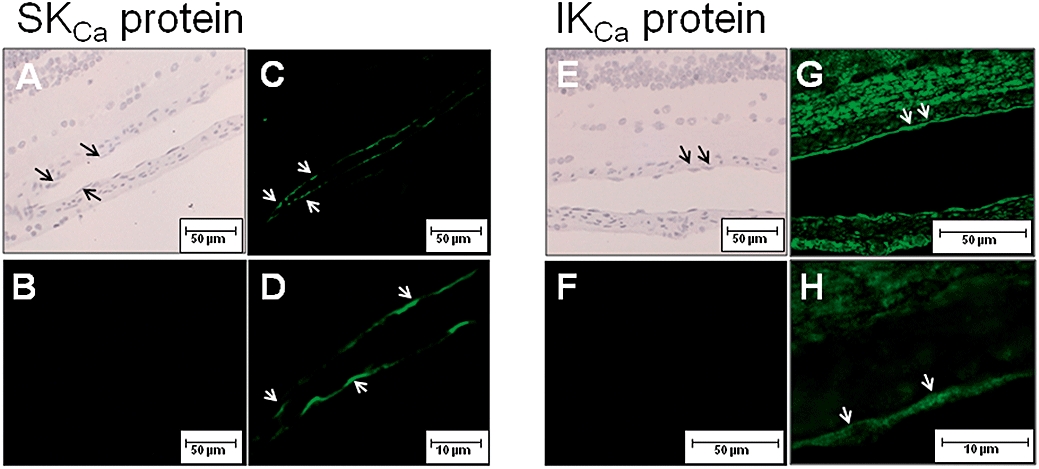
H&E staining (A, E) and localization of SKCa3 protein (C, D) and IKCa protein (G, H) by immunoreaction (green fluorescence, arrows) in retinal arterioles. B and F are negative controls without primary antibody. D and H are enlargements of C and G. The scale bars in A-C, E-F are 50 µm, and in D and H 10 µm.
Mechanisms involved in NS309 and CyPPA relaxation
In order to evaluate the selectivity of NS309 and CyPPA for SKCa and IKCa channels, concentration–response curves for NS309 and CyPPA were performed. Both NS309 (0.1–10 µM) and CyPPA (1–100 µM) induced concentration-dependent relaxations (EC50: 1.1 ± 0.5 µM and 7.5 ± 2.9 µM, maximum relaxation: 93 ± 3% and 96 ± 2%, n = 7 respectively) (Figure 2A,B). Removal of the endothelium or blocking SKCa channels with apamin (0.5 µM, n = 7) reduced NS309 and CyPPA relaxation (n = 7) (Figure 2A,B), and we have previously shown that blocking IKCa channels with charybdotoxin (0.1 µM, n = 7) has no effect on NS309 relaxation (Dalsgaard et al., 2009). To investigate whether NS309 and CyPPA in addition to opening potassium channels also have other effects, segments were contracted with 125 mM KPSS. In KPSS-contracted segments only high concentrations of NS309 (>6 µM) and CyPPA (>20 µM) induced relaxations (n = 7) (Figure 2A,B). Moreover, blocking SKCa channels (n = 7), removal of the endothelium (n = 7) or contraction with KPSS (125 mM) (n = 7) reduced CyPPA relaxations to similar levels (Figure 2B). NS309 relaxation in KPSS-contracted segments was reduced more than in segments where the SKCa channels were blocked (n = 7) or where the endothelium was removed (n = 7) (Figure 2A).
Figure 2.
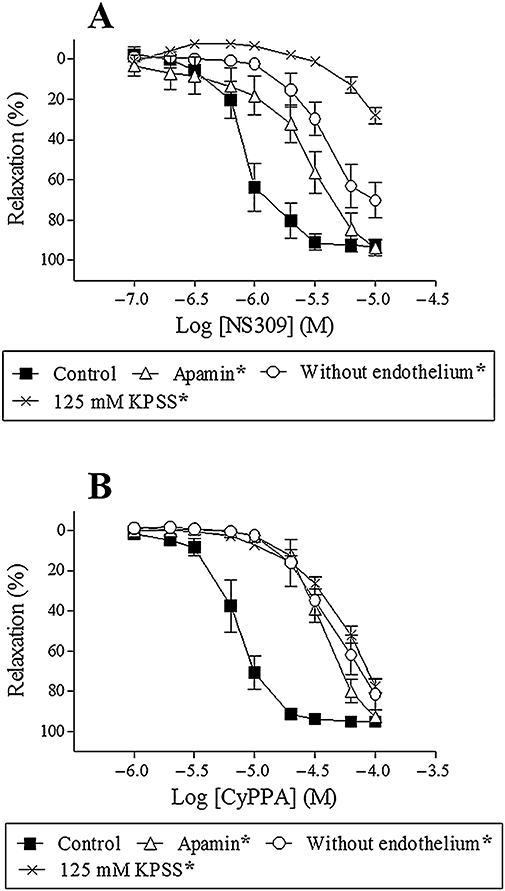
NS309 relaxation (A) or CyPPA relaxation (B) in the absence or presence of apamin (0.5 µM), in segments without endothelium, or contracted with 125 mM KPSS. Results are mean ± SEM (n = 7). Two-way anova. *P < 0.05 from control.
To address the relative contribution from NO and prostaglandins to CyPPA relaxation, segments were incubated with ADMA (300 µM) to inhibit NO synthesis, indomethacin (3 µM) to inhibit prostaglandin synthesis, and oxyhaemoglobin (25 µM), to scavenge NO. ADMA alone (n = 7) or in combination with indomethacin (n = 7) and/or oxyhaemoglobin (n = 7) reduced CyPPA relaxation. Indomethacin alone had no effect on CyPPA relaxation (n = 7) (Figure 3). We have previously found that NS309 relaxation is mainly mediated by NO (Dalsgaard et al., 2009).
Figure 3.
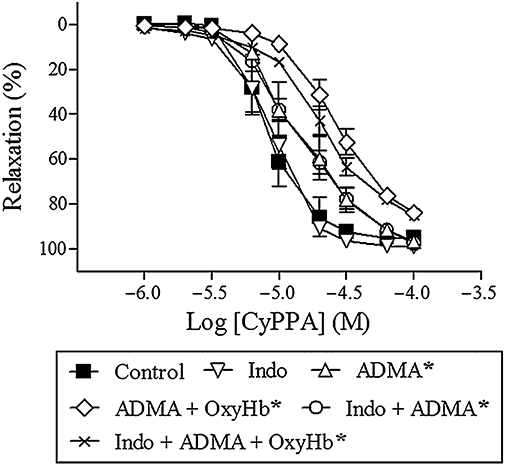
CyPPA relaxation in the presence of asymmetric dimethylarginine (300 µM, ADMA), ADMA + oxyhaemoglobin (25 µM, OxyHb), indomethacin (3 µM, Indo), Indo + ADMA and Indo + ADMA + OxyHb in porcine retinal arterioles contracted with U46619 (0.1 µM). Results are mean ± SEM (n = 7). Two-way anova. *P < 0.05 from control.
To address whether NS309 or CyPPA activated the bradykinin B2 receptor, segments were incubated with the bradykinin B2 receptor antagonist, HOE-140. This antagonist induced concentration-dependent reductions in bradykinin relaxation (n = 6–8) (Figure 4A). In contrast, HOE-140 (10 nM) failed to change NS309 (n = 7) or CyPPA relaxation (n = 7) (Figure 4B,C).
Figure 4.
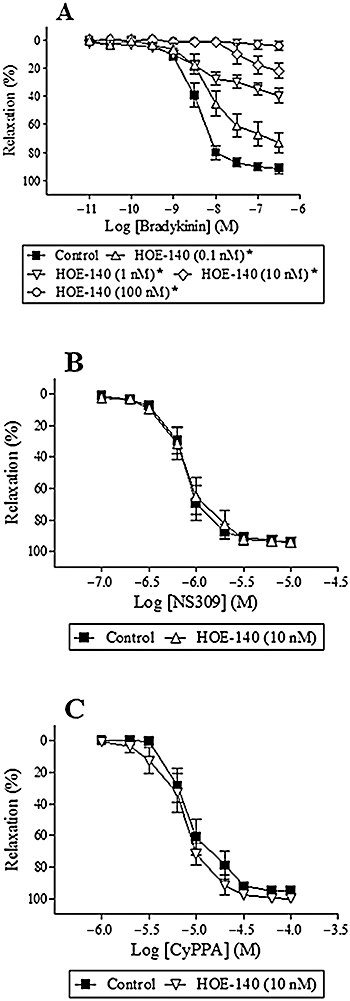
Effect of the bradykinin-receptor B2 antagonist, HOE-140 (0.1, 1.0, 10 and 100 nM), on bradykinin relaxation (A), HOE-140 (10 nM) on NS309 relaxation (B) and HOE-140 (10 nM) on CyPPA relaxation (C) in porcine retinal arterioles contracted with U46619 (0.1 µM). Results aremean ± SEM (n = 6–8). Two-way anova. *P < 0.05 from control.
Effect of NS309 and CyPPA on bradykinin relaxation
In U46619 (0.1 µM)-contracted segments, bradykinin (0.01 nM–300 nM) induced concentration-dependent relaxations (Figure 5A). To investigate the effect of opening SKCa and IKCa channels or opening SKCa channels alone on bradykinin relaxation, NS309 (1 µM) or CyPPA (6 µM) was added to the organ bath after the segments were contracted with U46619 (0.1 µM) and then concentration–response curves for bradykinin were performed. Addition of a single concentration of NS309 (1 µM) or CyPPA (6 µM) induced a relaxation of 14 ± 6% (n = 8) and 18 ± 8% (n = 7) respectively (Figure 5C,D). To exclude the effect of the reduced contraction level after addition of NS309 or CyPPA on bradykinin relaxation, a single concentration of the NO donor, SNP (1 µM), was used to reduce the contraction to the same level as with NS309 or CyPPA without activating the SKCa and IKCa channels. Addition of a single concentration of SNP (1 µM) induced a relaxation of 15 ± 8% (n = 7) (Figure 5B), and had no effect on bradykinin relaxation (n = 7) (Figure 6A). In contrast, both NS309 and CyPPA enhanced bradykinin relaxation (n = 7–8) (Figure 6A). Incubation with NS309 (1 µM) or CyPPA (6 µM) did not affect relaxation to SNP (n = 7) (Figure 6B). Blocking IKCa channels with charybdotoxin (0.1 µM, n = 8) had no effect on the enhanced bradykinin relaxation induced by NS309 (Figure 6C). In contrast, blocking SKCa channels with apamin (0.5 µM, n = 7–8) abolished the enhanced bradykinin relaxation induced by NS309 or CyPPA (Figure 6C,D). Myogenic tone after addition of apamin and/or charybdotoxin, U46619 (0.1 µM) contraction, EC50-values and maximum relaxation for bradykinin after addition of a single concentration of SNP (1 µM), NS309 (1 µM) and CyPPA (6 µM) are reported in Table 1. No increase in myogenic tone was found after treatment with apamin and/or charybdotoxin.
Figure 5.
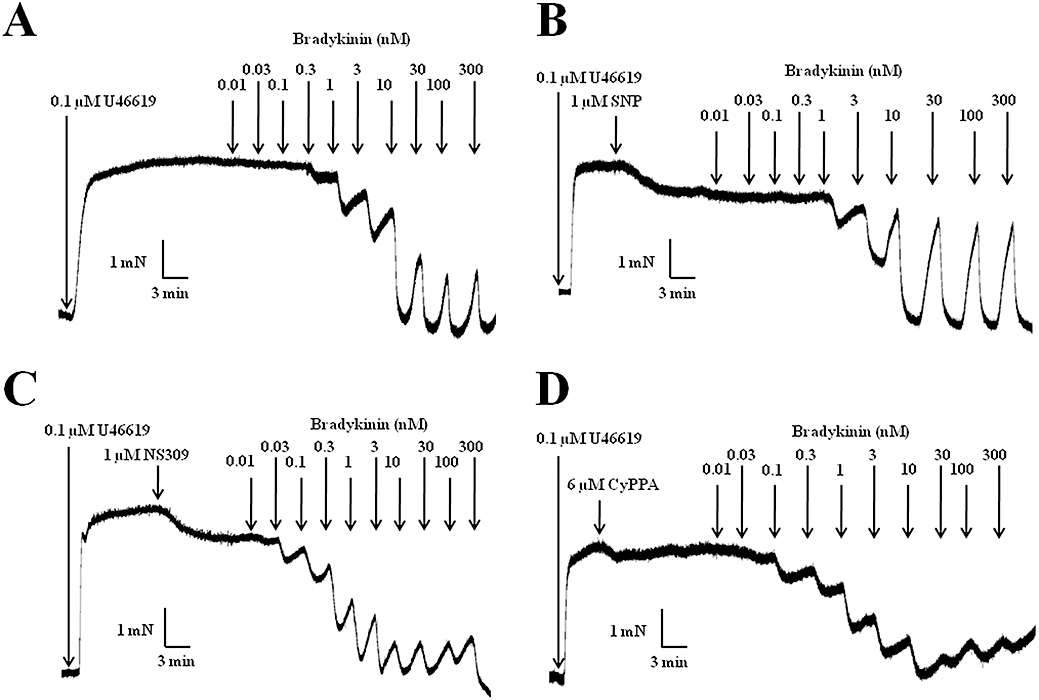
Original traces showing the response to increasing concentrations of bradykinin (A), bradykinin in the presence of sodium nitroprusside (SNP, 1 µM) (B), bradykinin in the presence of NS309 (1 µM) (C) and bradykinin in the presence of CyPPA (6 µM) (D), in porcine retinal arterioles contracted with U46619 (0.1 µM).
Figure 6.
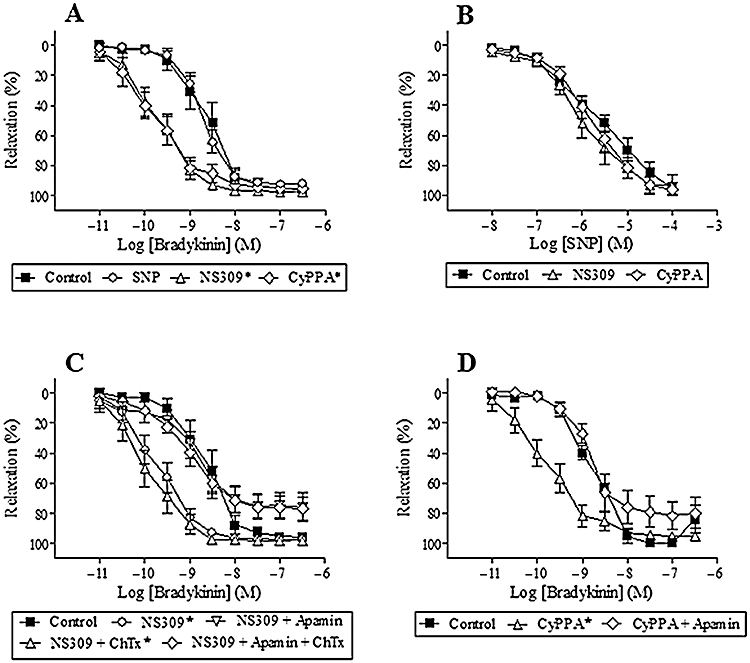
Effect of sodium nitroprusside (SNP, 1 µM), NS309 (1 µM) and CyPPA (6 µM) on bradykinin relaxation (A). Effect of NS309 (1 µM) and CyPPA (6 µM) on SNP relaxation (B). Effect of NS309 (1 µM) on bradykinin relaxation in the absence and presence of apamin (0.5 µM), and/or charybdotoxin (ChTx, 0.1 µM) (C). Effect of CyPPA (6 µM) on bradykinin relaxation in the absence and presence of apamin (0.5 µM) (D). Results are mean ± SEM (n = 6–8). Two-way anova. *P < 0.05 from control.
Table 1.
Myogenic tone after addition of blockers; EC50-values and maximum relaxation for bradykinin in the presence of SNP (1 µM), NS309 (1 µM) and CyPPA (6 µM)
| Treatment | n | Myogenic tone Contraction (Nm−1) | U46619 (0.1 µM) Contraction (Nm−1) |
Bradykinin |
|
|---|---|---|---|---|---|
| EC50 (nM) | Maximum relaxation (%) | ||||
| Control | 8 | 0.28 ± 0.06 | 1.0 ± 0.1 | 3.1 ± 1.1 | 96.3 ± 2.9 |
| SNP | 7 | 0.31 ± 0.04 | 1.0 ± 0.1 | 2.2 ± 1.4 | 92.2 ± 2.8 |
| Control | 8 | 0.30 ± 0.04 | 1.0 ± 0.1 | 3.9 ± 1.2 | 95.7 ± 1.9 |
| NS309 | 8 | 0.26 ± 0.05 | 1.0 ± 0.1 | 0.4 ± 0.2* | 97.4 ± 1.1 |
| NS309/charybdotoxin | 8 | 0.38 ± 0.10 | 1.0 ± 0.1 | 0.3 ± 0.2* | 98.3 ± 1.1 |
| NS309/apamin | 8 | 0.48 ± 0.12 | 0.9 ± 0.1 | 3.8 ± 1.4 | 75.8 ± 9.4* |
| NS309/charybdotoxin/apamin | 8 | 0.55 ± 0.13 | 1.0 ± 0.1 | 3.7 ± 1.5 | 76.8 ± 7.5* |
| Control | 7 | 0.26 ± 0.07 | 1.0 ± 0.1 | 2.6 ± 0.5 | 95.4 ± 4.6 |
| CyPPA | 7 | 0.31 ± 0.06 | 0.9 ± 0.1 | 0.3 ± 0.2* | 95.1 ± 1.9 |
| CyPPA/apamin | 7 | 0.45 ± 0.09 | 1.0 ± 0.1 | 3.2 ± 1.4 | 81.8 ± 9.3 |
Mean ± SEM Student's t-test.
P < 0.05 from their respective controls.
CyPPA, cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4- yl]-amine; NS309, 6,7-dichloro-1H-indole-2,3-dione 3-oxime; SNP, sodium nitroprusside; U46619, 9,11-dideoxy-9a,11a-epoxymethanoprostaglandin F2α.
Mechanisms involved in the enhanced bradykinin relaxation induced by NS309 and CyPPA
Investigating the calcium signalling in endothelial cells, concentrations of bradykinin above 1 nM increased the intracellular calcium concentration in endothelial cells (n = 5), whereas neither NS309 (1 µM) nor CyPPA (6 µM) changed the intracellular calcium concentration (n = 5). Furthermore, simultaneous addition of bradykinin (3 nM) and NS309 (1 µM) or CyPPA (6 µM) did not increase the intracellular calcium concentration further (Figure 7).
Figure 7.
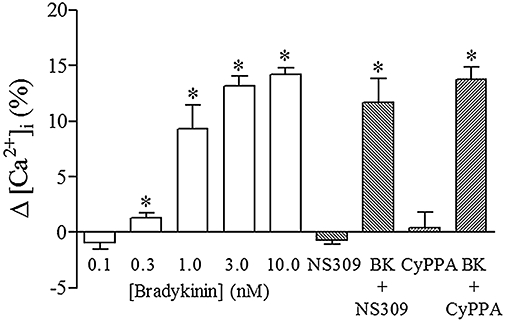
Relative change in intracellular calcium (Δ[Ca2+]i) for bradykinin (0.1 nM, 1 nM, 3 nM and 10 nM), NS309 (1 µM), NS309 (1 µM) + bradykinin (BK, 3 nM), CyPPA (6 µM) and CyPPA (6 µM) + BK (3 nM) in endothelial cells from porcine retinal arterioles. Results are mean ± SEM (n = 4–6). Wilcoxon matched pair test, *P < 0.05 from zero [Ca2+]i.
To address the relative contribution from NO and prostaglandins to bradykinin relaxation in the presence of NS309 or CyPPA, segments were incubated with ADMA (300 µM) to inhibit NO synthesis, indomethacin (3 µM) to inhibit prostaglandin synthesis, and oxyhaemoglobin (25 µM) to scavenge NO. ADMA (n = 7) and/or indomethacin (n = 8) reduced, but did not eliminate bradykinin relaxation, whereas ADMA, indomethacin and oxyhaemoglobin (n = 8) abolished bradykinin relaxation (Figure 8A,B). Myogenic tone after addition of ADMA and/or indomethacin, EC50 values and maximum relaxation for bradykinin are found in Table 2. Only those treatments involving ADMA increased myogenic tone.
Figure 8.
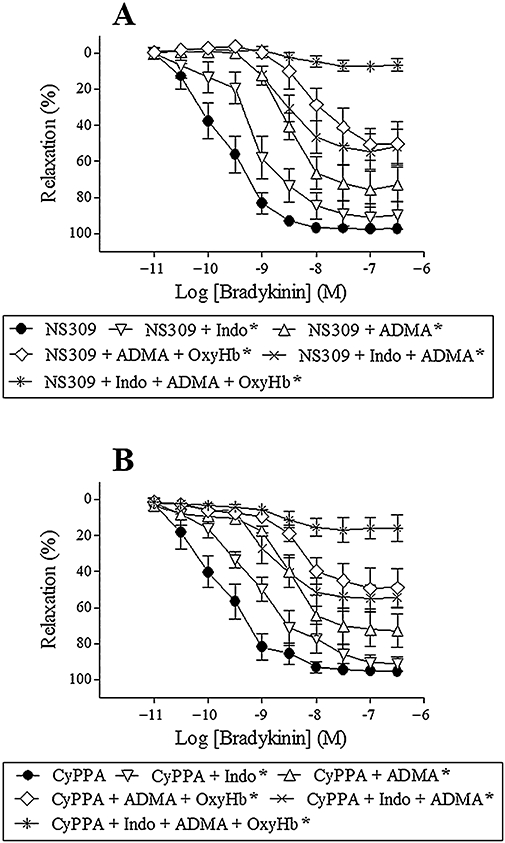
Bradykinin relaxation in the presence of NS309 (1 µM) (A) or CyPPA (6 µM) (B) and asymmetric dimethylarginine (300 µM, ADMA), ADMA + oxyhaemoglobin (25 µM, OxyHb), indomethacin (3 µM, indo), indo + ADMA and indo + ADMA + OxyHb in porcine retinal arterioles contracted with U46619 (0.1 µM). Results are mean ± SEM (n = 6–8). Two-way anova. *P < 0.05 from their respective controls.
Table 2.
Myogenic tone after addition of inhibitors; EC50-values and maximum relaxation for bradykinin in the presence of NS309 (1 µM) and CyPPA (6 µM)
| Treatment | n | Myogenic tone Contraction (Nm−1) | U46619 (0.1 µM) Contraction (Nm−1) |
Bradykinin |
|
|---|---|---|---|---|---|
| EC50 (nM) | Maximum relaxation (%) | ||||
| NS309 | 8 | 0.25 ± 0.06 | 0.9 ± 0.1 | 0.4 ± 0.2 | 97.4 ± 1.1 |
| NS309/ADMA | 7 | 0.63 ± 0.14* | 1.0 ± 0.1 | 8.4 ± 1.3* | 75.7 ± 10.0* |
| NS309/ADMA/oxyhaemoglobin | 8 | 0.56 ± 0.12* | 1.0 ± 0.1 | – | 50.5 ± 8.8* |
| NS309/indomethacin | 8 | 0.39 ± 0.09 | 1.0 ± 0.1 | 1.6 ± 0.7* | 91.0 ± 7.3 |
| NS309/indomethacin/ADMA | 8 | 0.78 ± 0.10* | 0.9 ± 0.1 | – | 54.7 ± 10.0* |
| NS309/indomethacin/ADMA/oxyhaemoglobin | 8 | 0.85 ± 0.12* | 0.9 ± 0.1 | – | 7.3 ± 2.5* |
| CyPPA | 8 | 0.30 ± 0.04 | 1.0 ± 0.1 | 0.5 ± 0.3 | 95.1 ± 2.0 |
| CyPPA/ADMA | 8 | 0.58 ± 0.12* | 1.0 ± 0.1 | 8.7 ± 0.9* | 72.7 ± 9.4* |
| CyPPA/ADMA/oxyhaemoglobin | 8 | 0.66 ± 0.16* | 1.0 ± 0.1 | – | 48.9 ± 10.6* |
| CyPPA/indomethacin | 8 | 0.45 ± 0.09 | 1.0 ± 0.1 | 1.6 ± 0.8* | 91.2 ± 4.0 |
| CyPPA/indomethacin/ADMA | 8 | 0.86 ± 0.12* | 1.0 ± 0.1 | – | 54.4 ± 5.8* |
| CyPPA/indomethacin/ADMA/oxyhaemoglobin | 8 | 0.80 ± 0.13* | 0.9 ± 0.1 | – | 16.0 ± 7.4* |
Mean ± SEM Student's t-test.
P < 0.05 from NS309 or CyPPA – indicates that EC50 values could not be calculated, as concentration-response experiments did not reach an asymptotic minimum.
ADMA, asymmetric dimethylarginine; CyPPA, cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4- yl]-amine; NS309, 6,7-dichloro-1H-indole-2,3-dione 3-oxime; U46619, 9,11-dideoxy-9a,11a-epoxymethanoprostaglandin F2α.
Investigating the effect of the constrictor on bradykinin relaxation, segments were contracted with U46619 (0.1 µM) or endothelin-1 (0.03 µM). No difference in bradykinin relaxations were observed using U46619 or endothelin-1 (EC50: 2.9 ± 0.8 nM vs. 3.6 ± 0.6 nM, maximum relaxation: 96 ± 2% vs. 94 ± 2%, n = 7). Furthermore, in segments contracted with either U46619 or endothelin-1, bradykinin relaxation was abolished by the combination of ADMA (300 µM), indomethacin (3 µM) and oxyhaemoglobin (25 µM) (n = 7) (Figure 9).
Figure 9.
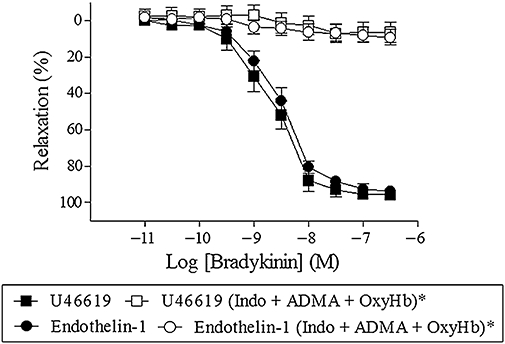
Bradykinin relaxation in the absence or presence of indomethacin (3 µM, Indo) + asymmetric dimethylarginine (ADMA; 300 µM) + oxyhaemoglobin (OxyHb; 25 µM) in porcine retinal arterioles contracted with U46619 (0.1 µM) or endothelin-1 (0.03 µM) (C). Results are mean ± SEM (n = 6–8). Two-way anova. *P < 0.05 from their respective controls.
To investigate if oxyhaemoglobin is a scavenger of only NO, or if it is also a scavenger of H2S that may be involved in EDHF-type relaxations (Feletou and Vanhoutte, 2009), relaxations to NO solution and H2S in the absence and the presence of oxyhaemoglobin were investigated. Both NO solution and H2S induced concentration-dependent relaxations (EC50: 0.090 ± 0.02 µM and 170 ± 30 µM, maximum relaxation: 95 ± 4% and 99 ± 1%, respectively n = 7–8) (Figure 10A,B). Oxyhaemoglobin (25 µM) completely abolished NO relaxation (n = 6) (Figure 10A), whereas H2S relaxation remained unchanged in the presence of oxyhaemoglobin (EC50: 300 ± 70 µM, maximum relaxation: 98 ± 2%, n = 7) (analysed by a two-way ANOVA) (Figure 10B). However, a sub-analysis (Student's t-test) showed that oxyhaemoglobin reduced the relaxation to 0.1 mM H2S. Investigating the contribution of H2S to bradykinin relaxation, inhibiting cystathionine γ-lyase with D,L-propargylglycine (PPG, 5 mM) in the presence of ADMA and indomethacin failed to change bradykinin relaxation (n = 7, data not shown).
Figure 10.
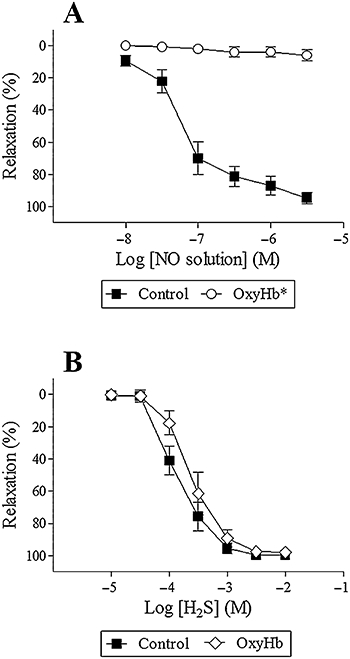
Relaxation to NO solution (A) and H2S (B) in the absence and presence of oxyhaemoglobin (25 µM, OxyHb) in porcine retinal arterioles contracted with U46619 (0.1 µM). Results are mean ± SEM (n = 6–8). Two-way anova. *P < 0.05 from control.
Discussion
In a previous study, we found that SKCa and IKCa channels were involved in endothelium-dependent bradykinin relaxation of retinal arterioles (Dalsgaard et al., 2009). The present study shows that SKCa3 and IKCa protein is localized in the vascular endothelium in porcine retinal arterioles, and that opening of SKCa channels with either NS309 or CyPPA, without causing changes in endothelial cell calcium, selectively enhance bradykinin relaxation in retinal arterioles. Moreover, our findings suggest that the enhanced bradykinin relaxation in the presence of SKCa openers can primarily be ascribed to increased formation of NO, as bradykinin relaxation, independent of contractile agonist applied, was abolished in the presence of a combination of inhibitors of cyclooxygenase and NO synthase, and the NO scavenger, oxyhaemoglobin.
In porcine coronary arteries, RT-PCR, Western blot and immunofluorescent labelling have shown that SKCa3 protein is expressed in endothelial cells and not in smooth muscle cells (Burnham et al., 2002). Single channel recordings from outside-out patches on isolated porcine coronary artery endothelial cells also confirm the existence of IKCa channels, and IKCa mRNA has been detected in intact porcine coronary artery endothelium (Bychkov et al., 2002). In agreement with these findings, SKCa3 and IKCa protein was expressed in the endothelium and not in the smooth muscle of the vascular segments, suggesting that SKCa3 and IKCa channels are important for endothelial function in porcine retinal arterioles.
In the present study, NS309 was applied to open SKCa and IKCa channels. NS309 has a slight selectivity for IKCa over SKCa, and no effect on large conductance calcium-activated potassium channels (BKCa or KCa1.1) (Strobaek et al., 2004). Previously, we have found that relaxation to NS309 was reduced upon inhibiting NO synthase and blocking SKCa channels in porcine retinal arterioles (Dalsgaard et al., 2009), suggesting that SKCa channels are involved in regulation of NO synthesis. These observations are further supported by other studies showing that blocking SKCa and IKCa channels inhibits ATP, histamine and NS309-induced NO synthesis in human umbilical vein endothelial cells (HUVECs) (Sheng and Braun, 2007), and noradrenaline and acetylcholine-induced NO formation in rat superior mesenteric artery (Stankevicius et al., 2006). Using a novel selective opener of SKCa2 and SKCa3 channels, CyPPA (Hougaard et al., 2007), the present study found that CyPPA induced endothelium-dependent relaxations. These relaxations were reduced by inhibition of NO synthase and a NO scavenger, but not by inhibiting cyclooxygenase, suggesting that opening SKCa channels indeed induce NO-mediated relaxation, and for the first time links SKCa channels to formation of NO in small arterioles.
In addition to opening of SKCa and IKCa channels, NS309 has been reported to block voltage-dependent Ca2+ (CaV) channels in bladder smooth muscle (Morimura et al., 2006) and voltage-dependent K+ (KV11.1) channels (Strobaek et al., 2004) at high concentrations, while CyPPA in HEK293 cells was reported also to block BKCa and voltage-dependent Na+ channels (NaV) channels in high concentrations (Hougaard et al., 2007). Therefore, to address the specificity of the channel openers in porcine retinal arterioles, we investigated their effects in segments contracted with high extracellular K+, which blocked the response to NS309 at concentrations below 6 µM and to CyPPA at concentrations below 20 µM. In the case of CyPPA no difference in relaxation was observed between blocking SKCa channels, removing the endothelium or contracting with high K+, suggesting that CyPPA is specific for SKCa2 and SKCa3 channels, but we cannot exclude that CyPPA may block other channels at high concentrations. However, NS309 relaxation was less pronounced in high K+ contracted segments compared with endothelium-denuded segments. Although we carefully removed adhering tissue in the present study, IKCa immunoreaction was observed in the majority of all retinal cells, and we cannot exclude that IKCa channels in residual perivascular tissue affected NS309 relaxation, explaining the difference in NS309 relaxation between high K+ contracted and endothelium-denuded segments.
Bradykinin binds to G-protein coupled B2 receptors in the endothelial cell and activates endothelium-dependent pathways leading to vasodilatation (Adams et al., 1989; Mombouli and Vanhoutte, 1995; Mombouli et al., 1996). To exclude that NS309 and CyPPA cause vasodilatation by activation of the bradykinin receptors, we investigated the effect of the bradykinin B2 receptor antagonist, HOE-140, and found that it concentration-dependently reduced bradykinin relaxation without changing NS309 and CyPPA relaxation, hence suggesting that NS309 and CyPPA induce relaxation independent of the bradykinin B2 receptor.
Previous studies have reported that opening of SKCa and IKCa channels with NS309 is associated with increased endothelium-dependent relaxation in mesenteric small arteries from Zucker lean (ZL) and Zucker diabetic fatty (ZDF) rats (Brondum et al., 2009), and also enhances acetylcholine relaxation in rat cremaster arterioles (Sheng et al., 2009). Moreover, another SKCa and IKCa opener, naphtho[1,2-d]thiazol-2-ylamine (SKA-31) enhanced EDHF-type relaxation in mouse carotid artery (Sankaranarayanan et al., 2009). In the present study, opening of SKCa and IKCa channels with NS309 or opening of only SKCa channels with CyPPA enhanced bradykinin relaxation. Bradykinin relaxation was not changed when a NO donor, SNP, was used to reduce the U46619 contraction to a similar level as caused by NS309 or CyPPA. Moreover, NS309 and CyPPA did not change SNP-induced relaxation. These results suggest that the reduction in U46619 contraction has no implications for the NS309- and CyPPA-enhanced bradykinin relaxation, and that NS309 or CyPPA do not change the sensitivity to NO. In addition, the pronounced leftward shifts of the concentration–response curves for bradykinin, induced by NS309 and CyPPA, were abolished by blocking SKCa channels with apamin, but not by blocking IKCa channels with charybdotoxin, suggesting that selective opening of SKCa channels is sufficient to enhance endothelium-dependent vasodilatation in porcine retinal arterioles.
Activation of the bradykinin B2 receptor leads to activation of phospholipase C, and thereby increase the intracellular calcium concentration in the endothelial cell through inositol 1,4,5-trisphosphate and diacylglycerol (Adams et al., 1989; Mombouli and Vanhoutte, 1995; Mombouli et al., 1996). NS309 and DC-EBIO have been reported to increase calcium in isolated HUVECs stimulated with ATP (Sheng et al., 2009). In contrast, endothelial cell calcium was not increased in intact rat cerebral arteries upon relaxation induced by the SKCa and IKCa channel opener, 1-EBIO (Marrelli et al., 2003). We have recently found that combined blockade of SKCa and IKCa channels fails to change the increase in endothelial cell calcium evoked by acetylcholine in rat superior mesenteric artery (Stankevicius et al., 2006), and that incubation with NS309 increases acetylcholine relaxation without changes in endothelial cell calcium from mesenteric small arteries from ZL and ZDF rats (Brondum et al., 2009). In the present study, bradykinin induced concentration-dependent increases in endothelial cell calcium, whereas neither NS309 nor CyPPA alone or in the presence of bradykinin increased endothelial cell calcium. Thus, the present study provides further support to the observation that the effect on endothelial cell calcium of SKCa and IKCa channel modulation is different in cultured isolated endothelial cells (Sheng and Braun, 2007; Sheng et al., 2009) versus intact arteries (Marrelli et al., 2003; McSherry et al., 2005; Stankevicius et al., 2006; Brondum et al., 2009). In arterial segments, calcium has been shown to be transported through gap junctions (Dora et al., 1997; Yashiro and Duling, 2000), and the adjacent endothelial cells may therefore rapidly buffer local calcium events in isolated arterial segments. Although, we cannot exclude that other approaches such as high velocity scanning or total internal reflection fluorescence (Navedo et al., 2006) developed for intact arteries may reveal local changes in endothelial cell calcium upon addition of SKCa and IKCa openers, the present results suggest that in intact arteries, NS309 and CyPPA rather than increasing endothelial cell calcium, sensitize the SKCa and IKCa channels for calcium, and hence enhance bradykinin relaxation. As contractile agonists may also increase endothelial cell calcium and lead to formation of endothelium-derived factors (Dora et al., 1997; Stankevicius et al., 2006), sensitization of the SKCa and IKCa channels may also explain the direct vasodilatatory effect of NS309 and CyPPA in porcine retinal arterioles.
Previous studies show that opening of IKCa and SKCa channels with either NS309 or SKA-31 enhances endothelium-dependent vasodilatation and ascribes this to an enhanced EDHF-type relaxation (Brondum et al., 2009; Sankaranarayanan et al., 2009; Sheng et al., 2009). In porcine retinal arterioles, we have previously found that NO and prostaglandins mediate bradykinin relaxation in porcine arterioles (Jeppesen et al., 2002; Dalsgaard et al., 2009). In agreement with these latter studies, this study found that in the presence of NS309 or CyPPA, bradykinin relaxation was still abolished by inhibiting cyclooxygenase, NO synthase and NO scavenging in porcine retinal arterioles. These findings suggest that NS309 and CyPPA through opening of SKCa channels enhance bradykinin relaxation by increasing mainly the formation of NO rather than increasing EDHF-type relaxations in porcine retinal arterioles.
In rat mesenteric arteries, it has been reported that stimulation of the thromboxane A2 receptor with U46619 leads to progressive loss of KCa channel activity, where the SKCa channel subtype is most sensitive, and ultimately loss of EDHF-linked changes in smooth muscle membrane potential and tension (Plane and Garland, 1996; Crane and Garland, 2004). However, in the present study the vascular segments were only contracted twice with U46619, and we have previously reported that SKCa channel blockade reduces bradykinin relaxation in U46619-contracted retinal arterioles (Dalsgaard et al., 2009). Moreover, in segments contracted with endothelin-1, bradykinin relaxation was not different to bradykinin relaxation in U46619-contracted segments, and relaxation was still abolished by inhibition of NO synthase, cyclooxygenase and in the presence of a NO scavenger. These results suggest that bradykinin relaxation is not affected by U46619 in porcine retinal arterioles.
Methaemoglobin, the product of oxidized haemoglobin, has been reported to scavenge H2S (Beauchamp, Jr et al., 1984), and as H2S has been suggested to be involved in EDHF-type relaxation (Feletou and Vanhoutte, 2009), the use of oxyhaemoglobin as a NO scavenger could block a potential relaxing effect of H2S in the arterioles. Therefore, the observed reduction in bradykinin relaxation upon addition of oxyhaemoglobin might also be related to the scavenging of not only NO, but also H2S. However, this study found that oxyhaemoglobin was a very poor scavenger of H2S compared with NO. Furthermore, inhibition of cystathionine γ-lyase failed to change bradykinin relaxation during inhibition of NO synthase and cyclooxygenase. Taken together these results support the findings that in porcine retinal arterioles, bradykinin relaxation does not involve H2S and is mainly mediated by NO.
In conclusion, in porcine retinal arterioles, SKCa3 and IKCa protein was localized to the vascular endothelium. Bradykinin increased endothelial cell calcium leading to opening of SKCa and IKCa channels. Without altering endothelial cell calcium, NS309 and CyPPA opened SKCa channels and enhanced the NO-mediated bradykinin relaxation. These findings imply that in retinal arterioles, opening of SKCa channels improves endothelium-dependent relaxation mediated by NO release. As a decreased endothelium-dependent relaxation is found in patients with glaucoma (Henry et al., 1999; Flammer et al., 2002; Galassi et al., 2004; Polak et al., 2007; Mozaffarieh et al., 2008) and diabetic retinopathy (Pemp and Schmetterer, 2008) treatment with specific SKCa channel openers may improve endothelium-dependent vasodilatation and restore retinal blood flow in these patients.
Acknowledgments
This study was supported by the Danish Research Council, the Lundbeck Foundation and the Danish Cardiovascular Research Academy.
We thank Henriette Johanson, Helle Zibrandtsen, Jane Rønn and Anna Malgorzata Jurkiewicz for excellent technical assistance.
Glossary
Abbreviations:
- 1-EBIO
1-ethyl-2-benzimidazolinone
- ADMA
asymmetric dimethylarginine
- ChTX
charybdotoxin
- CyPPA
cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine
- DC-EBIO
5,6-dichloro-1-ethyl-1,3dihydro-2H-benzimidazol-2-one
- DMSO
dimethylsulphoxide
- EDHF
endothelium-derived hyperpolarizing factor
- HOE-140
icatibant acetate
- IKCa (KCa3.1)
intermediate conductance calcium-activated potassium channel
- KPSS
125 mM K+ physiological saline solution
- NO synthase
nitric oxide synthase
- NS309
6,7-dichloro-1H-indole-2,3-dione 3-oxime
- PPG
D,L-propargylglycine
- PSS
physiological saline solution
- PSS0.0
physiological saline solution without CaCl2
- SKA-31
naphtho[1,2-d]thiazol-2-ylamine
- SKCa (KCa2.3)
small conductance calcium-activated potassium channel
- SNP
sodium nitroprusside
- U46619
9,11-dideoxy-9a,11a-epoxymethanoprostaglandin F2α
Conflicts of interest
None.
References
- Adams DJ, Barakeh J, Laskey R, Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989;3:2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- Brondum E, Kold-Petersen H, Simonsen U, Aalkjaer C. NS309 restores EDHF-type relaxation in mesenteric small arteries from type 2 diabetic ZDF rats. Br J Pharmacol. 2009;159:154–165. doi: 10.1111/j.1476-5381.2009.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, et al. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GJ, Garland CJ. Thromboxane receptor stimulation associated with loss of SKCa activity and reduced EDHF responses in the rat isolated mesenteric artery. Br J Pharmacol. 2004;142:43–50. doi: 10.1038/sj.bjp.0705756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard T, Kroigaard C, Bek T, Simonsen U. Role of calcium-activated potassium channels with small conductance in bradykinin-induced vasodilation of porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2009;50:3819–3825. doi: 10.1167/iovs.08-3168. [DOI] [PubMed] [Google Scholar]
- Delles C, Michelson G, Harazny J, Oehmer S, Hilgers KF, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke. 2004;35:1289–1293. doi: 10.1161/01.STR.0000126597.11534.3b. [DOI] [PubMed] [Google Scholar]
- Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Galassi F, Renieri G, Sodi A, Ucci F, Vannozzi L, Masini E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004;88:757–760. doi: 10.1136/bjo.2003.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E, Newby DE, Webb DJ, O’Brien C. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1710–1714. [PubMed] [Google Scholar]
- Hessellund A, Jeppesen P, Aalkjaer C, Bek T. Characterization of vasomotion in porcine retinal arterioles. Acta Ophthalmol Scand. 2003;81:278–282. doi: 10.1034/j.1600-0420.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- Hougaard C, Eriksen BL, Jorgensen S, Johansen TH, Dyhring T, Madsen LS, et al. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol. 2007;151:655–665. doi: 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P, Aalkjaer C, Bek T. Bradykinin relaxation in small porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2002;43:1891–1896. [PubMed] [Google Scholar]
- McSherry IN, Spitaler MM, Takano H, Dora KA. Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium. 2005;38:23–33. doi: 10.1016/j.ceca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Kinins and endothelial control of vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1995;35:679–705. doi: 10.1146/annurev.pa.35.040195.003335. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Bissiriou I, Agboton V, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: a key mediator of the vasodilator action of bradykinin. Immunopharmacology. 1996;33:46–50. doi: 10.1016/0162-3109(96)00083-5. [DOI] [PubMed] [Google Scholar]
- Morimura K, Yamamura H, Ohya S, Imaizumi Y. Voltage-dependent Ca2+-channel block by openers of intermediate and small conductance Ca2+-activated K+ channels in urinary bladder smooth muscle cells. J Pharmacol Sci. 2006;100:237–241. doi: 10.1254/jphs.sc0060011. [DOI] [PubMed] [Google Scholar]
- Mozaffarieh M, Grieshaber MC, Flammer J. Oxygen and blood flow: players in the pathogenesis of glaucoma. Mol Vis. 2008;14:224–233. [PMC free article] [PubMed] [Google Scholar]
- Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KR, Healy MJ, Qin Z, Skovgaard N, Vulesevic B, Duff DW, et al. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am J Physiol Regul Integr Comp Physiol. 2008;295:R669–R680. doi: 10.1152/ajpregu.00807.2007. [DOI] [PubMed] [Google Scholar]
- Pemp B, Schmetterer L. Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol. 2008;43:295–301. doi: 10.3129/i08-049. [DOI] [PubMed] [Google Scholar]
- Plane F, Garland CJ. Influence of contractile agonists on the mechanism of endothelium-dependent relaxation in rat isolated mesenteric artery. Br J Pharmacol. 1996;119:191–193. doi: 10.1111/j.1476-5381.1996.tb15970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak K, Luksch A, Berisha F, Fuchsjaeger-Mayrl G, Dallinger S, Schmetterer L. Altered nitric oxide system in patients with open-angle glaucoma. Arch Ophthalmol. 2007;125:494–498. doi: 10.1001/archopht.125.4.494. [DOI] [PubMed] [Google Scholar]
- Resch H, Garhofer G, Fuchsjager-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, et al. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmetterer L, Findl O, Fasching P, Ferber W, Strenn K, Breiteneder H, et al. Nitric oxide and ocular blood flow in patients with IDDM. Diabetes. 1997;46:653–658. doi: 10.2337/diab.46.4.653. [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Braun AP. Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C458–C467. doi: 10.1152/ajpcell.00036.2007. [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Simonsen U, Prieto D, Saenz de Tejada I, Garcia-Sacristan A. Involvement of nitric oxide in the non-adrenergic non-cholinergic neurotransmission of horse deep penile arteries: role of charybdotoxin-sensitive K+-channels. Br J Pharmacol. 1995;116:2582–2590. doi: 10.1111/j.1476-5381.1995.tb17211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen U, Wadsworth RM, Buus NH, Mulvany MJ. In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. J Physiol. 1999;516(Pt 1):271–282. doi: 10.1111/j.1469-7793.1999.271aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankevicius E, Lopez-Valverde V, Rivera L, Hughes AD, Mulvany MJ, Simonsen U. Combination of Ca2+-activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery. Br J Pharmacol. 2006;149:560–572. doi: 10.1038/sj.bjp.0706886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, et al. Activation of human IK and SK Ca2+– activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, et al. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Bonaventura D, de Oliveira AM. Functional characterization of the mechanisms underlying bradykinin-induced relaxation in the isolated rat carotid artery. Life Sci. 2007;80:1799–1805. doi: 10.1016/j.lfs.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro Y, Duling BR. Integrated Ca2+ signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]


