Abstract
Study Objectives:
Many patients with obstructive sleep apnea (OSA) are obese, and whether obesity itself explains the increased prevalence of cardiovascular disease in OSA is unknown.
We hypothesize that OSA, independent of obesity, contributes to abnormal vascular function.
Design:
Physiology study.
Setting:
Academic medical centers.
Patients:
Obese subjects, free of known comorbidities, were enrolled.
Measurements and Results:
Vascular function was assessed with brachial artery ultrasound for flow-mediated dilation (FMD) and in skin microcirculation by laser Doppler flowmetry. Arterial stiffness was measured by arterial tonometry. Seventy-two subjects (43/72 women, 38/72 with OSA) were studied. FMD was impaired in patients with OSA, compared with control subjects (5.7% ± 3.8% vs 8.3% ± 4.1%, P = 0.005). In step-forward regression analysis inclusive of age, sex, and body mass index, age (P = 0.013) was a significant independent predictor of FMD. In a subgroup of subjects younger than 50 years of age (n = 59), however, OSA was the only independent predictor of FMD (P = 0.04), adjusted for known covariates. OSA did not significantly influence vascular function in the skin microcirculation. The augmentation index, a measure of arterial stiffness, was similar between the OSA and control groups (16.2% ± 11.4% vs 20.4% ± 10.1%, respectively, P = 0.10). In step-forward regression analysis of younger men (≤ 50 years old, 23 subjects), OSA independently predicted the augmentation index in men only (P = 0.001).
Conclusions:
In obesity, both OSA and aging impair endothelial function and increase arterial stiffness. The influence of OSA on vascular function is most pronounced in young subjects. OSA, therefore, may be associated with functional impairment (“a premature aging effect”) on the endothelium and on arterial stiffness (in men), although skin microcirculatory function appears preserved.
Citation:
Yim-Yeh S; Rahangdale S; Nguyen ATD; Jordan AS; Novack V; Veves A; Malhotra A. Obstructive sleep apnea and aging effects on macrovascular and microcirculatory function. SLEEP 2010;33(9):1177-1183.
Keywords: Sleep, lung, breathing, vascular, apnea, airway, hypoxemia
WITH THE OBESITY EPIDEMIC, THE PREVALENCE OF OBSTRUCTIVE SLEEP APNEA (OSA) IS ALMOST CERTAINLY ON THE RISE.1 MANY HAVE THEORIZED THAT repetitive hypoxemia and sympathetic surges, which characterize OSA, can lead to oxidative stress, systemic inflammation, endothelial dysfunction, and, ultimately, cardiovascular disease.2–4 OSA causes hypertension5,6 and has been associated with other cardiovascular sequelae, such as stroke,7 ischemic heart disease, heart failure,8 and arrhythmias.9
Physiologic studies of OSA and cardiovascular disease have been of predominantly male populations with comorbid conditions who present to sleep clinics. Often, data from patients with OSA from sleep clinics are matched with those obese control subjects from the general population or from primary care clinics. Studies that include patients referred to sleep clinics with suspected OSA, however, may have a referral bias. We sought, therefore, to study the effect of OSA on an obese but healthy population recruited primarily from the community to assess OSA effects.
Because we wanted to assess subclinical effects of OSA in an obese population without comorbidities, we limited our study to correlates of cardiovascular endpoints, such as flow-mediated dilation (FMD), arterial stiffness, and skin microvascular reactivity. FMD is a measure of endothelial function derived by comparing arterial vessel dilation before and after hyperemic ischemia.10–14 Dysfunctional endothelium results in decreased arterial vessel dilation after hyperemic ischemia due to reduced endothelial nitric oxide production, likely a harbinger of atherosclerosis.15 Arterial stiffness is likely a composite measure of arterial elasticity, endothelial function, and sympathetic tone.16,17 Although important distinctions exist between various metrics of arterial stiffness (e.g., augmentation index [AIx] and pulse wave velocity),22,23 a number of studies have shown increases in OSA and improvements with treatment with continuous positive airway pressure.12,18,19,44 Finally, skin microvascular reactivity has been associated with poor wound healing and increased insulin resistance in diabetes, but the effect of OSA is unknown.24–26
We hypothesized that OSA would have an effect in this obese population on both macrovascular and microvascular reactivity and arterial stiffness (as estimated by the AIx). Furthermore, because age is a strong predictor of both endothelial function and arterial stiffness, we predefined a subgroup of younger subjects (< 50 years) for further analyses. We theorized that the effect of OSA on vascular function may be modulated by age and that such influences may be differentially expressed among our vascular measurements.27 As a result, we speculated that the effects of OSA on vascular function would be most pronounced in younger participants.
RESEARCH DESIGN AND METHODS
Subjects
Individuals with a body mass index (BMI) of at least 30 kg/m2 were recruited mainly from the community. Subjects were required to be aged 18 to 70 years, obese, nonsmokers, and without known cardiopulmonary, endocrine, or sleep disorders (other than OSA). Subjects were not eligible if they were taking any medications that could affect cardiovascular function or sleep. Of the 342 subjects who were interviewed by phone to determine eligibility, 103 were eligible for participation in the study. A licensed physician screened all subjects, including measurement of fasting blood glucose concentration, complete blood counts, thyroid stimulating hormone concentration, lipid panels, and blood pressure. Three patients with treated hypertension were included (2 OSA, 1 control). Out of 103 subjects, 15 subjects with abnormal findings on physical examination or laboratory values were excluded. Fourteen subjects withdrew consent or declined follow-up. Two subjects were withdrawn due to minor adverse events during testing. Prior to the study night, all subjects were asked to keep a 2-week sleep diary to exclude sleep deprivation. All subjects provided written informed consent. The study was approved by our ethics panels.
Experimental Protocol
Subjects arrived at the laboratory at 20:00 and underwent a standard in-laboratory overnight polysomnogram from 22:00 to 06:00. Height, weight, and blood pressure were taken upon arrival by a clinical research center nurse. At 06:00, the subjects were awakened. Subjects remained fasting from 22:00 to 11:00 the next morning and refrained from any important physical activity.
Polysomnogram
Recorded signals included electroencephalogram (C4-A1, C3-A2, O2-A1, and O1-A2), left and right electrooculogram, submental and bilateral tibial electromyogram, surface electrocardiogram, airflow, chest and abdominal excursion (piezo bands), oxyhemoglobin saturation, and body position. Polysomnograms were scored by an experienced sleep technologist according to standard criteria28; the technologist was not aware of the group status of the subjects. An apnea was scored if airflow was absent for 10 seconds, and a hypopnea was scored if there was at least a 50% reduction in airflow for 10 seconds or a detectable decrement in airflow for 10 seconds in association with a either an oxyhemoglobin desaturation of at least 3% or an arousal.
Laboratory Measurements
Blood samples were obtained using standard sterile technique from the antecubital vein. Plasma glucose, total serum cholesterol, and triglyceride concentrations were measured using the Synchoron CX analyzer (Beckman Systems, Fullerton, CA). High-density lipoprotein and serum cholesterol were measured directly (Sigma, St. Louis MO), and low-density lipoprotein was calculated. Glycated hemoglobin (HbA1c) was measured in whole blood with ion-exchange high-performance liquid chromatography. Complete blood count, thyroid stimulating hormone, and fasting glucose concentrations were measured in a core laboratory using standard laboratory techniques.
Vascular Reactivity Measurements
All measurements of vascular reactivity were made during the morning fast. All studies were performed in temperature-controlled rooms (24°-26°C). The vascular reactivity of the skin microcirculation was measured using laser Doppler flowmetry before and after the iontophoresis of acetylcholine (ACh, endothelium-dependent vasodilation) and sodium nitroprusside (SNp, endothelium-independent vasodilation). All measurements were taken from the ventral surface of the forearm. The reproducibility of our technique has been previously described.29
Vascular reactivity of the macrocirculation was measured in the brachial artery using a high-resolution ultrasound with a 10.0-MHz linear array transducer and an HDI Ultramark 9 system (Advanced Technology Laboratories, Signal Hill, CA). To measure endothelial-dependent vasodilation, the brachial-artery diameter was measured before and after flow-mediated dilation (FMD) during reactive hyperemia according to published guidelines.30 Reactive hyperemia was accomplished by inflating a pneumatic tourniquet distal to the brachial artery to 50 mm Hg above systolic blood pressure for 5 minutes followed by deflation.
To measure endothelial-independent vasodilation, the brachial-artery width was measured before and after administration of sublingual nitroglycerin (400 μg). All ultrasound images were analyzed by experienced personnel, blinded to other study results.
AIx Measurements
After removal of overnight monitoring equipment, the subjects were brought to a quiet temperature-controlled room. Blood pressure was measured manually with the subject seated. Arterial stiffness was measured in the opposite arm by trained investigators using a hand-held tonometer probe (Millar Instruments, Houston, TX) to measure peripheral pulse wave. The probe was placed over the radial artery in a nonocclusive manner. The peripheral pulse wave was analyzed using the SphygmoCor system (AtCor Medical, West Ryde, NSW, Australia), which generated an average peripheral pulse-wave contour over a 10-second period. The quality of the pulse waves were assessed visually and with the SphygmoCor system, which has a built-in quality index score. The 2 measurements that had a quality index of greater than 90% with a signal strength of more than 500 units were averaged and included in the analysis. Values were normalized to a heart rate of 75 beats per minute by the SphygmoCor system.
Statistical Analysis
Data are represented as means ± SD for normally distributed data (based on Shapiro-Wilk test) or medians with interquartile ranges for nonnormally distributed data. For univariate analysis, we used the t test for comparison of continuous variables and the Pearson χ2 test for categorical variables. The Wilcoxon rank sum test was used for nonparametrically distributed data.
To determine predictors of outcome measures (percentage of FMD and AIx), a step-forward multiple linear regression model with a stay criterion of 0.10 was built. Initially, variables associated with cardiovascular morbidity such as sex, age, and BMI, were included into the model. Subsequently, an AHI of 10 or more events per hour was forced into the model. To assess the association between an AHI of 10 or more events per hour in the subgroup of subjects who had a low risk of having cardiovascular morbidity, a predefined subgroup analysis in patients younger than 50 years was performed.
All reported P values are 2 sided, and a P value of less than 0.05 was considered significant.
RESULTS
Characteristics of Study Participants
The AHI ranged from 0 to 122.3 per hour across all study participants. OSA was defined as having an AHI of at least 10 per hour. Of the 53% subjects with OSA who met this criterion (38 subjects), the median AHI was 23.3 per hour, indicative of moderate disease severity. Baseline characteristics of OSA and control subjects (Table 1) were similar for most parameters, although subjects with OSA tended to be men, be older, have a higher waist-to-hip ratio, and have a somewhat higher blood pressure, compared with the non-OSA group.
Table 1.
Characteristics of participant
| Control Subjects without OSA (n = 34) | Patients with OSA (n = 38) | P Value | |
|---|---|---|---|
| Men, no (%) | 9 (26) | 20 (53) | 0.02 |
| AHI, events/h | 2.71 (1.29, 5.90) | 23.27 (15.81, 39.97) | < 0.001 |
| Age, y | 32.2 ± 11.6 | 44.1 ± 10.2 | < 0.001 |
| BMI, kg/m2 | 37.5 (33.4, 42.9) | 37.3 (32.6 45.3) | 0.6 |
| Waist:hip ratio | 0.85 ± 0.09 | 0.94 ± 0.09 | 0.0007 |
| Total cholesterol, mg/dL | 187 ± 33 | 197 ± 35 | 0.2 |
| LDL, mg/dL | 110.2 ± 28.5 | 115.5 ± 27.8 | 0.4 |
| HDL, mg/dL | 56.2 ± 14.9 | 51,41 ± 12.4 | 0.1 |
| Fasting glucose, mg/dL | 79 ± 12 | 83 ± 15 | 0.2 |
| HbA1c, % | 5.4 ± 0.4 | 5.7 ± 0.4 | 0.02 |
| ESS Score | 6 (3, 10) | 6 ± (4,14) | 0.6 |
| TST on night of study, min | 355 ± 68 | 337 ± 73 | 0.7 |
| Blood pressure, mm Hg | |||
| Systolic | 123 ± 11 | 131 ± 13 | 0.008 |
| Diastolic | 70 ± 8 | 77 ± 10 | 0.002 |
| TSH, mIU/L | 1.9 ± 0.8 | 1.9 ± 0.9 | 0.98 |
For normally distributed data, mean ± SD are shown; for nonparametric distributed data, medians (25th, 75th percentiles) are represented.
OSA refers to obstructive sleep apnea; AHI, apnea hypopnea index; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HbA1c, glycosylated hemoglobin, type A1c; ESS, Epworth Sleepiness Scale; TST, total sleep time; TSH, thyroid stimulating hormone.
Flow-mediated Dilatation
Table 2 describes the differences in vascular reactivity and the AIx between the OSA and control groups. FMD was measured in 71 subjects. One subject did not complete the study protocol. Mean baseline arterial diameter was slightly higher in the OSA group. In response to ischemic hyperemia, FMD was impaired in the OSA group, compared with the control group. In a step-forward linear regression model including an AHI of 10 or more events per hour, age, and BMI, age was the only predictor of percentage of change in FMD (P = 0.013).
Table 2.
Differences in vascular reactivity between the OSA and control groups
| Control Subjects without OSA (n = 34) | Patients with OSA (n = 38) | P Value | |
|---|---|---|---|
| Baseline brachial artery diameter, mm | 3.13 ± 0.63 | 3.49 ± 0.78 | 0.038 |
| % FMD | 8.3 ± 4.1 | 5.7 ± 3.8 | 0.005 |
| % NID | 17.79 ± 7.05 | 18.51 ± 6.73 | 0.66 |
| % Ach | 70.73 (46.03,107.07) | 59.77 (38.21,86.19) | 0.19 |
| % SNp | 65.75 (45.46, 88.03) | 57.14 (27.24,78.14) | 0.27 |
| AIx | 20.4 ± 10.1 | 16.2 ± 11.4 | 0.1 |
For normally distributed data, mean ± SD are shown; for nonparametric distributed data, medians (25th, 75th percentiles) are represented.
OSA refers to obstructive sleep apnea; %FMD, percentage change in brachial artery diameter after flow-mediated dilation; %NID, percentage change in brachial artery diameter after nitroglycerin-induced dilation; %Ach, percentage change in laser Doppler flowmetry of the skin microcirculation after iontophoresis of acetylcholine; %SNp, percentage change in laser Doppler flowmetry of the skin microcirculation after iontophoresis of sodium nitroprusside; AIx, augmentation index.
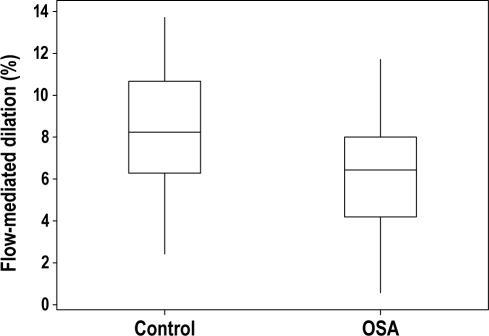
In analyses of subjects younger than 50 years of age (n = 59), the percentage of change in FMD remained impaired in the OSA group, compared with the control group (Figure 1, P = 0.009). In a step-forward linear regression model including OSA status, age, and BMI, only OSA status predicted the percentage of change in FMD (P = 0.04). For both multivariate analyses (entire group and age < 50 y), we reran these regressions including HbA1c, cholesterol, and systolic blood pressure as potential independent variables and found essentially identical results. There was no difference in FMD between control and OSA subjects older than 50 years of age (n = 13, 3.8% ± 7.0% vs 3.6% ± 4.6%; P = 0.96).
Figure 1.
Endothelial function, as measured by flow-mediated dilation of the brachial artery, is impaired in subjects with obstructive sleep apnea (OSA), as compared with control subjects, in a subgroup of subjects younger than 50 years of age (n = 59). The box encompasses the 25%-75% quartiles, and the median is represented by the horizontal line within the box. The whiskers extend to the highest and lowest values within the higher and lower limits, respectively.
There was no difference between the OSA and control groups in percentage of change of arterial diameter after systemic nitroglycerin otherwise known as nitroglycerin-induced dilation (Table 2).
There was also no difference in percentage of change in arterial diameter after FMD or after nitroglycerin-induced dilation between men and women with OSA (Table 3). There was no difference in percentage of change in FMD between the premenopausal and postmenopausal (n = 7) women (8.05% [ 5.2, 10.3] vs 7.39% [0.8, 9.4]; P = 0.55).
Table 3.
Differences in characteristics and vascular reactivity between men and women with OSAa
| Men (n = 20) | Women (n = 18) | P Value | |
|---|---|---|---|
| AHI, events/h | 25.0 (15.1, 44.5) | 21.5 (15.8, 122.3) | 0.75 |
| Age, y | 43.5 ± 10.5 | 44.7 ± 10.2 | 0.73 |
| BMI, kg/m2 | 33.3 (31.0, 37.6) | 41.7 (37.4, 47.3) | 0.003 |
| Waist-to-hip ratio | 0.99 ± 0.07 | 0.88 ± 0.07 | < 0.001 |
| %FMD | 5.7 ± 3.9 | 5.8 ± 3.7 | 0.91 |
| %NID | 17.4 ± 5.4 | 19.8 ± 8.0 | 0.27 |
| %Ach | 50.0 (33.3, 81.9) | 71.3 (38.8, 89.8) | 0.22 |
| %SNp | 39.3 (21.1, 77.3) | 69.7 (43.7, 90.2) | 0.035 |
| AIx (%) | 14.4 ± 8.2 | 27.1 ± 7.5 | < 0.001 |
Obstructive sleep apnea (OSA) was defined as an apnea-hypopnea index (AHI) ≥ 10 events/h.
For normally distributed data, mean ± SD are shown; for nonparametric distributed data, medians (25th, 75th percentiles) are represented. The augmentation index (AIx) is corrected to 75 bpm.
%FMD refers to the percentage change in brachial artery diameter after flow-mediated dilation; %NID, percentage change in brachial artery diameter after nitroglycerin-induced dilation; %Ach, percentage change in laser Doppler flowmetry of the skin microcirculation after iontophoresis of acetylcholine; %SNp, percentage change in laser Doppler flowmetry of the skin microcirculation after iontophoresis of sodium nitroprusside.
Endothelial Function in the Skin Microcirculation
Endothelial function of the skin microcirculation was measured with laser Doppler flowmetry before and after iontophoresis with acetylcholine and sodium nitroprusside. There was no difference in percentage of change of laser Doppler flowmetry after acetylcholine or sodium nitroprusside in the skin microcirculation between the OSA and control groups (Table 2). In a multiple linear regression model that included OSA status, age, sex, and BMI, no covariate predicted percentage of change in laser Doppler flowmetry after iontophoresis of acetylcholine or sodium nitroprusside.
Augmentation Index
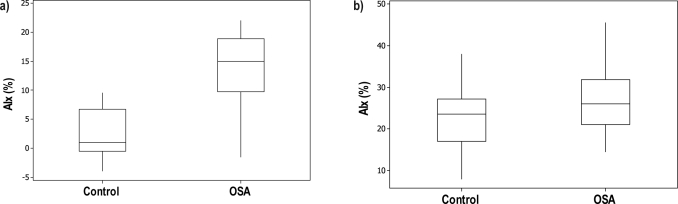
Arterial stiffness, as measured by AIx, was similar between the OSA and control groups (Table 2). However, there was a large difference in AIx between men and women when all subjects were analyzed (10.7 ± 9.0 vs. 23.7 ± 8.6, respectively P < 0.001) and when only subjects with OSA were analyzed (Table 3). Because women had higher baseline AIx values than men, we proceeded with a subgroup analysis by sex. When men were analyzed, there was a difference in AIx between subjects with OSA and without OSA (Figure 2a, P < 0.001). In a step-forward regression analysis that included OSA status, age, and BMI, OSA status (P < 0.001) and BMI (P < 0.039) predicted AIx. In a subset analysis of men aged 50 years and younger (n = 23), step-forward regression analysis showed that OSA status was the only predictor of AIx (P = 0.001). When data from only women were analyzed, there was no difference in AIx between women with OSA and without OSA (Figure 2b, P = 0.03). In a step-forward regression analysis that included OSA status, age, and BMI, age was the only predictor of AIx (P = 0.001). In a subset analysis of women aged 50 years and younger (n = 35), age still remained the only predictor of AIx (P = 0.001).
Figure 2.
Arterial stiffness, as measured by augmentation index (AIx), was higher in patients with obstructive sleep apnea (OSA), compared with control subjects, among men (Figure 2a, n = 29) and women (Figure 2b, n = 43). Differences between men with OSA and male control subjects were greater (Figure 2a) than the differences between women with OSA versus female control subjects (Figure 2b). The AIx was correct to a heart rate of 75 bpm for all subjects. The box encompasses the 25%-75% quartiles, and the median is represented by the horizontal line within the box. The whiskers extend to the highest and lowest values within the higher and lower limits respectively.
DISCUSSION
The present study had several findings. First, OSA is prevalent in a sample of obese, healthy, community-dwelling adults. Fifty-three percent of the subjects studied were diagnosed with OSA (AHI ≥ 10/h) during the course of the study, albeit they had mild disease. Second, within this obese but healthy cohort, aging predicted impaired FMD and increased arterial stiffness (the latter in men only) but OSA did not. Finally, the negative effect of OSA on vascular health is most prominent in younger (≤ 50 years) subjects. OSA, therefore, may theoretically have a premature aging effect on the vasculature in obesity.
Patients with OSA have a high prevalence of cardiovascular disease.31 However, many patients with OSA are obese, and whether obesity itself totally explains the increased prevalence is unknown.32 We sought to clarify the relationships among OSA, obesity, and vascular function by studying an obese but healthy cohort of individuals, recruited mainly from the community. Despite minimal symptoms (Epworth Sleepiness Scale score = 6/24), the prevalence of OSA was high. Fifty-three percent of our subjects, without a history of cardiovascular disease, had OSA. Given the obesity epidemic, the high prevalence of OSA in our obese but healthy cohort suggests that OSA may still be largely undiagnosed in the community.
One common way to assess cardiovascular risk in subjects with OSA has been to measure brachial artery dilation after FMD. OSA has been associated with impaired FMD in some but not all previous studies.33–35 Although our OSA group showed impaired FMD, compared with the control subjects, this association was influenced by older age. Multivariate modeling demonstrated that only age and not BMI or OSA status was an independent predictor of FMD. However, among younger participants (age ≤ 50 y), we found that FMD was predicted by OSA and not age or BMI. With increased age, all subjects have impaired endothelial function regardless of OSA status. OSA, therefore, may be associated with functional impairment (“a premature aging effect”) of the endothelium.
Differences between our finding—that FMD was predicted by age and not OSA status—and the findings of other studies may be due to multiple factors. First, previous studies rarely included many women, which may explain differences in our study results. Our female subjects with OSA had a higher BMI but lower waist-to-hip ratio than did male subjects with OSA. Women, in general, have been shown to be less susceptible to OSA36 and may have to be more obese to overcome the “protective” effect of being female. Second, the BMI for our cohort by design is higher than that used in previous studies. Obesity may blunt the effect of OSA on the endothelium because obesity alone can contribute to endothelial dysfunction.37 Prior studies examining obesity effects on the vasculature have largely overlooked OSA, making the independent effect of obesity on FMD unclear based on the prior literature. Finally, the exclusion of known cardiovascular comorbidities resulted in an obese “super-healthy” cohort, which may have decreased the effect of OSA on endothelial function. Previous studies have included other comorbid conditions, such as cigarette smoking and hypertension, making our findings an important advance.12,34
Our findings of FMD impairment in younger patients with OSA are consistent with the findings of Chung et al.,38 who showed that lowest O2 saturation predicted FMD impairment in young but not older (age > 60 y) subjects with OSA. In other words, the effect of OSA on the endothelium appears to be most apparent in younger populations. Older populations may also be adversely affected by OSA, but larger samples may be required to see such effects. For instance, the Sleep Health Heart Study of 1037 subjects older than 68 years of age showed worsening FMD with increasing severity of AHI.34 The differences between groups were small, however, with an absolute difference in the means of 0.3% between FMD of the lowest and highest AHI quintiles. In our analyses of younger subjects, the difference in median of percentage of change in FMD between the OSA and control groups was 1.82%. This finding supports the concept that younger subjects may be more susceptible to the effect of OSA on the endothelium. The mechanisms underlying this aging effect require further study but might include survivor effects, reductions in catecholamine receptor density, and ceiling effects of vascular risk factors.
We also assessed endothelial function in the skin microcirculation, which, to our knowledge, has not been attempted before in OSA. We did not find a significant effect of OSA on the skin microcirculation. Despite using a fairly large sample size for a physiologic study, we may have missed minor effects based on a nonsignificant trend toward decreased endothelial-dependent vasodilation of the skin microcirculation in OSA (P = 0.19). There was a difference in endothelial-independent vasodilation between men and women with OSA; however, it is not clear what clinical relevance this finding has. Thus, further research on the microcirculation in OSA is required.
Arterial stiffness (as estimated by AIx) is likely a composite measure of arterial elasticity, endothelial function, and sympathetic tone. In theory, the AIx is an estimate of arterial elasticity in the central aorta, but the results correlate poorly with other metrics of vessel stiffness, such as pulse wave velocity.22,23 AIx is influenced not only by the compliance of the less muscular aorta (which influences the velocity of reflected waves from the lower body), but likely also by changes in the tone of smaller more muscular peripheral arteries (which influences the amplitude of reflected waves). These latter changes in tone may, in turn, be influenced by sympathetic tone and endothelial function. AIx has been shown to be an independent predictor of cardiovascular events in people with hypertension, people with diabetes, and in the general population.17,21,39,40 Furthermore, the results of the AIx have been negatively correlated with endothelial function as measured by FMD.41 Whether arterial stiffness is a modifiable risk factor is debated. Aspects of arterial stiffness, such as changes in arterial wall elastic lamina, are irreversible with age. However, smooth muscle function as mediated by the endothelium may be reversible with improved cardiovascular health. For instance, in a study of end-stage renal disease, patients on hemodialysis showed a decrease in AIx after hemodialysis, which was associated with a decrease in endogenous asymmetric dimethylarginine levels, a known inhibitor of endothelial nitric oxide synthase.42 Although arterial stiffness has been shown to be increased in OSA,43–45 many prior reports largely examined the effect of OSA in men and in some cases excluded women altogether. In our cohort, AIx was higher in women than in men, independent of OSA status. This observation, that women have systematically higher AIx than men, has been reported before in large cohort studies,22,23,46 although normative data in obese populations are sparse. Women are generally shorter than men, which decreases the distance between the heart and sites of wave reflection and may increase AIx as a result. However, even when normalized for height, women have higher AIx than do men. Moreover, height is a poor predictor of AIx in several cohorts, including our own. Further research is clearly required regarding why obese women have higher AIx than obese men.
AIx increases with age, but the relationship is nonlinear. AIx has also been shown to increase more in younger individuals (≤ 50 y), compared with older individuals, and may therefore be a more sensitive measure of arterial stiffness in younger groups.46 In our cohort, we found that OSA was associated with increased AIx and that this relationship was strongest for men younger than 50 years of age. In women, however, age was the only significant predictor of increased Aix, even in younger subjects. Younger women may be less susceptible to this vascular effect of OSA than are younger men. Sex, therefore, may need to be more carefully analyzed in studies of AIx and OSA.
Although there were differences in baseline characteristics between our control and OSA groups, we argue that our cohort is representative of an obese, community-sampled population that was otherwise free of known cardiovascular risk factors. This group was at high risk for having OSA. Differences that we found in age, sex, and BMI are reflective of a “real-world” population. However, because the effect of age on endothelial function predominates, we may have missed minor abnormalities in our older participants; however, very large sample sizes are not logistically feasible in physiologic studies. The study was powered to detect only major differences in vascular reactivity of the skin microcirculation, which likely warrants further investigation. We, however, have probably defined important effects by studying a reasonably large, well-characterized, and carefully controlled sample. We acknowledge that our cohort consisted of subjects with primarily mild apnea, and, thus, the results may be quite different in patients with severe apnea, who are commonly referred to sleep clinics.
In conclusion, we have found that, in a cohort of obese but healthy individuals, OSA is highly prevalent. Furthermore, we found that the effect of OSA on vascular function is modulated by age. In young populations, OSA has a major effect on FMD and AIx (the latter in men only). OSA, therefore, may be associated with a premature aging effect on the endothelium. The cardiovascular risk of a young obese patient with OSA may be higher than previously thought. These findings warrant further mechanistic research and may help to explain the heterogeneous results seen in observational cohort studies.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Malhotra has received consulting and/or research income from Philips, Apnex, NMT, Pfizer, Cephalon, Sepracor, SGS, SHC, Itamar, Ethicon, and Medtronic. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
All authors participated in the design of the research, data acquisition, and manuscript preparation.
AM has received funding from NIH K24 HL 093218, R01 HL090897, R01 HL085188 and R01 HL73146 as well as an Established Investigator Award of the American Heart Association. SY is the recipient of the National Sleep Foundation's Pickwick Fellowship. SR is supported by a grant from the American Sleep Medicine Foundation. AV is funded by R01-HL075678, R01 DK076937, R01 DK076937. The Harvard Catalyst is funded by UL1 RR 025758-01.
ABBREVIATIONS
- AHI
apnea hypopnea index
- BMI
body mass index
- FMD
flow-mediated dilation
- OSA
obstructive sleep apnea
- RDI
respiratory disturbance index
REFERENCES
- 1.Young T, Peppard P, Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra A, Loscalzo J. Sleep and cardiovascular disease: an overview. Progress in cardiovascular diseases. 2009;51:279–84. doi: 10.1016/j.pcad.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavie L. Oxidative stress--a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–12. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177:369–75. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. The Journal of clinical investigation. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 8.Naughton MT, Lorenzi-Filho G. Sleep in heart failure. Progress in cardiovascular diseases. 2009;51:339–49. doi: 10.1016/j.pcad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–55. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grebe M, Eisele HJ, Weissmann N, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 11.Köhler D, Schönhofer B. How important is the differentiation between apnea and hypopnea? Respiration. 1997;64(Suppl 1):15–21. doi: 10.1159/000196731. [DOI] [PubMed] [Google Scholar]
- 12.Köhler M, Craig S, Nicoll D, Leeson P, Davies RJ, Stradling JR. Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. Am J Respir Crit Care Med. 2008;178:984–8. doi: 10.1164/rccm.200805-717OC. [DOI] [PubMed] [Google Scholar]
- 13.Köhler M, Lushington K, Couper R, et al. Obesity and risk of sleep related upper airway obstruction in Caucasian children. J Clin Sleep Med. 2008;4:129–36. [PMC free article] [PubMed] [Google Scholar]
- 14.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 15.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton PK, Lockhart CJ, Quinn CE, McVeigh GE. Arterial stiffness: clinical relevance, measurement and treatment. Clin Sci (Lond) 2007;113:157–70. doi: 10.1042/CS20070080. [DOI] [PubMed] [Google Scholar]
- 17.Weber T, Auer J, O'Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–9. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 18.Phillips C, Hedner J, Berend N, Grunstein R. Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep. 2005;28:604–9. doi: 10.1093/sleep/28.5.604. [DOI] [PubMed] [Google Scholar]
- 19.Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16:217–25. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 20.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–5. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 21.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 22.Yasmin, Falzone R, Brown MJ. Determinants of arterial stiffness in offspring of families with essential hypertension. Am J Hypertens. 2004;17:292–8. doi: 10.1016/j.amjhyper.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. Q J Med. 1999;92:595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 24.Arora S, Pomposelli F, LoGerfo FW, Veves A. Cutaneous microcirculation in the neuropathic diabetic foot improves significantly but not completely after successful lower extremity revascularization. J Vasc Surg. 2002;35:501–5. doi: 10.1067/mva.2002.121126. [DOI] [PubMed] [Google Scholar]
- 25.Arora S, Veves A, Caballaro AE, Smakowski P, LoGerfo FW. Estrogen improves endothelial function. J Vasc Surg. 1998;27:1141–6. doi: 10.1016/s0741-5214(98)70016-3. discussion 7. [DOI] [PubMed] [Google Scholar]
- 26.Greenman RL, Panasyuk S, Wang X, et al. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet. 2005;366:1711–7. doi: 10.1016/S0140-6736(05)67696-9. [DOI] [PubMed] [Google Scholar]
- 27.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;68:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in adults. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 29.Veves A, Saouaf R, Donaghue VM, et al. Aerobic exercise capacity remains normal despite impaired endothelial function in the micro- and macrocirculation of physically active IDDM patients. Diabetes. 1997;46:1846–52. doi: 10.2337/diab.46.11.1846. [DOI] [PubMed] [Google Scholar]
- 30.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 31.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra A, Hillman D. Obesity and the lung: 3. Obesity, respiration and intensive care. Thorax. 2008;63:925–31. doi: 10.1136/thx.2007.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27:1113–20. doi: 10.1093/sleep/27.6.1113. [DOI] [PubMed] [Google Scholar]
- 34.Nieto F, Herrington D, Redline S, Benjamin E, Robbins J. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–60. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 35.Kato J, Isono S, Tanaka A, et al. Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest. 2000;117:1065–72. doi: 10.1378/chest.117.4.1065. [DOI] [PubMed] [Google Scholar]
- 36.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–6. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 37.Arkin JM, Alsdorf R, Bigornia S, et al. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. J Am Cardiol. 2008;101:98–101. doi: 10.1016/j.amjcard.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Ahn HJ. Endothelial dysfunction and inflammatory reactions of elderly and middle-aged men with obstructive sleep apnea syndrome. Sleep Breath. 2009;13:11–7. doi: 10.1007/s11325-008-0210-x. [DOI] [PubMed] [Google Scholar]
- 39.Mattace-Raso FU, van den Meiracker AH, Bos WJ, et al. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25:1421–6. doi: 10.1097/HJH.0b013e32811d6a07. [DOI] [PubMed] [Google Scholar]
- 40.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 41.Soga J, Nakamura S, Nishioka K, et al. Relationship between augmentation index and flow-mediated vasodilation in the brachial artery. Hypertens Res. 2008;31:1293–8. doi: 10.1291/hypres.31.1293. [DOI] [PubMed] [Google Scholar]
- 42.Soveri I, Lind L, Wikstrom B, Zilmer M, Zilmer K, Fellstrom B. Improvement in central arterial pressure waveform during hemodialysis is related to a reduction in asymmetric dimethylarginine (ADMA) levels. Nephron. 2007;106:c180–6. doi: 10.1159/000104429. [DOI] [PubMed] [Google Scholar]
- 43.Tanriverdi H, Evrengul H, Kara CO, et al. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: non-invasive indicators of atherosclerosis. Respiration. 2006;73:741–50. doi: 10.1159/000093531. [DOI] [PubMed] [Google Scholar]
- 44.Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379–86. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- 45.Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep. 2002;25:850–5. [PubMed] [Google Scholar]
- 46.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–60. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]