Abstract
Patients with idiopathic small fibre neuropathy (ISFN) have been shown to have significant intraepidermal nerve fibre loss and an increased prevalence of impaired glucose tolerance (IGT). It has been suggested that the dysglycemia of IGT and additional metabolic risk factors may contribute to small nerve fibre damage in these patients.
25 patients with ISFN and 12 aged-matched control subjects underwent a detailed evaluation of neuropathic symptoms, neurological deficits (Neuropathy deficit score (NDS); Nerve Conduction Studies (NCS); Quantitative Sensory Testing (QST) and Corneal Confocal Microscopy (CCM) to quantify small nerve fibre pathology.
8 (32%) of patients had IGT. Whilst all patients with ISFN had significant neuropathic symptoms, NDS, NCS and QST except for warm thresholds were normal. Corneal sensitivity was reduced and CCM demonstrated a significant reduction in corneal nerve fibre density (NFD) (P<0.0001), nerve branch density (NBD) (P<0.0001), nerve fibre length (NFL) (P<0.0001) and an increase in nerve fibre tortuosity (NFT) (P<0.0001). However these parameters did not differ between ISFN patients with and without IGT, nor did they correlate with BMI, lipids and blood pressure.
Corneal confocal microscopy provides a sensitive non-invasive means to detect small nerve fibre damage in patients with ISFN and metabolic abnormalities do not relate to nerve damage.
Keywords: small fibre neuropathy, impaired glucose tolerance, corneal confocal microscopy
Introduction
Patients with impaired glucose tolerance (IGT) or impaired fasting glucose (IFG) are at substantially increased risk of developing diabetes and cardiovascular disease (Unwin, et al., 2002). The incidence of microalbuminuria (Barr, et al., 2006), and retinopathy (Wong, et al., 2005) is higher in patients with IFG and IGT compared to subjects with normal glucose metabolism. The evidence that there is a similar link between IGT and neuropathy is less clear. The majority of studies supporting this contention have demonstrated a greater than expected prevalence of impaired glucose tolerance (IGT) in patients with idiopathic small fibre neuropathy (ISFN) (Smith, et al., 2001, Smith, et al., 2006, Sumner, et al., 2003) with an improvement in IENF following an improvement in weight, lipid and glucose levels with diet and exercise in ISFN patients with IGT (Smith, et al., 2006). However, recently the design, interpretation and hence the contributory role of IGT in the development of nerve damage in ISFN has been questioned (Dyck, et al., 2007). Furthermore, a European study has not shown as high a prevalence of IGT in patients with ISFN (Nebuchennykh, et al., 2008), suggesting that the higher prevalence of IGT in the previous US studies may well have reflected the higher background incidence of IGT in the US population.
If patients are selected for having IGT only as opposed to ISFN, then a recent population based study from Germany, has shown that the prevalence of polyneuropathy assessed using the Michigan Neuropathy Screening Instrument was only slightly increased in individuals with IGT compared to those with NGT (Ziegler, et al., 2008). More detailed studies in IGT subjects have shown no abnormality in either nerve conduction velocity and amplitude (Cappellari, et al., 2005) or sural nerve myelinated nerve fibre density (Sundkvist, et al., 2000). Although, a recent study in 46 subjects with IGT demonstrated a significantly greater abnormality for heart rate variability and a higher frequency of hyperesthesia and hypoesthesia as well as increased heat detection thresholds (Putz, et al., 2009), suggestive of predominantly small fibre damage.
With regard to causation, ISFN patients with normal glucose levels have recently been shown to have a significantly higher total and LDL cholesterol, and a higher prevalence of abnormal HDL and triglycerides (Smith, et al., 2008), suggesting that dyslipidaemia may be as important as glucose dysmetabolism in the development of neuropathy (Smith and Singleton, 2008). This notion is supported in Type 1 diabetic patients, as in addition to hyperglycaemia; BMI, lipids and blood pressure have also been shown to contribute to the development of neuropathy (Tesfaye, et al., 2005). Studies also suggest that the earliest damage in patients with ISFN or metabolic syndrome may occur in the small fibres, and accumulating evidence suggests IENF loss predates large fibre damage, in early diabetic polyneuropathy (Loseth, et al., 2008, Umapathi, et al., 2007). Hence assessment of IENF in skin biopsies has been endorsed recently in the detection of distal small fiber sensory polyneuropathy (Ebenezer, et al., 2007, England, et al., 2009). However, this is an invasive procedure and cannot be advocated for use in patients with minor metabolic abnormalities and no overt evidence of neuropathy.
Previously we have shown that corneal confocal microscopy (CCM), a non-invasive means of quantifying small fibre damage in patients with diabetic neuropathy (Kallinikos, et al., 2004, Malik, et al., 2003) may relate to intra epidermal nerve fibre loss (Quattrini, et al., 2007). It also relates to a loss of corneal sensitivity (Tavakoli, et al., 2007), and detects early small nerve fibre regeneration following pancreas transplantation in patients with Type 1 diabetes (Mehra, et al., 2007). We have also recently shown that CCM detects nerve damage in patients with Fabry disease (Tavakoli, et al., 2009).
In the present study patients with ISFN underwent CCM in addition to conventional neurophysiology and quantitative sensory testing. Furthermore nerve fibre damage was related to a range of metabolic risk factors for nerve damage.
Methods
The study was approved by Central Manchester Ethics committee and written informed consent was obtained from each patient according to the declaration of Helsinki.
Patient description
25 patients referred with ISFN from the department of neurology and 12 age-matched healthy volunteers were studied. All patients were examined carefully by a neurologist and other causes of neuropathy were excluded based on a detailed medical history, no family history of neuropathy, toxin exposure and normal renal function, complete blood count, thyroid function, B12 levels and plasma electrophoresis. An Oral Glucose Tolerance Test (OGTT) was performed according to ADA and WHO guidelines in all patients and Impaired Glucose Tolerance (IGT) was defined on the basis of a 2-h plasma glucose ≥7.8 and < 11.1 mmol/l. Fasting lipids and blood pressure were also evaluated.
Assessment of Neuropathy
All patients and control subjects underwent a detailed evaluation of neurological symptoms using the Neuropathy Symptom Profile (NSP) (Dyck, et al., 1986, Young, et al., 1993). Neurological deficits were assessed using the neuropathy disability score (NDS) (Abbott, et al., 2005) which included tuning fork vibration perception, pin prick perception and temperature perception as well as the presence or absence of ankle reflexes to give a combined score between 0 and 10. Electro diagnostic studies were undertaken using a Dantec “Keypoint” system (Dantec Dynamics Ltd, Bristol, UK) equipped with a DISA temperature regulator to keep limb temperature constantly between 32–35°C. Electrophysiological studies were performed in Sural, Peroneal, Tibial, Radial and Ulnar nerves. All studies were performed according to a standardised protocol using surface electrodes of 9mm diameter. Stimulation was supra-maximal and motor studies were recorded using a belly-tendon electrode placement. Motor nerve conduction was investigated in the following nerves: Ulnar (Recording, Abductor Digiti Minimi (ADM); Stimulation, Wrist (60mm proximal to active recording electrode), 4cm distal to elbow and 5mm proximal to elbow) and Peroneal (Recording, Extensor Digitorm Brevis (EDB); Stimulation, Ankle (60mm proximal to active recording electrode), 5cm distal to the fibular head and 5cm proximal to the fibular head). Sensory nerve conduction was investigated in the following nerves: Sural (recording, ankle; stimulation calf (140mm proximal to active recording electrode)) and Radial (recording, anatomical snuffbox; stimulation, wrist (80 mm proximal to active recording electrode)). The DML reflects the time taken from supramaximal stimulation of the nerve 60mm proximal to the active recording electrode to the onset of the compound muscle action potential (i.e. the evoked motor response). Vibration Perception Threshold (VPT) was measured using a Neurothesiometer (Horwell, Scientific Laboratory Supplies, Wilford, Nottingham, UK). Quantitative sensory testing included warm sensation and heat induced pain (WS, HIP) to assess C fibres and cold sensation and cold induced pain (CS, CIP) to assess Að fibres, using the MEDOC TSA II (Medoc Ltd., Ramat Yishai 30095, Israel) device on the dorsum of the left foot. Sensory thresholds were determined by the method of limits.
Corneal sensitivity
Corneal sensitivity was quantified using a non contact corneal aesthesiometer (NCCA, Glasgow, Caledonian University, UK) which uses a puff of air on the centre of the cornea, lasting 0.9 seconds and exerting a force expressed in millibars (mbars). The stimulus jet is mounted on a slit lamp and is positioned 1 cm from the eye and the air jet is aligned to the centre of the cornea. The sensation described by the subject varies with subjects most commonly describing a “cold” sensation and others a “breeze” like sensation which is not regarded as unpleasant (Tavakoli, et al., 2007). The coefficient of variation for NCCA is 5.6%.
Corneal Confocal Microscopy
Patients underwent examination with a Tomey Confoscan corneal confocal microscope model P4 (Erlangen, Germany). One eye of each subject was selected at random for examination and anaesthetized with one drop of benoxinate hydrochloride 0.4% (oxybuprocaine hydrochloride, Minims). The objective lens of the confocal microscope was disinfected (isopropyl alcohol 70% v/v, Swabs). A large drop of Viscotears liquid gel (carbomer 940, Ciba Vision Ophthalmics) was applied onto the tip of the lens and advanced forwards until the gel touched the cornea, allowing optical but not physical contact between the objective lens and corneal epithelium during the examination. Several scans of the entire depth of the cornea were recorded by turning the fine focus of the objective lens backwards and forwards for approximately 2 min to acquire satisfactory images of all corneal layers providing en face two dimensional images with a lateral resolution of approximately 1 to 2 μm and final image size of 768 pixels × 576 pixels. Our recently published study compared 5 different regions of the cornea and demonstrated that the density in the centre was the highest and was only reduced in the extreme periphery (Patel, et al., 2009). Therefore we selected three to five high quality images of Bowman’s layer from the centre of the cornea in each patient and control subject. This layer is of particular relevance for defining neuropathic changes since it is the location of the main nerve plexus that supplies the overlying corneal epithelium. The images were coded and the observer quantified the abnormalities in a randomised and masked fashion.
We have established four parameters as potential indicators of corneal nerve fiber damage and repair (Malik, et al., 2003, Quattrini, et al., 2007): (i) Corneal nerve fiber density (CNFD) the total number of major nerves/mm2 of corneal tissue; (ii) Corneal nerve fiber length (CNFL) the total length of all nerve fibers and branches (mm/mm2) of corneal tissue; and (iii) Corneal nerve branch density (CNBD) the number of branches emanating from major nerve trunks/mm2 of corneal tissue and (iv) Corneal nerve fibre tortuosity (NFT). Measures (i) and (iii) were determined using morphometric software incorporated within the Tomey instrument. Measure (ii) was determined using third party image analysis software (Scion Image for Windows, Scion Corporation, Frederick, Ma., USA) and measure (iv) was calculated using a MATLAB function (MATLAB, Mathworks, USA, version 6.5) that was created for this purpose (Kallinikos, et al., 2004). To estimate the error in measuring CNFD, CNFL and CNBD, we acquired images and determined each of these parameters in 15 subjects on two occasions separated by at least 48 h. The coefficient of variation of these parameters was: 12% for CNFD, 9% for CNFL and 24% for CNBD.
Statistics
SPSS 11.05.0 for Windows was used to compute the results. Analysis included descriptive and frequency statistics. All data are expressed as mean ± SEM. One-way analysis of variance (ANOVA) with Scheffe Post-hoc tests was used to study differences between means. The Pearson test was used to analyze correlations between potentially related variables.
Results
25 patients (age: 60.2 ± 3.3) with ISFN and 12 healthy volunteers (age: 59.3 ± 3.9) were studied. The clinical characteristics of ISFN patients and controls are summarized in Table 1.
Table 1.
Clinical assessments
| Controls | ISFN Patients with NGT | ISFN Patients with IGT | |
|---|---|---|---|
| Number | 12 | 17 | 8 |
| Sex (Female/Male) | 9/3 | 5/12 | 2/6 |
| Age (years) | 59.3 ± 3.9 | 57.5 ± 3.1 | 63 ± 3.6 |
| Fasting glucose (mmol/l) | <5.5 | 5.3 ± 0.1 | 5.6 ± 0.2 |
| 2h glucose (mmol/l) | ≤7.7 | 5.5 ± 0.4 | 8.8 ± 0.8 |
| BMI (kg/m2) | <25 | 26.3 ± 4.2 | 31.6 ± 2.8 |
| Systolic BP (mmHg) | - | 147 ± 7 | 139 ± 5.5 |
| Diastolic BP (mmHg) | - | 69.5 ± 0.5 | 72.5 ± 3.5 |
| Total Cholesterol (mmol) | - | 4.7 ± 0.4 | 5.1 ± 0.4 |
| TG (mmol) | - | 2.9 ± 0.8 | 2.9 ± 0.3 |
Glucose Tolerance Test
The OGTT demonstrated that 8 (32%) of patients (age: 63 ± 3.6 yrs) had IGT (2h: 8.8 ± 0.8 mmol/l) and 17 patients (age: 57.5 ±3.1 yrs) had NGT (2h: 5.5 ± 0.4 mmol/l).
Symptoms and neurological deficits
Neuropathic symptoms as assessed by the NSP were significantly increased in ISFN patients with IGT (11.3 ± 2.8, P=0.002) and NGT (10.7 ± 1.8, P<0.0001) compared to controls, but no difference was observed between patients with NGT and IGT. The severity of neurological deficit according to the NDS was significantly increased in patients with IGT (4.9 ± 1.8, P=0.01) and NGT (3.9 ±0.9, P=0.01) compared to controls, but with no difference between IGT and NGT.
Electrophysiology
By definition i.e. ‘idiopathic small fibre neuropathy’ ISFN patients had normal sensory (Sural and Radial) and motor (Peroneal, Tibial and Ulnar) nerve electrophysiology and only sural nerve conduction velocity was marginally but significantly reduced (Table 2).
Table 2.
Neurological assessment
| Controls (n=12) | ISFN Patients with NGT (n=17) | ISFN Patients with IGT (n=8) | |
|---|---|---|---|
| NSP (0–38) | 0* | 10.65±1.83 | 11.29±2.83 |
| NDS (0–10) | 0.11±0.11† | 3.94±0.87 | 4.86±1.75 |
| Sural Amplitude (micro volts) | 14.20±2.23 | 12.53±2.09 | 8.42±2.06 |
| Sural Velocity (m/sec) | 51.80±2.50† | 46.78±1.13 | 44.22±1.97 |
| Peroneal Amplitude (millivolts) | 3.92±0.66 | 3.35±0.83 | 2.02±0.14 |
| Peroneal Velocity (m/sec) | 47.63±1.28 | 44.38±2.00 | 41.32±1.41 |
| DML Peroneal (ms)( Ankle-EBD) | 3.76±0.27 | 4.10±0.25 | 4.02±0.55 |
| Tibial Amplitude (millivolts) | 6.20±0.62 | 6.73±1.06 | 4.28±1.15 |
| Tibial Velocity (m/sec) | 45.20±1.39 | 46.92±1.87 | 43.08±2.65 |
| Tibial DML (ms) | 4.26±0.23 | 4.35±0.16 | 4.50±0.27 |
| Radial Amplitude (micro volts) | 32.45±3.47 | 25.28±4.80 | 26.50±6.51 |
| Radial Velocity (m/sec) | 61.41±1.80 | 56.28±1.87 | 60.82±1.89 |
| Ulnar Amplitude (millivolts) | 7.72±0.30 | 8.84±0.55 | 8.03±0.29 |
| Ulnar Velocity (m/sec) | 60.63±1.81 | 57.60±0.93 | 53.42±2.25 |
| Ulnar DML (ms)(wrist-ADM) | 2.58±0.21 | 2.24±0.09 | 2.43±0.13 |
| VPT (volts) | 11.90±2.18 | 17.61±3.43 | 22.76±6.21 |
| CS (°C) | 21.33±2.31 | 15.42±2.45 | 14.30±5.10 |
| WS(°C ) | 40.18±2.10† | 46.13±0.86 | 44.71±1.38 |
| CIP(°C ) | 8.17±4 | 1.04±0.82 | 3.60±4.35 |
| HIP(°C ) | 43.51±5.46 | 49.82±0.33 | 49.43±0.41 |
Data are expressed as mean ± SE for ISFN patients and control subjects.
Statistically significant difference between patients and controls using ANOVA:
P<0.0001,
P<0.05.
Quantitative Sensory Testing
Vibration perception threshold did not differ significantly between patients with IGT (22.8 ± 6.2) and NGT (17.6 ± 3.4) compared to controls (13.3 ± 2.4). The threshold for cold sensation (CS), Cold Induced Pain (CIP) and heat induced pain (HIP) did not differ significantly between patients and control subjects. However, the threshold for warm sensation (WS) was increased in ISFN patients with NGT (P=0.005) but not in those with IGT (P=0.08) compared to controls.
Corneal nerve sensitivity
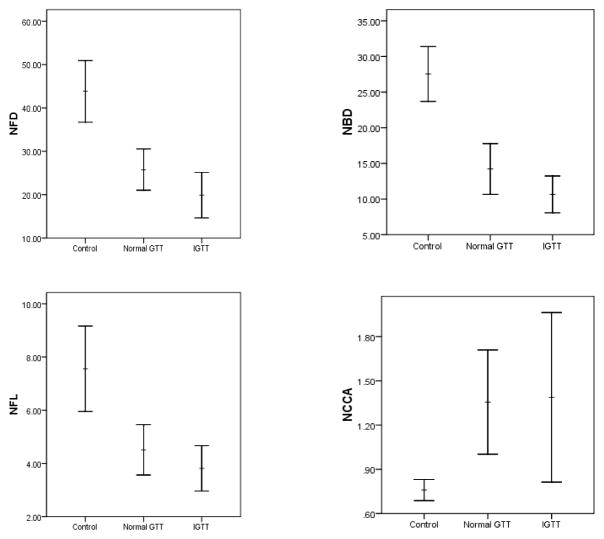
Although the threshold of corneal sensitivity was increased in patients, it did not differ significantly in those with IGT (1.4 ± 0.3) and NGT (1.2 ± 0.2) compared to controls (0.8 ±0.1) (Table 3).
Table 3.
Corneal sensitivity and nerve morphology assessed by CCM (Mean ± SEM)
| Controls | ISFN Patients with NGT | ISFN Patients with IGT | |
|---|---|---|---|
| NCCA (mili bars) | 0.76±0.07 | 1.15±0.18 | 1.38±0.34 |
| NFD (no/mm2) | 43.61±4.26* | 24.65±3 | 19.89±3.19 |
| NBD (no/mm2) | 26.57±2.70* | 12.98±2.07 | 10.66±1.57 |
| NFL ( mm/mm2) | 9.28±0.55* | 4.36±0.62 | 3.81±0.52 |
| NFT (TC) | 15.91±0.40‡ | 21.76±1.01‡ | 20.39±0.44‡ |
Statistically significant difference between patients and controls using ANOVA:
P<0.0001,
P<0.05,
P<0.01. Post hoc results significantly different between controls and patients with NGT and IGT: ||P<0.05, ‡P<0.01
Corneal nerve morphology
In patients with ISFN there was a highly significant reduction in CNFD (P<0.0001) (Table 3, Figure 1a), CNBD (P<0.0001), (Figure 1b) and CNFL (P<0.0001) (Figure 1c) with an increase in CNFT (P<0.0001) compared to controls. However, there was no difference for any parameter between ISFN patients with IGT compared to NGT.
Figure 1.
CCM nerve fiber density (a), nerve fiber branch density (b) nerve fiber length (c) and sensitivity of cornea (d) in control subjects and patients with ISFN with NGT and IGT. Horizontal bars illustrate the mean and vertical bars illustrate the SE.
Correlation
NCCA correlated with NPS (r=0.657, P<0.002) and NDS (r=0.598, P=0.007. Corneal NFD correlated significantly with NSP (r=−0.47, P=0.03), NDS (r=−0.50, P=0.02), sural conduction velocity (r=0.59, P=0.007) but not WS (r=−0.38, P=0.13). Similarly, corneal NBD correlated significantly with NSP (r=−0.83, P=0.01), NDS (r=−0.48, P=0.03), sural conduction velocity (r=0.63, P=0.004) but not WS (r=−0.38, P=0.13). NFL correlated with NSP (r=−0.46, P=0.04), NDS (r=−0.58, P=0.007), sural conduction velocity (r=0.55, P=0.01) and WS (r=−0.49, P=0.04). CNFD correlated with other measures of corneal nerve pathology (NBD (r=0.92, P<0.0001), NFL (r=0.90, P<0.0001) and NFT (r=−0.59, P=0.009). CNFD did not correlate with either fasting (r=−0.396, p=0.180) or 2 hour glucose levels in the OGTT (r=−0.034, P=0.911), BMI (r=−0.293, p=0.382), total cholesterol (r=−0.208, p=0.539), triglycerides (r= −0.529, p=0.116) or systolic (r= −0.316, p=0.542) and diastolic (r= 0.471, p=0.345) blood pressure. Although NBD (r=−0.653, P=0.04) and NFL (P=−0.618, P=0.06) correlated with the triglycerides this was an inverse correlation (Table 4).
Table 4.
Correlation between corneal nerve morphology and neurological parameters
| NSP | NDS | Sural Vel. | WS | 2-hrs glucose | BMI | Total Cholestrol | TG | |
|---|---|---|---|---|---|---|---|---|
| NFD | r=−0.473* P=0.03 |
r= −.0504* P= 0.02 |
r=0.594 ** P=0.007 |
r=−0.375 P=0.13 |
r=−0.03 P=0.91 |
r=−0.293 P=0.382 |
r=−0.208 P=0.539 |
r=−0.529 P=0.116 |
| NBD | r=−0.832* P=0.01 |
r=−.483* P= 0.03 |
r=0.631** P=0.004 |
r=−0.376 P=0.13 |
r=−0.254 P=0.41 |
r=−0.517 P=0.104 |
r=−0.215 P=0.526 |
r=−0.653** P=0.041 |
| NFL | r=−0.462* P=0.04 |
r=−0.581** P= 0.007 |
r=0.549* P=0.01 |
r=−0.484* P=0.04 |
r=0.210 P=0.49 |
r=−0.212 P=0.531 |
r=−0.362 P=0.275 |
r=−0.618 P=0.057 |
| NCCA | r=0.657** P=0.002 |
r=0.598 ** P= 0.007 |
r=−0.381 P=0.119 |
r=0.205 P=0.447 |
r=−0.414 P=0.15 |
r=0.401 P=0.221 |
r=−0.010 P=0.978 |
r=0.229 P=0.525 |
Correlation is significant at 0.01 level,
Correlation is significant at 0.05 level
NSP: Neuropathy Symptom Profile; NDS: Neuropathy Deficit Score; WS: Warm Sensation; TG: Triglyceride
Discussion
Patients with ISFN have been shown to have significant small nerve fibre pathology using the novel non-invasive technique of CCM. Whilst we confirm the significantly increased prevalence of IGT (32%), comparable to previous studies in patients with ISFN, suggesting that IGT may be important in the genesis of nerve damage in this condition (Cappellari, et al., 2005, Novella, et al., 2001, Singleton, et al., 2001), our data differ from recent data in a cohort of Norwegian patients with ISFN, where only 13% had IGT (Nebuchennykh, et al., 2008). A potential reason for this difference may be that we studied only 25 subjects which formed a small sample of all the patients with ISFN in the clinic and therefore there was potential for selection bias.
However, in patients selected for having IGT as opposed to ISFN, the prevalence of polyneuropathy is only slightly increased in individuals with IGT compared to those with NGT (Ziegler, et al., 2008). And subjects with IGT consistently show no abnormality in either nerve conduction velocity and amplitude (Cappellari, et al., 2005) or sural nerve myelinated nerve fibre density (Sundkvist, et al., 2000, Thrainsdottir, et al., 2003). Although, in a recent study subjects with IGT demonstrated both autonomic and small fibre damage (Putz, et al., 2009). The studies in patients with ISFN have also demonstrated normal electrophysiology and QST but with IENF loss (Smith, et al., 2001, Smith, et al., 2006, Sumner, et al., 2003) which improves with weight, lipid and glucose control (Smith, et al., 2006). Several others studies confirm nerve fibre damage primarily to small nerve fibres in this group of patients (Periquet, et al., 1999, Smith, et al., 2008, Smith, et al., 2006) suggesting that the earliest damage in patients with ISFN or metabolic syndrome may occur to the small fibres. Accumulating evidence also suggests IENF loss predates large fibre damage, in early diabetic polyneuropathy (Loseth, et al., 2008, Umapathi, et al., 2007). Hence assessment of IENF in skin biopsies has been endorsed recently in the detection of distal small fiber sensory polyneuropathy (Ebenezer, et al., 2007, England, et al., 2009).
As expected patients with ISFN have intact large fibre function, although the NDS a neurological exam was abnormal as was sural nerve conduction velocity. The warm threshold, suggestive of a small fibre deficit, was significantly increased in patients with ISFN. However, the key finding of this study is that we have shown significant small nerve fibre pathology using the rapid and non-invasive technique of CCM, providing a novel diagnostic means to diagnose small fibre damage in patients with ISFN. These data are supported by our recent findings of abnormal CCM findings in patients with Fabry disease (Tavakoli et al 2009).
CCM may also allow one to undertake more readily further studies to address the mechanistic basis of ISFN in relation to risk factors. Thus with regard to causation of nerve damage in ISFN patients, the early focus was in relation to impaired glucose tolerance (Singleton, et al., 2001, Singleton, et al., 2001). However, in a recent study a significantly higher total and LDL cholesterol, and a higher prevalence of abnormal HDL and triglycerides (Smith, et al., 2008) has been demonstrated in normoglycaemic patients with ISFN, suggesting that factors such as dyslipidaemia may be as important in the development of neuropathy in these patients (Smith and Singleton, 2008). Similarly in a recent population based study which did not preselect patients with ISFN polyneuropathy was associated with not only diabetes but also waist circumference (Ziegler, et al., 2008). Furthermore, not only hyperglycaemia but BMI, lipids and blood pressure have been shown to contribute independently to the development of neuropathy in Type 1 diabetes (Tesfaye, et al., 2005). In the present study although the incidence of IGT was higher in patients with ISFN than one might expect in the general population, the premise that IGT may cause nerve damage is not sustained as despite marked corneal nerve damage in patients with ISFN this did not differ between patients with IGT and NGT. Furthermore there was also no correlation between corneal nerve fibre loss and a range of metabolic parameters. Although, clearly a lack of correlation does not necessarily mean a lack of association and therefore these conclusions must be guarded due to the small sample size studied.
In conclusion CCM provides a novel non-invasive means to demonstrate significant small fibre abnormalities in patients with ISFN. This study questions the role of impaired glucose tolerance in the genesis of this condition. Larger cross sectional or longitudinal mechanistic studies are warranted and CCM may facilitate this.
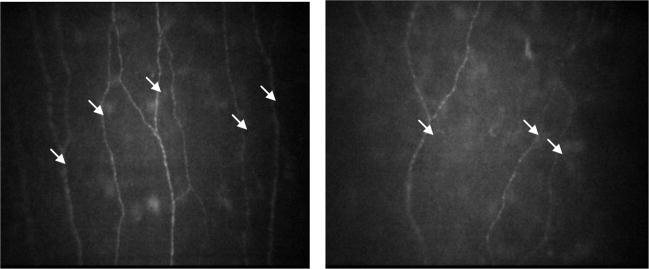
Figure 2.
CCM images of the corneal nerves, original magnification ×700. (a) Control subject with 5 fibers and 3 major branches compared to a patient with ISFN with 3 fibres and 1 branch. The white arrows indicate nerve fibers.
Acknowledgments
This work was supported by Juvenile Diabetes Research Foundation International Grant 5-2002-185 and National Institute of Health Grant 1 R01 NS46259-01. Support for the NIHR Manchester Biomedical Research Centre is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott CA, Garrow AP, Carrington AL, Morris J, Van Ross ER, Boulton AJ. Foot ulcer risk is lower in South-Asian and african-Caribbean compared with European diabetic patients in the U.K.: the NorthWest diabetes foot care study. Diabetes Care. 2005;28:1869–1875. doi: 10.2337/diacare.28.8.1869. [DOI] [PubMed] [Google Scholar]
- 2.Barr EL, Wong TY, Tapp RJ, Harper CA, Zimmet PZ, Atkins R, Shaw JE. Is peripheral neuropathy associated with retinopathy and albuminuria in individuals with impaired glucose metabolism? The 1999–2000 AusDiab. Diabetes Care. 2006;29:1114–1116. doi: 10.2337/diacare.2951114. [DOI] [PubMed] [Google Scholar]
- 3.Cappellari A, Airaghi L, Capra R, Ciammola A, Branchi A, Levi Minzi G, Bresolin N. Early peripheral nerve abnormalities in impaired glucose tolerance. Electromyogr Clin Neurophysiol. 2005;45:241–244. [PubMed] [Google Scholar]
- 4.Dyck PJ, Karnes J, O’Brien PC, Swanson CJ. Neuropathy Symptom Profile in health, motor neuron disease, diabetic neuropathy, and amyloidosis. Neurology. 1986;36:1300–1308. doi: 10.1212/wnl.36.10.1300. [DOI] [PubMed] [Google Scholar]
- 5.Dyck PJ, Klein CJ, Weigand SD. Does impaired glucose metabolism cause polyneuropathy? Review of previous studies and design of a prospective controlled population-based study. Muscle Nerve. 2007;36:536–541. doi: 10.1002/mus.20846. [DOI] [PubMed] [Google Scholar]
- 6.Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M. Assessment of epidermal nerve fibers: a new diagnostic and predictive tool for peripheral neuropathies. J Neuropathol Exp Neurol. 2007;66:1059–1073. doi: 10.1097/nen.0b013e31815c8989. [DOI] [PubMed] [Google Scholar]
- 7.England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann D, Howard JF, Lauria G, Miller RG, Polydefkis M, Sumner AJ. Evaluation of distal symmetric polyneuropathy: The role of laboratory and genetic testing (an evidence-based review) Muscle Nerve. 2009;39:116–125. doi: 10.1002/mus.21226. [DOI] [PubMed] [Google Scholar]
- 8.Kallinikos P, Berhanu M, O’Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–422. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 9.Loseth S, Stalberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008 doi: 10.1007/s00415-008-0872-0. [DOI] [PubMed] [Google Scholar]
- 10.Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, Boulton AJ. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–688. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 11.Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30:2608–2612. doi: 10.2337/dc07-0870. [DOI] [PubMed] [Google Scholar]
- 12.Nebuchennykh M, Loseth S, Jorde R, Mellgren SI. Idiopathic polyneuropathy and impaired glucose metabolism in a Norwegian patient series. Eur J Neurol. 2008;15:810–816. doi: 10.1111/j.1468-1331.2008.02197.x. [DOI] [PubMed] [Google Scholar]
- 13.Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve. 2001;24:1229–1231. doi: 10.1002/mus.1137. [DOI] [PubMed] [Google Scholar]
- 14.Patel DV, Tavakoli M, Craig JP, Efron N, McGhee CN. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea. 2009;28:735–740. doi: 10.1097/ICO.0b013e318193e0e3. [DOI] [PubMed] [Google Scholar]
- 15.Periquet MI, Novak V, Collins MP, Nagaraja HN, Erdem S, Nash SM, Freimer ML, Sahenk Z, Kissel JT, Mendell JR. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology. 1999;53:1641–1647. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- 16.Putz Z, Tabak AG, Toth N, Istenes I, Nemeth N, Gandhi RA, Hermanyi Z, Keresztes K, Jermendy G, Tesfaye S, Kempler P. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care. 2009;32:181–183. doi: 10.2337/dc08-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–2154. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 18.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24:1448–1453. doi: 10.2337/diacare.24.8.1448. [DOI] [PubMed] [Google Scholar]
- 19.Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve. 2001;24:1225–1228. doi: 10.1002/mus.1136. [DOI] [PubMed] [Google Scholar]
- 20.Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001;57:1701–1704. doi: 10.1212/wnl.57.9.1701. [DOI] [PubMed] [Google Scholar]
- 21.Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci. 2008 doi: 10.1016/j.jns.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, Singleton JR. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 23.Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist. 2008;14:23–29. doi: 10.1097/NRL.0b013e31815a3956. [DOI] [PubMed] [Google Scholar]
- 24.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- 25.Sundkvist G, Dahlin LB, Nilsson H, Eriksson KF, Lindgarde F, Rosen I, Lattimer SA, Sima AA, Sullivan K, Greene DA. Sorbitol and myo-inositol levels and morphology of sural nerve in relation to peripheral nerve function and clinical neuropathy in men with diabetic, impaired, and normal glucose tolerance. Diabet Med. 2000;17:259–268. doi: 10.1046/j.1464-5491.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 26.Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 2007;30:1895–1897. doi: 10.2337/dc07-0175. [DOI] [PubMed] [Google Scholar]
- 27.Tavakoli M, Marshall A, Thompson L, Kenny M, Waldek S, Efron N, Malik R. Corneal confocal microscopy: A novel non-invasive means to diagnose neuropathy in patients with Fabry disease. Muscle and Nerve. 2009 doi: 10.1002/mus.21383. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 29.Thrainsdottir S, Malik RA, Dahlin LB, Wiksell P, Eriksson KF, Rosen I, Petersson J, Greene DA, Sundkvist G. Endoneurial capillary abnormalities presage deterioration of glucose tolerance and accompany peripheral neuropathy in man. Diabetes. 2003;52:2615–2622. doi: 10.2337/diabetes.52.10.2615. [DOI] [PubMed] [Google Scholar]
- 30.Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve. 2007;35:591–598. doi: 10.1002/mus.20732. [DOI] [PubMed] [Google Scholar]
- 31.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 32.Wong TY, Barr EL, Tapp RJ, Harper CA, Taylor HR, Zimmet PZ, Shaw JE. Retinopathy in persons with impaired glucose metabolism: the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Am J Ophthalmol. 2005;140:1157–1159. doi: 10.1016/j.ajo.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–154. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31:464–469. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]