Abstract
Introduction: To improve our understanding of the various clinical phenotypes in inflammatory bowel disease (IBD)-associated colorectal cancer (CRC) and provide potential targets for early diagnosis and future therapy, we sought to identify new candidate genes and molecular pathways involved in the pathogenesis and progression of this disorder. Recent evidence has implicated the actin-cytoskeleton pathway in the development of metastatic sporadic CRC through cytoskeletal proteins such as fascin-1. We hereby propose that similar genetic polymorphisms and mutations among regulatory genes of the actin-cytoskeleton pathway may also be associated with increased dysplasia, carcinogenesis, and susceptibility for invasion and metastasis in IBD-associated CRC, as compared with sporadic CRC. Materials and Methods: To test this hypothesis, we identified three patients with IBD-associated CRC. We subsequently retrieved normal, dysplastic, and cancerous tissue from within the same surgical colonic specimen. Messenger RNA was subsequently isolated from fresh frozen tissue, and oligonucleotide arrays were carried out to identify genes that were differentially expressed between the three various tissue types (normal, dysplasia, and cancer). By utilizing the same specimen to obtain each of the three various tissue types, we excluded intersubject variability during the analysis. Finally, we performed bioinformatic interaction pathway analysis using the “Ingenuity Pathway Analysis” software. Results: Computerized pathway analysis revealed that the actin-cytoskeleton pathway was significantly dysregulated in the progression of normal cells, via dysplasia, to IBD-associated CRC (p < 0.05). Significantly up-regulated genes identified in the analysis included the fibroblast growth factor, Abelson interactor gene-2, profilin-2, and radixin genes. Conversely, the diaphanous homolog gene appeared to be significantly down-regulated. Conclusion: Via the dysregulation of these five genes within the actin-cytoskeleton pathway, we propose that this molecular pathway provides a potential mechanism for the malignant transformation and progression of normal tissue, via dysplasia, to IBD-associated CRC.
Introduction
Inflammatory bowel disease (IBD) encompasses several inflammatory disorders affecting the gastrointestinal tract, and it is currently thought to include ulcerative colitis (UC), Crohn's disease, and indeterminate colitis. These disorders are thought to affect over 1 million Americans today and pose significant health and lifestyle concerns. Patients with extensive IBD, particularly patients with UC, have an up to 20-fold increased risk of developing colorectal cancer (CRC). Further, the age of onset is thought to be approximately 20 years younger than sporadic CRC in the general population (Harpaz and Talbot, 1996). Despite advanced investigative modalities and stringent surveillance programs, advanced CRC is still annually diagnosed in many patients with IBD. The risk of cancer increases dramatically with disease duration and is likely independent of clinical disease activity. From 8 years after diagnosis, patients with UC undergo annual colonoscopic screening in the United States, in an effort to identify dysplastic changes. Although dysplastic lesions are thought to represent unequivocal neoplastic proliferation equivalent to an adenoma, which may represent a marker or premalignant precursor of carcinoma, these lesions may also possess malignant characteristics, such as the potential to invade the basement membrane (Riddell et al., 1983).
Although there are a number of well-described risk factors for CRC development in patients with IBD, relatively little is known about the underlying genetic changes that constitute the transformation from inflammation, through dysplasia, to malignant cancer. Two primary theories exist as to how UC-related cancer develops. According to one theory, UC-related cancer is acquired by virtue of the on-going regeneration and repair process, which is associated with a high cell turnover and, thus, an increased risk of inflammation-induced DNA mutations (Harpaz and Talbot, 1996). The alternative theory proposes that common primary genetic events, such as dysregulation of cell cycle checkpoints, result in both inflammation and subsequent cancer formation.
In contrast to IBD-associated CRC, sporadic CRC is a multistep process involving the progressive transition of genetic abnormalities along an adenoma-dysplasia-carcinoma-metastasis sequence that has been extensively studied and is well described. Events such as p53 mutations, which occur late in sporadic CRC, appear to occur early in the course of IBD-associated CRC. This may be in keeping with potentially similar underlying molecular pathways that are essential in the development of the two different types of CRC (Leedham et al., 2009).
Hypothesis
Recent evidence has implicated the actin-cytoskeleton pathway in the development of metastatic sporadic CRC. Important genes within the pathway, such as fascin-1, were found to be up-regulated and are thought to promote the grade and severity of dysplasia, tumor invasiveness, and the migratory potential of tumor cells.
We hereby propose that the genetic polymorphisms and mutations in regulatory genes of the actin-cytoskeleton pathway may also be similarly associated with dysplasia, carcinogenesis, and an enhancement of cells' invasive and metastatic abilities in IBD-associated CRC.
We present our preliminary findings in the next paragraph.
Materials and Methods
Three patients with UC in whom IBD-associated CRC was diagnosed underwent surgery for tumor excision. The excised samples were analyzed by a specialist gastrointestinal pathologist who identified areas of normal colonic mucosa, dysplastic mucosa, and IBD-associated CRC within each specimen. To minimize contamination of abnormal tissue with normal tissue, we took scrapes of the specific areas (normal, dysplasia, and cancer) from the frozen samples as identified by the gastrointestinal pathologist. In addition, we used different slides in the cell-scraping procedure and eventually compared the slides with the pathology reports before pursuing further. Samples from each segment were harvested and stored at − 80°C for subsequent analysis. Before analysis, samples were stored in formalin for transport between the pathologist and laboratory.
Messenger RNA (mRNA) was extracted from total RNA using Qiagen Oligotex mRNA isolation columns (Valencia, CA) and subsequently converted to double-stranded complimentary DNA using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and an oligo(dT) primer linked to the T7 RNA polymerase-binding site sequence. Double-stranded complimentary DNA was then converted to biotin-labeled cRNA using T7 RNA polymerase in the presence of biotinylated uridine 5′-triphosphate (UTP) and cytidine triphosphate (CTP). The Enzo BioArray High Yield RNA Transcript Labeling Kit was utilized and the manufacturer's instructions were closely followed (Enzo Diagnostics, Farmingdale, NY). Labeled cRNA was then purified (RNeasy column; Qiagen) and fragmented. Samples were hybridized to a set of 9 Affymetrix Human U95Av2 arrays at 45°C for 16 h and were finally washed and stained. Signal amplification was carried out with biotinylated antistreptavidin antibody.
Alterations in RNA levels were initially analyzed using the Affymetrix Analysis Suite software version 5.0 (Santa Clara, CA). The analysis was conducted such that both cancer and dysplastic regions were compared with benign tissue (control). To minimize variability between multiple comparisons, chips that did not include Affymetrix control probes, expressed sequence tags, and probes were excluded. The remaining 8089 probe sets were each fitted using a repeated-measure design across tissue type and analyzed using the comparatively new Proc Mixed procedure (SAS, v 9.0; SAS Institute, Cary, NC), as previously described (Colliver et al., 2006). For data analysis, we utilized the Ingenuity Pathway Analysis (IPA) Program version 7.6© (Redwood, CA). Array data were uploaded into the IPA system, which is capable of analyzing genetic dysregulation based on 89 signaling and 80 metabolic “canonical pathways.” Diagrammatic illustrations were generated using the IPA software, and copyright permission to reproduce the images was successfully sought.
Results
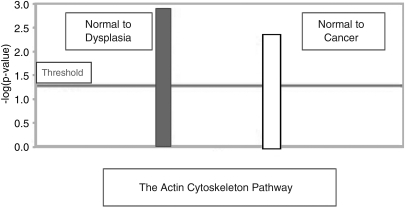
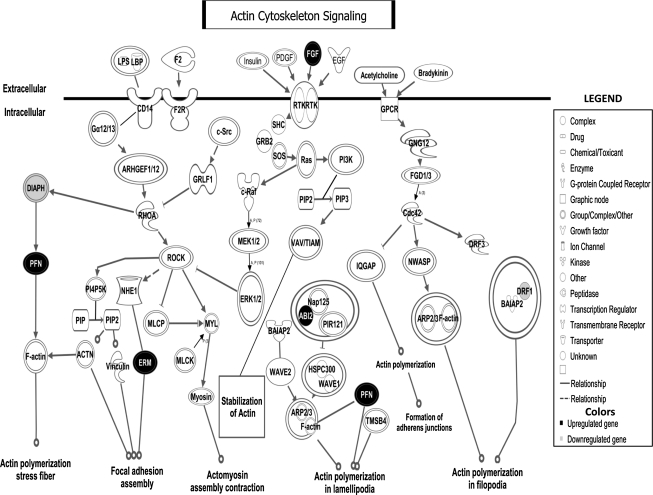
Preliminary canonical pathway analyses identified the actin-cytoskeleton pathway to be the most relevant and significantly disrupted in the progression of normal colonic mucosa, via dysplasia, to IBD-associated CRC (p < 0.05) (Fig. 1). Five genes were found to be dysregulated, including the fibroblast growth factor-2 (FGF-2), diaphanous homolog (DIAPH1), Abelson interactor-2 (Abi-2), profilin-2 (Pfn-2), and radixin (Rdx) genes (Figs. 2 and 3).
FIG. 1.
The actin-cytoskeleton “Canonical Pathway” dysregulation from normal colonic mucosa to dysplasia (−log p-value >1.3 meaning a p-value <0.05) and from normal colonic mucosa to inflammatory bowel disease-associated colorectal cancer (−log p-value >1.3 meaning a p-value <0.05).
FIG. 2.
The actin-cytoskeleton signaling pathway and the candidate genes involved in colorectal cancer progression in patients with ulcerative colitis. FGF-2, Pfn-2, Rdx, and Abi-2 are up-regulated (black). DIAPH1/DRF1 is down-regulated (light gray). FGF-2, fibroblast growth factor-2; Pfn-2, profilin-2; Rdx, radixin; Abi-2, Abelson interactor-2; DIAPH1, diaphanous homolog.
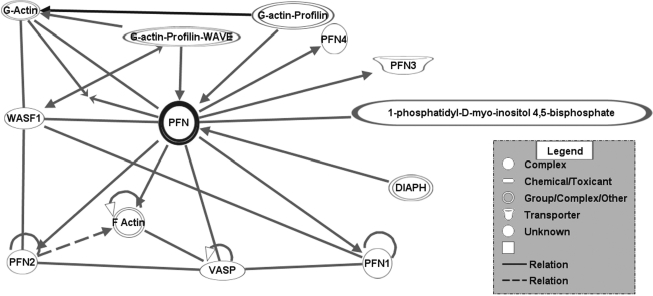
FIG. 3.
Pfn gene neighborhood and its interacting pathway. A neighborhood is a network consisting of the relationships between a molecule and its nearest neighbors based on the connections available in the Ingenuity knowledge base. It displays the molecule of interest at the center, with its interacting partners and the biological relationships between them. Essentially, the molecules that are displayed in the neighborhood regulate, are regulated by, or physically interact with the molecule of interest.
The FGF-2, or basic FGF, gene was up-regulated 34-fold in patients with IBD-associated CRC as compared with patients who were normal. The DIAPH1 gene was down-regulated 245-fold in IBD-associated colonic mucosa as compared with normal mucosa. The Abi-2 gene of the actin-cytoskeleton pathway was up-regulated 34-fold in IBD-associated cancer as compared with normal colonic mucosa. The Pfn-2 gene, which is interestingly regulated by DIAPH1, was found to be up-regulated 268-fold in IBD-associated CRC as compared with normal colonic mucosa. Finally, Rdx, a member of the ERM family, was up-regulated 65-fold in IBD-associated CRC as compared with normal colonic mucosa.
Discussion
The actin-cytoskeleton pathway is a key component in the movement of cells, and it is thought to represent the backbone of cells that allow migration and mobility of cells within the body. In carcinogenesis, the ability of cells to invade and migrate past the basement membrane may be dependent on conformational changes within this cellular backbone.
The actin-cytoskeleton pathway has already been implicated in the development and pathogenesis of invasive metastatic sporadic CRC. In a study by Buda and Pignatelli (2004), the authors found that tumor progression and tissue invasiveness require increased cell motility and enhanced plasticity of the cell's actin-cytoskeleton. Both invasion and subsequent migration of carcinoma cells are highly coordinated processes that depend, in large part, on alterations in cell-to-cell and cell-to-extracellular matrix adhesion properties as well as on alterations in the molecular composition and organization of the actin-cytoskeleton (Carragher and Frame, 2004; Guo and Giancotti, 2004).
Fascin is a cross-linking protein within the cellular backbone and is a member of the actin-cytoskeleton pathway. It is known to localize to the actin bundle core of spikes and to filopodia at the leading edge of migratory cells (Hashimoto et al., 2007). Recent reports have suggested that the actin-cytoskeleton pathway might be readily involved in the metastasis of sporadic CRC through cytoskeletal proteins such as Fascin-1 (Vignjevic et al., 2007; Parsons and Adams, 2008). Up-regulation of Fascin-1 within the actin-cytoskeleton pathway is thought to promote the migratory potential and, therefore, the invasive phenotype of malignant cancer cells, which can ultimately lead to metastasis (Vignjevic et al., 2007). Further, in a separate study by Tsai et al. (2007), the authors were able to show that the resultant CRC tumor grade and tumor node metastasis (TNM) staging system for tumors directly correlated to the degree of Fascin-1 up-regulation. These findings are not exclusive to CRC and have similarly been shown to exist in serous ovarian carcinoma, wherein up-regulated Fascin levels within a dysregulated actin-cytoskeleton pathway were directly related to the invasive and metastatic potential of the ovarian tumor (Wen et al., 2009).
Although previous studies that investigated the role of the actin-cytoskeleton network showed a link in the development of sporadic CRC, our early results show a significant role for this pathway in both IBD-associated dysplasia and IBD-associated CRC, when compared with normal colonic mucosal cells. Our preliminary results are from three patients with UC with identified CRC progression stages. It is a clinically challenging task recruiting patients having distinct phases (normal, dysplasia, and cancer) identified in CRC progression. In support of this point, a study done by Winther et al. (2004) reported that the cumulative probability of CRC was 0.4% after 10 years of a UC diagnosis. In a separate study, the probability of detecting a precancerous lesion could be even more difficult (Thomas et al., 2007). According to the metaanalysis by Thomas et al. (2007), the incidence of any dysplastic lesion was 0.03% of person-years' duration of patients with UC.
The importance of dysregulation of the actin-cytoskeleton pathway in both cancer and dysplasia is very interesting, particularly since detection of this dysregulated pathway in early dysplasia may provide a novel screening and therapeutic target. Early detection and treatment may thus offer an opportunity for curative therapy before advancement of the disease. In addition, the combined presence of the same dysregulated pathway in both sporadic and IBD-associated CRC may indicate a universal link through the actin-cytoskeleton pathway, which may later be shown to underlie other types of cancer. If so, these cancers may subsequently share an equally positive outlook related to early detection and treatment.
Putative oncogenes and tumor suppressor genes in the actin-cytoskeleton pathway
By utilizing the IPA software to analyze the pathways and genes involved in the progression from normal tissue to dysplasia and from dysplasia to cancer, five different genes within the actin-cytoskeleton pathway appeared to be dysregulated. These included FGF-2, DIAPH1, Abi-2, Pfn-2, and Rdx genes (IPA Website [Help Manual]; https://analysis.ingenuity.com/pa/info/help).
Fibroblast growth factor-2
FGF-2 was up-regulated 34-fold in patients with IBD-associated CRC as compared with patients who were normal (Fig. 2). The protein encoded by this gene is a member of the FGF family. Proteins of the FGF family bind heparin and possess broad mitogenic and angiogenic activities. This protein has been implicated in diverse biological processes, such as tumor growth, limb and nervous system development, and wound healing (Böttcher and Niehrs, 2005).
The mRNA for this gene contains multiple polyadenylation sites and is unusually translated from non-AUG (CUG) as well as traditional AUG initiation codons. The ability to initiate the translation from non-AUG initiation codons results in the formation of different isoforms with distinct properties. For example, CUG-initiated isoforms are localized in the nucleus and are responsible for an intracrine cellular effect. The AUG-initiated isoform is predominantly cytosolic and is responsible for paracrine and autocrine effects of FGF, which include proliferation, migration, growth, apoptosis, differentiation, mitogenesis, angiogenesis, and general survival of the cell. The mitogenic and angiogenic properties of FGF-2 are essential in the ability of malignant cells to survive and subsequently metastasize from the primary site to more distant sites through the newly enhanced network of capillaries and blood vessels (Katoh and Katoh, 2005). The significant up-regulation of this gene is important, and its up-regulation in IBD-associated CRC is a novel finding that we describe within the significantly implicated actin-cytoskeleton pathway.
Diaphanous homolog gene (DIAPH1/DRF1)
DIAPH1/DRF1 was down-regulated 245-fold in IBD-associated colonic mucosa as compared with normal mucosa. DIAPH1, a member of the actin-binding family, is a homolog of the Drosophila diaphanous gene and has been previously implicated in the autosomally dominant, fully penetrative, and nonsyndromic sensorineural progressive low-frequency hearing loss. Alteration of DIAPH1 function has been documented in many diseases, including Waldenstrom's macroglobulinemia and sporadic CRC (Yamana et al., 2006; Hatjiharissi et al., 2007). DIAPH1 has also been implicated in the expression of smooth muscle element (Staus et al., 2007), DIAPH3 (an enzyme found in the nucleus that is involved in actin binding) (Peng et al., 2003), PAX6 (a transcription regulator found in the nucleus) (Tominaga et al., 2002), and S-phase kinase associated protein 2 (Mammoto et al., 2004). Further, its importance has also been highlighted in the nucleation of F-actin (Li and Higgs, 2005); the activation of c-fos serum response element-binding transcription factor (Geneste et al., 2002); the activation of mitogen-activated protein kinase (MAPK1/3) (Eisenmann et al., 2007); and the accumulation of adenomatosis polyposis coli (APC) (Yamana et al., 2006), F-actin (Geneste et al., 2002), and short for sarcoma (SRC) (Yamana et al., 2006). These elements may all possess important functions in the development of CRC and other diseases. Most importantly, however, DIAPH1 has been found to be heavily involved in the assembly of actin, the loss of the function of protein kinase D-2 that disassembles actin, and the release of calcium. In our study, DIAPH1/DRF1 was significantly down-regulated. We believe that there could be a negative, yet unidentified, feedback pathway that may be involved in down-regulating DIAPH1/DRF1. The three cancer samples in our study did have a previous dysplastic stage in which many mutations could have occurred; however, we speculated that the cell could have employed a cell-salvage mechanism through down-regulating DIAPH1/DRF1 to oppose further dysregulation of the actin-cytoskeleton pathway.
These functions of DIAPH1, particularly within the actin-cytoskeleton pathway, provide possible mechanisms by which the actin-cytoskeleton pathway is modified to promote the invasion and metastasis by cancer cells (Gavert et al., 2007). Its significant down-regulation in IBD-associated CRC may be a crucial addition to the hypothesized involvement of the actin-cytoskeleton pathway in IBD-associated CRC.
Abelson interactor-2 gene
The Abi-2 gene of the actin-cytoskeleton pathway, which ultimately forms the cytosolic Abi protein, was up-regulated 34-fold in IBD-associated cancer as compared with normal colonic mucosa. The Abi-2 gene belongs to the cytoskeletal adaptor family and is also involved in DNA binding as well as binding of Abi tyrosine kinase, a nonreceptor tyrosine kinase whose activation results in regulation of cytoskeleton function, cell growth, and apoptosis in response to a variety of biological stimuli (Hernandez et al., 2004). The Abi gene exists in two forms in mammalian cells, Abi-1 and Abi-2. These isoforms share an overall homology of 69% (Lin et al., 2004). Abi-2 is known to regulate c-abl (Juang and Hoffmann, 1999), ABL1 L-tyrosine (Hirao et al., 2006), and WASF1 genes (Echarri et al., 2004). Abi is linked to the Abi tyrosine kinase signaling pathway that regulates Abi-mediated transformation (Tani et al., 2001). It is also linked to kinase activity and signal transduction between Ras and Rac, the two small GTP-binding proteins (Tani et al., 2001). The expression of activated Ras genes has been implicated as a contributing factor to the radio-resistance of some colorectal tumor cells (Russell et al., 1999).
At the cellular level, Abi proteins are localized to sites of actin polymerization. This is a dynamic event affecting cell morphogenesis and migration (Stradal et al., 2001). Altered expression of Abi due to a germ-line mutation, somatic mutation, or epigenetic changes could explain transition of normal mucosa, via dysplasia, to cancer through dysregulation of the actin-cytoskeleton pathway. Its 34-fold up-regulation in IBD-associated CRC is a novel preliminary finding that may be highly important.
Profilin-2
The Pfn-2 gene, which is interestingly regulated by DIAPH1, was found to be up-regulated 268-fold in IBD-associated CRC as compared with normal colonic mucosa. The protein encoded by this gene is a ubiquitous actin monomer-binding protein that belongs to the Pfn family. It is thought to regulate actin polymerization in response to extracellular signals. There are two alternatively spliced transcript variants encoding different isoforms of the gene, Pfn-1 and Pfn-2. Pfn's molecular function includes actin binding and binding of phosphatidylinositol (4,5)-bisphosphate (Fig. 3). Pfn is biologically involved in cellular cytoskeleton organization and regulation of actin polymerization or depolymerization. It has previously been observed to bind actin and form a complex that localizes to sites of dynamic actin polymerization at the leading edge of migrating cells and pathogen-induced actin tails (Li et al., 2008). Previous findings by Bae et al. (2008) demonstrated that reduced expression of Pfn-1, a pro-migratory molecule, could promote breast cancer cell motility via Ena/VASP proteins. This could lead to increased cell motility and thus enhance ability of malignant cells to metastasize. In a similar fashion, this finding of Pfn up-regulation within the actin-cytoskeleton pathway may be highly relevant in the increase in invasive and metastatic potential of IBD-associated tumors.
Radixin
Rdx was up-regulated 65-fold in IBD-associated CRC as compared with normal colonic mucosa. Rdx is a member of the ERM protein family. It is a cytoskeletal protein in the actin-cytoskeleton pathway that is important in linking actin to the plasma membrane. It is very similar in sequence homology to both ezrin and moesin, which link actin-cytoskeleton proteins. The Rdx gene has been localized by fluorescence in situ hybridization to 11q23. Rdx, along with other “linking proteins,” forms a flexible framework for the cell, which offers attachment points for organelles and formed bodies. These attachment points subsequently provide an intrinsic route for communication between different parts of a cell. Rdx has been shown to be involved in general cell reorganization, cell morphology, linkage and anchorage, retraction and ruffling, and cell death. Rdx has also been implicated in leukocyte extravasation signaling. The up-regulation of Rdx, through its ability to influence cell infrastructure, communication, flexibility, and motility, may thus play a crucial role in allowing colon cancer cells to undergo necessary changes to acquire metastatic potential.
Although the initiating and exact mechanisms by which cells acquire invasive and metastatic properties were not elucidated by this study, our findings indicate a preliminary role for several genes in the development of IBD-associated CRC and which may provide molecular screening and therapeutic targets for early detection and treatment. Further, processes related to Crohn's disease-associated CRC may differ significantly from UC-associated CRC, which could provide an avenue for further investigation and even the possibility of differentiating between the two, pathologically similar precursor disease entities.
Conclusion
FGF-2, Abi-2, DIAPH1, Pfn-2, and Rdx are crucial key messengers in the actin-cytoskeleton pathway that interact and transmit external signals to modulate cytoskeleton activity. The dysregulation of these key elements is likely to play a role in promoting dysplasia, carcinogenesis, and subsequent ability of cancerous cells to invade and metastasize. Through our preliminary and previously undescribed findings, we propose that dysregulation of these key genes within the actin-cytoskeleton pathway may specifically promote malignant transformation in IBD-associated CRC. These findings need to be further validated with studies having larger sample size and employing laser capture microdissection technique for better isolation of cell populations. The clinical implication of unraveling new genetic pathways in IBD-associated CRC may provide enhanced targeted screening techniques that allow earlier identification of cancerous change or even provide new targets for molecular gene therapy directed against elements of the actin-cytoskeleton pathway.
Acknowledgments
This work was generously funded in part by the John and Caroline Price Family Trust and the Joint Royal College of Surgeons of Edinburgh and James and Emmeleine Ferguson Fellowship. These funding sources had no role in the production of this manuscript. Additionally, this work was supported by a National Institute of Environmental Health Sciences' grant (1P30ES014443-01A1).
Disclosure Statement
None of the authors have any financial or other conflicts of interest to disclose. In addition, all authors have seen and approved the final version of this manuscript for submission.
References
- Bae YH. Ding Z. Zou L, et al. Loss of profilin-1 expression enhances breast cancer cell motility by Ena/VASP proteins. J Cell Physiol. 2008;219:354–364. doi: 10.1002/jcp.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher RT. Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Buda A. Pignatelli M. Cytoskeletal network in colon cancer: from genes to clinical application. Int J Biochem Cell Biol. 2004;36:759–765. doi: 10.1016/j.biocel.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Carragher NO. Frame MC. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Colliver DW. Crawford NP. Eichenberger MR, et al. Molecular profiling of ulcerative colitis-associated neoplastic progression. Exp Mol Pathol. 2006;80:1–10. doi: 10.1016/j.yexmp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Echarri A. Lai MJ. Robinson MR. Pendergast AM. Abl interactor 1 (Abi-1) wave-binding and SNARE domains regulate its nucleocytoplasmic shuttling, lamellipodium localization, and wave-1 levels. Mol Cell Biol. 2004;24:4979–4993. doi: 10.1128/MCB.24.11.4979-4993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann KM. West RA. Hildebrand D, et al. T Cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem. 2007;282:25152–25158. doi: 10.1074/jbc.M703243200. [DOI] [PubMed] [Google Scholar]
- Gavert N. Sheffer M. Raveh S, et al. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res. 2007;67:7703–7712. doi: 10.1158/0008-5472.CAN-07-0991. [DOI] [PubMed] [Google Scholar]
- Geneste O. Copeland JW. Treisman R. LIM kinase and diaphanous cooperate to regulate serum response factor and actin dynamics. J Cell Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. Giancotti FG. Integrin signaling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Harpaz N. Talbot IC. Colorectal cancer in idiopathic inflammatory bowel disease. Semin Diagn Pathol. 1996;13:339–357. [PubMed] [Google Scholar]
- Hashimoto Y. Parsons M. Adams JC. Dual actin-bundling and protein kinase C-binding activities of fascin regulate carcinoma cell migration downstream of Rac and contribute to metastasis. Mol Biol Cell. 2007;18:4591–4602. doi: 10.1091/mbc.E07-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatjiharissi E. Ngo H. Leontovich AA, et al. Proteomic analysis of Waldenstrom macroglobulinemia. Cancer Res. 2007;67:3777–3784. doi: 10.1158/0008-5472.CAN-06-3089. [DOI] [PubMed] [Google Scholar]
- Hernandez SE. Krishnaswami M. Miller AL. Koleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 2004;14:36–44. doi: 10.1016/j.tcb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hirao N. Sato S. Gotoh T, et al. NESH (Abi-3) is present in the Abi/WAVE complex but does not promote c-Abl-mediated phosphorylation. FEBS Lett. 2006;580:6464–6470. doi: 10.1016/j.febslet.2006.10.065. [DOI] [PubMed] [Google Scholar]
- Juang JL. Hoffmann FM. Drosophila Abelson interacting protein (dAbi) is a positive regulator of Abelson tyrosine kinase activity. Oncogene. 1999;18:5138–5147. doi: 10.1038/sj.onc.1202911. [DOI] [PubMed] [Google Scholar]
- Katoh Y. Katoh M. Hedgehog signaling pathway and gastric cancer. Cancer Biol Ther. 2005;4:1050–1054. doi: 10.4161/cbt.4.10.2184. [DOI] [PubMed] [Google Scholar]
- Leedham SJ. Graham TA. Oukrif D, et al. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136:542–550. doi: 10.1053/j.gastro.2008.10.086. .e6. [DOI] [PubMed] [Google Scholar]
- Li F. Higgs HN. Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J Biol Chem. 2005;280:6986–6992. doi: 10.1074/jbc.M411605200. [DOI] [PubMed] [Google Scholar]
- Li Y. Grenklo S. Higgins T. Karlsson R. The profilin:actin complex localizes to sites of dynamic actin polymerization at the leading edge of migrating cells and pathogen-induced actin tails. Eur J Cell Biol. 2008;87:893–904. doi: 10.1016/j.ejcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Lin TY. Huang CH. Chou WG. Juang JL. Abi enhances Abl-mediated CDC2 phosphorylation and inactivation. J Biomed Sci. 2004;11:902–910. doi: 10.1007/BF02254375. [DOI] [PubMed] [Google Scholar]
- Mammoto A. Huang S. Moore K, et al. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- Parsons M. Adams JC. Rac regulates the interaction of fascin with protein kinase C in cell migration. J Cell Sci. 2008;121(Pt 17):2805–2813. doi: 10.1242/jcs.022509. [DOI] [PubMed] [Google Scholar]
- Peng J. Wallar BJ. Flanders A, et al. Disruption of the diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr Biol. 2003;13:534–545. doi: 10.1016/s0960-9822(03)00170-2. [DOI] [PubMed] [Google Scholar]
- Riddell RH. Goldman H. Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Russell JS. Lang FF. Huet T, et al. Radiosensitization of human tumor cell lines induced by the adenovirus- mediated expression of an anti-Ras single-chain antibody fragment. Cancer Res. 1999;59:5239–5244. [PubMed] [Google Scholar]
- Staus DP. Blaker AL. Taylor JM. Mack CP. Diaphanous 1 and 2 regulate smooth muscle cell differentiation by activating the myocardin-related transcription factors. Arterioscler Thromb Vasc Biol. 2007;27:478–486. doi: 10.1161/01.ATV.0000255559.77687.c1. [DOI] [PubMed] [Google Scholar]
- Stradal T. Courtney KD. Rottner K, et al. The Abl interactor proteins localize to sites of actin polymerization at the tips of lamellipodia and filopodia. Curr Biol. 2001;11:891–895. doi: 10.1016/s0960-9822(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Tani K. Sato S. Sukezane T, et al. Abl interactor 1 promotes tyrosine 296 phosphorylation of mammalian enabled (Mena) by c-Abl kinase. J Biol Chem. 2001;278:21685–21692. doi: 10.1074/jbc.M301447200. [DOI] [PubMed] [Google Scholar]
- Thomas T. Abrams KA. Robinson RJ. Mayberry JF. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther. 2007;25:657–668. doi: 10.1111/j.1365-2036.2007.03241.x. [DOI] [PubMed] [Google Scholar]
- Tominaga T. Meng W. Togashi K, et al. mDia inhibits the DNA-binding ability of Pax6 and changes the pattern of neurite extension in cerebellar granule cells through its binding to Pax6. J Biol Chem. 2002;277:47686–47691. doi: 10.1074/jbc.M207539200. [DOI] [PubMed] [Google Scholar]
- Tsai WC. Chao YC. Sheu LF, et al. Overexpression of fascin-1 in advanced colorectal adenocarcinoma: tissue microarray analysis of immunostaining scores with clinicopathological parameters. Dis Markers. 2007;23:153–160. doi: 10.1155/2007/685163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignjevic D. Schoumacher M. Gavert N, et al. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- Wen YH. Yee H. Goswami S. Shukla PS. Fascin expression in serous tumors of ovary correlates with aggressiveness of malignancy. Int J Gynecol Pathol. 2009;28:187–192. doi: 10.1097/PGP.0b013e318183cfde. [DOI] [PubMed] [Google Scholar]
- Winther KV. Jess T. Langholz E, et al. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–1095. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- Yamana N. Arakawa Y. Nishino T, et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol. 2006;26:6844–6858. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]